Abstract
We investigated relationships between tree-ring δ13C and growth, and flux tower estimates of gross primary productivity (GPP) at Harvard Forest from 1992 to 2010. Seasonal variations of derived photosynthetic isotope discrimination (Δ13C) and leaf intercellular CO2 concentration (ci ) showed significant increasing trends for the dominant deciduous and coniferous species. Δ13C was positively correlated to growing-season GPP and is primarily controlled by precipitation and soil moisture indicating that site conditions maintained high stomatal conductance under increasing atmospheric CO2 levels. Increasing Δ13C over the 1992–2010 period is attributed to increasing annual and summer water availability identified at Harvard Forest and across the region. Higher Δ13C is coincident with an enhancement in growth and ecosystem-level net carbon uptake. This work suggests that tree-ring δ13C could serve as a measure of forest GPP and be used to improve the calibration and predictive skill of ecosystem and carbon cycle models.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Our ability to project the future carbon cycle is limited by our lack of understanding of terrestrial carbon (C) cycle dynamics and the feedbacks that constrain C budgets. Projections of C-flux and C-sequestration from current coupled terrestrial carbon cycle models give widely divergent results (Friedlingstein et al 2006). The lack of agreement among projections is, in part, related to poorly constrained model parameters. Constraining carbon budget projections is an important issue given the current mitigation of anthropogenic emissions by terrestrial ecosystems sequestering C (Le Quéré et al 2009). Terrestrial ecosystems sequestered approximately 30% of anthropogenic emissions from 2000 to 2006, and there is interest in the potential to manage forest ecosystems to increase the strength of the carbon sink (Hurtt et al 2002, Canadell et al 2007, Woodbury et al 2007). Longer periods of observational data on C-cycle variability, in particular, would help constrain model parameters and improve model performance. CO2 flux measurements between ecosystems and the atmosphere from eddy covariance flux towers (Baldocchi 2003) are valuable for model evaluation (Suzuki and Ichii 2010, Richardson et al 2012, Schaefer et al 2012, Keenan et al 2012a, Raczka et al 2013), but they only measure CO2 flux conditions over short periods (maximum 20 years, most ⩽10 years) in a small footprint (1 km2) and the regional tower density is low (e.g., five sites in northeastern US forests with >10 years of data). Consequently, a variety of other types of measurements are needed to constrain C-cycle model parameters and to improve projections of interannual variability in C-cycle dynamics in terrestrial ecosystems (Pan et al 2006, Sims et al 2006, Randerson et al 2009, Babst et al 2013).
Annual measurements of forest carbon production can be obtained from tree rings that integrate the influence of climate and site factors on forest growth (Babst et al 2013). Attempts to associate tree-ring width with net ecosystem exchange (NEE) and gross primary productivity (GPP) measurements from flux towers have proven inconclusive (Rocha et al 2006). Integrals of annual growth over a few to several years have yielded similar net carbon fluxes as continuous flux tower measurements, but annual increment and flux tower measurements do not show strong inter-annual correlations (Barford et al 2001).
The composition of carbon isotopes (δ13C) of an annual tree ring records the proportions of assimilated carbon and is closely linked to photosynthetic capacity and stomatal regulation during tree growth (Farquhar et al 1989, Ogée et al 2009). Therefore, the distribution of stable carbon isotopes within a tree ring reflects the carbon assimilation and environmental conditions experienced by a tree through the growing season. The δ13C is widely used to study the linkage between environmental conditions and the physiological processes that control tree growth at inter- and intra-annual time resolutions (Loader et al 1995). Tree rings, particularly wood cellulose δ13C, contain valuable records of past climate, leaf-gas exchange, and carbon allocation within trees (e.g. Barbour et al 2002). Several studies used tree-ring δ13C to reconstruct the intrinsic water use efficiency (iWUE) of trees (e.g. Duquesnay et al 1998), and δ13C within a tree ring has been shown to track the physiological changes at the canopy level as recorded by the flux towers (Walcroft et al 1997, Michelot et al 2011). This approach has shown the potential to better understand the link between canopy-level physiology, tree-ring isotopic signature and climate drivers (Offermann et al 2011).
In this paper, we identify the relationships between interannual variability in tree-ring δ13C, tree-ring increment, flux tower measurements of CO2, and climate for a temperate forest in the northeastern United States. The period of analysis spans 18 years (1992–2010) and is from the longest available record of continuous flux tower measurements (Harvard Forest, Petersham, Massachusetts; Urbanski et al 2007). We analyzed the δ13C (of α-cellulose) from tree-ring latewood (LW) because it is less dependent on carbon stored during the previous growth year and therefore corresponds to the isotopic signal of recently assimilated C during the growing season. We conducted this analysis for one broad-leaf deciduous and one evergreen needle-leaf co-dominant species in the flux-tower footprint to contrast species response. Specifically, we: (1) identified the relationship between tree-ring δ13C, GPP, and tree growth, and, (2) assessed the responses of tree physiology and growth to rising atmospheric CO2 mole fraction and climate variation. We also discuss how changes in climate conditions and atmospheric CO2 concentration over the period of record may have modulated growth and forest productivity over the last 18 years.
2. Materials and methods
2.1. Study site and tree-ring chronologies
The forest within the flux-tower footprint (1 km2; Harvard Forest-EMS tower) is mainly deciduous and is dominated by Quercus. rubra (L.) (northern red oak; 52% of basal area), Acer rubrum L. (red maple; 22% of basal area) and Tsuga canadensis (L.) (eastern hemlock; 17% of basal area). Increment core sampling focused on canopy dominant trees. To identify interannual variability in tree growth and tree-ring δ13C, we cored Q. rubra and T. canadensis with a 5 mm increment borer at 1.37 m stem height (table 1). Two cores were taken from each tree to build ring-width chronologies. Additionally, we cored five and four trees respectively of each species at the same height with a 12 mm increment borer. The large cores were used to analyze the δ13C in the LW of the tree rings. A sample of four trees is sufficient to achieve good precision of the δ13C sample mean (McCarroll and Loader 2004, Leavitt 2008).
Table 1. Sample characteristics for Quercus rubra and Tsuga canadensis from the Harvard Forest site.
| Species | Quercus rubra | Tsuga canadensis |
|---|---|---|
| No. of dated cores | 42 (16 trees) | 55 (22 trees) |
| No. of isotopic cores | 5 (5 trees) | 4 (4 trees) |
| Mean intercorrelation RW | 0.67 | 0.61 |
| Mean intercorrelation δ13C | 0.71 | 0.67 |
| EPS RW (1992–2010) | 0.95 | 0.96 |
| EPS δ13C (1992–2010) | 0.85 | 0.86 |
Numbers of dated cores and trees for chronology and isotopic analyses. Mean intercorrelation for isotopic cores. Note that only one core per tree was sampled for isotopic analyses as the intra-tree variability is small in comparison with the inter-tree variability (Daux et al 2011).
Ring-width chronologies were developed for each species using standard dendrochronological techniques (Speer 2010). For the site, Q. rubra mean age was 97 years (range 71–115 years) and T. canadensis mean age was 145 years (range 89–221 years). For isotope cores, Q. rubra mean age was 103 years (range 87–113 years) and T. canadensis mean age was 142 years (range 93–188 years). All ages were estimated from inner ring dates.
Basal area increment (BAI) was calculated for each tree and species using the dplR package in R (Bunn 2008). We used the 'outside in' function to convert raw ring-width measurements to BAI based on the diameter of the tree and the width of each ring moving towards the pith of the tree. The method assumes a circular growth pattern. BAI was used instead of ring width as a surrogate of radial growth and carbon gain because it represents more accurately tree annual biomass increment without the need for standardization (Biondi and Qeadan 2008). A mean BAI value was computed for each species at an annual resolution, averaged over all the trees cored for both carbon isotope analyses and ring-width chronologies (i.e., 16 trees/42 cores for Q. rubra and 22 trees/55 cores for T. canadensis).
2.2. Stable istope analyses
We analyzed the LW portion of each tree ring from individual trees over the 1992–2010 period (table 1). The samples were milled using an ultra-centrifugation mill (Qiagen TissueLyserII) and α-cellulose was extracted from each wood sample following the Soxhlet method elaborated by Green (1963) and modified by Leavitt and Danzer (1993). δ13C ratios were measured on the CO2 produced by α-cellulose combustion in a Costech elemental analyzer coupled with a Thermo Delta V-IRMS. The sample accuracy was determined to be ±0.08‰ (1 σ standard deviation calculated from the average difference between measured and true internal standard, n = 13). The isotopic value is expressed in the delta (δ) notation relative to the VPDB (‰ VPDB).
2.3. Calculation of Δ, ci and iWUE
We used δ13C to determine carbon isotope discrimination (Δ) by the plant against atmospheric δ13C, and variation in plant iWUE. The Δ describes the isotopic difference between the δ13C of air (δ13Cair) and the plant (δ13Cplant) and results from the preferential use of 12C over 13C during photosynthesis. Δ is calculated using:
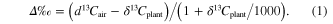
Records of δ13Cair were obtained from Mauna Loa from 1992 to 2002, and from aircraft measurements collected in Worcester, Massachusetts at 500 m above ground and available from 2003 to 2010 (White and Vaughn 2011). The model of Farquhar et al (1982) describes isotopic Δ for C3 plants via:
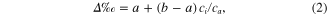
where a is the fractionation during CO2 diffusion through the stomata (4.4‰: O'Leary 1981); b is the fractionation by RuBP carboxylase (27‰: Farquhar and Richards 1984); and ci and ca , are the leaf intercellular space and ambient CO2 concentrations (μmol Mol−1), respectively. We used the data of atmospheric CO2 concentration (ca) measured at 29 m height on the eddy-covariance tower to calculate ci from equation (2). The fractionations due to diffusion and carboxylation are constant but additive; therefore, the δ13C records variations in ci as regulated by two main processes: stomatal conductance (ca/ci) and photosynthetic assimilation rate (A). The iWUE is the ratio of the net photosynthetic assimilation rate (A) and water vapor conductance (gH2O) and is described by Ehleringer and Cerling (1995) as:
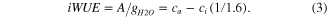
The iWUE derived from plant isotope data is used to compare photosynthetic properties independent from evaporative demand (Osmond et al 1980), and is therefore, often applied as an indicator of long-term trends in the internal regulation of carbon uptake and water loss of plants (Seibt et al 2008). We used summer (June, July and August) values of δ13Cair and ca to calculate Δ, and to reconstruct ci and iWUE for the period corresponding to LW formation.
2.4. Meteorological and CO2 flux data and data analyses
We used hourly gap-filled meteorological data from the eddy-covariance tower measured by sensors above the canopy at 29 m height (Urbanski et al 2007, Harvard Forest Data Archive HF004) to compute monthly mean temperature, vapor pressure deficit (VPD), incident photosynthetically active radiation, relative humidity as well as monthly total precipitation from 1992 to 2010. Hourly gap-filled GPP was used to provide monthly cumulative GPP. We used the Palmer Drought Severity Index (PDSI) for the central Massachusetts climate region from the National Climatic Data Center.
The association between climate variables and tree-ring Δ chronologies were assessed using correlation analyses. The GPP time series during the growing season (from May to October) were compared to tree-ring Δ chronologies for each species. Correlations were calculated between monthly GPP and LW-Δ using the Pearson (pairwise) product-moment correlation. Trends over the 1992–2010 period were estimated using linear regression.
3. Results
We assessed the number of trees required to extract a common reliable climate signal (thus, the degree to which isotopic composition series vary in parallel) using the expressed population signal (EPS, Robertson et al 1997, McCarroll and Pawellek 1998). The EPS values calculated using the 1992–2010 isotopic compositions are ⩾0.85 for two trees from each species. An EPS value of ⩾0.85 suggests that the sample size is adequate (Wigley et al 1984). At the high frequency investigated in this study (year to year), the δ13C series display a high common variance within each species (table 1). We assessed the confidence interval (CI) around annual mean values to account for the differences in the absolute isotopic values (McCarroll and Loader 2004). The sample size of 4 T. canadensis and 5 Q. rubra provided a CI95% = 0.3‰ and 0.18‰ respectively. The mean values of 1992–2010 individual series were calculated for all tree cores to produce one main series for each species (Shi et al 2011) hereafter called δ13CTSCA for T. canadensis and δ13CQURU for Q. rubra.
δ13C measurements were significantly different between species (t = 2.02, P < 0.05). The mean δ13CTSCA display higher ratios than δ13CQURU, this difference is probably linked to hydraulic conductivity in conifers which is less efficient than in ring-porous deciduous species (Stuiver and Braziunas 1987, McCulloh et al 2010) or other factors such as differences in the maximum photosynthetic capacity or leaf morphology (Barbour et al 2002). The species specific time series of annual δ13C variations are positively correlated with each other (r = 0.61 P < 0.001). The derived Δ and ci series for each species reveal similar and significant increasing trends over time, although the increase was more pronounced for T. canadensis (figure 1). The iWUE over the period of record remained stable which translates into a constant ca − ci or an increasing ci /ca . A few years did display a higher iWUE in comparison to most years; in particular, iWUE spikes in 1999 in both species records.
Figure 1. From top to bottom: trends in the δ13C discrimination Δ (‰), the leaf intercellular CO2 concentration (ci, ppm), intrinsic water use efficiency (iWUE) and basal area increment (BAI) for T. canadensis (Left) and Q. rubra (Right) throughout time. The slopes of the linear regressions fitted to the data, the coefficients of determination and the P-values are reported for each parameter (see also table 2). The upper and lower levels of the 95% confidence interval for each linear trend are shaded in gray.
Download figure:
Standard image High-resolution imageThe mean BAI also showed a significant increasing trend over the period of record for Q. rubra (R2 = 0.64, P = 0.0001) but the trend was less pronounced for T. canadensis (R2 = 0.32, P < 0.05). Overall, the trends analysis using linear regression shows that Δ, ci and BAI have increased for each species. The changes were significant (p < 0.05) and the slopes were positive (table 2).
Table 2. Trend statistic, per species, for isotopic discrimination (Δ), intercellular CO2 concentration (ci ), intrinsic water use efficiency (iWUE) and basal area increment (BAI). All units are given per year (yr). The slopes are estimated using linear regression.
| Slope | Sig | |
|---|---|---|
| ΔTSCA (‰ yr–1) | 0.06 | <0.0001* |
| ΔQURU (‰ yr–1) | 0.03 | 0.01** |
| ci TSCA (ppm yr–1) | 2.01 | <0.0001* |
| ci QURU (ppm yr–1) | 1.72 | <0.0001* |
| iWUETSCA (μmol mol yr–1) | −0.018 | 0.9*** |
| iWUEQURU (μmol mol yr–1) | 0.16 | 0.2*** |
| BAITSCA (cm2 yr–1) | 0.22 | 0.01** |
| BAIQURU (cm2 yr–1) | 0.40 | <0.001* |
The significance of each variable slope is given * p < 0.01, ** p < 0.05 and *** Positive or negative but non-significant.
The Δ was strongly correlated with July (0.55, P < 0.05) and October (0.53, P < 0.05) GPP for T. canadensis, and with July (0.49, P < 0.05), August (0.58, P < 0.01), and October (0.55, P < 0.05) GPP for Q. rubra. Online supplementary table S1 (available at stacks.iop.org/ERL/9/074011/mmedia) summarizes the Pearson correlation obtained between monthly climate variables and ΔQURU and ΔTSCA. Overall, the strongest positive correlations were found with PDSI from May to August for T. canadensis and for May for Q. rubra. A positive correlation was found between Δ of both species and early-growing season precipitation (April and May).
4. Discussion
4.1. Relationship between Δ, growth, and flux tower estimates of GPP
The carbon contribution to stem growth during LW formation should primarily originate from the immediate product of carboxylation thus reflecting the δ13C signature of recent assimilates (Helle and Schleser 2004). The interannual variability of Δ was compared with monthly GPP to identify the seasonality of carbon uptake and allocation to aboveground woody biomass. The correlations shown in figure 2 indicate that recent photosynthates provided the substrates for stem radial growth occurring in the second part of the growing season (Gessler et al 2009, Offermann et al 2011). The Δ-GPP correlations are logical based on the timing of LW formation for both T. canadensis and Q. rubra. LW tracheid formation and cell wall thickening in T. canadensis begins later in the growing season (late July–October, Skene 1972) coincident with the significant correlations. In contrast, LW formation in Quercus species begins approximately five weeks after the unfolding of first leaves and continues until radial growth cessation in late summer or early fall (Zasada and Zahner 1969, Voelker et al 2012).
Figure 2. Tree-ring Δ chronologies and monthly GPP data from April to October. Pearson correlations from 1992 to 2010 between Δ and mean monthly GPP data for T. canadensis (a) and Q. rubra (b). May–October GPP and the Δ chronologies for T. canadensis (c) and Q. rubra (d) versus time.
Download figure:
Standard image High-resolution imageThe Δ-GPP correlations are consistent with the seasonality of carbon uptake from eddy flux measurements and C storage estimates at the deciduous forest-EMS tower site and the Hemlock Forest tower site located 0.5 km from the EMS tower (Hadley and Schedlbauer 2002, Hadley et al 2009). Net C uptake at the deciduous forest site occurs between the end of May and mid-October. At the hemlock site, the peak in net C uptake and storage occurs in April and May. This period is not influencing the δ13C in the LW, although it shows the effect of conifers on the annual pattern of the C exchange. Conifers currently represent 17% of the total basal area at the deciduous forest site. After C uptake declines in June, it increases in July, and falls in August before a second uptake peak in October, consistent with the Δ-GPP correlations for T. canadensis.
The species seasonality of carbon allocation is further reflected in the relationship between LW-Δ and growth. The correlation for Q. rubra LW-Δ and mean BAI was significant (r = 0.72, P < 0.001), and was slightly higher with the mean Q. rubra BAI of all sampled trees (r = 0.75, P < 0.001). To illustrate the relationship between aboveground carbon gain and photosynthetic discrimination we regressed BAI against Δ, and a strong positive trend was obtained for Q. rubra (R2 = 0.39 P < 0.01). The growth increase of Q. rubra could be interpreted as the result of a greater photosynthetic rate induced by increased ci concentrations (von Caemmerer and Farquhar 1981, Schubert and Jahren 2012), and a substantial allocation of carbohydrates to LW formation and radial growth (Keel et al 2006, Palacio et al 2011). However stage of stand development may also be contributing to this increase in BAI (e.g. Foster et al 2014, figure S1). A relationship between Δ and BAI was not found for T. canadensis, probably because carbon sequestered after June contributes little to the current year radial growth or that carbon allocation differs by species (Richardson et al 2013). The trend in the BAI growth enhancement for Q. rubra is consistent with changes in the measured annual increment of aboveground biomass in the flux-tower footprint from 1993–2010 (∼20% increase, Data Archive: HF069), and provides a quantification of carbon stored in aboveground woody biomass. Interestingly, the aboveground biomass over the last decade accounted for ∼50% of the total carbon sequestered (Keenan et al 2012a) confirming the relationship we found between Q. rubra BAI and Δ, and growing season GPP (figure 2). The remaining carbon uptake could be attributed to litter or soil pools; however, these carbon proportions and stocks are not accounted for in the tree-rings δ13C.
The inter-annual variability and fraction of carbon uptake and sequestration explained by the tree-ring derived Δ (figures 2(c), (d)) provides a quantitative proxy that can be used to constrain models that estimate forest productivity. Process-based models fail to accurately reproduce the observed inter-annual variability of C-fluxes (Keenan et al 2012b) mainly because of inaccurate model allocation structure and lagged effects of climate variability on tree growth and physiology (Gough et al 2009). In contrast, the Δ integrates biotic factors which modulate the δ13C fractionation and the isotopic composition of the total pool of carbon fixed during photosynthesis at seasonal and annual cycles (Leavitt 1993).
4.2. Climate drivers and physiological implication of the Δ trend
We examined the climatic drivers of Δ using meteorological data from the eddy-covariance tower and PDSI. The climate signal within both species Δ series is dominated by monthly precipitation and PDSI during the growing season (table S1), although they have a stronger influence on T. canadensis due to its particularly drought-sensitive nature caused by a relatively shallow rooting-depth (Cook and Cole 1991).
The carbon isotope discrimination properties can be used to assess how trees are responding to increasing atmospheric concentration ca and changes in climate. Three physiological response scenarios described by Saurer et al (2004) identify possible changes in iWUE and ci following ca increase: (1) ci remains constant such that ci/ca decreases and iWUE increases; (2) ci increases proportional to ca such that ci/ca remains constant and iWUE increases; (3) ci increases at the same rate as ca, ci/ca increases and iWUE remains constant. In this study, Δ and ci increased and there was no increase in iWUE for both species (figure 2, table 2). The ci/ca calculated for both species increases indicating ci follows the increase in ca at a rate of 2 ± 0.21 ppm yr–1 for T. canadensis, and 1.7 ± 0.17 ppm yr–1 for Q. rubra. The ci/ca increase and no change in iWUE suggest a weak stomatal response to ca increase and is described as a passive response to changes in ca (McCarroll et al 2009) where neither stomatal conductance nor photosynthetic rate change. The most common response documented in trees is an active response as stomatal conductance is reduced (Bert et al 1997, Saurer et al 2004, Peñuelas et al 2011). The iWUE trends derived from the tree-rings δ13C in our study show a divergence from the instantaneous WUE (the ratio of carbon assimilation to transpiration, Farquhar and Richards 1984) trend documented at the ecosystem level using continuous measurements of CO2 and water vapor fluxes at HF (Keenan et al 2013). The canopy-integrated water use efficiency calculated as the ratio between gross ecosystem photosynthesis (GEP) and Ecosystem evapotranspiration (E) shows a significant increase over the measurements period (3.5% change yr−1, P = 0.01). The latter was calculated taking into account the atmospheric evaporative demand and therefore is referred to as 'inherent' water use efficiency (Wei). The 'intrinsic' water use efficiency as derived from tree-rings δ13C is often used as an indicator of long-term trends in the internal regulation of carbon uptake and water loss independently from evaporative demand (Osmond et al 1980). Therefore iWUE inferred from δ13C may not be representative of instantaneous or inherent WUE. Although both are affected by similar processes (i.e. stomatal conductance), they can vary independently because they are influenced by additional factors like mesophyll conductance, leaf N-content and C-respiratory losses (Griffiths et al 1999, Seibt et al 2008). We note that when iWUE is calculated from the flux tower measurements (as the ratio between GEP and canopy water conductance), the magnitude of the trend is lower and the statistical strength is no longer significant compared to those calculated for Wei (1% change yr−1, P = 0.2, Keenan et al 2013).
The Δ trend found in this study during the last 18 years is unusual but not unique. Results from literature often reported a decrease in Δ inferred from tree-ring records, and thus a strong improvement of iWUE during the last 100 years under increased atmospheric CO2 (Arneth et al 2002). However, Marshall and Monserud (1996) showed that iWUE has remained static in sites from western US and a switch in iWUE from an active to a passive response around AD 1970 has been noted in several European sites (Gagen et al 2011). The spatial variability of stomatal regulation and iWUE response to increasing ca is explained by the strong dependence of water and carbon usage on moisture stress experienced at a specific site and species sensitivity to moisture availability (Warren et al 2001). The increased discrimination is a reflection of the availability of CO2. When the level of CO2 (ci ) is high, large discrimination is observed reflecting the RuBisCO enzymatic preference for 12CO2 (Stewart et al 1995). Clearly, the Δ chronologies at HF indicate that despite higher ca concentrations, factors like physiology and site condition contributed to stomatal openness and conductance.
The strong correlation between Δ and growing season PDSI and precipitation for both species suggest a link between water availability and stomatal conductance (Dupouey et al 1993). The climatic data for central Massachusetts indicates that precipitation and moisture (PDSI) are high at HF and are rarely limiting tree growth (Voelker 2011). In fact, the region became wetter over the period of analysis. The mean annual precipitation from 1898 to 2010 was 107.45 cm and increased 0.28 ± 0.06 cm yr–1 (p < 0.0001). The trend in precipitation is also reflected in a trend of increasing PDSI of 0.03 ± 0.005 units yr–1 (P < 0.0001) over the same period. Since 1992, precipitation and PDSI have increased at rates of 1.30 ± 0.67 cm yr–1 (P < 0.07) and 0.15 ± 0.05 units yr–1 (P < 0.006), respectively. The increase in regional scale water availability (Wang et al 2013) is also evident at the HF site. Annual precipitation and growing season precipitation have increased by 10% and 15% over the last 18 years, respectively (Keenan et al 2013).
We conclude on the basis of the photosynthetic discrimination sensitivity to water availability that the long-term increase in moisture which controls Δ drove the increase in stomatal conductance and carbon photosynthetic discrimination. A positive correlation between Δ and mean annual and summer precipitation has been confirmed for modern leaf tissues from multiple biomes in North America (Diefendorf et al 2010). This correlation is explained by the effect of the mean annual and summer precipitation levels on both soil water status and VPD (Hartman and Danin 2010). It is noteworthy that despite the mesic conditions of the HF site, precipitation and soil moisture levels remain very strong predictors of CO2 discrimination. Similar to our findings, drought sensitivity has been demonstrated for radial growth of T. canadensis and Q. rubra growing in mesic sites located in the New York City watershed (Pederson et al 2013).
The sensitivity to water availability is further illustrated by iWUE data for the year 1999. Both species exhibited a higher iWUE reflecting lower Δ values (figure 1). The summer of 1999 was very dry with decreased soil moisture levels resulting in the restriction of the CO2 supply to the leaf (Brugnoli et al 1988) because of stomatal closure, and in a weaker carbon uptake observed in the summertime NEE record (Urbanski et al 2007).
5. Conclusion
An enhancement in photosynthetic isotopic 13C discrimination and ci was observed over the last 18 years for two co-dominant species at the Harvard Forest. The combined effect of increased water availability and higher atmospheric CO2 concentration lead to increased plant CO2 assimilation. The higher net photosynthesis can in part explain the growth enhancement of Q. rubra and above-ground GPP (e.g. Linares and Camarero 2012) and is consistent with the observed higher CO2 uptake and C storage documented in the flux-tower measurements over the same period. The tree-ring Δ-GPP relationship identified in this study can be used as a quantitative proxy to reconstruct and interpret past forest productivity, as driven by climate variability and in response to long-term atmospheric CO2 increase.
The photosynthetic carbon isotopic discrimination model is embedded in most of the models of forest carbon cycling (Richardson et al 2012) that are coupled with earth-system models to project terrestrial carbon cycle and feedbacks to climate change (Sitch et al 2008). We show that the data provided by tree-ring δ13C, recording the seasonal and interannual patterns of plant carbon assimilation, can be used to inform forest ecosystem model parameterization (Bodin et al 2013), to improve simulations of monthly scale ecosystem-function (e.g. Medvigy et al 2013), and to constrain longer term terrestrial carbon dynamics in response to climate fluctuations and forest management (e.g. Brooks and Mitchell 2011).
Whether the documented isotopic trends in the present study are merely temporary or a site specific phenomenon remains to be seen. Further investigations of tree-ring δ13C and ecosystem physiology measurements from other flux-tower sites in northeastern US forests need to be explored to assess physiological responses at the regional scale.
Acknowledgements
This study was supported by the US Department of Energy through the Northeast Regional Center of the National Institutes for Climatic Change Research, the College of Earth and Mineral Science's Earth and Environmental Sciences Institute at The Pennsylvania State University, Penn State's Institutes of Energy and the Environment and the National Science Foundation grant NSF-BCS- ♯ 1229887. The Harvard Forest EMS tower is a component of the Harvard Forest LTER, supported by National Science Foundation, and is additionally supported by the Office of Science (BER), US Department of Energy. We thank the three anonymous reviewers for their helpful comments and suggestions to improve this manuscript.


