Abstract
Water resources, including groundwater and prominent rivers worldwide, are under duress because of excessive contaminant and nutrient loads. To help mitigate this problem, the United States Department of Energy (DOE) has supported research since the late 1980s to improve our fundamental knowledge of processes that could be used to help clean up challenging subsurface problems. Problems of interest have included subsurface radioactive waste, heavy metals, and metalloids (e.g. uranium, mercury, arsenic). Research efforts have provided insights into detailed groundwater biogeochemical process coupling and the resulting geochemical exports of metals and nutrients to surrounding environments. Recently, an increased focus has been placed on constraining the exchanges and fates of carbon and nitrogen within and across bedrock to canopy compartments of a watershed and in river–floodplain settings, because of their important role in driving biogeochemical interactions with contaminants and the potential of increased fluxes under changing precipitation regimes, including extreme events. While reviewing the extensive research that has been conducted at DOE's representative sites and testbeds (such as the Oyster Site in Virginia, Savannah River Site in South Carolina, Oak Ridge Reservation in Tennessee, Hanford in Washington, Nevada National Security Site in Nevada, Riverton in Wyoming, and Rifle and East River in Colorado), this review paper explores the nature and distribution of contaminants in the surface and shallow subsurface (i.e. the critical zone) and their interactions with carbon and nitrogen dynamics. We also describe state-of-the-art, scale-aware characterization approaches and models developed to predict contaminant fate and transport. The models take advantage of DOE leadership-class high-performance computers and are beginning to incorporate artificial intelligence approaches to tackle the extreme diversity of hydro-biogeochemical processes and measurements. Recognizing that the insights and capability developments are potentially transferable to many other sites, we also explore the scientific implications of these advances and recommend future research directions.
Export citation and abstract BibTeX RIS
1. Introduction
Water security is critical for food and energy production, economic development, and national security. Yet water security is under severe duress globally because of climate change, growing population, and human activities (Alley et al
2002, Rodell et al
2009, Heathwaite 2010, Famiglietti 2014). A recent study projected that more than 65% of the human population (∼ billion people) live in water-insecure regions (Vörösmarty et al
2010). Exacerbating the problem, the demand for freshwater (
billion people) live in water-insecure regions (Vörösmarty et al
2010). Exacerbating the problem, the demand for freshwater ( 3% of all water on Earth) is increasing, challenging our ability to meet food and energy needs globally. Although future technologies may allow us to increase clean water supplies, it is imperative to protect the available freshwater resources from numerous threats, such as a range of chemicals, including metals, metalloids, radionuclides, and nutrients that reduce usable supply.
3% of all water on Earth) is increasing, challenging our ability to meet food and energy needs globally. Although future technologies may allow us to increase clean water supplies, it is imperative to protect the available freshwater resources from numerous threats, such as a range of chemicals, including metals, metalloids, radionuclides, and nutrients that reduce usable supply.
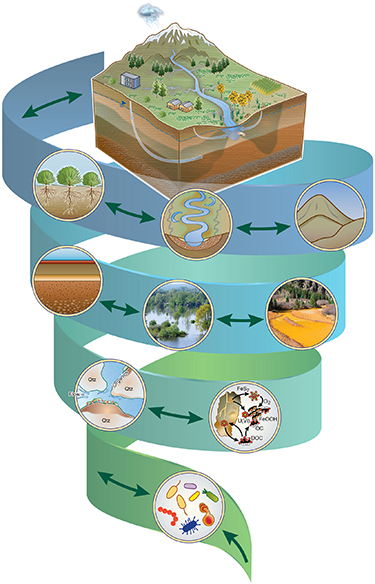
Increasing contamination of freshwater poses a serious problem to both surface water and groundwater (e.g. Varol and Şen 2012, Brender et al 2013, Dwivedi et al 2013, 2016b), particularly because most of the world's prominent rivers, supporting several million people, are under threat and experiencing contamination from a variety of chemicals, including uranium (U), chromium (Cr), arsenic (As) and excessive amounts of nutrients (e.g. Brown and Halweil 1998, Dodds 2006, Paddison 2016, Tripathi et al 2016, Gross 2017, Lu et al 2018). To understand the behavior and reactivity of a plethora of contaminants and nutrients, the United State Department of Energy (DOE) has funded extensive research across several sites in the United States of America (USA) for several decades now (www.energy.gov/). Below, we provide how scientific progress has evolved through these efforts (figure 1).
Figure 1. The spiral shows the progression of DOE-supported science that evolved in both scale and complexity. Significant scientific progress has been achieved from fundamental subsurface microbiology to geochemistry and biogeochemistry to hydro-biogeochemistry and now to eco-hydro-biogeochemistry over the past two decades.
Download figure:
Standard image High-resolution imageThe DOE's mission is 'to ensure America's security and prosperity by addressing its energy, environmental and nuclear challenges through transformative science and technology solutions.' The DOE was created in 1977, succeeding various energy-related programs previously dispersed throughout various federal agencies, including the Manhattan Project effort to develop the atomic bomb during World War II. Over fifty years of nuclear weapons production, testing, and energy research generated a vast volume of legacy contamination and created contaminated soil and water across the USA. The DOE has supported research and cleanup associated with several challenging subsurface problems since the late 1980s, such as treating radioactive waste (e.g. U, plutonium (Pu)) and heavy metals and metalloids (e.g. Cr, mercury (Hg), As) in the subsurface. Notably, since its inception in 1989, the DOE's Environmental Management (EM) and Environmental Remediation (ER) programs have been responsible for the restoration of as many as 107 sites across the country—an area equal to Rhode Island and Delaware combined (figure 2). Within the DOE's Office of Science, the Biological and Environmental Research (BER) program has led the fundamental science research associated with water and energy security at the watershed, continental, and global scales through various projects and programs. The DOE-BER, its predecessors, and other related programs have significantly contributed to the progress of environmental sciences, setting the stage for this paper's thematic organization (figure 1).
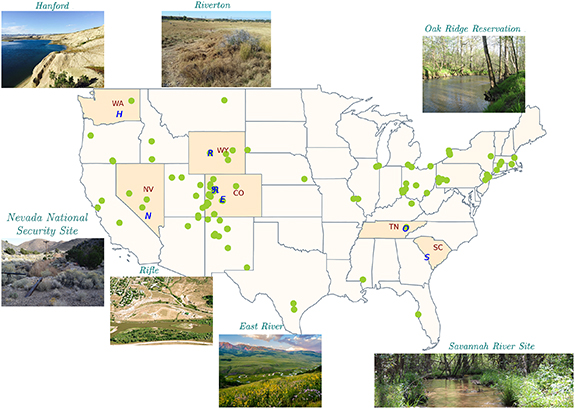
Figure 2. The DOE's EM and ER programs have been responsible for the restoration of as many as 107 sites across the country. The sites also exist outside the contiguous states in the USA (not shown here). Here we show only DOE-LM sites to demonstrate the extent of DOE cleanup activities spreading across the nation. In this review, we chose seven representative DOE sites and testbeds, including Savannah River Site in South Carolina, Oak Ridge Reservation in Tennessee, Hanford in Washington, Nevada National Security Site in Nevada, Riverton in Wyoming, and Rifle and East River in Colorado. Note that the Riverton and Rifle are DOE-LM sites, whereas the Savannah River Site, Oak Ridge Reservation, Hanford, and Nevada National Security Site are DOE-EM sites. The East River is not associated with any DOE historical contamination site. We have synthesized these seven sites as representative DOE sites and testbeds in this review.
Download figure:
Standard image High-resolution imageNext, we briefly describe the history of various DOE programs that have supported water-security-research. The DOE-BER pioneered genomics, system biology, and biotechnology research through the Human Genome Project in 1990. In the late eighties, the Subsurface Science program initiated biological and co-contaminant geochemistry research through its deep microbiology and later bacterial transport projects. The Natural and Accelerated BIoRemediation (NABIR) program subsequently pioneered the 'bio' part of biogeochemistry (e.g. metal-reducing bacteria, in-silico modeling). The Subsurface Biogeochemical Research (SBR) program then continued that linkage, emphasizing the interplay between transport and biogeochemistry. Within the DOE's Office of Science, the Advanced Scientific Computing Research (ASCR) brings together researchers from various fields to tackle some of the most challenging scientific problems, leveraging DOE's leadership-class supercomputers and developing high-end computational science. In the environmental sciences, the ASCR's Scientific Discovery Through Advanced Computing (SciDAC) program has boosted subsurface research like multiscale science and reactive transport modeling. We use 'DOE research' and 'DOE-supported science' interchangeably to broadly reflect scientific advances pioneered through BER–SBR, their predecessors, and other related programs (e.g. EM, ASCR). This review paper follows the evolution of DOE research in this context, focusing primarily on the subsurface environmental sciences from the nineties to recent times.
Research efforts during the 1990s and early 2000s led to significant developments in our understanding of microbial communities, metals geochemistry, and co-contaminant interactions. These included the development of novel techniques for sampling and cultivating subsurface microbial communities (Ghiorse and Wobber 1989, Phelps et al 1989), identification of colloids as an important mechanism for mobilizing low solubility metals and radionuclides (e.g. Kersting et al 1999, Santschi et al 2002, Suzuki et al 2002), understanding of the influence of natural organic matter (NOM) on metal speciation and sorption on mineral surfaces (Gu et al 1994, 1995), application of synchrotron-based x-ray absorption spectroscopies to elucidate the nature of metal and organic matter complexes on mineral surfaces (e.g. Bargar et al 2000) and bacterial cells (Kelly et al 2002, Boyanov et al 2003), computational approaches to understanding the fundamental properties of natural minerals as metal sorbents (e.g. Zachara et al (1995), Felmy and Rustad (1998), Steefel et al (2003)), examination of microbial impacts on metal immobilization (e.g. Fredrickson et al 2000, Labrenz et al 2000, Kemner et al 2004), and implementation of mechanistic processes in reactive transport codes to simulate transport behavior (e.g. Steefel et al 2005).
At the same time, it was realized that natural and accelerated bioremediation held great potential for solving a wide spectrum of contamination problems (Lovley and Phillips 1992). These research efforts led to significant advances in natural and active stabilization of metals in the subsurface (Bender et al 2000, Lovley 2003). Coupled hydrological and biogeochemical processes such as reactive, colloidal, and advective–dispersive transport were investigated within the view of biological availability, transformation, and movement of radionuclides and metals (Wang et al 2003). Some notable examples include the abiotic (O'Loughlin et al 2003a) and microbially mediated reductive precipitation and stabilization of U, iron (Fe) reduction, Pu surface-mediated reduction (Powell et al 2005), and the influence of NOM (McCarthy et al 1998), and other competing processes that can limit metal immobilization in the subsurface (Brooks et al 2003). Subsequent studies (e.g. Zheng et al (2003), Wan et al (2005), Tokunaga et al (2008)) questioned whether the microbially mediated redox changes resulting in radionuclide immobilization were sustainable.
Motivated by the potential of natural and accelerated bioremediation as an effective cleanup strategy for DOE, the Oyster Virginia Subsurface Bacterial Transport Project was initiated in 1999 as the first large, team-based, multidisciplinary, multi-institutional, DOE-supported scientific project that was specifically tied to a field research testbed. Through iterative integration of information gained through lab experiments, field characterization, field experiments, and numerical modeling associated with the seminal South Oyster Site, the project greatly advanced a predictive understanding of bacterial transport in physically, chemically, and biologically heterogeneous aquifers. The project led to several scientific firsts, including the first:
- (a)
- (b)
- (c)
- (d)
As summarized by Scheibe et al (2011), not only has the body of literature resulting from the Oyster Site research been widely cited, but the project has served as a model for subsequent DOE-supported team-based research using data from highly-instrumented field testbeds with model-guided experimental design (Scheibe et al 2001). Many such representative DOE sites and testbeds are described below (section 3), together with advanced characterization and simulation modeling approaches to advance predictive understanding of complex hydro-biogeochemical phenomena and water security research.
In the late 2000s, much emphasis was placed on linking the interplay between transport and biogeochemistry (e.g. diffusion-limited mass transfer, kinetic sorption-desorption). Indeed, this was the time when a paradigm shift ensued in multiscale, multiphysics science, and reactive transport modeling (e.g. White and Oostrom 2000, 2003, Pruess 2004, Steefel et al 2005, Hammond et al 2007). These advances subsequently paved the way for fully exploiting the DOE's unique computational capabilities in high-performance computing. In unison, subsurface environmental simulation capabilities were enhanced by explicitly representing multiphase, multicomponent process dynamics, and by code parallelization.
Up until the past decade, the investigations of small-scale (molecular to millimeter) processes had been a primary focus. This small-scale process understanding was used to upscale and predict behavior at the field scale by deriving scaling laws and mathematical models. Although this reductionist (bottom-up) approach was remarkable in advancing basic research, it was inadequate for addressing scaling behavior in the presence of a range of complex as well as coupled processes, nonlinearity, and a wide range of landscape heterogeneity. Consequently, large uncertainties existed in predictions that lacked a holistic, unifying approach to capture large-scale responses. There was a need to link small-scale process understanding with the larger-scale responses systematically (i.e. treating several ecosystem subsystems, components, and compartments, from the bedrock to the canopy, as a complex system). This perspective aims to comprehensively couple and model key processes across the critical zone (National Research Council 2001), and to link these processes to deep hydrological flow paths and atmospheric circulation systems.
To address this need, at the beginning of the last decade, researchers associated with DOE fate and transport challenges recommended new approaches that melded select strengths of mechanistic, bottom-up approaches that were prevalent at the time with top-down approaches, with an aim to improve characterization and prediction of complex hydro-biogeochemical behavior across scales (DOE-Complexity 2009). New emphasis was placed on actively linking sub-compartments of the Earth system, including atmospheric and biospheric processes, from the bedrock to the canopy. Multiple aspects of the system, such as hydrologic and carbon (C) cycles as well as microbiological and geochemical processes, were explicitly represented, linked, and allowed to interact with each other. This approach encouraged researchers to recognize scale transitions in a hierarchical subsurface system, link local and regional chemical fluxes, and investigate collective system behavior.
Subsequently, a more holistic system science approach evolved through active contaminant remediation-type studies. The system science approach tackles questions such as how watersheds respond to changes over the long term (e.g. Hubbard et al 2018). These include developing insights into the surface–subsurface hydrology; groundwater–surface water interactions; C, nutrient, and trace element transformations; ecosystem disturbances and resilience; impacts on earth systems, development of Earth System Models, and pioneering interdisciplinary community science (e.g. Stegen and Goldman 2018, Arora et al 2019b, Hubbard et al 2020).
Here we summarize the important findings developed from several decades of research work at DOE sites that have contributed to our understanding of contaminants and nutrient cycling and their transport in the environment. These DOE sites have been heavily tracked, monitored, and investigated for legacy contamination and have subsequently served as testbeds for exploring the exchange of materials between terrestrial and aquatic ecosystems—and how this exchange influences element export at river-basin scales. Therefore, these sites and their associated scientific discoveries provide an opportunity for us to review the nature and distribution of legacy contamination and their coupled interactions with C, nitrogen (N), and sulfur (S) dynamics, as well as the timescales of biogeochemical exports. Finally, with this article, we expect to raise awareness of the water-security problems arising from the dispersal of a range of chemicals in the environment and to provide a rich body of literature to address these problems.
2. An outline of DOE-supported science
A central focus of the DOE-supported science has been identifying the dominant biogeochemical processes controlling the migration of metals and radionuclides in the environment. Given the interrelationships between metals' behaviors and the presence of other elements (e.g. C, N, Fe, S), investigations into the geochemistry of metals and radionuclides have necessarily evolved to include detailed studies into the behavior of a broader range of elements that impact water quality (e.g. C, N). In the past decade, detailed mechanistic studies of metals geochemistry have included both laboratory and field investigations. Particular focus was initially placed on elements such as U (e.g. Rifle, Hanford, Oak Ridge), Hg (e.g. Oak Ridge), Pu (e.g. Savannah River Site, Nevada National Security Site), and metal co-contaminants (e.g. Rifle, Hanford), which later expanded to a broader set of biogeochemically complex elements, such as C and N, that are relevant to the ecosystem's health and water security at a national scale. Although it is not possible to capture all the scientific progress made at the DOE offices and associated sites in the past several decades, our goal is to provide a broad understanding from all the contaminated sites in the past that lead to current ecosystem science.
We first describe the salient features of the DOE's representative sites used to achieve important successes in subsurface environmental sciences over the past two decades in section 3. Section 4 chronicles scientific progress in subsurface microbiology, geochemistry and biogeochemistry, and hydro-biogeochemistry over the past two decades. We primarily discuss the water security issues concerning the fate and transport of legacy contaminants, critical elements, and nutrients at representative DOE sites.
We then describe the capability development achieved while making many scientific advancements in section 5. We choose Hg as a use case to describe watershed biogeochemical processes holistically in section 6. Hg is emblematic in its complexity, unique to DOE challenges, and is particularly sensitive to extreme events that are increasingly expected in the future.
Then section 7 briefly reviews a few constructs such as multiscale, multiphysics, hybrid modeling approaches, hot spots and hot moments (HSHMs), and functional zonation that evolved over the years to transfer knowledge across sites. We then review legacy contamination and their coupled interactions with C, N, and S dynamics in the backdrop of the HSHM construct. After that, in section 8, we identify the current ecosystem science, eco-hydro-biogeochemistry, that links ecology, hydrology, and biogeochemistry. Following this, we highlight global water security issues and offer possible solutions in section 9. Finally, section 10 discusses the implications of scientific advances in ecosystem science and provides concluding thoughts and future research directions.
3. Description of the DOE's representative sites
To summarize the broad understanding that has emerged from DOE research, we choose seven DOE representative sites or testbeds: Savannah River Site in South Carolina, Oak Ridge Reservation in Tennessee, Hanford in Washington, Nevada National Security Site in Nevada, Riverton in Wyoming, and Rifle and East River in Colorado (figure 2). The Savannah River Site, Oak Ridge Reservation, Hanford, and Nevada National Security Site are DOE-EM sites. The Riverton and Rifle sites have been managed by the Office of Legacy Management (LM), which was established in 2003 to manage the remaining legacy of World War II (i.e. radioactive and chemical waste, contaminants, and hazardous material) at over 100 sites across the country. The East River is not associated with any DOE historical contamination, but represents evolution of DOE research toward broader watershed systems science. In addition, all of these sites were later funded by the SBR program to study ecosystem function, thereby providing an excellent opportunity to learn how near-surface and subsurface legacy contaminant transport is modified by terrestrial and aquatic ecosystems.
The Savannah River Site, Oak Ridge Reservation, Hanford, Nevada National Security Site, and Riverton are respectively located in the South Atlantic-Gulf Region, Tennessee, Pacific Northwest, Lower Colorado regions, and Missouri regions. The Rifle and East River sites are located in the Upper Colorado River Basin, the principal water source in the southwestern USA. The Colorado River, one of the major rivers in the region, supplies water for 40 million people in seven states: Arizona, California, Colorado, Nevada, New Mexico, Utah, and Wyoming. These sites span across a wide range of hydro-climatic and geologic conditions. Table 1 describes contrasts in climate, geology, hydrogeology, surface water bodies, and groundwater depths across the representative sites.
Table 1. Geologic and climatologic variability across the DOE sites (National Research Council 2000, Zachara et al 2013, Dam et al 2015, Dwivedi et al 2018b).
| DOE site | Research focus | Climate | Geology and hydrogeology | Surface water body | Approximate depth to groundwater |
|---|---|---|---|---|---|
| Savannah River Site | Wetland hydro-biogeochemistry | Humid, subtropical; average annual rainfall 122 cm | Atlantic Coastal Plain with clay soils; the strata are deeply dissected by creeks, and most groundwater eventually seeps into and is diluted by creeks. | Savannah River and its tributaries | 0 m–46 m |
| Oak Ridge Reservation | Stream corridor hydro-biogeochemistry | Humid, typical of the southern Appalachian region; average annual rainfall 138 cm | Valley and ridge province bordering the Cumberland Plateau; primary porosity is low, but fracture porosity present; high clay content; shallow water table. | Clinch River | 1 m–37 m |
| Hanford Site | River corridor eco-hydro-biogeochemistry | Arid, cool, mild winters and warm summers; average rainfall 16 cm | Alluvial plain of bedded sediments with sands and gravels; Groundwater flows toward the Columbia River. | Columbia River | 10 m–90 m |
| Nevada National Security Site | Hydro-biogeochemistry of actinides | Arid, mild winters and warm summers; average rainfall 13–32 cm | Alluvium, volcanic, and carbonate geology that is part of the Death Valley regional flow system. | Ephemeral streams, transient ponding, springs | 210 m–610 m |
| Riverton Site | Floodplain hydro-biogochemistry | Arid to semi-arid, steppe; average annual rainfall 25 cm | Gravel bed alluvial floodplain overlain by redox-active fine sediments; Wind River Formation. | Little Wind River | 0 m–2.5 m |
| Rifle Site | Contaminants, C and N cycling | Semiarid; average annual rainfall 35 cm | Wasatch Formation overlain by Quaternary floodplain deposit. | Colorado River | 1 m–5 m |
| East River | Watershed function | Arid to semiarid; annual rainfall 30 cm and 70–90 cm as snow | A diverse suite of Paleozoic and Mesozoic sedimentary rocks intruded by Tertiary igneous laccoliths and ore-rich stocks; Cretaceous Mancos Shale bedrock overlain by glacial moraine deposits. | East River and several tributaries representative of a headwater system | 0 m–100 m |
Although several other DOE-funded testbeds have advanced the environmental sciences, they were not included in this review to keep the discussion manageable. This was particularly the case for sites with an extensive focus on aboveground processes, while this contribution's primary focus is to review the progress in understanding belowground processes and ecosystem function.
3.1. Savannah River Site
The Savannah River Site, located in south-central South Carolina, near Aiken, is an 800 square kilometer area where facilities were constructed in the early 1950s to produce special radioactive isotopes (e.g. Pu and tritium (3H)) for the Department of Defense nuclear weapons stockpile. The major facilities constructed included production reactors, chemical processing plants, and solid and liquid waste storage sites. It is estimated that the Savannah River Site has approximately  m3 of groundwater, soil, and debris contaminated with metals, radionuclides, and organics (National Research Council 2000). Contamination of the environmental resources is the result of disposal practices conducted on-site. For example, the F-Area Seepage Basins (located in the north-central portion of Savannah River Site) consists of three unlined, earthen surface impoundments that received approximately 7.1 billion liters of acidic low-level radioactive (e.g. U, iodine (I)) waste solutions.
m3 of groundwater, soil, and debris contaminated with metals, radionuclides, and organics (National Research Council 2000). Contamination of the environmental resources is the result of disposal practices conducted on-site. For example, the F-Area Seepage Basins (located in the north-central portion of Savannah River Site) consists of three unlined, earthen surface impoundments that received approximately 7.1 billion liters of acidic low-level radioactive (e.g. U, iodine (I)) waste solutions.
3.2. Oak Ridge Reservation Site
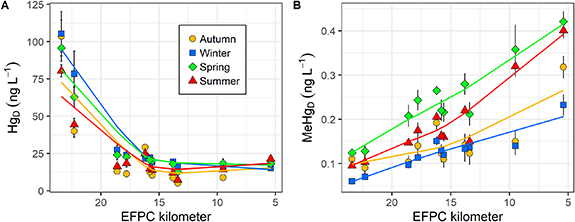
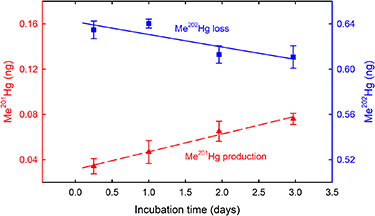
The Oak Ridge Reservation (formerly known as Clinton Engineer Works) was established in the early 1940s as part of the Manhattan Project on 239 square kilometers of land in eastern Tennessee, approximately 40 kilometers west of Knoxville, Tennessee. Its original missions, conducted at three large facilities, included U isotope enrichment, construction, and operation of the world's first continuously operating nuclear reactor to demonstrate Pu production and separation, and radiochemical research and development. Today, the Oak Ridge Reservation covers an area of approximately 134 square kilometers and includes three major DOE complexes: the Oak Ridge National Laboratory (ORNL), the East Tennessee Technology Park, and the Y-12 National Security Complex (Y-12 NSC). Over the past 70 years, historical activities have resulted in significant releases of contaminants into the environment on the Oak Ridge Reservation, contaminating soils, sediments, groundwater, surface water, and biota. Waste disposal areas, unlined infiltration pits, trenches, and spills and leaks have created extensive subsurface contamination. Additionally, from 1950 through 1963, lithium (Li) isotope separation processes at the Y-12 NSC resulted in the release of about 212 000 kg of Hg into the environment, of which about 108 000 kg were estimated to have been lost to the East Fork Poplar Creek (EFPC). Both historical and ongoing Hg releases continue to negatively impact downstream waters, including many kilometers of the river–reservoir system outside the Oak Ridge Reservation.
3.3. Hanford Site
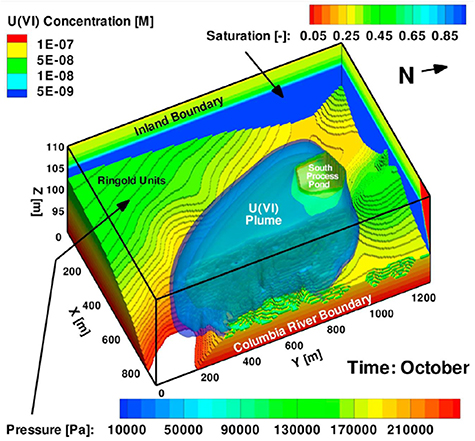
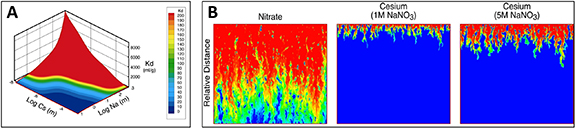
The Hanford Site, located in southeastern Washington State, was established by the federal government to conduct defense-related nuclear research, development, and weapons production activities. As a result of activities at the Pu production complex and processing facilities (chemical separations plants and solid–liquid disposal and waste storage sites), it is estimated that the 1517 square kilometer area has approximately  m3 of groundwater, soil, and debris contaminated with metals, radionuclides, and organics (National Research Council 2000). Contamination of the sites' environmental resources occurred as a result of 1.3 trillion liters of wastewater from chemical processing operations being intentionally discharged into the ground through settling ponds and other subsurface drainage structures (DOE 1997). Additionally, DOE estimates that 67 of the 177 underground high-level radioactive waste storage tanks have leaked 3.8 million liters or more of the highly radioactive waste into the subsurface (DOE 1997). Most of Hanford's subsurface contamination is concentrated in two locations: the Columbia River Corridor (100 Area and 300 Area) and the Central Plateau (200 Area). The 544 square kilometer Columbia River Corridor contained the production reactors, several waste burial sites, and major research facilities (DOE 1996). The 194 square kilometer Central Plateau, near the middle of the Hanford Site, contained the chemical processing facilities for extracting U and Pu from irradiated reactor fuel, associated waste storage facilities (18 tank farms), and waste disposal facilities (i.e. surface settling basins and underground drainage cribs) (DOE 1996). It is estimated that more than 220 square kilometers of groundwater at the Hanford Site is contaminated above current standards, mostly from operations in the 100 and 200 Areas. Section 5 briefly discusses the modeling of U contamination in the Hanford 300 Area and cesium (Cs) migration at the Hanford 200 Area.
m3 of groundwater, soil, and debris contaminated with metals, radionuclides, and organics (National Research Council 2000). Contamination of the sites' environmental resources occurred as a result of 1.3 trillion liters of wastewater from chemical processing operations being intentionally discharged into the ground through settling ponds and other subsurface drainage structures (DOE 1997). Additionally, DOE estimates that 67 of the 177 underground high-level radioactive waste storage tanks have leaked 3.8 million liters or more of the highly radioactive waste into the subsurface (DOE 1997). Most of Hanford's subsurface contamination is concentrated in two locations: the Columbia River Corridor (100 Area and 300 Area) and the Central Plateau (200 Area). The 544 square kilometer Columbia River Corridor contained the production reactors, several waste burial sites, and major research facilities (DOE 1996). The 194 square kilometer Central Plateau, near the middle of the Hanford Site, contained the chemical processing facilities for extracting U and Pu from irradiated reactor fuel, associated waste storage facilities (18 tank farms), and waste disposal facilities (i.e. surface settling basins and underground drainage cribs) (DOE 1996). It is estimated that more than 220 square kilometers of groundwater at the Hanford Site is contaminated above current standards, mostly from operations in the 100 and 200 Areas. Section 5 briefly discusses the modeling of U contamination in the Hanford 300 Area and cesium (Cs) migration at the Hanford 200 Area.
3.4. Nevada National Security Site (formerly Nevada Test Site)
The Nevada National Security Site (NNSS), located in southern Nevada, was the primary site for USA's underground nuclear testing. It led to the deposition of substantial quantities of 43 different radionuclides into the environment (Smith et al
2003). While 3H is the most abundant anthropogenic radionuclide deposited in the NNSS subsurface from an activity standpoint, Pu is the most abundant anthropogenic element by mass. Between 1951 and 1992, 828 underground nuclear tests (and 100 aboveground tests) were performed, and approximately 2.8 metric tons of Pu remains in the NNSS subsurface (DOE, Nevada Operations Office 2000). The site is approximately  km northwest of Las Vegas, Nevada, and covers approximately 3500 square kilometers. The DOE-EM program has been developing a monitoring strategy that involves identifying contaminant boundaries, restricting access to contaminated groundwater, and implementing a long-term monitoring program.
km northwest of Las Vegas, Nevada, and covers approximately 3500 square kilometers. The DOE-EM program has been developing a monitoring strategy that involves identifying contaminant boundaries, restricting access to contaminated groundwater, and implementing a long-term monitoring program.
3.5. Riverton site
The Riverton, Wyoming DOE legacy site is a riparian floodplain that was the location of a U and vanadium (V) ore processing mill that conducted operations between 1958 to 1963 (DOE-LM 1998). It is situated at ∼ m elevation along the gravel bed of Little Wind River and has a semiarid to arid climate (25 cm mean annual precipitation). Temperatures exceed 0 ∘C 154 days per year. The floodplain is vegetated with steppe flora, including sagebrush, grasses, and willows. Roots are relatively abundant, and robust upward solute transport through evapotranspiration has resulted in extensive evaporite mineralization of unsaturated soils (Dam et al
2015). The upper 2.5 m of soil is predominantly loam with abundant clay lenses, deposited abruptly over the underlying gravelly–cobbly–sandy alluvial bed. Groundwater contains persistent U, molybdenum (Mo), and sulfate (
m elevation along the gravel bed of Little Wind River and has a semiarid to arid climate (25 cm mean annual precipitation). Temperatures exceed 0 ∘C 154 days per year. The floodplain is vegetated with steppe flora, including sagebrush, grasses, and willows. Roots are relatively abundant, and robust upward solute transport through evapotranspiration has resulted in extensive evaporite mineralization of unsaturated soils (Dam et al
2015). The upper 2.5 m of soil is predominantly loam with abundant clay lenses, deposited abruptly over the underlying gravelly–cobbly–sandy alluvial bed. Groundwater contains persistent U, molybdenum (Mo), and sulfate ( ) contamination from an upgradient legacy U ore processing facility. Soils are loamy, but clay layers and sand lenses are present above and within the unconfined shallow sandy–gravelly aquifer (2.5 m–3 m below the ground surface). The site experiences intense seasonal redox activity triggered by rising and ebbing water tables and frequent flood events. Clay lenses exhibit molecular oxygen (O2) depletion upon water saturation and the establishment of reducing conditions (including iron sulfide (FeS) precipitation). Significantly, reducing conditions extend into the surrounding sandy aquifer and persist in proximity to clay lenses.
) contamination from an upgradient legacy U ore processing facility. Soils are loamy, but clay layers and sand lenses are present above and within the unconfined shallow sandy–gravelly aquifer (2.5 m–3 m below the ground surface). The site experiences intense seasonal redox activity triggered by rising and ebbing water tables and frequent flood events. Clay lenses exhibit molecular oxygen (O2) depletion upon water saturation and the establishment of reducing conditions (including iron sulfide (FeS) precipitation). Significantly, reducing conditions extend into the surrounding sandy aquifer and persist in proximity to clay lenses.
3.6. Rifle Site
The Rifle, Colorado DOE legacy site is a riparian floodplain that was the location of a former U and V ore processing facility that operated from 1924 through 1958 (DOE-LM 1998). The Rifle Site is approximately 750 m in length along the Colorado River shore and 250 m at the widest point. The former processing facility contained large piles of mill tailings on-site, from which residual U and other associated contaminants leached into the subsurface. All surface structures and contaminated soil were subsequently removed, and the site was capped with fill material and vegetated. However, the potential for infiltration of groundwater contaminants remained until that time (Williams et al 2011, Long et al 2012). Site-specific investigations revealed that the groundwater contained dissolved U at the micromolar concentration level. Other contaminants of concern were identified as As, selenium (Se), and V. Groundwater at the site flows through alluvial flood plain deposits, which are 6 m–7 m thick and underlain by the relatively impermeable Wasatch formation. Groundwater enters the alluvial aquifer from upgradient sources above the floodplain and exits into the Colorado River.
Given this background, earlier research was focused on in situ immobilization of contaminants. More recently, an increased focus was placed on constraining the exchanges and fates of different forms of C and N in river–floodplain settings because of their important roles in driving biogeochemical interactions with contaminants. More broadly, efforts were dedicated to developing a fundamental understanding of microbial communities and how they mediate biogeochemical cycles in the terrestrial subsurface. More than 15 years of focused investigations at the site have created a rich legacy of understanding subsurface contaminant transport and provided transferable insights.
3.7. East River Site
The East River Watershed, located in western Colorado, is a mountainous headwater testbed for exploring how climatic perturbations impact downgradient water availability and quality. The East River Watershed is located approximately 160 kilometers southeast of the Rifle Site and covers approximately 300 square kilometers. The East River is a pristine watershed. The Berkeley Lab's Watershed Function Scientific Focus Area (SFA; https://watershed.lbl.gov/) is examining the contribution to the riverine budgets of C and N that were neglected in previous assessments of contaminated sites like Rifle. The East River testbed began operation in 2014. Since then, it has enhanced process understanding of watershed dynamics and developed new approaches (such as monitoring strategies using sensor networks and scale-adaptive approaches) to improve watershed-dynamics simulation (Hubbard et al 2018, Arora et al 2020).
4. Advances and innovations through DOE science in the last 20 years
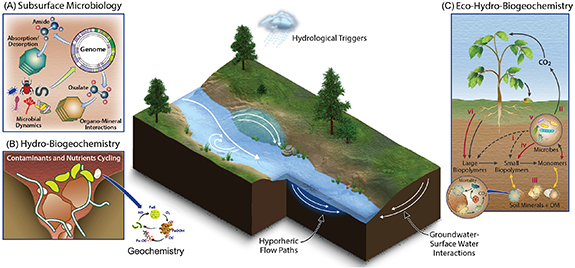
DOE research has evolved in scale and complexity, beginning with fundamental microbiology and geochemistry, and then linking them into biogeochemistry. Further, this research expanded to hydro-biogeochemistry (figure 3) in unison with characterization and monitoring strategies. A detailed description of these is highlighted below.
Figure 3. Schematic depiction of processes within (A) subsurface microbiology (B) geochemistry, biogeochemistry, and hydro-biogeochemistry, and (C) eco-hydro-biogeochemistry. These processes are intimately coupled, and their aggregated responses drive ecosystem function. These processes occur from the genome to watershed scales, and perturbations such as hydrologic triggers lead to the formation of HSHMs through groundwater–surface water interactions, particularly hyporheic flow paths.
Download figure:
Standard image High-resolution image4.1. Advances in subsurface microbiology
It is widely understood that microbial communities, not individual microbes, are the drivers of most subsurface biogeochemical cycles (figure 3(A)). Yet, in the early period of biological research at the Rifle Site, the focus was primarily on one family of bacteria, the Geobacteraceae, which have a laboratory-demonstrated capacity for U reduction (Gorby and Lovley 1992). This body of work motivated in situ acetate injection experiments to test the potential of a Geobacter-based bioremediation strategy at the Rifle Site (Anderson et al 2003). Proteomics of subsurface-derived cells (metaproteomics) were attempted to assay for Geobacter-specific proteins in amended aquifer groundwater. The only reference protein sequences available for this analysis were from Geobacter isolate genomes (Wilkins et al 2009). Thus, the effectiveness of protein identification was limited because the aquifer Geobacter genotypes differed from those in pure cultures.
The first proteomics experiment motivated one of the first cultivation-independent genomics (metagenomic) analyses of groundwater to improve the representation of Geobacter protein sequences. DNA from a Geobacter isolate not present at the Rifle Site provided an internal standard that was used to verify the accuracy of the recovered sequences. The experiment uncovered the very substantial microbial diversity of groundwater microbiomes. Draft genomes were reconstructed for over 80 bacteria, most of which were from previously unsampled or unknown lineages. Approximately half were from a group of novel bacteria whose gene content suggested they were symbionts of other microorganisms (Wrighton et al
2012). These groups were subsequently deeply sampled in a larger acetate injection experiment at the site and from unamended groundwater, and are now recognized as essentially ubiquitous in groundwaters worldwide (He et al
2021). The lineages share a common ancestry, so they were described as the Candidate Phyla Radiation (CPR) and were shown to comprise a substantial fraction (15% to ∼ %) of all bacterial diversity (Brown et al
2015, Hug et al
2016). The uniformly small genomes for CPR bacteria (Wrighton et al
2012, Kantor et al
2013) predicted their small cell size and thus potential to pass through the filters routinely used for cell recovery. Imaging of cells from the smallest filtrate fraction from acetate-amended groundwater revealed that the cells are around the theoretical minimum size for life (Luef et al
2015).
%) of all bacterial diversity (Brown et al
2015, Hug et al
2016). The uniformly small genomes for CPR bacteria (Wrighton et al
2012, Kantor et al
2013) predicted their small cell size and thus potential to pass through the filters routinely used for cell recovery. Imaging of cells from the smallest filtrate fraction from acetate-amended groundwater revealed that the cells are around the theoretical minimum size for life (Luef et al
2015).
Also first detected in Rifle groundwater were novel archaea with genomic features reminiscent of CPR bacteria. These diverse archaea, now part of the DPANN group, substantially extended the breadth of known archaeal diversity (Castelle et al 2013). Given that both CPR bacteria and DPANN archaea are generally predicted to grow as surface-attached symbionts of other cells, it has been inferred that they have the potential to impact biogeochemical cycles through their impacts on host cells as well as via their own capacities for C, H, N, and S compound transformations. Subsequent research identified numerous bacteriophages (phages), the virus of bacteria, in subsurface communities (Al-Shayeb et al 2020), bringing them into focus as important contributors to C compound turnover.
Sampling of subsurface microbial communities from pumped pore fluids overlooks particle-associated cells. This motivated the study of sediment-associated consortia and uncovered very significant microbial diversity (Castelle et al 2013). One of the first applications of long-read sequencing to complex microbiomes revealed the presence of thousands of different organisms, most of which are at similar, very low abundance levels in Rifle sediments (Sharon et al 2015). Metagenomic analyses of saturated whole sediment samples recovered from 4, 5, and 6 m depths identified numerous new lineages, one of which is the bacterial phylum Zixibacteria. From a complete, curated genome, Zixibacteria were predicted to have an incredible diversity of metabolic capacities that enable growth despite shifts in conditions and the available aquifer resources (Castelle et al 2013). Another study targeting sediment-associated Chloroflexi revealed, among other traits, an unexpected pathway for carbon dioxide (CO2) fixation that brings into focus the potential for this process in C cycling in the aquifer (Hug et al 2013).
A study that utilized groundwater and sediment metagenomes sampled from below and above the water table indicated that microbial cohorts consistently establish across the aquifer (Hug et al 2015). This, along with the desire to develop models for integrated microbial community function (Zhuang et al 2011), motivated a major genomes recovery effort. Genomes from over 2000 different microbial community members were analyzed simultaneously (an unprecedented effort). The major conclusion from this study was that biogeochemical cycles are attributed not to single organisms with capacities for specific pathways but to consortia whose metabolisms are interlinked by handoffs of pathway intermediates (Anantharaman et al 2016). This ecosystem structure probably confers resilience and has since been documented by genomics-based studies in other ecosystems, including soil (Diamond et al 2019).
Important limitations of the Rifle research site were its comparatively small size, lack of access to undisturbed soil and riparian zone sediments, and limited vegetation, slope, and aspect types. Thus, methods developed at the Rifle Site were scaled up to tackle the challenge of watershed ecosystem-scale microbial biogeochemical process analyses that incorporate genome-derived information. Initial studies focused on the soils surrounding the meanders of the East River Site in Colorado. Motivated by the need to link micron-scale microbial analyses to a transect that is many kilometers in length, it was hypothesized that soils within the arcs of successive meanders would represent a system representative of meanders along the river length.
Metagenomic datasets were generated from a sampling grid at three sites in the river's upper, middle, and lower reaches. Extensive genome recovery enabled documentation of biogeochemically important capacities across each site. A core floodplain microbiome was identified and found to be enriched in capacities for aerobic respiration, aerobic carbon monoxide oxidation, and thiosulfate oxidation. Metatranscriptomic data revealed that the most highly transcribed genes were amoCAB and nxrAB (for nitrification). Low soil organic C correlates with the high activity of genes involved in methanol, formate, sulfide, hydrogen, and ammonia oxidation, nitrite oxidoreduction, and nitrate and nitrite reduction. Overall, it was concluded that the meander-bound regions serve as scaling motifs that predict aggregate capacities for biogeochemical transformations in floodplain soils (Carnevali et al 2021). Similar research has addressed patterns of organism distribution and function across hillslopes and under four different vegetation types.
4.2. Advances in geochemistry and biogeochemistry
DOE's research in water and energy security arose from its historical and present-day need to resolve the many environmental management issues across its vast nuclear facilities, and its responsibility to develop a long-term solution to the USA's growing nuclear waste stockpile associated primarily with civilian nuclear energy production. The range of radiologic contaminants quite literally spans the entire periodic table: from 3H to the short-lived heavy elements (e.g. curium-244 (244Cm)) (Kurosaki et al 2014). More traditional contaminants of concern include Cr, Hg (discussed in section 6), and highly radioactive Cs and radioactive U (discussed in section 5.2), and more recently C and nutrients, including N and phosphorus (P). Below, we briefly describe DOE advancements in understanding the biogeochemical processes impacting contaminant fate and transport, as well as the major elements and nutrients (i.e. C, N, Fe, S, and manganese (Mn)) that drive subsurface biogeochemistry.
4.2.1. Contaminant biogeochemistry in redox-dynamic environments
U is a major risk driver at many of DOE's legacy waste sites, and DOE-funded programs have made major contributions to our knowledge of molecular species and processes controlling the movement of U in natural waters. The mobility of U in the DOE legacy waste sites is largely controlled by its speciation. The speciation includes dissolved, adsorbed, and mineralized species, predominantly in the +4 or +6 valence states. UV is generally unstable and disproportionates to UVI and UIV (O'Loughlin et al
2011); however, UV has been detected in environmentally relevant systems (Ilton et al
2005, Nico et al
2009b), and stable UV associated with FeII-containing clays has been reported (Boyanov et al
2016, 2017a). Hexavalent U is stable and soluble under oxic conditions as the uranyl cation ( ) (Ragnarsdottir et al
2000). Tetravalent U is stable and sparingly soluble under reducing conditions, but can be mobilized as complexes with organic or inorganic C (Frazier et al
2005, Luo and Gu 2009, Stoliker et al
2013).
) (Ragnarsdottir et al
2000). Tetravalent U is stable and sparingly soluble under reducing conditions, but can be mobilized as complexes with organic or inorganic C (Frazier et al
2005, Luo and Gu 2009, Stoliker et al
2013).
UVI can be reduced by microbial organisms (Wall and Krumholz 2006, and references therein) and structural FeII in minerals (O'Loughlin et al 2003a, 2010, Ilton et al 2005, Ithurbide et al 2009, Veeramani et al 2011, Singer et al 2012, Latta et al 2012b, Boyanov et al 2017a). These reactions generally produce amorphous UIV products (Kelly et al 2008, Sharp et al 2011, Latta et al 2012a, Bargar et al 2013, Wang et al 2014a, Morin et al 2016, Bone et al 2017a, 2017b, 2020, Stetten et al 2018). For example, adsorbed UIV was found to be the dominant form of U in naturally reduced sediments at legacy DOE sites across the Upper Colorado River Basin (Noël et al 2017a, 2017b). NOM functional groups, phosphate or phosphoryl groups, and high-affinity titanium (Ti)- or Fe-based binding sites in minerals have been cited as important ligands for UIV in natural systems (Fletcher et al 2010, Boyanov et al 2011, 2017b, Sivaswamy et al 2011, Veeramani et al 2011, Stylo et al 2013, Latta et al 2014, Wang et al 2015, Bone et al 2017b, 2020). U redox cycling also can lead to the production of UV in FeIII oxyhydroxides (Kerisit et al 2011, Massey et al 2014, Dewey et al 2020). The finding that sorbed species are a major mode of occurrence of UIV was initially unexpected and has subsequently informed the development of reactive transport models (Yabusaki et al 2017).
Oxidation of UIV by O2 has been studied extensively for uraninite (Gu et al 2005, Boyanov et al 2007, Senko et al 2007, Ulrich et al 2009, Lezama-Pacheco et al 2015). Oxidation of UO2 by FeIII oxides and clays has also been documented within the DOE programs (Sani et al 2005, Ginder-Vogel et al 2006, Senko et al 2007, Stewart et al 2013). Oxidation of non-uraninite UIV species by O2 has also been investigated to a limited extent, producing mixed results regarding their stability relative to uraninite (Sharp et al 2011, Cerrato et al 2013, Bi et al 2016, Latta et al 2016). Another recent finding is that the presence of reduced C and Fe phases inhibits oxidative release of non-uraninite UIV (Newsome et al 2015, Bone et al 2017a).
Unlike U, which is regulated at picomolar concentrations, many radionuclides are regulated at femtomolar concentrations (e.g. The United States Environmental Protection Agency (EPA) Maximum Contaminant Levels for drinking water of  ,
,  , and
, and  molL−1 for strontium-90 (90Sr), 137Cs, and 238Pu, respectively) (Deblonde et al
2020). The body of environmental radiochemistry research supported by the DOE is much too vast to summarize here; however, we have selected a few examples to highlight key advances in this area. Much of DOE's research in environmental radiochemistry has focused on a process-level understanding of radionuclide reactive transport. Examination of colloid-facilitated Pu transport revealed the importance of both organic (Xu et al
2008, Zhao et al
2011) and inorganic colloids (Zhao et al
2020), the nature of these colloid associations (Powell et al
2011), the importance of redox cycling (Pan et al
2021), and the sorption–desorption kinetics that limit colloid-facilitated transport (Begg et al
2017, 2018). A combination of molecular dynamics simulations revealed the importance of Cs interaction with minerals via ion exchange (Zaunbrecher et al
2015) along with experiments that determined the retardation properties controlling its migration (Zachara et al
2002, Steefel et al
2003, 2005, Durrant et al
2018). The complex chemistry and reactive transport of I were found to be controlled by a combination of redox chemistry and organic matter associations (Neeway et al
2019). Indeed, the importance of redox conditions in the transport behavior of radionuclides is a theme that spans across several radionuclides of concern to the DOE (
molL−1 for strontium-90 (90Sr), 137Cs, and 238Pu, respectively) (Deblonde et al
2020). The body of environmental radiochemistry research supported by the DOE is much too vast to summarize here; however, we have selected a few examples to highlight key advances in this area. Much of DOE's research in environmental radiochemistry has focused on a process-level understanding of radionuclide reactive transport. Examination of colloid-facilitated Pu transport revealed the importance of both organic (Xu et al
2008, Zhao et al
2011) and inorganic colloids (Zhao et al
2020), the nature of these colloid associations (Powell et al
2011), the importance of redox cycling (Pan et al
2021), and the sorption–desorption kinetics that limit colloid-facilitated transport (Begg et al
2017, 2018). A combination of molecular dynamics simulations revealed the importance of Cs interaction with minerals via ion exchange (Zaunbrecher et al
2015) along with experiments that determined the retardation properties controlling its migration (Zachara et al
2002, Steefel et al
2003, 2005, Durrant et al
2018). The complex chemistry and reactive transport of I were found to be controlled by a combination of redox chemistry and organic matter associations (Neeway et al
2019). Indeed, the importance of redox conditions in the transport behavior of radionuclides is a theme that spans across several radionuclides of concern to the DOE ( I, technetium (Tc), U, neptunium (Np), and Pu) (O'Loughlin et al
2011). Section 5.2 describes the reactive migration of U and Cs at the Hanford and Rifle sites further illustrating the importance of redox conditions and demonstrating the comprehensive and rigorous treatment of geochemistry in contamination modeling, as part of the simulation capability development.
I, technetium (Tc), U, neptunium (Np), and Pu) (O'Loughlin et al
2011). Section 5.2 describes the reactive migration of U and Cs at the Hanford and Rifle sites further illustrating the importance of redox conditions and demonstrating the comprehensive and rigorous treatment of geochemistry in contamination modeling, as part of the simulation capability development.
In addition to U and other radionuclides, Cr is a contaminant of concern at many DOE legacy waste sites. Cr is a naturally occurring, redox-active transition metal that can exist in a range of oxidations states, with CrIII and CrVI being the most stable in environmental systems. Furthermore, CrVI is one of the most mobile forms of Cr, which is transported in surface and subsurface waters as a negatively charged oxyanion. Although hexavalent Cr is generally more soluble, mobile, and toxic, CrIII is significantly less soluble than CrVI and is an essential micronutrient. At DOE sites, such as the Hanford 100 Area (near reactor D, see www.hanford.gov/page.cfm/100Area), sodium dichromate was used during reactor operations to retard corrosion in reactor cooling systems. Consequently, Cr was released to the environment by spills and/or leaks from pipes, resulting in CrVI contamination in the vadose zone and groundwater adjacent to the Columbia River. For over two decades, DOE has performed groundbreaking research on factors that control Cr fate and transport at the Hanford 100 Area. For example, researchers investigated Cr interactions with sediments (Zachara et al 1987, 1988, Beller et al 2014) and the use of various forms of Fe and biostimulation to convert CrVI to CrIII (Powell et al 1995, Fendorf and Li 1996, Jardine et al 1999, Mayes et al 2000, Hazen and Tabak 2005), illustrating techniques for transforming mobile CrVI to immobile CrVI in subsurface systems.
The fate and mobility of As in a heterogeneous redox environment are difficult to predict because it is influenced by mineralogy, chemical speciation, and biological processes. Arsenic has five oxidation states (−III, −I, 0, III, V), with AsIII and AsV being the most prevalent oxidation states in surface and groundwater, where it typically is present as oxyanion (arsenate and arsenite) and thioanion species. Thioarsenic can dominate in sulfidic environments that contain large amounts of reduced S, such as organic-rich surface water and groundwater (Stucker et al
2014, Boye et al
2017, Kumar et al
2020a). Stucker et al (2013, 2014) observed the presence of thioarsenic species under  -reducing conditions while conducting a biostimulation experiment at the Rifle Site in Colorado to treat the U-contaminated groundwater plume. NOM is also an important consideration in understanding As fate and mobility in environmental systems, such as wetlands, riparian areas, and groundwater aquifers, where redox conditions vary over space and time. This type of redox heterogeneity can have an outsized influence on As speciation, release, and retention. Recently, Kumar et al (2020a) showed that As release and retention in natural aquifer sand is governed by the presence of thin organically rich clay lenses, which generate redox heterogeneities in sediments and thus alter the relative concentrations of Fe and S and promote the formation of mobile thioarsenate species.
-reducing conditions while conducting a biostimulation experiment at the Rifle Site in Colorado to treat the U-contaminated groundwater plume. NOM is also an important consideration in understanding As fate and mobility in environmental systems, such as wetlands, riparian areas, and groundwater aquifers, where redox conditions vary over space and time. This type of redox heterogeneity can have an outsized influence on As speciation, release, and retention. Recently, Kumar et al (2020a) showed that As release and retention in natural aquifer sand is governed by the presence of thin organically rich clay lenses, which generate redox heterogeneities in sediments and thus alter the relative concentrations of Fe and S and promote the formation of mobile thioarsenate species.
4.2.2. Biogeochemical critical elements that mediate contaminant behavior
Mn is one of the most abundant trace metals in soils, sediments, and natural waters. Its complex redox chemistry, consisting of three commonly occurring oxidation states of MnII, MnIII, and MnIV. The MnII state is highly soluble, while the MnIII and MnIV states are sparingly soluble and occur most frequently as mixed hydr(oxides). While oxidation of MnII by O2 is highly thermodynamically favorable, it is kinetically limited, leading to significant concentrations of dissolved Mn in many natural waters and leading control of Mn oxidation by a number of key processes. The most dominant of these is biological Mn oxidation, in which microorganisms including both bacterial and fungi catalyze the oxidation of MnII to MnIII/MnIV (Spiro et al 2010, Santelli et al 2011, Geszvain et al 2012). These organisms oxidize Mn directly through enzymes such as multicopper oxidase as well as indirectly through the production of reactive oxygen species (ROS) (Hansel et al 2012). ROS produced abiotically, e.g. through photochemistry or Fenton chemistry, also provide an abiotic pathway to MnII oxidation (Nico et al 2002, Learman et al 2011, van Genuchten and Peña 2016). The strong coupling of the Mn cycle with the ROS cycle is also thought to protect organisms from ROS-driven oxidative damage (Spiro et al 2010).
The oxidation of MnII can produce a wide variety of Mn (hydr)oxides with different structures and reactivity (Bargar et al 2009, Tan et al 2010, Droz et al 2015, Ling et al 2020). Given the ubiquitous nature of Mn (hydr)oxides, they play important roles at DOE sites. Some of the most common Mn (hydr)oxides have layered type structures consisting predominantly of MnO6 octahedra, but also contain multiple vacancies and defects, including inclusions of MnIII centers (Ilton et al 2005, Bargar et al 2009, Spiro et al 2010). The domain particle size is frequently on the nanoscale, making them natural nanoparticulate materials (Bargar et al 2009). Overall, Mn (hydr)oxides are both powerful oxidants of other elements and strong, high surface area adsorbents. In terms of sorption behavior, they have been shown to strongly sorb other key elements, including cobalt (Co), zinc (Zn), nickel (Ni), Mn, copper (Cu), Cr, cadmium (Cd), lead (Pb), and U (Duckworth et al 2009, Kwon et al 2009, 2013, Wang et al 2013a, Fuller and Bargar 2014, Peña et al 2015, Simanova et al 2015, van Genuchten and Peña 2016). This sorption behavior is frequently associated with vacancy sites within the oxides and can also include sorption at edges and structural incorporation. Mn (hydr)oxides frequently act as oxidants of other environmentally important metals and metalloids, including As, Cr, S, U, Tc, Co (Nico et al 2009a, Plathe et al 2013, Wang et al 2013a, 2013b, 2014a, 2014b, Duckworth et al 2014, Fan et al 2014, Tang et al 2014, McClain et al 2017). The oxidation of these species by Mn hydr(oxides) is important in the efficacy of contamination remediation approaches focused on reductive immobilization of critical elements. In the case of Cr, Mn (hydr)oxides appear to be the critical pathway by which CrVI is generated in natural systems (Plathe et al 2013, Wang et al 2013b, 2014b, Keiluweit et al 2015, Hausladen and Fendorf 2017, McClain et al 2017). More recently, Mn has been shown to play an important role in natural C cycling both in waters as well as soils and sediments, whereby either through direct reaction with Mn (hydr)oxides or by biological utilization of an Mn complex, organic material is oxidatively decomposed into smaller molecules (Keiluweit et al 2015, Estes et al 2017, Jones et al 2018, 2020). Microorganisms can directly utilize Mn (hydr)oxides as terminal electron acceptors, thereby coupled with C and other elemental redox cycles.
Fe oxides and Fe-bearing clay minerals are common constituents of soils and sediments at DOE sites. The biogeochemistry of Fe in most aquatic and terrestrial environments is driven largely by microbial activity, particularly in Fe-rich soils and sediments where Fe redox cycling by microorganisms is a significant component of C cycling and energy flux (Nealson and Saffarini 1994, Roden and Wetzel 1996, Lovley 2000). As a group, dissimilatory iron-reducing bacteria (DIRB) can use a wide range of FeIII forms as terminal electron acceptors for anaerobic respiration, including soluble FeIII complexes, FeIII oxides, and clay minerals containing varying amounts of structural FeIII (Fredrickson et al 1998, Zachara et al 1998, Kukkadapu et al 2001, O'Loughlin et al 2021). DIRB activity can yield a suite of FeII species including soluble FeII complexes, FeII complexes with the surfaces of organic and inorganic solid phases, and a host of mineral phases containing structural FeII (Lovley et al 1987, O'Loughlin et al 2013, 2019, Dong et al 2020). Many of these FeII species effectively reduce a wide range of organic and inorganic contaminants of concern at DOE sites, including chlorinated hydrocarbons, nitrate, CrVI, UVI, TcVII, NpV, and PuV (Kelly et al 2002, Bond and Fendorf 2003, O'Loughlin et al 2003a, 2003b, 2020, Fredrickson et al 2004, Kemner et al 2004, Peretyazhko et al 2008, Wiatrowski et al 2009, Felmy et al 2011, Veeramani et al 2011, Latta et al 2012a). Finally, FeII (bio)oxidation can result in the formation of a variety of FeIII-bearing minerals, depending on geochemical conditions.
Because of the relative insolubility of most Fe-bearing minerals in typical aquatic and terrestrial environments (i.e. circumneutral pH), their use for respiration by DIRB and iron-oxidizing bacteria (IOB) as terminal electron acceptors and donors, respectively, requires different mechanisms for electron transfer relative to soluble terminal electron acceptors/donors that are easily transported into the cell (e.g. O2,  ,
,  ). DOE-funded research has advanced understanding of extracellular electron transfer to solid-phase electron acceptors. Some DIRB such as Geobacter and Shewanella can transfer electrons directly to FeIII oxide surfaces by means of reductases located on their outer cell membrane (Shi et al
2009) or via electrically conductive pili or nanowires (Reguera et al
2005, Gorby et al
2006). The need for physical contact between FeIII oxide minerals and microbial cells, however, can be readily overcome. The dissolution of FeIII oxides is promoted by exogenous and endogenous ligands, and the resulting soluble FeIII complexes can diffuse away and be reduced by DIRB at a distance (Nevin and Lovley 2002, Taillefert et al
2007). Likewise, the transfer of electrons from the cell to external electron acceptors (e.g. FeIII oxides) can be facilitated by soluble electron shuttles, i.e. compounds that can be reversibly oxidized and reduced, which include a wide variety of endogenous and exogenous organic and inorganic compounds, including quinones, flavins, humic substances, and reduced S species (Nevin and Lovley 2000, 2002, Royer et al
2002, O'Loughlin 2008, Roden et al
2010, Flynn et al
2014).
). DOE-funded research has advanced understanding of extracellular electron transfer to solid-phase electron acceptors. Some DIRB such as Geobacter and Shewanella can transfer electrons directly to FeIII oxide surfaces by means of reductases located on their outer cell membrane (Shi et al
2009) or via electrically conductive pili or nanowires (Reguera et al
2005, Gorby et al
2006). The need for physical contact between FeIII oxide minerals and microbial cells, however, can be readily overcome. The dissolution of FeIII oxides is promoted by exogenous and endogenous ligands, and the resulting soluble FeIII complexes can diffuse away and be reduced by DIRB at a distance (Nevin and Lovley 2002, Taillefert et al
2007). Likewise, the transfer of electrons from the cell to external electron acceptors (e.g. FeIII oxides) can be facilitated by soluble electron shuttles, i.e. compounds that can be reversibly oxidized and reduced, which include a wide variety of endogenous and exogenous organic and inorganic compounds, including quinones, flavins, humic substances, and reduced S species (Nevin and Lovley 2000, 2002, Royer et al
2002, O'Loughlin 2008, Roden et al
2010, Flynn et al
2014).
Although S is often less abundant than Fe in soils and sediments at DOE sites, its biogeochemical transformations are more complex because of the variety of S oxidation states (VI, V, IV, III, II, I, 0, −I, and −II). In oxic environments SVI (e.g.  ) is typically the most thermodynamically stable valence state, while S−II (e.g. H2S and metal sulfides) is the most stable in anoxic environments. Unlike FeIII, SVI as
) is typically the most thermodynamically stable valence state, while S−II (e.g. H2S and metal sulfides) is the most stable in anoxic environments. Unlike FeIII, SVI as  is a highly soluble anion found commonly in groundwater, as a consequence of the dissolution of
is a highly soluble anion found commonly in groundwater, as a consequence of the dissolution of  minerals or as a product of the oxidation of iron sulfide minerals (e.g. pyrite). Like DIRB, dissimilatory sulfate-reducing bacteria (DSRB) and archaea are anaerobes that can obtain energy by coupling the oxidation of organic compounds or molecular H2 with the reduction of
minerals or as a product of the oxidation of iron sulfide minerals (e.g. pyrite). Like DIRB, dissimilatory sulfate-reducing bacteria (DSRB) and archaea are anaerobes that can obtain energy by coupling the oxidation of organic compounds or molecular H2 with the reduction of  to sulfide. This process is a key component of S and C cycles in anoxic soils and sediments (Megonigal et al
2004). In addition, sulfide generated by DSRB can reduce FeIII oxides (Kwon et al
2014a, 2014b, Johnson et al
2021), resulting in the oxidation of sulfide, primarily to elemental S (as molecular S0 and polysulfides) with minor amounts of thiosulfate. These pathways are important links coupling Fe and S biogeochemical cycles (Howarth et al
1992, Lovley 1993, Nealson and Saffarini 1994, Flynn et al
2014, Hansel et al
2015).
to sulfide. This process is a key component of S and C cycles in anoxic soils and sediments (Megonigal et al
2004). In addition, sulfide generated by DSRB can reduce FeIII oxides (Kwon et al
2014a, 2014b, Johnson et al
2021), resulting in the oxidation of sulfide, primarily to elemental S (as molecular S0 and polysulfides) with minor amounts of thiosulfate. These pathways are important links coupling Fe and S biogeochemical cycles (Howarth et al
1992, Lovley 1993, Nealson and Saffarini 1994, Flynn et al
2014, Hansel et al
2015).
4.2.3. C and N biogeochemistry
C biogeochemistry in soils is linked with redox reactions (e.g. aerobic respiration, denitrification, methanogenesis) in a simplified manner by conceptualization of a single solid phase soil organic matter (SOM) that provides a sustained supply of dissolved organic carbon (DOC). However, DOC availability that drives redox reactions is not unlimited and is also influenced by SOM dynamics. The traditional modeling approach for belowground C cycling does not explicitly consider the primary underlying processes and agents (e.g. microbes, aggregation) important for SOM cycling. Instead, SOM is classified into multiple C pools based on their degradability and is qualitatively named—for instance, recalcitrant versus labile or active pools. An emergent modeling approach recognizes that SOM comprises many complex molecules (Schmidt et al 2011, Dwivedi et al 2019) and that SOM decomposition is a function of a wide range of ecosystem properties and mechanisms such as temperature, thermodynamics, redox status, moisture content, exoenzyme production, decomposition, organo-mineral interactions, microbial necromass, ecology of belowground biota, and root contributions, as well as the mobilization of nutrients (as shown in figure 3(C), Schmidt et al 2011, Keiluweit et al 2016, Boye et al 2017, Dwivedi et al 2019, and references therein). As a result, the DOC supply that drives redox reactions varies significantly in space and time, and has implications for redox processes.
Motivated by this emerging understanding, Riley et al (2014) developed a complex reaction network of SOM, including above- and belowground organic inputs, multiple DOC species, and heterotrophic microbes. They captured vertical SOM variability reasonably well. However, they did not include explicit organo-mineral interactions in their reaction network, and used a Langmuir isotherm to represent sorption (Riley et al 2014), which does not adequately account for the suite of minerals present in the subsurface. Dwivedi et al (2017a) later enhanced this reaction network by including a Surface Complexation Model (SCM) and demonstrated controls exerted by plant roots and mineral surface area on availability and persistence of DOC in the soil.
Maggi et al (2008) developed a reaction network primarily consisting of ammonia oxidation and denitrification to examine N cycling in near-surface soil. Maggi et al (2008) did not consider other redox species, like Fe and S, in their reaction network as their investigation was limited to N cycling for an agricultural field. However, Arora et al (2016b) developed a reaction network representing a redox staircase for the Rifle Site, where redox species (e.g. Fe, S) and naturally reduced sediments were predominantly present. Dwivedi et al (2018a) subsequently used this reaction network at the Rifle Site and investigated the N dynamics in a floodplain environment. They demonstrated that, although the Rifle Site experiences little precipitation (being in an arid climate), short and episodic summer rain events led to the formation of HSHMs of N species due to nitrification resulting from the percolation of oxic water. Yabusaki et al (2017) also examined N biogeochemistry at the Rifle Site; however, they did not explicitly consider nitrification processes and instead focused on the reductive pathways. Moving away from a contaminated site, Dwivedi et al (2018b) also examined N biogeochemistry within intra-meander regions of the East River site. The East River, a pristine watershed, is low in nitrate. N cycling in the East River is primarily linked with snowmelt, vegetation, and atmospheric deposition (Newcomer et al 2021).
4.2.4. Coupling of contaminant and nutrient redox cycles
Redox-active U in natural sediments is coupled to the C cycle through metabolic oxidation of organic matter by microbes to CO2. Electrons released from this process are transferred to UVI, reducing it to UIV (see section 4.2.1). This process is the basis for stimulated bio-reduction, in which exogenous DOC is injected into groundwater, stimulating the reduction of UIV and resulting in net attenuation of dissolved U (Williams et al 2011, Long et al 2012). Another mode by which contaminant and nutrient cycles interact is via complexation reactions with functional groups produced via metabolic and anabolic processes. For example, NOM coatings, abundant on clay and other minerals, strongly complex UIV (see section 4.2.1). As pointed out elsewhere in this paper, methyl groups are profoundly important as complexing ligands Hg in natural waters, and organic functional groups containing reduced S are important as complexing agents for As (see section 4.2.1) and Hg (see section 6).
Nitrate-facilitated oxidation of UVI has been extensively investigated and provides numerous examples of strong coupling between the U and N cycles, as well as of the impacts of the Fe and S cycles on U-N redox coupling. Researchers have noted that when groundwater enriched in nitrate is amended with DOC (e.g. acetate), UVI will not be reduced until nitrate has been consumed, and metal reduction or  reduction commences (Finneran et al
2002, Senko et al
2002, Istok et al
2004). Furthermore, UVI can be recovered from aquifer sediments that have previously been reduced when groundwater is amended with nitrate (Finneran et al
2002, Istok et al
2004, Wu et al
2010). There are multiple pathways through which nitrate reduction can lead to UIV oxidation. It has been suggested that some bacteria can couple denitrification to UIV oxidation (Finneran et al
2002, Beller 2005). However, nitrate-dependent microbial oxidation of UIV may be slower than abiotic oxidation of UIV under nitrate-reducing conditions (Senko et al
2005b); for instance, UIV is oxidized more quickly by nitrite formed during denitrification (Senko et al
2002). UIV is oxidized most rapidly by FeIII oxides that are produced by the oxidation of FeII by nitrite (Senko et al
2005a). The extent (Ginder-Vogel et al
2006) of UIV oxidation by FeIII (hydr)oxides depends strongly on the type of FeIII(hydr)oxide; nanocrystalline/amorphous FeIII oxides may react rapidly with UIV due to high surface areas (Senko et al
2005a). In support of these conclusions, recent studies performed under diffusion-limited conditions suggest that denitrification occurs rapidly in soils and aquifers that host reduced U and is unlikely to overwhelm the reducing capacity of sediments that resides in the form of FeII, sulfide, and other reduced species.
reduction commences (Finneran et al
2002, Senko et al
2002, Istok et al
2004). Furthermore, UVI can be recovered from aquifer sediments that have previously been reduced when groundwater is amended with nitrate (Finneran et al
2002, Istok et al
2004, Wu et al
2010). There are multiple pathways through which nitrate reduction can lead to UIV oxidation. It has been suggested that some bacteria can couple denitrification to UIV oxidation (Finneran et al
2002, Beller 2005). However, nitrate-dependent microbial oxidation of UIV may be slower than abiotic oxidation of UIV under nitrate-reducing conditions (Senko et al
2005b); for instance, UIV is oxidized more quickly by nitrite formed during denitrification (Senko et al
2002). UIV is oxidized most rapidly by FeIII oxides that are produced by the oxidation of FeII by nitrite (Senko et al
2005a). The extent (Ginder-Vogel et al
2006) of UIV oxidation by FeIII (hydr)oxides depends strongly on the type of FeIII(hydr)oxide; nanocrystalline/amorphous FeIII oxides may react rapidly with UIV due to high surface areas (Senko et al
2005a). In support of these conclusions, recent studies performed under diffusion-limited conditions suggest that denitrification occurs rapidly in soils and aquifers that host reduced U and is unlikely to overwhelm the reducing capacity of sediments that resides in the form of FeII, sulfide, and other reduced species.
Although O2 can oxidize UIV, nitrate-dependent UIV oxidation has been shown to occur much more rapidly (Moon et al 2007, 2009). Using sediments from the background area of the Rifle Site, Moon et al (2007, 2009) demonstrated that slower oxidation of UIV under oxic conditions was due to O2 reacting quickly with numerous other reduced sediments species. In contrast, nitrate was less reactive towards the reduced species and could travel farther into the sediments and reach a greater total amount of UIV. In fact, O2 breakthrough did not occur for several weeks, whereas nitrate- breakthrough occurred within hours (Moon et al 2007, 2009). Furthermore, in the presence of FeS, only 1% of U in the sediment columns was solubilized in the presence of O2 over the same time period as 11%–60% U was solubilized in the presence of nitrate (Moon et al 2009). Buffering of UIV against oxidation by reduced species including FeII, S−II, and organic C has been suggested by several other researchers (e.g. Abdelouas et al 1999, N'Guessan et al 2010, Spycher et al 2011).
4.3. Advances in hydro-biogeochemistry
Hydro-biogeochemistry is essential for tracking and monitoring contaminants and nutrients that affect river water quality through subsurface geochemical fluxes. The subsurface geochemical behavior of contaminants and nutrients differs across sites due to distinct environmental, geological, and hydroclimatic conditions (Zachara et al 2013, Neeway et al 2019). In particular, sediments, mineral composition, geology, and hydroclimatic conditions give rise to contrasting mechanisms controlling plume persistence in the subsurface and riparian corridors. For example, several studies have demonstrated that the effect of mineral surfaces and sediments is important for the transformation and export of geochemical species (Keppler et al 2000, Fox et al 2012, Allard and Gallard 2013, Maher et al 2013, Cumberland et al 2016, Dwivedi et al 2017a, 2019), while hydrology plays a critical role in mobilizing contaminants (Li et al 2010, Flores Orozco et al 2011, Williams et al 2013, Dangelmayr et al 2017, Jemison et al 2020).
In a comparative study, Zachara et al (2013) outlined key differences in the geochemical nature of residual contaminant U at the Rifle and Hanford 300 Area sites. They suggested that the major differences between the sites and the kinetic processes influencing the U distribution in space primarily resulted from the spatial geochemical heterogeneity of detrital organic matter and the magnitude of groundwater hydrologic dynamics controlled by river stage fluctuations.
At DOE sites, including the Rifle and Columbia River Corridor at Hanford, it was found that the interplay between groundwater dynamics and river stage oscillations may result in gradient reversal, changes in flow velocities, redox zonation, and alterations in groundwater composition (Greskowiak et al 2010, Yabusaki et al 2011, Lezama-Pacheco et al 2015, Dwivedi et al 2018a, Noël et al 2019). The relative variations in groundwater levels and river stages can alter the mixing zone's extent, where groundwater meets the surface waters and exchanges solutes (Ellis et al 2007, Fritz and Arntzen 2007). The mixing zone may span over distances of the meter- to kilometer scales (Dwivedi et al 2017b, 2018b, Zachara et al 2020). For example, Arora et al (2016b), Dwivedi et al (2018a), and Yabusaki et al (2017) demonstrated that the subsurface geochemical exports, and thus river water quality, were influenced by the spatial geochemical heterogeneity of detrital organic and temporal variability in groundwater flow direction at the Rifle Site. Similarly, the Columbia River Corridor site at Hanford also experiences frequent hydrologic gradient reversals, primarily due to dam operations and distinct subsurface hydrogeology control (Shuai et al 2019). Overall, the gradient reversal at sites like Rifle and Hanford can result in episodic oxic and anoxic conditions in the subsurface (Yabusaki et al 2011, Lezama-Pacheco et al 2015, Dwivedi et al 2018a, Chen et al 2021). Consequently, changes in the oxic environment due to groundwater mixing and microbially mediated sequential biogeochemical processes can lead to distinct redox gradients through a succession of electron acceptors.
4.3.1. Influence of hyporheic exchange flows and water-table fluctuations on mobilizing contaminants
The hyporheic zone, an active dynamic ecotone where nutrient-rich groundwater and oxic river water interact, extends below the saturated sediments adjacent to the flowing channels of streams and rivers and signifies the elevated reaction rates for nutrients and contaminants (e.g. Mulholland et al 2008, Dwivedi et al 2018b). The hyporheic exchange flows, referring to the water movement between hyporheic zones and river channels, are important controls on contaminant and nutrient transformations and ultimately export to downstream surface water bodies. To illustrate this further, the dynamic redox behavior in the hyporheic zone influences the fate and transport of Cr, a common contaminant in subsurface and surface water. Liu et al (2017) investigated Cr behavior in the Columbia River hyporheic zone at the Hanford Site. They found that the hyporheic zone acts as a natural redox barrier for reductively immobilizing Cr under dynamic hydrological conditions.
In the Upper Colorado River Basin, seasonal water-table elevations can vary by up to 2 m over the course of the year (Noël et al 2019, Tolar et al 2020). During wet spring conditions, invasion of water into sediment pore space displaces air (and O2), allowing reducing conditions to develop in shallow sediments that can persist into late summer in fine-grained clay-rich sediments (Tolar et al 2020). Subsequently, drainage of water from sediments in late summer allows air to perfuse in, facilitating re-oxidation of UIV, as well as FeII and sulfides (Lezama-Pacheco et al 2015, Lefebvre et al 2019, Noël et al 2019). Under repeated annual cycles, complex patterns of U, as well as S and Fe redox cycles, overlap, creating distinctive signatures of redox conditions (Noël et al 2017a, 2017b).
4.3.2. Influence of hydro-climatic conditions on contaminant and nutrient dynamics
Hydro-climatic conditions specific to precipitation and evapotranspiration are equally important from the contaminant and nutrient dynamics standpoint. Frequent and intense rainfall in subtropical and humid climates like the Savannah River Site leads to prolonged flooding and stormwater infiltration (O'Reilly et al 2012). Changing hydro-climatic and geochemical conditions and wet-dry redox cycles exert significant controls on contaminant cycling and mobilization–immobilization in the subsurface (Noël et al 2017b, Song et al 2018). For example, redox processes such as nitrification can be significant for mobilizing contaminants (e.g. U), particularly when oxic river water or rainwater infiltration alters the redox gradients (Bone et al 2017a, Dwivedi et al 2018a, Cardarelli et al 2020, Tolar et al 2020). Noël et al (2019) showed that U accumulated as UIV in shallow riparian floodplain sediments at the contaminated DOE Shiprock Disposal Site in New Mexico under water-saturated spring conditions, but was oxidized and precipitated as UVI carbonates or silicates during summer drought.
Climate change is causing precipitation to decrease and evapotranspiration to increase in the Upper Colorado River Basin (Christensen and Lettenmaier 2007, Prein et al
2016), leading to decreasing water tables and exposure of reduced sulfidic sediments to oxygenated conditions. It is thus plausible that climate change will drive regional remobilization of accumulated U and other metals. In support of this conclusion, Janot et al (2016) estimated that oxidative release of U from a single moderate-sized naturally reduced zone at the Rifle Site could sustain a U plume at the regulatory concentration limit (0.044 mg L−1) for hundreds if not thousands of years.
L−1) for hundreds if not thousands of years.
Developing insights into the interplay of hydrology, geochemistry, and biology requires developing insights into processes controlling the cycling of contaminants, nutrients, and elements such as oxygen (O), Fe, S, C, and N in the groundwater and floodplain. High-fidelity multicomponent reactive transport modeling has been found to advance a robust predictive understanding of this interplay across the DOE representative sites (Yabusaki et al 2011, Li et al 2017, Dwivedi et al 2018a, Steefel 2019). Significant progress has been made in modeling complex multidirectional flow processes and their biogeochemistry interactions using these DOE representative sites. We describe these modeling developments in greater detail in section 5.
4.4. Characterization and monitoring strategies
Over the last several decades, there have been significant advances in the characterization of above- and belowground properties and dynamics necessary to parameterize, calibrate, and validate reactive transport models. At the point and single-borehole scales, there have been rapid advances in in situ sensors to measure groundwater and surface qualities, such as soil moisture, pH, water table, and electrical conductivity. These sensors are typically placed inside wells to continuously monitor groundwater or surface water.
Geophysical methods—including electrical resistivity, seismic, and radar—have been increasingly used to characterize the subsurface in a noninvasive manner (e.g. Hubbard and Rubin 2005, Binley et al 2015). Geophysics can bridge the gap in sparse wellbore locations by providing high-resolution and spatially extensive information in a minimally invasive manner. It can image subsurface contaminant plumes (e.g. Johnson et al 2010, 2012, Dafflon et al 2011), as well as map flow and biogeochemical properties that are important for predictive modeling and understanding (e.g. Johnson et al 2010, 2012, Dafflon et al 2011, Wainwright et al 2014). In particular, geophysics has been powerful in identifying biogeochemical hot spots (Wainwright et al 2016a). For the watershed or regional scale, airborne geophysics—particularly airborne electromagnetic surveys—was originally developed for mineral exploration, but is now increasingly used for water-resources applications (e.g. Barfod et al 2018, Ball et al 2020). While a number of different field experiments have demonstrated the feasibility of such subsurface imaging techniques (e.g. Johnson et al 2015), real-time monitoring was still challenging until recently. In particular, autonomous electrical resistivity and phase tomography (ERT) monitoring have the potential to achieve rapid and automated detection and identification of changes in the subsurface (Dafflon et al 2011, Johnson et al 2015).
In parallel, recent advances in remote sensing have revolutionized how we characterize and monitor ecosystem and watershed dynamics. A high-resolution digital elevation model (DEM) derived from the Light Detection and Ranging (LiDAR) method is increasingly available across the USA, which has been applied to understand better the relationship between geomorphology and hydrology (Prancevic and Kirchner 2019), as well as to measure snow depths and snow-water-equivalent (SWE) over a basin scale (Painter et al 2016b). Because LiDAR data has been shown to inform near-surface soil properties (Gillin et al 2015, Patton et al 2018), hydrological connectivity (Jencso et al 2009) and biogeochemical hot spots (Duncan et al 2013) in the literature, it is being increasingly used in several DOE sites, including the East River (Hubbard et al 2018) and Columbia River Corridor Site at Hanford (Chen et al 2021). Similarly, hyperspectral remote sensing is used extensively in DOE- and non-DOE supported research to map plant traits (e.g. Asner et al 2015), leaf water contents (e.g. Colombo et al 2008), leaf chemistry (e.g. Feilhauer et al 2015) and other properties, which are also proxies for soil biogeochemistry (e.g. Madritch et al 2014). The East River is making use of these methods to characterize a range of properties, including leaf area, wet, and dry weights for leaf sample, strait models developed independently for needle and non-needle leaf species (Chadwick et al 2020a, 2020b, 2020c). For capturing the spatiotemporal heterogeneity, time-lapse images from satellites have enabled the monitoring of vegetation dynamics associated with hydrological disturbances (e.g. Wainwright et al 2020). The remote sensing images are available at increasingly high resolution and high frequencies (Falco et al 2018, Devadoss et al 2020).
Representativeness and quality of collected environmental datasets (e.g. groundwater, precipitation) impact accuracy and precision of climate, hydrological, and biogeochemical analyses and predictions. Often, these are incomplete and may contain unspecified or missing entries due to unavoidable reasons, such as unfavorable weather conditions or delays in collecting sensor data. Several statistical and machine learning-based methods have been developed to render Quality Assurance (QA)/Quality Control (QC) and address related issues using datasets at the East River (Dafflon et al 2020, Mital et al 2020, Faybishenko et al 2021, Dwivedi et al 2022).
5. Simulation capability developments (HPC, RTM and other advances)
With the evolution of research on the DOE sites, several important advances have been made, and new computational capabilities have been developed that enable a more robust predictive understanding of the complex processes at work. A more detailed description of these processes is presented below.
5.1. Developments in hydrological and reactive transport modeling tools
The need to understand and predict contaminant transport and the export of nutrients and other important constituents has driven the development of much-improved modeling tools for multiphase (variably saturated) hydrological flow, such as TOUGH (Pruess 1987, 2004, Finsterle et al 2014, Jung et al 2018, Sonnenthal et al 2021), STOMP (White and Oostrom 2000, 2003), PFLOTRAN (Hammond et al 2007, 2014, Lichtner et al 2015), and reactive transport such as Crunch (Steefel et al 2005, 2015), ToughReact (Sonnenthal et al 2021), PFLOTRAN (Hammond et al 2007, 2014, Lichtner et al 2015). In turn, the availability of the new and evolving software has made it possible to address many scientific and engineering issues quantitatively at levels that were not possible previously.
5.1.1. Multiphase hydrology software
The multiphase (variably saturated) hydrological flow software developed for the DOE sites has historically been intended primarily for simulation of subsurface processes based on continuum formulations for porous media Darcy flow. Several hydrological codes such as HP1/HPx (Jacques and Šimŭnek 2005, Jacques et al 2006, 2012, 2013), PHT3D (Appelo and Rolle 2010, Greskowiak et al 2010), OpenGeoSys (Kolditz et al 2012, Nagel et al 2013), MIN3P (Mayer et al 2002), and HYDRUS (Šimŭnek et al 2006, 2008, Šimŭnek and van Genuchten 2008) have been developed globally since the late 1980s and early 1990s. However, many of the multiphase codes for variably saturated flow were developed within the DOE Laboratory system, including the TOUGH family of codes (Finsterle et al 2014), PFLOTRAN (Hammond et al 2012), STOMP (White et al 2012), NUFT (Hao et al 2012), FEHM (Zyvoloski et al 1997a, 1997b, Dempsey et al 2012), and Amanzi–Advanced Terrestrial Simulator (ATS) (Garimella et al 2014, Coon et al 2016, 2020). Variably saturated flow has been represented by the Richards equation, which assumes a passive gas phase, as well as with a full multiphase treatment in which multiple phases are subject to gradients and allowed to flow. Enhancements over the years have focused on improving the numerical stability of the Richards or multiphase flow simulations, which are notoriously difficult because of the nonlinearity of the problems they address. In addition, implementation of massively parallel computer architectures has been the focus of much of the effort (e.g. Hammond et al 2012, Garimella et al 2014, Jung et al 2017), although the details of this effort are beyond the scope of this review.
In order to extend beyond the subsurface so as to consider integrated surface and subsurface water, as required for analyzing terrestrial systems that consist of watersheds and river basins, much additional effort has been required. DOE-supported researchers have made important advancements to the state of the art in these integrated surface–subsurface hydrological models, with a focus on highly parallel open-source community codes. For example, extensions (Wu et al 2021) of PFLOTRAN (Hammond et al 2014) to include surface water and recent updates (Kuffour et al 2020) to the integrated surface–subsurface hydrology model PARFLOW (Kollet and Maxwell 2008, Kollet et al 2010) were supported by DOE projects. The Amanzi–ATS code (Coon et al 2016, 2020) was initiated as subsurface flow and reactive transport code (Amanzi) to support work at DOE legacy sites, and then extended as Amanzi–ATS to include surface flow and reactive transport processes to support research across DOE's network of watershed research testbeds.
The state of the art with respect to reactive transport modeling was summarized recently in Steefel et al (2015). In this summary of a special issue on benchmarking of reactive transport software for environmental applications emphasizing continuum formulations, such codes as PHREEQC, TOUGHReact, OpenGeoSys, PFLOTRAN, MIN3P, eStomp, HydroGeochem, and CrunchFlow were described. The first of these benchmarking workshops was supported by DOE-BER, although at a bare-bones level. The codes were shown to be capable of achieving similar or identical results for a range of complex environmental problems that required numerical solutions, i.e. those with nonlinear or coupled processes that are well beyond what can be described with analytical solutions subject to many simplifying assumptions. A significant portion (but not all) of this software development was undertaken with funding from the DOE, while the applications of the software were motivated in many cases by environmental problems on DOE lands and later by the desire to understand and predict biogeochemical cycling in ecosystems.
The development of biogeochemical reaction capabilities that could be used in reactive transport codes has followed a somewhat separate path. These efforts have followed closely the fundamental scientific work on understanding the role of microbes in surface and subsurface environments, particularly as they contribute to contaminant retardation through their control of the redox environment (NABIR 2006). Thus, the focus in reactive transport development here has been on incorporating arbitrarily complex biogeochemical reaction networks mediated by one or more competing or collaborating microbial communities. The microbial communities mediate rates that depend on the local environmental conditions and thermodynamics and contribute to reaction pathways that affect contaminant sorption (and thus retardation) and mineral dissolution + precipitation, as well as gas exchange. One example of such a biogeochemical reaction network with multiple microbially mediated pathways is shown in figure 4 (Arora et al 2016b).
Figure 4. Example of a biogeochemical reaction network used to simulate processes in the Rifle flood plain (from Arora et al 2016b).
Download figure:
Standard image High-resolution imageAs might be expected for complex environmental problems like those facing the DOE Hanford Site, where several processes come into play over a large spatial extent, massively parallel computing for reactive transport is now playing an important role. Arguably the first parallel code for reactive transport was the MCTracker code described and verified in Yabusaki et al (1998). Following this by a few years was the parallel software PFLOTRAN (Hammond et al 2012) that built on many of the capabilities found in FLOTRAN. The power of this new code and its application to the real-world environmental problem of U contamination in the Hanford 300 Area was highlighted in Hammond and Lichtner (2010), discussed below (figure 5). As part of the DOE Advanced Simulation Capability for EM (ASCEM) program, the high-performance computing code Amanzi was developed to simulate subsurface flow and transport. This Amanzi framework was later integrated into the ATS code to bring in surface processes like freeze and thaw (Painter et al 2016a), overland flow, and river flow (Coon et al 2020).
Figure 5. Example of a 3D flow and reactive transport simulation of the Hanford 300 Area, with hydrological and geochemical transients driven by changing water stages in the Columbia River (from Hammond and Lichtner 2010). Hammond and Lichtner (2010). John Wiley & Sons. Copyright 2010 by the American Geophysical Union.
Download figure:
Standard image High-resolution image5.1.2. Integrated surface–subsurface reactive transport software
Although spatially distributed models that fully integrate surface and subsurface flow representations are increasingly available (e.g. Paniconi and Putti 2015), extensions to include reactive transport in surface and subsurface water are relatively rare, Amanzi–ATS includes the capability for integrated surface–subsurface transport and accesses mature biogeochemical reaction capabilities in PFLOTRAN or CrunchFlow (see Dwivedi et al 2016a) through an Application Programming Interface called Alquimia (github.com/LBL-EESA/alquimia-dev). This relatively new reactive transport capability for integrated surface water-groundwater systems is an important complement to observational studies, with significant potential for aiding in the interpretation of field observations.
Reactive transport in the integrated surface water–groundwater system is important for understanding transport and biogeochemical transformation in river corridors. The hyporheic zone often contains biogeochemical hot spots where many biogeochemical transformations occur. However, the open channel is the primary pathway for downstream movement and allows for O2 exchange with the atmosphere, emphasizing the importance of an integrated modeling capability. Integrated surface–subsurface reactive transport models have great potential for helping understand the field-scale implications of relatively small-scale hydrologic and geochemical processes occurring in the hyporheic zone. However, it is first necessary to address the mismatch between the native spatial and temporal scales of fundamental hydrologic and biogeochemical processes, and the systems that they impact (e.g. watersheds). For example, locally anoxic conditions within an otherwise O2-rich environment are important for nutrient and trace-metal processing in stream corridors, but bringing that fundamental understanding into field-scale models remains challenging. DOE-funded researchers have recently introduced multiscale models that confront that scale mismatch by using relatively coarse discretization for the channel network, with subgrid models for hyporheic zone transport and reactions. Painter (2018), and Painter (2021) introduced the ADELS (Advection Dispersion equation with Lagrangian Subgrid) model, which associates a one-dimensional advection-reaction subgrid model with each channel grid cell to represent the ensembles of streamlines that are diverted from the channel into the biogeochemically active hyporheic zone before returning to the channel. The computationally challenging simulations are made tractable by formulating the subgrid models in stochastic Lagrangian form, with hyporheic age replacing travel distance. Key model inputs—hyporheic exchange rates and hyporheic lifetime distributions—can be inferred from nonreacting tracer tests (Rathore et al 2021) or synthesized data products (Gomez-Velez and Harvey 2014). The ADELS model is implemented in Amanzi–ATS (Jan et al 2021). Motivated by similar considerations, Fang et al (2020) implemented a multirate transient storage model in PFLOTRAN. In contrast to the advection-based conceptualization of the ADELS model, the multirate transient storage model (mTSM) considers the hyporheic zone to be a collection of well-mixed reactors that are each coupled to the channel but not to each other. The ADELS and mTSM models have been shown to be mathematically equivalent for nonreacting tracers but yield very different results for reactive transport, because of the different conceptualization of hyporheic exchange (Painter 2021). These model developments clearly demonstrate that field-scale models that honor fine-scale understanding are possible and have significant potential, but the identified differences in field-scale reactive transport also underscore the importance of the observational studies afforded by DOE's network of watershed research testbeds.
5.2. Advances in contaminant transport modeling
Modern reactive transport methods have made significant contributions to the topic of contaminant transport in recent years, and many of these applications are to DOE legacy sites or to sites where DOE contributed funding (e.g. Naturita, Colorado). The contributions can be broadly divided into two important themes:
- (a)The comprehensive and rigorous treatment of geochemistry in contaminant transport models, and
- (b)The role of aquifer heterogeneity in transport and mixing rates.
The second of these topics is not considered further here, since it is primarily the purview of hydrology and transport disciplines and is beyond the scope of this review paper. We focus here on the geochemical and mineralogical aspects of the problem.
The starting point for contaminant hydrogeology has always been the linear distribution coefficient (or  ) models for sorption, which have the advantage of being easily incorporated into transport equations without rendering them nonlinear. It has also been asserted that they have an advantage in that the data required for the implementation is minimal, or at least less than that required by the multicomponent models discussed below. However, it should be pointed out that the
) models for sorption, which have the advantage of being easily incorporated into transport equations without rendering them nonlinear. It has also been asserted that they have an advantage in that the data required for the implementation is minimal, or at least less than that required by the multicomponent models discussed below. However, it should be pointed out that the  models, while potentially being as simple as the determination of the sorbed and aqueous concentration under a set of environmental conditions, typically require unique constraints for each and every site to which they are applied. In addition, as will be apparent from the discussion below, such
models, while potentially being as simple as the determination of the sorbed and aqueous concentration under a set of environmental conditions, typically require unique constraints for each and every site to which they are applied. In addition, as will be apparent from the discussion below, such  models may not even apply to a single site where conditions (temperature, salinity, competing ion concentrations, sorption site density) change over time and space. In other words, there is no real generality in the case of linear distribution coefficients, in contrast to the more rigorous ion exchange and surface complexation models (like that of metals on Fe-hydroxides) that can be applied quite widely.
models may not even apply to a single site where conditions (temperature, salinity, competing ion concentrations, sorption site density) change over time and space. In other words, there is no real generality in the case of linear distribution coefficients, in contrast to the more rigorous ion exchange and surface complexation models (like that of metals on Fe-hydroxides) that can be applied quite widely.
The use of linear  coefficients came under strong attack when it became apparent that 137Cs had migrated further than expected as a result of highly saline tank leaks in the Hanford 200 tank farm (Zachara et al
2002, Steefel et al
2003, 2005). To address this issue, multicomponent ion exchange and surface complexation models were then considered in the context of reactive transport. Below, we briefly review them.
coefficients came under strong attack when it became apparent that 137Cs had migrated further than expected as a result of highly saline tank leaks in the Hanford 200 tank farm (Zachara et al
2002, Steefel et al
2003, 2005). To address this issue, multicomponent ion exchange and surface complexation models were then considered in the context of reactive transport. Below, we briefly review them.
5.2.1. Ion exchange and Cs migration at Hanford 200 Area
The problem of understanding and predicting 137Cs migration was made more complicated as a result of the multiple exchange sites that occur in sediments below the Hanford 200 tanks (Zachara et al
2002, Steefel et al
2003). In the Hanford case, the effective  depends on the Cs+ concentration in addition to the competing sodium ion (Na+) concentration (figure 6(A)), since the ion exchange sites with a very high preference for Cs+ are present in only low concentrations in the sediment. The low concentration of the high affinity exchange sites means that they could be completely filled, resulting in a reliance on relatively lower affinity sites for the 137Cs sorption.
depends on the Cs+ concentration in addition to the competing sodium ion (Na+) concentration (figure 6(A)), since the ion exchange sites with a very high preference for Cs+ are present in only low concentrations in the sediment. The low concentration of the high affinity exchange sites means that they could be completely filled, resulting in a reliance on relatively lower affinity sites for the 137Cs sorption.
Figure 6. Example of 137Cs transport in the Hanford 200 Area tank farm. (A) Effective linear distribution coefficient ( ) for Cs+ (from Steefel et al
2003). The
) for Cs+ (from Steefel et al
2003). The  depends on both the competing cation concentration (Na+ associated with NaNO3 in the tank wastes), and on Cs+ itself because of the presence of at least two sites with different selectivities for Cs+ versus Na+; (B) Relative migration of nitrate (nonreactive), and Cs+ at 1 M NaNO3 and 5 M NaNO3. The high Na+ concentrations in the tank leak explain the enhanced migration and thus weaker-than-expected retardation of 137Cs at the Hanford 200 tanks (from Steefel et al
2005).
depends on both the competing cation concentration (Na+ associated with NaNO3 in the tank wastes), and on Cs+ itself because of the presence of at least two sites with different selectivities for Cs+ versus Na+; (B) Relative migration of nitrate (nonreactive), and Cs+ at 1 M NaNO3 and 5 M NaNO3. The high Na+ concentrations in the tank leak explain the enhanced migration and thus weaker-than-expected retardation of 137Cs at the Hanford 200 tanks (from Steefel et al
2005).
Download figure:
Standard image High-resolution imageAn example 2D reactive transport simulation was presented in Steefel et al (2005) to demonstrate the effect of the competing sodium nitrate (NaNO3) (a major component of the leaking tank fluids) concentration. This simulation was carried out using ion exchange selectivities and site concentrations determined from Hanford bulk sediment (Steefel et al
2003). Figure 6(B) shows the transport of the nonreactive nitrate, with the plume extending over the entire domain size considered. The fractional migration of 137Cs at 1 M NaNO3 is significantly less, in keeping with the expectation that some retardation of this contaminant will occur (although still more than would be the case for 137Cs in a typically dilute soil or vadose zone water). At 5 M NaNO3, the migration of the 137Cs is significantly farther, even if still retarded relative to the nitrate. The enhanced 137Cs observed below many of the Hanford 200 tanks can thus be explained largely by a classical ion exchange model that accounts for the competing NaNO3 concentrations in the plume, and by the elevated concentrations of Cs+ that exceed the number of high affinity sites that are available for strong sorption. The model also suggests that as leaking of highly concentrated NaNO3 tank fluids ceases, further migration of the 137Cs is unlikely. This is because infiltration by more normal rainwater is not capable of displacing the 137Cs presently sorbed on the Hanford sediment. In any case, the use of constant linear  to describe the transport fails completely, so either the more rigorous ion exchange models need to be used, or at minimum, a model that accounts for environmental condition-dependent sorption (i.e. a 'smart
to describe the transport fails completely, so either the more rigorous ion exchange models need to be used, or at minimum, a model that accounts for environmental condition-dependent sorption (i.e. a 'smart  ').
').
5.2.2. Surface complexation and U migration
Perhaps an even more convincing demonstration of the inadequacy of the classical  models is provided by reactive transport analyses of metal and radionuclide migration influenced by surface complexation on Fe-hydroxides. In fact, an entire generation of geochemists has investigated the surface complexation behavior of metals on ferric hydroxides over many years (Dzombak and Morel 1990, Davis et al
1998, 2004), but only more recently has the use of surface complexation models been demonstrated convincingly in reactive transport frameworks at the field scale.
models is provided by reactive transport analyses of metal and radionuclide migration influenced by surface complexation on Fe-hydroxides. In fact, an entire generation of geochemists has investigated the surface complexation behavior of metals on ferric hydroxides over many years (Dzombak and Morel 1990, Davis et al
1998, 2004), but only more recently has the use of surface complexation models been demonstrated convincingly in reactive transport frameworks at the field scale.
Most likely, the first successful demonstration of the use of surface complexation models to describe field-scale reactive transport was presented by Davis and co-workers for the DOE-LM U-contaminated site at Naturita, Colorado (Curtis et al
2004, 2006, Davis et al
2004). Davis et al (2004) used both electrostatic and nonelectrostatic surface complexation models to describe U sorption on the Naturita sediment, but finally settled on the nonelectrostatic models because of their flexibility in treating natural and complex multimineralic sediments (figure 7(A)). In order to match the pH dependence of sorption, in particular, it was necessary to use a three-site SCM consisting of weak, strong, and very strong sites. Figure 7(B) shows the match with the experimental data at a variety of CO2 partial pressures, an important variable because of the strong competition between U carbonate complexes in solution and the surface complexes developed on the Naturita sediment surfaces (Hsi and Langmuir 1985, Prikryl et al
2001, Davis et al
2004). Calcium uranium carbonate complexes in solution can further reduce the sorption of U, especially Ca2UO2(CO3) (Bernhard et al
2001, Brooks et al
2003, Fox et al
2006).
(Bernhard et al
2001, Brooks et al
2003, Fox et al
2006).
Figure 7. Example demonstrating inadequacy of  (linear sorption) models to describe U transport. (A)
(linear sorption) models to describe U transport. (A)  values for Naturita sediment as a function of partial pressure of CO2, pH, and solid/liquid ratio (from Davis et al
2004). (B) Simulated values of field scale dissolved U, alkalinity, and spatially variable
values for Naturita sediment as a function of partial pressure of CO2, pH, and solid/liquid ratio (from Davis et al
2004). (B) Simulated values of field scale dissolved U, alkalinity, and spatially variable  at the Naturita site (From Curtis et al
2006). (A) Reprinted from Davis et al (2004), Copyright (2004), with permission from Elsevier. (B) Curtis et al (2006). John Wiley & Sons. Copyright 2006 by the American Geophysical Union.
at the Naturita site (From Curtis et al
2006). (A) Reprinted from Davis et al (2004), Copyright (2004), with permission from Elsevier. (B) Curtis et al (2006). John Wiley & Sons. Copyright 2006 by the American Geophysical Union.
Download figure:
Standard image High-resolution imageCurtis et al (2006) used the nonelectrostatic surface complexation model presented in Davis et al (2004) to investigate field-scale transport at the Naturita, Colorado Site (figure 7(B)). They demonstrated that the surface complexation model accurately reproduced the available data when combined with the realistic flow and transport parameters for the aquifer. They also demonstrated the inadequacy of a constant  model (figure 7(B)).
model (figure 7(B)).
Many other studies have been carried out that demonstrate the ability of the surface complexation models to describe field-scale behavior, including those at the U-contaminated Rifle Site (Yabusaki et al 2007, Zachara et al 2013), and at the contaminated Savannah River Site (Arora et al 2018).
5.2.3. Modeling of microbially mediated reaction effects on U migration at the Rifle Site
The fundamental studies of biogeochemistry mediated by microbial communities undertaken as part of the DOE research portfolio over the last 10–15 years have contributed to a much improved ability to model redox processes in the subsurface. This is a key development for predicting the dependence of redox-sensitive contaminants like U and Tc in the subsurface. The redox state of the subsurface depends on the classical sequence of electron acceptor-donor microbially mediated pathways from aerobic respiration under oxidizing conditions to methanogenesis under reducing conditions. The redox state of the subsurface can be manipulated with the injection of a suitable electron donor that drives the system towards reducing conditions, as was demonstrated at the Rifle Integrated Field Research Challenge in western Colorado (Anderson et al 2003, Dullies et al 2010, Williams et al 2011). The engineered biostimulation of indigenous microorganisms like Geobacter (see section 4.1) to catalyze the conversion of UVI to a reduced immobile form of U, UIV, was demonstrated convincingly at the field scale, although the longevity of such treatments has remained in question.
In the field experiments undertaken at the Rifle Site, acetate was injected into a series of wells upgradient of a series of monitoring wells (figure 8(A)). However, to simulate the acetate injection and resulting U reduction and immobilization as a result of microbial (primarily Geobacter) activity, it proved useful to begin with 1D column experiments (Li et al 2009). With this approach, it was possible to show that the reactive transport modeling could capture both the sequence and distribution of redox processes (figure 8(B)).
Figure 8. Acetate injection-induced reduction and immobilization of UVI at the Rifle Site. (A) Well layout for the 2008 field experiment at the Rifle Site (from Yabusaki et al 2011). (B) Comparison of column experimental data (symbols) and 1D reactive transport modeling (from Li et al 2009). (C) Comparison of simulated (red solid line) and observed dissolved UVI in groundwater as monitored during 2008 acetate injection experiment (from Yabusaki et al 2011). (A) Reprinted from Yabusaki et al (2011), Copyright (2011), with permission from Elsevier.
Download figure:
Standard image High-resolution imageTo capture the combined behavior of the acetate, U, and other electron acceptors/donors in the 2008 field experiment, it was necessary to use a fully 3D model for the site (Yabusaki et al 2011). The injection of acetate drives the system to reducing conditions, with the result that the UVI is immobilized as solid UIV, and the ability to capture this behavior (along with associated redox processes) at the field scale is demonstrated convincingly with the simulations as shown in figure 8(C) (Yabusaki et al 2011).
Similarly, to understand the biogeochemical behavior of U within the groundwater and aquifer solids at the Oak Ridge Field Research Center, and as part of their Integrated Field Research Challenge, a conceptual model for simulating an emulsified vegetable oil injection was developed and incorporated into the geochemistry code PHREEQC (Tang et al 2013a, 2013b). Numerous field experiments and subsequent interpretation of pyrosequencing and qPCR results in conjunction with geochemical data provided the basis for this hybrid microbial-geochemical conceptual model development (Gihring et al 2011). Consistent with the field observations, the model demonstrated the biologically mediated reductive immobilization of U in groundwater with various electron donors and under diverse geochemical conditions (Wu et al 2006a, 2006b, Watson et al 2013).
6. Hg: a use case to study watershed biogeochemical processes
Hg is a pollutant of global concern (Assessment, UNEP Global Mercury 2019). Hg has a complex environmental cycle that has been significantly altered by anthropogenic activity. Elemental Hg (Hg0) has a significant vapor pressure and long residence time in the atmosphere, facilitating long-range transport from source areas. Given that Hg mining dates back over 3000 years, there may be no place on the Earth's surface that has not been affected by this altered cycle.
Inorganic Hg has well-documented deleterious health effects, and its transformation in the environment to the more toxic organomercurial compounds (primarily monomethylmercury, MeHg) poses increased risks to human and environmental health. The MeHg measured in environmental samples is the net result of the counteracting processes of Hg methylation and MeHg demethylation. Research into Hg environmental cycling has a long history, yet fundamental questions remain unanswered, particularly with respect to the watershed biogeochemical conditions that promote net MeHg generation. For example, although the gap is closing, research on lotic ecosystems (rivers and streams) is relatively underrepresented in comparison to lentic (lakes and ponds) ecosystems.
DOE-supported research on watershed-scale biogeochemistry has been conducted using an iterative pattern-to-process approach, in which the interactions and feedbacks among pattern and process were investigated across a range of spatiotemporal scales. These are interpreted to inform our understanding of watershed-scale function. This is being achieved through a combination of long-term observations, targeted event sampling, studies of seasonal and intraday patterns, and conducting field-informed experiments under controlled conditions in the laboratory. An important study site is EFPC (see section 3.2), which has a well-documented history of Hg contamination that began at the headwaters and affected the creek and floodplain along its entire length (Brooks and Southworth 2011, Loar et al 2011).
6.1. Baseflow sampling identifies additional sources of Hg to the creek
Long-term (∼ year) observations made at baseflow along the length of EFPC demonstrate consistent opposing patterns of Hg and MeHg concentration. Total and dissolved Hg (HgT and HgD, respectively) concentrations decrease from the headwaters downstream. Conversely, total and dissolved MeHg (MeHgT and MeHgD) concentrations increase with distance downstream. Additionally, the MeHg concentrations show a seasonal dependence, with higher concentrations in spring and summer and lower concentrations in autumn and winter (figure 9).
year) observations made at baseflow along the length of EFPC demonstrate consistent opposing patterns of Hg and MeHg concentration. Total and dissolved Hg (HgT and HgD, respectively) concentrations decrease from the headwaters downstream. Conversely, total and dissolved MeHg (MeHgT and MeHgD) concentrations increase with distance downstream. Additionally, the MeHg concentrations show a seasonal dependence, with higher concentrations in spring and summer and lower concentrations in autumn and winter (figure 9).
Figure 9. Filter passing total Hg (A) and MeHg (B) along EFPC sampled under baseflow conditions as a function of season. Creek flow is from left to right. Smoothed curves are added to help visualize the data.
Download figure:
Standard image High-resolution imageDischarge in EFPC increases with distance downstream, suggesting that one explanation for the decreasing HgT and HgD concentration is that the additional water is diluting it in the channel. Nevertheless, dilution or particle settling cannot account for the concentration changes. In other words, the fact that the flux of Hg increases with distance downstream suggests that legacy sources of Hg in the watershed contribute additional Hg mass to EFPC (Peterson et al 2018). These mass-balance-based estimates are supported by natural abundance Hg stable isotope signature studies (Donovan et al 2014, Demers et al 2018). By comparing Hg stable isotope signatures from different possible source areas and along the creek, hyporheic water was identified as the likely source of the added Hg, and the reduction of HgII to Hg0 by both microbially mediated and photochemical mechanisms was indicated.
Two controlling variables on Hg speciation and transport are NOM and dissolved organic matter (DOM), respectively, and total suspended solids (TSS). Much of the Hg transported downstream is associated with suspended particles comprising a mixture of mineral particles, particulate organic detritus, and algae, predominantly diatoms. The nature of the association between Hg and particle surfaces has broader implications with respect to the reactivity and biogeochemical cycling of Hg in the creek ecosystem, including its release from particles and availability for methylation. Synchrotron-based x-ray fluorescence mapping of the suspended particles indicated that the mineral-bound Hg was closely associated with Fe and NOM (Gu et al 2014). In conjunction with complementary Fourier-transform infrared spectroscopy (FTIR) analysis, these results suggested NOM-facilitated Hg bonding to Fe oxides, in which Hg was tightly bound to reduced S groups in the NOM, and the NOM was bound to Fe-oxides via its carboxyl and hydroxide functional groups. The diatom-associated Hg was found on the outer cell surface, suggesting passive sorption onto the cell surface rather than active uptake.
The filter-passing, or dissolved, Hg is strongly complexed by reduced S groups in DOM (Skyllberg et al
2006). Using ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) coupled with electrospray ionization, investigators found that molecules containing multiple N and S atoms dominated the complexation of Hg (Chen et al
2017). From both EFPC and Suwanee River, a 2:1 complex ([C5HNS Hg−S2NHC5]+) that dominated Hg complexation was identified in DOM isolates based on both molecular mass and Hg stable isotope distribution. Possible molecular structures of this complex were estimated using density functional theory calculations.
Hg−S2NHC5]+) that dominated Hg complexation was identified in DOM isolates based on both molecular mass and Hg stable isotope distribution. Possible molecular structures of this complex were estimated using density functional theory calculations.
MeHg flux also increases with downstream distance, which is expected given that both concentration and discharge increase. As is the case for EFPC, MeHg is typically not the initial form of Hg entering the ecosystem; rather, it is formed in the environment from an inorganic Hg precursor via a microbially mediated reaction carried out by a diverse group of anaerobic bacteria possessing the hgcAB two-gene cluster (Gilmour et al 2013, Parks et al 2013, Podar et al 2015).
Numerous studies have demonstrated that watershed wetland abundance is positively correlated with MeHg concentrations in the water bodies that drain those watersheds (Wentz et al 2014). Nevertheless, the EFPC watershed has 3% wetland abundance, but EFPC has MeHg concentrations typical of watersheds with 30% wetland abundance. Like inorganic Hg, MeHg in the water column is either particle associated or complexed with NOM. An overarching goal of the research is to understand where and under what biogeochemical conditions Hg is methylated and MeHg demethylated.
6.2. Flood sampling reveals terrestrial–aquatic connections and MeHg sources
Targeted sampling of multiple storm-driven flood events revealed specific linkages between the terrestrial and aquatic environments. For instance, precipitation events delivered DOM and associated Hg to the creek, but not MeHg or particulate phases. Additionally, the recovery of dissolved solute concentrations to pre-flood levels closely followed the flood hydrograph, except for MeHgD which lagged significantly behind other solutes. Recovery of MeHgD concentrations closely followed the re-establishment of the diel cycle of dissolved O2 concentration (figure 10). Taken together, the results of targeted flood sampling implied that in-stream biological mechanisms are primarily responsible for MeHg generation in EFPC (Riscassi et al 2016).
Figure 10. Flood hydrograph from EFPC illustrating (A) creek discharge and specific conductance as a proxy measure of dissolved ions, and (B) dissolved MeHg and DO dynamics before, during, and after the storm event. The specific conductance mirrors the flood hydrograph diluting during higher flow and returning to pre-flood levels approximately one and a half days after the start of the flood. In contrast, DO and MeHgD do not return to pre-flood conditions until ten days after the flood start (from Riscassi et al 2016).
Download figure:
Standard image High-resolution image6.3. Seasonal and intraday patterns highlight the importance of algal biofilms
Natural cycles and anthropogenic activity affect stream ecology, water quality, and water quantity on timescales as short as months to hours. For example, the progression of the seasons leads to cyclic variation in creek discharge, water temperature, sunlight intensity, and duration. Well-established diel patterns of dissolved oxygen (DO) concentration and pH are driven by photosynthetic activity (Allan and Castillo 2007). Wastewater treatment plants and hydroelectric dam operations are anthropogenic causes of short-time-scale water quality variations and river discharge.
Do Hg and MeHg concentrations have analogous cyclic variations to things like the daily photocycle, pH, and DO, and if so, how do they compare with other water-quality variations? Multiple sampling campaigns have been conducted to monitor changes in water chemistry over 30 to 48 hours to address this question. Previous research has documented the photochemical demethylation of MeHg and the role that various photosensitizers, including NOM, play in enhancing or inhibiting the reaction (Qian et al 2014). Given these earlier results, lower MeHg concentrations were anticipated during the day, driven by photodemethylation. Instead, MeHg concentrations were positively correlated with the daily photocycle increasing during the day with overnight minima. While photodemethylation may have been occurring, the rate of MeHg production outpaced that of all demethylation reactions, resulting in net positive MeHg production (Brooks et al 2021).
Long-term observations demonstrated seasonally dependent watershed sources of MeHg to EFPC. Targeted flood event sampling pointed to in-stream as opposed to out-of-stream sources of MeHg, and that the recovery of MeHg concentrations parallels the recovery of diel patterns in DO. Diel MeHg concentration variations are closely correlated with the daily photocycle. Together, these observations led us to hypothesize that periphyton biofilms are a source of MeHg in EFPC.
6.4. Field-informed laboratory experiments identify a dominant source of MeHg
Periphyton, or benthic algae, biofilms are complex assemblages comprising biotic (e.g. algae, fungi, bacteria, and archaea, insect larvae), abiotic (mineral grains, entrained sediment particles), and detrital (coarse and fine particulate organic matter) components often embedded in an extracellular gel-like matrix. These biofilms are in-stream, and their photosynthetic inhabitants produce O2 in a pattern driven by the daily photocycle. During floods, the biofilms are buried and scoured, disrupting the daily DO cycle, which is gradually re-established as the biofilms return. However, bacterial MeHg production is only carried out by anaerobic bacteria. Specifically, the only known methylators are iron-reducing bacteria,  -reducing bacteria, fermentative microorganisms, and methanogenic archaea. For Hg methylation to occur in periphyton biofilms, these oxygenic systems must also harbor O2-free zones where these anaerobic microorganisms can be active.
-reducing bacteria, fermentative microorganisms, and methanogenic archaea. For Hg methylation to occur in periphyton biofilms, these oxygenic systems must also harbor O2-free zones where these anaerobic microorganisms can be active.
Chemical gradients within intact periphyton biofilms collected from EFPC were studied in the lab using microelectrode voltammetry. Using this technique, concentrations of DO, FeII, and S−II were measured vertically from the periphyton-water interface down into the biofilm. Over a vertical distance of a few millimeters, conditions progressed from O2-saturated to iron-reducing and  -reducing. The steep biogeochemical gradients within the biofilms were consistent with the biofilms harboring conditions conducive to MeHg production.
-reducing. The steep biogeochemical gradients within the biofilms were consistent with the biofilms harboring conditions conducive to MeHg production.
To further explore the role of periphyton biofilms in Hg cycling, biofilms were grown in the creek, returned to the lab, and amended with enriched stable isotopes of inorganic 201Hg and Me202Hg (Olsen et al 2016, Schwartz et al 2019). The production of isotopically labeled Me201Hg and loss of Me202Hg was monitored over time as measures of Hg methylation and MeHg demethylation, respectively (figure 11). Experiments were conducted in each of the four seasons. Although the biofilms were a net positive source of MeHg during most incubations, MeHg production was greater during spring and summer and for samples collected downstream versus upstream. Both results are consistent with the long-term field observations that show higher concentrations in those seasons and increased MeHg flux with downstream distance. Biofilms grown or incubated in the dark had substantially lower MeHg production, and in some cases, were net demethylating, which is consistent with the diel sampling in which MeHg concentrations increase during the day and decrease at night. Finally, biofilm structure was important to the measured activity and function of these communities; in biofilms with disrupted structure, methylation potentials decreased by 50%.
Figure 11. Hg methylation (production of Me201Hg) and MeHg demethylation (loss of Me202Hg) by periphyton biofilms over time. Samples were grown in winter and incubated at ambient stream temperature. These biofilms are a net positive source of MeHg to the creek (from Olsen et al 2016).
Download figure:
Standard image High-resolution imageHigh-throughput amplicon sequencing has been applied to methylating periphyton communities to understand better the complex interactions that occur within them (Carrell et al 2021). Bacterial and archaeal richness was lower in summer relative to biofilms in the autumn, while fungal richness showed the opposite pattern. Interestingly, Hg methylation potential correlated with numerous bacterial families that do not contain the Hg-methylation genes hgcAB, suggesting that overall microbiome structure and relationships within periphyton communities influence rates of Hg transformation.
Hg and MeHg in contact with periphyton are subject to other reactions in addition to methylation and demethylation, including sorption, desorption, and, in the case of HgII, reduction to Hg and its reoxidation. These ancillary reactions were measured, and a novel model was created—the transient availability model (TAM)—to more accurately describe the periphyton methylation-demethylation results (Olsen et al
2018). Consistent with lab incubations data of field-derived samples from EFPC, the TAM reproduced concentration versus time behavior that had previously been thought to be anomalous. Importantly, the TAM predicts faster Hg methylation rates than previous models, with broad implications for net MeHg generation in lotic ecosystems.
and its reoxidation. These ancillary reactions were measured, and a novel model was created—the transient availability model (TAM)—to more accurately describe the periphyton methylation-demethylation results (Olsen et al
2018). Consistent with lab incubations data of field-derived samples from EFPC, the TAM reproduced concentration versus time behavior that had previously been thought to be anomalous. Importantly, the TAM predicts faster Hg methylation rates than previous models, with broad implications for net MeHg generation in lotic ecosystems.
Using Hg biogeochemistry as a use case, we demonstrated that long-term observations in the EFPC, a DOE site (see section 3.2, Oak Ridge Reservation Site), coupled with natural abundance stable isotope fractionation, identified sources and locations of Hg entering the creek. Additionally, it identified seasons of the year that supported higher MeHg concentration. Targeted event sampling demonstrated that discrete and short-duration events connected the terrestrial and aquatic ecosystems in the watershed. In other words, these floods constitute hot moments with major implications for material and energy exchange. Event sampling also eliminated the floodplain as a source of MeHg to the creek and focused attention on in-stream sources. High temporal resolution sampling over daily periods produced the unexpected result of increasing MeHg concentration during the day. Controlled laboratory experiments confirmed that periphyton biofilms are a net positive source of MeHg and constitute hot spots of Hg, Fe, and S cycling. Further, biofilms with a higher degree of connectivity among its populations also have higher Hg methylation potential. This combination of approaches buttressed some ideas, but more importantly, highlighted previous misconceptions, identified HSHMs of governing processes, and opened new avenues of discovery to advance further our understanding of these systems that support diverse and vital ecosystem services for the global economy.
7. Evolution of scientific approaches and strategies to tackle scale and complexity
Significant progress has been made in process understanding at the hillslope and field scales, which is relevant in particular for watersheds such as the mountainous East River Site. Yet watershed-scale modeling has remained challenging because of the scale and wide underlying range of heterogeneity in watershed processes and parameters. Hyper-resolution watershed-scale models capable of exploiting high-performance computing are needed to resolve process complexity (level of details needed) and the spatial gradients that typically drive flow. However, these computationally expensive models pose a serious impediment to scientific progress, requiring abundant CPU hours. The variable resolution mesh or more sophisticated adaptive mesh refinement methods make it possible to focus on functionally dynamic regions compared to more conventional approaches. Efforts are underway to advance watershed-scale modeling using leadership-class computers, big data, and machine learning combined with learning-assisted physics-based simulation tools (e.g. https://exasheds.org/). However, the details of these efforts are well beyond the scope of this review. As an alternative, we have developed strategies to tackle scale and complexity over the years. Some examples of these strategies include multiscale, multiphysics, hybrid modeling, incorporating HSHMs, and functional zonation as part of scale-adaptive modeling.
7.1. Multiscale, multiphysics and hybrid modeling
Historically there has been a mismatch between the spatial and temporal scales at which fundamental hydrologic and biogeochemical processes are most readily studied and understood (molecular to laboratory) and those of the ecosystems that they impact (field to watershed/basin). Because of nonlinear process interactions and complex heterogeneity in natural ecosystems, small-scale (e.g. laboratory) studies do not directly translate to field settings. Key reactive transport parameters, including permeability, dispersivity, and reaction-rate coefficients are all well known to be scale dependent and, in some cases, boundary-condition dependent. These challenges have motivated a variety of modeling approaches that are aimed at integrating information across scales and representing ecosystem processes at multiple levels of fidelity and complexity through DOE- and non-DOE supported research.
Multiple approaches have been developed to address this challenge, each with advantages and limitations. A summary of selected approaches used in DOE-supported research is provided here; for broader reviews, see Keyes et al (2013) and Scheibe et al (2015a).
Multiresolution methods use variable discretizations to capture effects of small-scale processes and properties on large-scale phenomena (e.g. Özgen-Xian et al 2020). These methods include adaptive grid refinement (refining grids dynamically to put higher resolution where and when needed); multigrid methods (hierarchical sets of grids used to speed numerical convergence of a fine-grid solution), and nested or spatially varying grids that enhance resolution in specific subdomains (e.g. figure 12).
Figure 12. Several scale-aware constructs have emerged over the years to tackle scale and complexity of ecosystem processes. Examples illustrate multiresolution mesh, HSHMs, and functional zonation. Zoomed-in view of the mesh figure courtesy of Ilhan Özgen-Xian (LBNL).
Download figure:
Standard image High-resolution imageUpscaling methods use assumptions about the nature of subgrid variability to derive effective parameters valid at the model resolution. These methods include numerical upscaling (direct solution of subgrid domains to derive approximate grid-scale parameters), various forms of homogenization and volume averaging, and perturbation solutions to stochastic partial differential equations.
Hybrid multiscale methods are similar to multiresolution methods. However, they use fundamentally different models in selected subdomains (for example, pore-scale simulations in portions of a domain where mixing-limited precipitation occurs; Scheibe et al 2015b) and are sometimes referred to as 'adaptive physics' models. These include hierarchical methods (e.g. the heterogeneous multiscale method; E et al 2003) in which overlapping domains exchange information and concurrent methods (e.g. mortar methods; Balhoff et al 2008), within which distinct subdomains pass information at domain boundaries.
7.2. HSHM: a construct to cope with intense biogeochemical activity
The HSHM construct provides the basis for developing a robust predictive understanding of how contaminant behavior in subsurface heterogeneous media varies across spatiotemporal scales. Subsurface heterogeneities are sites of intense biogeochemical activity and are critical to watershed function. Recent studies have highlighted in greater detail the roles of the naturally reduced zones (or 'redox heterogeneities') on biogeochemical function. For example, heterogeneity caused by clay lenses in high permeability soils provides opportunities to sorb contaminants in place (Campbell et al 2012, Janot et al 2016), while fracture networks can lead to rapid infiltration of contaminants to groundwater (e.g. Berkowitz 2002, Arora et al 2019a). Thus, high contaminant concentrations can be spatially diffused across large scales or concentrated at higher rates in specific areas, thereby forming contaminant hot spots. Similarly, these concentrations can be temporally diffused or more concentrated during specific times, thereby exhibiting contaminant hot moments. In addition, hydrological perturbations in transiently saturated zones drive changes in soil microbial communities, triggering a range of geochemical responses, thereby forming dynamic HSHMs. Figure 12 illustrates static hot spots (i.e. fixed in space) and dynamic hot spots (i.e. migrating in space).
Although the HSHM construct simplifies the characterization of complex heterogeneous systems and time scales at which biogeochemical processes occur—by subdividing the region and time into a finite number of relatively homogeneous units—it lacks a quantitative definition. Moreover, the HSHM construct is underutilized because of its dynamic nature. In response to that, Bernhardt et al (2017) argued that the temporal dynamics of a putative hot spot are a fundamental trait that should be used in their description and, therefore, stressed the merger of HSHMs into a single descriptive term 'ecosystem control points.' This definition indicates that knowledge of the rate distributions has more relevance than knowledge of maximum rates. Partly supported by DOE, Bernhardt et al (2017) suggested four distinct categories of ecosystem control points: (a) permanent control points, experiencing high rates of biogeochemical activity relative to their proximity (e.g. floodplain), (b) activated control points, where higher biogeochemical activity is set off when certain substrates are delivered (e.g. low-lying topographic positions), (c) export control points that accumulate reactants until some hydraulic or diffusion threshold is not surpassed (e.g. geochemical exports through meanders), and (d) transport control points having extremely high transport capability without their direct intervention in exhibiting high biogeochemical activity (e.g. preferential flow paths). Table 2 presents some examples of HSHMs from DOE research in the backdrop of ecosystem control points.
Table 2. Examples of HSHMs in the context of ecosystem control points.
| Examples | Study | Type of ecosystem control point |
|---|---|---|
| Storm events constituted hot moments of Hg and MeHg in EFPC and U discharge in Tims Branch, resulting in orders-of-magnitude higher concentrations than observed during baseflow conditions | Batson et al (1996), Riscassi et al (2016) | Activated control points |
| Seasonal river stage fluctuations of more than 2 m resulted in U hot moments at the Hanford Site by promoting water intrusion and increasing residence times | Zachara et al (2020) | Activated control points |
| Naturally reduced zones or zones enriched with Fe and S minerals in sediments adjacent to the riverbanks at the Rifle Site constituted hot spots of C, N, S, and U | Campbell et al (2012), Janot et al (2016), Arora et al (2016a), Wainwright et al (2016b), Boye et al (2017), Noël et al (2017b), Dwivedi et al (2018a) | Permanent control points |
| Seasonal meltwater-driven water-table rise at the Rifle Site creates short-lived carbonate solutes (calcium ions) for 2-week, but intense oxidizing hot moments, during which the majority of annual U oxidation occurs | Lezama-Pacheco et al (2015) | Activated control points |
| Microtopographic features such as low-centered polygons act as CH4 hot spots in arctic tundra environments and have a significant impact on ecosystem-scale C fluxes | Vaughn et al (2016), Arora et al (2019c) | Activated control points |
| Meander acts as a sink for organic and inorganic C as well as Fe during the extended baseflow and high-water conditions | Dwivedi et al (2018b) | Export control points |
| Microtopographic features such as gullies at the East River Site exert significant impacts on redox processes in the hyporheic zone and thereby controlling subsurface geochemical exports of Fe and C | Dwivedi et al (2018b), Rogers et al (2021) | Transport control points |
7.2.1. Impact of HSHMs on biogeochemistry of legacy contaminants and nutrients
DOE investigations at former U ore processing sites within the Upper Colorado River Basin show the importance of HSHMs on legacy contamination (e.g. U) and nutrient cycling (e.g. Fe, S). Clayey lenses containing elevated organic C are common within coarse alluvium. These lenses host intense reducing activity (i.e. hot spots) and sustain halos of reducing conditions within the surrounding coarser aquifer sediments (Kumar et al 2020a). Precipitation of iron monosulfides within these lenses and reductive attenuation of UVI to UIV cause S and U to become enriched to levels 25 and 200 fold higher, respectively, than surrounding sediments (Janot et al 2016, Noël et al 2017a, 2017b, Lefebvre et al 2019). As and non-redox active species such as Zn have also been shown to accumulate under these conditions through multiple chemical mechanisms involving association with reduced S species, as well as precipitation of double hydroxides (Kumar et al 2020a, Engel et al 2021). When clayey sulfidic lenses reside within the zone of annual water-table excursion, exposure to O2 perfusion during summer drought conditions can cause SOM-complexed UIV species to be oxidized to UVI, providing a mechanism of release and contamination of groundwater. Desiccation of sediments during summer drought leads to precipitation of uranyl carbonates and silicates (Noël et al 2019).
Although the relationship between hydrological change and microbial function is centrally important for predicting watershed function, only a few studies have explored the microbial ecology of dynamic HSHMs. Cardarelli et al (2020) examined the microbial ecology of N-cycling archaea within transiently saturated and reduced zones at five contaminated DOE legacy floodplain sites (Shiprock in New Mexico, Rifle, Grand Junction, and Naturita in Colorado, and Riverton in Wyoming), which spans a 900 km N–S transect of the Upper Colorado River Basin. This study showed that ammonia-oxidizing 'ecotypes' are strongly influenced by water table position, the location of reducing zones, or both. This study revealed that archaeal ammonia oxidation predominates within the terrestrial subsurface beyond a meter belowground. Recently, (Tolar et al 2020) characterized microbial community diversity at the U-contaminated Riverton, Wyoming, legacy across transiently reduced zones. These results showed that microbial communities are surprisingly stable, despite robust flood-to-drought fluctuation and accompanying redox inversion. These findings were interpreted to indicate that microorganisms oscillate between 'active' and 'dormant' states, contingent upon prevailing environmental conditions, and their metabolic products play a more significant role in contaminant mobility than the organisms themselves. Taken together, these results indicate that microbial community responses to drought and flood are complex and require more study.
7.3. Functional zonation
Modeling eco-hydro-biogeochemical processes requires the characterization of the bedrock-to-canopy properties across watersheds. The tremendous heterogeneity of these properties is often a significant obstacle toward scaling modeling efforts from the hillslope and floodplain scales to a larger scale. Although various spatially extensive characterization techniques such as remote sensing and geophysics are available, it is still challenging to estimate each property distribution individually over the watershed scale, particularly soil and subsurface properties in the subsurface.
A functional zonation approach holds tremendous potential for tractably estimating individual property distributions over the watershed scale. In this approach, zones or spatial units within a landscape with unique distributions of multiple properties relative to neighboring regions can be identified using machine-learning-based spatial clustering approaches (e.g. figure 12). The zonation approach essentially builds on a hydrofacies approach, which has been used in groundwater hydrology to define hydrological parameters in each facies (e.g. Fogg et al 1998, Klingbeil et al 1999). The reactive facies (Sassen et al 2012, Wainwright et al 2014) defined both hydrological and geochemical properties in each facies and stochastically mapped the reactive facies based on multiple geophysical data. Bea et al (2013) documented the value of incorporating estimated reactive facies information within a reactive transport model to improve predictions of long-term U plume transport. These concepts have proved valuable in identifying zones and hot spots of biogeochemical activity (Flores Orozco et al 2014, 2018, Wainwright et al 2015).
More recently, the availability of enhanced remote-sensing (including hyperspectral) products and advanced machine-learning approaches has led to a paradigm shift in the zonation approach and demonstrated possibilities for characterizing the bedrock-to-canopy properties across watersheds. With this new paradigm shift, the zonation approach harnesses a suite of remote sensing datasets for identifying and distributing zones relevant to biogeochemistry and/or ecosystem functioning. In addition, the zonation approach takes advantage of the current scientific understanding of critical zone processes and insights into water and nutrient cycling and their exports within a watershed and its different compartments, particularly through the Critical Zone Observatory (CZO) network (Brantley et al 2017).
Next, we briefly describe some prominent applications of the zonation approach at DOE sites. Hubbard et al (2013) and Wainwright et al (2015) used geophysical and remote sensing datasets to identify zones representing the heterogeneity of key properties and ecosystem functions—such as soil properties and C fluxes—in Arctic tundra. Devadoss et al (2020) defined the ecosystem functioning zones (each of which has distinct time series signatures of soil moisture and plant dynamics) and investigated the relationship among soil, plant, and snow dynamics. Wainwright et al (2016b) subsequently incorporated U reactive facies (e.g. Bea et al 2013) into the zonation approach to identify biogeochemical hot spots (e.g. naturally reduced sediments) based on electrical geophysical methods at the Rifle Site. Several additional modeling studies at the Rifle Site represented naturally reduced sediments in their models to investigate the HSHMs of N, C, Fe, and DO and quantified their subsurface geochemical exports (e.g. Arora et al 2016b, Yabusaki et al 2017, Dwivedi et al 2018a).
Recent investigations have been focused on examining how properties associated with specific functional zones exert controls on water and N exports in response to snow dynamics and contribute to the aggregated watershed concentration–discharge signature in mountainous regions like the East River (Hubbard et al 2018). A suite of remotely sensed bedrock-through-canopy watershed data layers and machine-learning approaches were utilized at the East River to identify watershed zonation. The zonation approach explored the sensitivity of different identified zones to foresummer drought (Wainwright et al 2022). Zonation is also powerful for understanding the co-variability of above- and belowground processes, as well as identifying relevant field experimental sites throughout the domain. For example, research at the Hanford Site is using river channel morphology zonation (hydromorphic units, Ren et al (2021)) as a framework for a transferable understanding of river–groundwater exchange fluxes and transit times.
8. Current ecosystem science and legacy contamination
Current ecosystem science comprehensively aims to couple and model ecological, hydrological, biogeochemical processes across the critical zone, ranging from the bedrock to the canopy. Water security challenges have motivated current ecosystem science, which has continued to emerge and expand well beyond traditional contaminant remediation-type studies. Recent severe weather events and hydro-climatic conditions have only added to these challenges, raising important concerns about geochemical exports of metals, C, N, and nutrients to the environment. In past assessments, the contribution to the riverine budgets of C and N were largely neglected, as part of the monitored natural attenuation strategy exclusively focused on stabilizing contaminants in place. More recently, an increased focus has been placed on constraining the exchanges and fates of different forms of C and N in river-floodplain settings, because of their important role in driving biogeochemical interactions with contaminants, and the potential of increased fluxes under changing precipitation regimes. Several studies have emphasized strong interlinkages between subsurface legacy contamination and hydrologically driven biogeochemical exports of metals, C, N, and nutrients in watersheds (e.g. Stucker et al 2013, Zhang et al 2018, Kumar et al 2020b).
To illustrate this further, riparian zones and wetlands, where the contaminated groundwater interfaces with surface water, play a significant role in transforming contaminants because the presence of plant roots may facilitate the sequestration of trace metals. Large biogeochemical gradients exist in wetland ecosystems influenced by the primary production activities of plants that release large quantities of labile organic matter, and by the transport of O2 in roots to maintain aerobic respiration. Plant roots also modulate mineralogy and promote contaminant immobilization through altering contaminant binding environment (e.g. Kaplan et al 2016). Several studies have explored in situ immobilization of contaminants for remediation at several DOE sites (e.g. Szecsody et al 1998, Dai et al 2002, Pawloski et al 2009, Zachara et al 2013). Notably, the long-term sustainability of in situ reductive bioimmobilization of CrVI was examined under different biogeochemical regimes at the Hanford 100 Area (e.g. Faybishenko et al 2008, Beller et al 2014, Varadharajan et al 2017). Alternatively, plants can offer an effective solution for the removal of hexavalent Cr by transforming it to less mobile, reduced Cr species as shown in the literature (Xu and Jaffé 2006). Further, O2 diffused from the wetland plant roots into their surrounding sediments can result in distinctive Fe plaques that precipitate on the surface of roots (Blute et al 2004), which may consequently facilitate active Fe cycling by stimulating Fe-reducing bacteria such as Geobacter spp. in anaerobic wetland sediments of the Savannah River Site (Chang et al 2014). The enhanced Fe cycling can impact U attenuation by accumulating U near the plant roots in more oxidized states (UVI or UV), which are less susceptible to remobilization during episodic reoxidation events compared to UIV, as observed at the Savannah River Site (Gilson et al 2015, Kaplan et al 2016).
Wetlands and river corridors experience episodic oxidation events associated with wetting and drying cycles Noël et al (2017a), Tolar et al (2020). Such cycling may facilitate the ongoing formation of fresh Fe oxyhydroxide, which can sorb or co-precipitate with U. The flow and transport of water links vegetation, soil, and contaminant transformations at the contaminated sites. The fraction of soil pore space that is water-filled vs. air-filled, i.e. the soil saturation, determines the rate at which water and O2 can be transmitted to meet the transpiration and respiration demands of plant roots and aerobic soil microbes, thereby influencing soil redox potential (Yan et al 2018). Aerobic microsites may form around the roots of wetland plants growing in saturated soils that are otherwise anoxic, leading to reaction hot spots. Redox reactions and cycling of redox-sensitive elements can alter the solubility and availability of key nutrients and metals, shifting microbial metabolism from largely oxidative reactions to methanogenesis, denitrification, and other less energetically efficient processes (Megonigal and Neubauer 2019, Cardarelli et al 2020). In turn, the history of water availability and rooting zone redox potential have direct and indirect effects on vegetation's distribution, structure, physiological state, and health.
9. Global water security issues and possible solutions through DOE-supported science
The existence of contaminants and the presence of high levels of nutrients in the environment is a major threat to water security. The problem of freshwater contamination is more pressing than ever, because most of the world's prominent rivers, supporting several million people, are under threat. For instance, India's Ganges River is considered one of the most polluted rivers globally: it contains many chemicals, including toxic Cr (Tripathi et al 2016). A major drinking water source in China, the Yellow River, the second-longest in Asia and the sixth-longest river globally, has been suffering from severe pollution and is now on the verge of becoming unusable even for agricultural or industrial use (Brown and Halweil 1998, Lu et al 2018). The Citarum, the longest and largest river in West Java, Indonesia, faces extreme pollution from several contaminants, including Hg and As. The Hg concentrations in some parts of the river are as much as 100 times higher than levels considered acceptable by the EPA (maximum contaminant level is 2 ppb) (Paddison 2016). As another example, every year the Mississippi River in the USA receives millions of pounds of toxic waste, poisonous chemicals (e.g. As), and excess nutrients (e.g. N), thereby turning the river's mouth into a dead zone (Dodds 2006). The Colorado River and its tributaries, supporting over 36 million people in seven states in the western USA, have also experienced contamination from a variety of chemicals, including U and As (Gross 2017). Groundwater contamination by As in Bangladesh represents one of the largest mass poisonings in history, with over a million people exposed to elevated As levels in their drinking water (Islam et al 2010).
An accurate accounting of the fate and transport of these chemicals in the environment is key to ensuring water security. Many insights developed from several decades of research work at DOE sites described in this paper can be used globally to examine the fate and transport of various contaminants and nutrient cycling. Capabilities developed as part of DOE research include hydrological and reactive transport modeling tools. These tools can comprehensively address water security problems and are transferable across DOE sites and worldwide. For example, several DOE codes such as PFLOTRAN and CRUNCH are currently being applied for various environmental applications globally (e.g. Alt-Epping et al 2015, Knabe et al 2021).
Finally, current ecosystem science is evolving through emerging and promising developments that include the use of artificial intelligence methods, such as machine learning to support zone identification, the association of system properties and functions with zonal types, HSHM detection, and the development of macroscale reduced-complexity models (Painter 2018, 2021, Fang et al 2020). Collaborations among hydrologists, geochemists, and microbiologists have led to advances in the use of increasingly abundant data from molecular biology studies ('omics') to inform reactive transport models that are beginning to mature (Meile and Scheibe 2019). Multiscale heterogeneity and nonlinear process interactions in natural systems continue to challenge our predictive understanding, but these enhanced models and other recent advances offer promise for the future.
10. Summary, conclusions, and future directions
Within the DOE's Office of Science, the BER and other related DOE programs (e.g. ASCR, EM, ER) have contributed significantly to environmental sciences' progress since the late 1980s. They have addressed several challenging subsurface problems, including treating radioactive waste (e.g. U, Pu) and heavy metals and metalloids (e.g. Cr, Hg, As). These efforts have provided transferable insights into process understanding, designed novel monitoring strategies to collect valuable field information, developed robust predictive capabilities, and devised scale-aware approaches to tackle underlying processes and the complexity resulting from the ecosystems' scales and magnitudes. To document these scientific advances that are generalizable and applicable to a range of water security problems worldwide, we have synthesized research activities conducted at representative DOE sites and testbeds, including the Savannah River Site in South Carolina, Oak Ridge Reservation in Tennessee, Hanford in Washington, Nevada National Security Site in Nevada, Riverton in Wyoming, and Rifle and East River in Colorado, over the last two decades. In particular, we have described the progress made in subsurface environmental sciences through DOE programs. To demonstrate the range of topics that have been studied in the environmental sciences through DOE-supported research, we show a word cloud highlighting key contributions (figure 13), including keywords such as 'subsurface, environment, contaminant, redox, reactive transport modeling, and watershed'.
Figure 13. Word cloud shows a spectrum of science topics covered through DOE research over the past two decades. Font sizes show topical importance.
Download figure:
Standard image High-resolution imageEfforts through DOE-supported research have marked several breakthroughs, such as isolation of archaea from groundwater and expansion of the microbial 'tree of life' using genomic methods in the subsurface microbiology; understanding the geochemical and biogeochemical behavior of S, Fe, Mn, U, Cr, Hg, C, and N in redox dynamic environments; and generating a robust predictive understanding of the relevant hydro-biogeochemistry and eco-hydro-biogeochemistry through novel field measurements and simulations. These findings have led the way in predicting the behavior and formation of HSHMs in the environment. Multiscale, multiphysics, and hybrid models have provided a framework for rigorous reactive flow and transport simulation capabilities. These modeling capabilities have the potential to telescope into watershed subsystems to simulate fine-scale detail, taking advantage of advanced meshing workflow, machine learning, and functional zonation concepts.
We also discussed a paradigm shift in the ways that environmental science was done in the early 2010s. Until then, investigations of small-scale (molecular to the millimeter) processes had been a primary focus. This approach was inadequate for addressing scaling behavior in the presence of a range of complex, coupled, nonlinear processes and a wide range of landscape heterogeneity. A holistic system science approach evolved to address these concerns, standing on the shoulders of active contaminant remediation-type studies. Since then, the DOE has supported the system science approach through sustained investments and bringing in multidisciplinary, multi-institutional teams together to unravel the intricacies and interconnectedness of system components that influence the behavior of both contaminated and natural systems across a range of spatial and temporal scales. This system science approach has investigated watershed science, including surface–subsurface hydrology; groundwater-surface water interactions; C, nutrient, and trace element transformations; ecosystem disturbances and resilience; Earth systems impacts; and Earth system models' development. Again, these relatively more recent science questions took advantage of capabilities developed over the past two decades for contaminant transport and transformation modeling. Modeling capabilities included using high-performance computers to simulate hydro-biogeochemistry, representing complex environmental biogeochemical cycles within diverse subsurface environments. We also detailed parallel development in monitoring strategies that started from the column scale and evolved through the field to watershed-scale investigation. These scientific advances and scale-aware modeling approaches have led to watershed and basin simulation capabilities that include processes from the bedrock to the canopy. In this context, we also described eco-hydro-biogeochemistry development across various representative DOE sites and testbeds.
To date, although great strides have been made in understanding the coupled biogeochemical cycling of Fe, S, and C, as well as their role in the transformation of nutrient and contaminant elements through bottom-up (i.e. reductionist) and top-down (complex system) approaches, knowledge gaps remain for addressing water security challenges. However, the science underlying water security challenges—such as water availability in drought-prone regions, excess nutrients, hypoxia, and extreme events—is complex and requires community-wide efforts to address such challenges. The crux of such efforts is to transfer the data and knowledge across sites, testbeds, and watershed systems globally. These efforts entail interagency coordination (e.g. collaborations across DOE, United States Geological Survey (USGS), National Science Foundation (NSF), national and international universities, and observational networks) and mission alignment. In essence, future water security requires collective action; it will take a collective effort to tackle it.
To maximize community efforts, the DOE has promoted open watershed system science, which involves identifying community needs, challenges, and opportunities in the areas of multiscale integration, measurement, computation, and cyberinfrastructure (collectively known as the Integrated Coordinated Open Networked (ICON) Science). As the data are highly valuable for advancing science, the DOE has advocated the FAIR (i.e. findable, accessible, interoperable, reusable) principle for data management. More details can be found in the open watershed science report (U.S. DOE 2019).
Finally, through collaborations and the advent of newer technologies, data in Earth system science will grow in magnitude, scale, complexity, and diversity. Our ability to collect and create data has already far exceeded our ability to assimilate them with predictive models (Luo et al 2015, Gentine et al 2018, Reichstein et al 2019). This data deluge will require improvements to interact with the growing observations and model outputs. Automated data-model analytics will be needed to enable the assimilation of diverse multiscale data into models for near-real-time prediction, the rapid identification of system-tipping-point precursors, and the development of models that inform the real-time optimization of autonomous sensing systems—from watershed to water basin to continental scales (Hubbard et al 2020). Advanced machine learning approaches have the potential to provide actionable intelligence, which will enable efficient data management, smooth workflows, and interdisciplinary collaborations, all contributing to the scientific advancements needed to tackle water security problems in the future.
Acknowledgments
The work highlighted here was chosen because it was overwhelmingly supported by the DOE and would not have existed otherwise. However, the authors did not specifically require that each cited manuscript have a specific DOE funding source acknowledgment. The efforts of DD, CIS, BA, JB, SSH, PN, HMW, and KHW were supported by the Watershed Function Scientific Focus Area (SFA) funded by the US Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-AC02-05CH11231. DD and CIS also acknowledge support from the ExaSheds Project at Lawrence Berkeley National Laboratory funded by the United States Department of Energy, Office of Science, Biological and Environmental Research under Contract No. DE-AC02-05CH11231. HMW also acknowledges support from the Department of Energy, Office of Environmental Management, ALTEMIS—Advanced Long-Term Environmental Monitoring Systems project. This research used resources of the National Energy Research Scientific Computing Center (NERSC), a United States Department of Energy Office of Science User Facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DE-AC02-05CH11231. The efforts of MIB, DIK, KMK, and EJO were supported by the Wetlands Hydrobiogeochemistry Scientific Focus Area (SFA) at Argonne National Laboratory, which is supported by the Earth and Environmental System Science Program, Office of Biological and Environmental Research (BER), Office of Science, US Department of Energy (DOE), under Contract DE-AC02- 06CH11357. JB's effort was supported by the SLAC Floodplain Hydro-Biogeochemistry SFA, funded by the US Department of Energy, Office of Biological and Environmental Research, Earth and Environmental Systems Sciences Division. SLAC National Accelerator Laboratory is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The efforts of EP, SP, and SB were sponsored in part by the Office of Biological and Environmental Research within the Office of Science of the US Department of Energy (DOE), as part of the Hg Science Focus Area (Critical Interfaces SFA) and IDEAS-Watersheds projects at the Oak Ridge National Laboratory (ORNL). The DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan). ORNL is managed by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with DOE. XC and TS were supported by the United States Department of Energy, Office of Science, Office of Biological and Environmental Research, Environmental System Science (ESS) Program through the River Corridor Scientific Focus Area project at Pacific Northwest National Laboratory. MZ's contribution was performed with funding from the Department of Energy, Office of Science, Biological and Environmental Research, Subsurface Biogeochemical Research program (SCW1053) and performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. We thank Diana Swantek (LBNL) and Adam Malin (ORNL) for assistance with preparing figures 1, 3, and 8(A). We express our gratitude to Dan Hawks (LBNL) for helping with technical editing. Finally, we thank the anonymous reviewer(s) for their insightful comment that helped improve the manuscript.
Data availability statement
No new data were created or analysed in this study.