Abstract
Three-dimensional porous titanium alloys printed via electron beam melting have low stiffness similar to that of cortical bone and are promising scaffolds for orthopedic applications. However, the bio-inert nature of titanium alloy is poorly compatible with bone ingrowth. We previously observed that simvastatin/poloxamer 407 thermosensitive hydrogel induces endogenous angiogenic/osteogenic growth factors and promotes angiogenesis and osteogenesis, but the mechanical properties of this hydrogel are poor. The purpose of this study was to construct 3D-printed porous titanium scaffolds (pTi scaffolds) filled with simvastatin/hydrogel and evaluate the effects of this composite on osseointegration, bone ingrowth and neovascularization using a tibial defect rabbit model. Four and eight weeks after implantation, the bone volume, bone mineral density, mineral apposition rate, and push-in maximum force of the pTi scaffolds filled with simvastatin/hydrogel were significantly higher than those without simvastatin (p < 0.05). Moreover, filling with simvastatin/hydrogel significantly enhanced vascularization in and around the pTi scaffolds, and a significant correlation was observed between the volume of new bone and neovascularization (p < 0.01). In conclusion, incorporating simvastatin/poloxamer 407 hydrogel into pTi scaffolds significantly improves neovascularization, osseointegration and bone ingrowth.
Export citation and abstract BibTeX RIS
1. Introduction
Titanium alloys have great advantages in orthopedic metal implants due to their excellent biocompatibilities, high strength-to-weight ratios, and corrosion resistance [1]. However, the stiffness of wrought titanium alloys is much higher than those of cortical and cancellous bone [2]. A promising approach to alleviate biomechanical mismatch is to fabricate porous structures with significantly reduced modulus [2]. The use of additive manufacturing (AM) technologies in bone tissue engineering (TE) has increased in recent years, and 3D printing is becoming popular due to the ability to directly print porous scaffolds with the desired shapes and interconnected porosities [3].
3D porous titanium alloy scaffolds (pTi scaffolds) with additives that are printed using electron beam melting (EBM) have superior properties compared with traditional solid titanium alloys scaffolds, such as higher surface areas and lower stiffness similar to that of cortical bone, and are therefore useful for bone defect repair [4]. An ideal bone graft or substitute should provide a template for osteoconduction, growth factors for osteopromotion, and scaffolding for internal osteogenesis [5]. Therefore, additive osteogenic factors could be combined with pTi scaffolds to improve local osteogenesis [6]. We previously found that the incorporation of exogenous bone morphogenic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) significantly enhanced both angiogenesis and osteogenesis inside pTi scaffolds [7]. However, these factors are expensive and have relatively short half-lives. Small molecule drugs that can be processed into sustained delivery vehicles and that can stimulate endogenous cells to increase the production of BMPs and/or VEGF are valuable in the TE field [8].
Statins have been widely used to lower cholesterol and reduce the risk of heart attack for many years. Although statins stimulate bone formation in vitro and in vivo [9], controversies remain [8]. The observed discrepancies in statin effectiveness on bone formation might be due to the low bioavailability from oral administration; only approximately 5% of the active compound remains in circulation [10], with even less at the local site. Local administration of simvastatin has an excellent effect on bone formation [11]. In addition, the local application of simvastatin in polylactic acid can promote bone formation and bone defect healing [12]; a single local injection of simvastatin using poloxamer 407, a thermosensitive hydrogel with low toxicity and weak immunogenicity [10, 13], as the vehicle induced autogenous BMP-2 and VEGF expression and promoted bone formation in ovariectomized minipigs [12]. However, injectable thermosensitive simvastatin/hydrogel has poor mechanical properties. The 3D-printed porous titanium alloy has good mechanical properties and an internal porous structure, which provides cavities ideal for filling with the injectable thermosensitive simvastatin/poloxamer 407 hydrogel.
The goal of the present work was to construct composites of pTi scaffolds filled with simvastatin/poloxamer 407 hydrogel and evaluate the effects of bone ingrowth, osseointegration, and neovascularization using an in vivo tibial defect rabbit model.
2. Materials and methods
2.1. Fabrication of porous Ti6Al4V scaffolds
Porous cylinder Ti6Al4V scaffolds (figure S1(a)) (Ø5 mm × L6 mm, pore size of 640 μm, strut diameter of 400 μm) were fabricated using an EBM S12 system (Arcam AB, Sweden) as previously described [7]. Briefly, a cylindrical 3D model was designed, converted into a standard triangulation language (STL) file, and transferred to the EBM machine. Medical-grade Ti6Al4V powder (particle size 45–100 μm) was melted layer by layer according to the STL data, and solidification occurred by cooling. All samples were ultrasonically cleaned successively in acetone, ethyl alcohol and deionized water for 15 min.
2.2. Preparation of simvastatin/poloxamer 407 hydrogel and incorporation into pTi scaffolds
The poloxamer 407 hydrogels were prepared as previously described [10]. Briefly, poloxamer 407 (BASF, Ludwigshafen, Germany) was added to 0.01 M phosphate-buffered saline (PBS) (pH 7.4, at 4 °C) with stirring; the final concentration was 25% (w/w). After incubation overnight at 4 °C, the gels completely dissolved and formed clear, viscous solutions. Poloxamer 407 gels loaded with simvastatin (National Institutes for Food and Drug Control, Beijing, China) were prepared by adding the drug to the prepared poloxamer 407 solutions. The final simvastatin concentrations were 0, 0.1, or 0.5 mg ml−1. The scaffold was then placed inside a custom-made gel chamber, and equal volumes of simvastatin/hydrogel were injected at 4 °C. The samples were warmed to room temperature prior to implantation. The components are shown in table 1.
Table 1. The components of the simvastatin, poloxamer 407 hydrogel, and pTi scaffolds.
| Material | Components |
|---|---|
| Simvastatin | Simvastatin (purity: 98.5%) |
| Poloxamer 407 Hydrogel | Poloxamer 407 (25%), PBS (pH 7.4) (75%) |
| Ti6Al4V Scaffolds | Ti (90%), Al (5.7%), V (3.8%) |
2.3. Microstructural characterization of the simvastatin/poloxamer 407 hydrogel/pTi scaffolds
The microstructure of the simvastatin/poloxamer 407 hydrogel/pTi scaffolds was characterized in multiple experiments. In brief, the microstructure was observed using a stereoscope, and the surface morphology of the pTi scaffolds alone and the composites were detected using scanning electron microscopy (SEM) (S-3000N, Hitachi, Japan). The chemical composition of the scaffold surface was characterized by energy dispersive spectroscopy (EDS) attached to the SEM apparatus [14]. The crystallographic characteristics of the pTi scaffolds were determined using x-ray diffraction (XRD) (Bruker D8 Advance TXS, Germany) [15]. The porosity of the pTi scaffolds was analyzed using a mercury porosimeter (PoreMasterGT 60, Quantachrome Instruments, Boynton Beach, Florida, USA) [16]. The specific surface area was calculated by the N2-BET method. The 3D surface roughness was investigated by micro-CT as previously described [17]. The mechanical strength was explored by a mechanical testing system (Landmark, MTS Inc., Eden Prairie, MN, USA). Compression tests were performed at an initial strain rate of 10−3 s–1 at room temperature [18].
To evaluate the degradation behavior of the poloxamer 407 hydrogel in porous Ti6Al4V scaffolds, hydrogel degradation experiments were performed in vitro. First, the transparent poloxamer 407 hydrogel was stained with calcein for improved visualization. Next, simvastatin/poloxamer 407 hydrogel/pTi scaffolds were constructed as described in section 2.2, and the composites were immersed in PBS at 37 °C. The degradation behavior of the poloxamer 407 hydrogel was observed and imaged every 3 days.
To explore the simvastatin release profile delivered by the poloxamer 407 hydrogel in the 3D printed porous titanium scaffold, in vitro simvastatin release experiments were performed as previously described [10]. Briefly, the 3D-printed porous Ti6Al4V scaffolds with incorporated simvastatin/poloxamer 407 hydrogel were placed in glass tubes and immersed in the same volume of PBS solution at 37 °C. The bathing buffer was collected and replaced with fresh buffer every 12 h. The released amounts of simvastatin were evaluated by a μQuant microplate spectrophotometer at 240 nm (Perkin Elmer Life Sciences, Waltham, MA, USA). The percentage of simvastatin released over time was calculated according to a standard calibration curve. Samples were measured in triplicate.
2.4. Cytotoxicity tests
To evaluate the cytotoxicity of the pTi scaffolds, cytotoxicity tests based on the international standard ISO 10993-5 were performed as previously described [15, 19]. In brief, to obtain leaching liquor from specimens, the scaffolds were immersed in MEM at a rate of 3.6 cm2 ml−1 at 37 °C for 3 days. Next, the L-929 fibroblast cells (ATCC, Manassas, VA, USA) were seeded in the leaching liquor at a density of 104 cells per well and incubated in a humidified atmosphere with 5% CO2 at 37 °C for 24, 48, and 72 h. Normal medium was used as a negative control. The cells were then incubated with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (50 μl well–1, 1 mg ml−1) for 4 h. The solution was removed, and dimethyl sulfoxide (DMSO) (100 μl well–1) was added. The absorbance values were read on the μQuant microplate spectrophotometer at 570 nm (Perkin Elmer Life Sciences, Waltham, MA, USA). Cell viability was calculated by the following equation: Cell viability (%) = (ODtest/ODcontrol) ×100, where ODtest and ODcontrol represent the absorbance values of the test and the control groups, respectively.
2.5. Animals and surgical procedures
The Peking University Third Hospital Committee on Ethics in the Care and Use of Laboratory Animals approved all animal experimental protocols (Project Number: 2016-0010). Sixty adult male New Zealand white rabbits (25 weeks, 3.5 ± 0.3 kg) were randomly assigned to three groups (20 rabbits for each group): pTi scaffolds with (i) simvastatin 0 mg, (ii) simvastatin 0.1 mg, and (iii) simvastatin 0.5 mg. Each scaffold was implanted in both the left and right tibiae of the rabbits.
The surgical procedures were performed under general anesthesia using pentobarbital sodium (30 mg kg−1, i.p.) [20]. The left and right lateral proximal tibiae were exposed, and cylindrical defects with a diameter of 5 mm and a depth of 5 mm were drilled. The pTi scaffolds were filled with simvastatin (0, 0.1, and 0.5 mg) and immediately inserted into the predrilled defects as shown in figures S1(b) and (c).
Four weeks after surgery, thirty randomly selected rabbits were euthanized (10 rabbits from each group). Five rabbits randomly selected from each group (10 bilateral tibia specimens) were prepared for micro-CT analyses. The left tibiae (5 specimens) were cut into undecalcified histological slices, and the right tibiae (5 specimens) were prepared for mechanical push-in tests. The other 5 rabbits in each group (10 bilateral tibia specimens) were studied for vascular infusion and then analyzed by micro-CT and preparation of undecalcified histological slices. The scheme at 8 weeks was the same as that at 4 weeks.
2.6. Lead tetroxide (Pb3O4) infusion
Five randomly selected rabbits received subcutaneous injections of 1000 IU kg–1 heparin. The proximal inferior vena cava and artery were ligatured, and 24-gauge indwelling needles were cannulated into the distal inferior vena arteries. Using a peristaltic pump (Harvard Apparatus, South Natick, Massachusetts, USA), approximately 150 ml of pre-warmed normal saline, 100 ml of neutral formalin, and 50 ml of contrast solution were infused successively through the aorta [21]. The contrast agent was 40% Pb3O4 and 5% gelatin in normal saline [22]. After incubation overnight at 4 °C, the tibiae from each animal were dissected and fixed in 10% neutral-buffered formalin. The samples were subsequently used for micro-CT and histology of the undecalcified bones with pTi scaffolds.
2.7. Micro-CT analyses
To evaluate the bone mass and vascular volumes (VVs), micro-CT was performed using an Inveon MM system (Siemens, Munich, Germany) [23]. In brief, the specimens were located and scanned in whole, with 360° rotation in 360 equiangular steps. Images were acquired at an effective pixel size of 9.08 μm, voltage of 80 kV, current of 500 μA and exposure time of 2000 ms. The images consisted of 1512 slices and a voxel size of 9.08 μm × 9.08 μm × 9.08 μm. Two-dimensional images were used to construct 3D reconstructions using multimodal 3D visualization software (Inveon Research Workplace, SIEMENS, Munich, Germany). The bone volume/tissue volume (BV/TV), which was calculated as the ratio of the total amount of bone region present the analyzed total volume, and the bone mineral density (BMD) were calculated using an Inveon Research Workplace (Siemens, Munich, Germany). The threshold value (1000-3885) was adjusted as appropriate for the mineralized bone phase. The analytical zone was new bone in the pTi scaffolds and the 1 mm regions surrounding the pTi scaffolds at 4 and 8 weeks.
To evaluate neovascularization, the rabbits with Pb3O4 infusions were also scanned by micro-CT as described above. The VVs (threshold value: 6156-14586) of the pTi scaffolds and the 1 mm regions surrounding the pTi scaffolds were calculated.
2.8. Histology
The rabbits without vascular perfusion were injected intravenously with alizarin red (30 mg kg−1) and calcein (20 mg kg−1) (Sigma, St. Louis, MO, USA) 21 and 7 days before euthanasia as double-fluorochrome labels to evaluate the mineral apposition rate (MAR). After the tibiae were scanned by micro-CT, the samples were fixed as described above and dehydrated in ethanol, followed by embedding in destabilized methyl methacrylate resin [24]. Next, the sections were ground and polished to 40–60 μm using an EXAKT precision cutting and grinding system (EXAKT Cutting & Grinding System, Norderstedt, Germany). After MARs analysis using BioQuant software (BioQuant, San Diego, CA, USA), the sections were stained with toluidine blue.
To further evaluate neovascularization, the specimens with Pb3O4 infusions were cut into undecalcified histological sections as described above, and the slices were examined using a stereoscope.
2.9. Push-in test
To measure the fixation strengths of the scaffolds, the right tibiae of the five rabbits without vascular infusions were subjected to mechanical push-in tests using a mechanical testing system (Landmark, MTS Inc., Eden Prairie, MN, USA) [25]. Mechanical testing was accomplished at a rate of 0.25 mm min−1 along the longitudinal axis of the scaffold. The maximum load (Fmax) at the ultimate load was recorded during each push-in test.
2.10. Statistical analyses
The data are the means ± SD. SPSS v16.0 (IBM, Armonk, NY, USA) was used for the statistical analyses. One-way ANOVA was conducted to assess the differences among the groups, followed by appropriate least significant difference (LSD) tests. Linear regression and correlation were used to evaluate the relationships between the BV/TV in new bone and the volume of neovascularization; p < 0.05 was considered statistically significant.
3. Results
3.1. Microstructural characterization of the simvastatin/poloxamer 407 hydrogel/pTi scaffolds
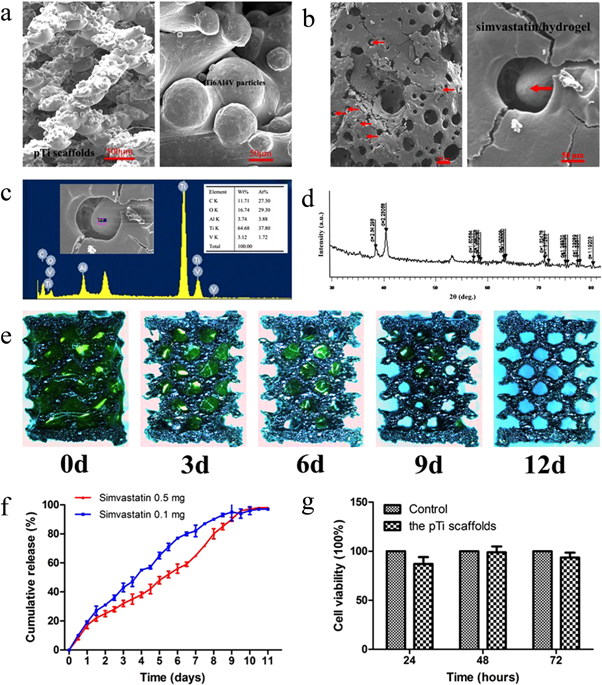
As shown in figure 1(a), SEM images of the pTi scaffold surface revealed that the pTi scaffold had a highly aligned porous structure. Moreover, the pTi scaffold was closely wrapped in simvastatin/hydrogel on the surface and interior (red arrows represent pTi scaffolds) (figure 1(b)), with relatively large pores with a size of ∼400 μm. EDS (figure 1(c)) indicated that titanium element was the main material in the scaffold and that the simvastatin/hydrogel was successfully enclosed on the scaffold.
Figure 1. Characterization of the simvastatin/ poloxamer 407 hydrogel/ pTi scaffolds. (a) Representative SEM images of the pTi scaffold surface. The pTi scaffold has a highly aligned porous structure. (b) Representative SEM images of the simvastatin/poloxamer 407 hydrogel/pTi scaffold surface. The pTi scaffold was filled with simvastatin/poloxamer 407 hydrogel. The red arrows indicate pTi scaffolds wrapped in poloxamer 407 hydrogel. (c) EDS indicated that the dominant element of the scaffold was titanium and that the simvastatin hydrogel was fully enclosed in the scaffolds. (d) XRD patterns of the pTi scaffolds. (e) Photo images of the degradation behavior of the poloxamer 407 hydrogel in the porous Ti6Al4V scaffolds in vitro. Green represents the poloxamer 407 hydrogel (the transparent hydrogel was stained with calcein to improve visualization). (f) Release profile of simvastatin from the simvastatin/poloxamer 407 hydrogel in vitro. (g) Evaluation on the cytotoxicity of the pTi scaffolds. The cell viability of L-929 fibroblast cells was determined in medium pretreated with leaching liquor from the pTi scaffolds after culture for 24, 48, and 72 h.
Download figure:
Standard image High-resolution imageThe characteristics (porosity, specific surface area, surface roughness, and nominal stress) of the pTi scaffolds are shown in table 2. The XRD patterns of the pTi scaffolds are presented in figure 1(d) and provide more detailed structural information.
Table 2. Characteristics of the pTi scaffolds.
| Characteristic | Value |
|---|---|
| Porosity | 75.61% ± 0.23% |
| Specific surface area | 2.25 ± 0.36 m2 g−1 |
| Surface roughness | 20.59 ± 5.43 μm |
| Nominal stress | 10.16 ± 2.77 MPa |
3.2. Evaluation of the degradation of poloxamer 407 and release of simvastatin in vitro
The degradation behavior of the poloxamer 407 hydrogel in porous Ti6Al4V scaffolds in vitro is shown in figure 1(e). The poloxamer 407 hydrogel was fully degraded after 12 days in the porous Ti6Al4V scaffolds.
The release kinetics of simvastatin in vitro were evaluated (figure 1(f)). In vitro, both the simvastatin 0.1 and 0.5 mg group exhibited slightly faster initial release. However, no initial burst release was detected during the first 24 h in simvastatin/poloxamer 407 hydrogels. Release was slightly faster in the simvastatin 0.1 mg group than in the simvastatin 0.5 mg group after 24 h. Simvastatin release was complete by day 9 in both groups in vitro.
3.3. Cytotoxicity of the pTi scaffolds
After culture for 24 h, 48 h, and 72 h, the cell viabilities were 87.0%, 98.9%, and 93.5%, respectively. There were no significant differences in cell viability between the experimental groups and the control groups (100%), indicating that the pTi scaffolds had excellent biocompatibility (figure 1(g)).
3.4. Micro-CT evaluation of the new bone in and around the pTi scaffolds
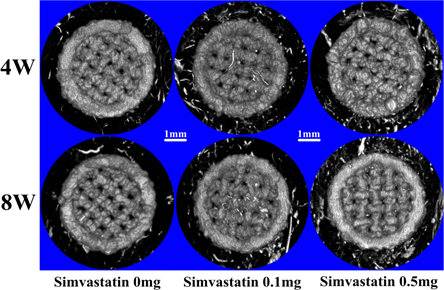
Micro-CT revealed that filling with simvastatin promoted bone ingrowth in and osseointegration around the pTi scaffolds (figure 2) (table 3).
Figure 2. Representative images of bone ingrowth (a) and osseointegration (b) in/around the implanted pTi scaffolds. (a) Bone ingrowth in the pTi scaffolds was evaluated ex vivo via micro-CT at 4 and 8 weeks (yellow represents new bone in the 3D micro-CT images). (b) Osseointegration around the pTi scaffolds was evaluated ex vivo by micro-CT at 4 and 8 weeks (gray represents new bone in 3D micro-CT images).
Download figure:
Standard image High-resolution imageTable 3. The BV/TV, BMD of new bone, and volume of neovascularization in and 1 mm around the pTi scaffolds at 4 and 8 weeks.
| In the pTi scaffolds | Around the pTi scaffolds | |||||
|---|---|---|---|---|---|---|
| Parameters | Simvastatin 0 mg | Simvastatin 0.1 mg | Simvastatin 0.5 mg | Simvastatin 0 mg | Simvastatin 0.1 mg | Simvastatin 0.5 mg |
| 4 weeks | ||||||
| BV/TV of new bone (%) | 26.70 ± 0.95 | 29.29 ± 1.97* | 25.37 ± 1.77## | 3.05 ± 0.61 | 3.36 ± 0.42 | 4.42 ± 0.93**,# |
| BMD of new bone (g cm−3) | 0.88 ± 0.01 | 0.90 ± 0.02* | 0.87 ± 0.01## | 0.72 ± 0.01 | 0.69 ± 0.01* | 0.72 ± 0.02## |
| Volume of neovascularization (mm3) | 1.55 ± 0.21 | 4.00 ± 0.47** | 2.40 ± 0.24**,## | 3.18 ± 0.12 | 3.82 ± 0.20** | 4.04 ± 0.06** |
| 8 weeks | ||||||
| BV/TV of new bone (%) | 28.89 ± 1.35 | 33.54 ± 1.54** | 37.95 ± 2.88**,## | 0.74 ± 0.22 | 4.68 ± 0.49** | 6.69 ± 1.39**,## |
| BMD of new bone (g cm−3) | 0.85 ± 0.01 | 0.88 ± 0.01* | 0.89 ± 0.01** | 0.64 ± 0.01 | 0.69 ± 0.01** | 0.70 ± 0.02** |
| Volume of neovascularization (mm3) | 5.10 ± 0.26 | 7.13 ± 0.36** | 10.00 ± 1.19**,## | 2.50 ± 0.17 | 3.80 ± 0.31** | 4.46 ± 0.38**,# |
*p < 0.05, **p < 0.01 versus 0 mg simvastatin group and #p < 0.05, ##p < 0.01 versus 0.1 mg simvastatin group.
At 4 weeks, the 0.1 mg simvastatin group exhibited significantly increased BV/TV (p < 0.05) and BMD (p < 0.05) of the new bone in the pTi scaffolds compared to the 0 mg simvastatin group (figure 2(a)). Additionally, the BV/TV of the new bone around the pTi scaffolds in the 0.5 mg simvastatin group was dramatically increased by 44.78% compared with the 0 mg simvastatin group (p < 0.01) (figure 2(b)). However, the BMD of new bone surrounding the pTi scaffolds in the 0.1 mg simvastatin group was significantly decreased by 4.46% compared with the 0 mg simvastatin group (p < 0.05), which was inconsistent with the BV/TV of the new bone surrounding the pTi scaffolds. The BV/TV and BMD of the new bone in the pTi scaffolds in the 0.5 mg simvastatin group were lower than those in the 0.1 mg simvastatin group.
Generally, the volumes of new bone at 8 weeks were increased compared with those at 4 weeks, except for the new bone surrounding the pTi scaffolds in the 0 mg simvastatin group. In the pTi scaffolds, the BV/TV in the 0.1 and 0.5 mg simvastatin groups was significantly increased by 16.09% and 31.35% compared with the 0 mg simvastatin group (p < 0.01, p < 0.001) (figure 2(a)). The BMD of the new bone in the pTi scaffolds in the 0.1 and 0.5 mg simvastatin groups was significantly increased by 2.57% and 3.94% compared with the 0 mg simvastatin group (p < 0.05, p < 0.01). For the new bone surrounding the pTi scaffolds, the BV/TV of the 0.1 and 0.5 mg simvastatin groups was increased by 530.64% and 800.34% compared to the 0 mg simvastatin group (p < 0.001, respectively), and the BMD of new bone surrounding the pTi scaffolds in the 0.1 and 0.5 mg simvastatin groups was significantly increased by 7.90% and 10.34% compared to the 0 mg simvastatin group (p < 0.001, respectively) (figure 2(b)). In contrast to the characteristics at 4 weeks, the bone volumes and BMD in and around the pTi scaffolds in the 0.5 mg simvastatin group are higher than those in the 0.1 mg simvastatin group at 8 weeks.
3.5. Histological evaluation of the new bone in and around the pTi scaffolds
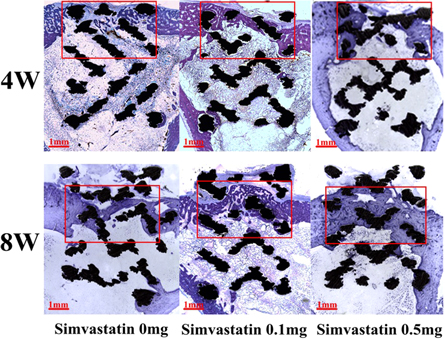
Undecalcified histological analysis of the bone samples with implants also confirmed that filling with simvastatin/hydrogel promoted bone ingrowth and was replaced by mature woven bone. More interestingly, there was no bone ingrowth in the porous titanium alloys in the bone medullary compartments. Additionally, osseointegration was promoted by filling with simvastatin, and there was extensive new bone formation around the pTi scaffolds (figure 3).
Figure 3. Representative images of new bone in and around the pTi scaffolds. New bone in and around the pTi scaffolds was evaluated ex vivo by slicing undecalcified tissues (toluidine blue staining) at 4 and 8 weeks. The mazarine zone inside the red box represents the new bone; mazarine zone outside the red box represents the cortical bone; the black latticed zone represents the pTi scaffold.
Download figure:
Standard image High-resolution image3.6. Mineral apposition rates
Consistent with the analyses of bone mass, dynamic histomorphometric analyses also revealed that the MAR in the 0.5 mg simvastatin group was decreased by 25.78% and 27.63% compared with the 0 and 0.1 mg simvastatin groups at 4 weeks (p < 0.01, respectively) (figure 4).
Figure 4. Dynamic histomorphometric analyses of new bone in the pTi scaffolds. Representative fluorescence images obtained from the pTi scaffolds at 4 and 8 weeks after double labeling with alizarin red and calcein are shown (left). Scale bar = 20 μm. MAR of new bone in the pTi scaffolds (right). The data are the mean ± SD. *p < 0.05 versus 0 mg simvastatin group, #p < 0.05 versus 0.1 mg simvastatin group.
Download figure:
Standard image High-resolution imageAt 8 weeks, the MARs were generally higher than those at 4 weeks. The 0.1 mg simvastatin group had a significantly increased MAR by 37.36% compared with the 0 mg simvastatin group (p < 0.01). Moreover, the MAR of the 0.5 mg simvastatin group dramatically increased by 75.23% and 27.57% compared to those of the 0 and 0.1 mg simvastatin groups (p < 0.001, p < 0.01) (figure 4).
3.7. Fixation strengths of the pTi scaffolds
At 4 weeks, the maximum load of the pTi scaffolds demonstrated that the 0.1 mg simvastatin group had a significantly increased Fmax by 24.81% and 18.10% compared with the 0 and 0.5 mg simvastatin groups (p < 0.01, respectively) (table 4).
Table 4. The maximum push-in force of the pTi scaffolds measured using a mechanical testing system at 4 and 8 weeks.
| Maximum push-in force (N) | |||
|---|---|---|---|
| Simvastatin 0 mg | Simvastatin 0.1 mg | Simvastatin 0.5 mg | |
| 4 weeks | 59.42 ± 2.52 | 74.17 ± 6.15* | 62.80 ± 4.04# |
| 8 weeks | 291.75 ± 16.90 | 348.16 ± 15.25* | 415.05 ± 42.69*,# |
*p < 0.05 versus 0 mg simvastatin group and #p < 0.05 versus 0.1 mg simvastatin group.
At 8 weeks, the Fmax of the pTi scaffolds dramatically increased by 4- to 6-fold compared with 4 weeks. The Fmax of the 0.5 mg simvastatin group was dramatically increased by 42.26% and 19.21% compared to the 0 and 0.1 mg simvastatin groups (p < 0.01, p < 0.05). Additionally, Fmax increased significantly in the 0.1 mg simvastatin group by 19.34% compared with the 0 mg simvastatin group (p < 0.05) (table 4).
3.8. Neovascularization in and around the pTi scaffolds
Pb3O4 infusion and micro-CT scanning demonstrated that neovascularization was greater in and around the pTi scaffolds filled with simvastatin/hydrogel than in the 0 mg simvastatin group (figure 5) (table 3).
Figure 5. Representative images of infused vessels in and around the pTi scaffolds. The infused vessels in and around the pTi scaffolds were evaluated ex vivo by micro-CT (the silvery line represents vessels in 3D micro-CT images) at 4 weeks (a) and 8 weeks (b).
Download figure:
Standard image High-resolution imageAt 4 weeks, the neovascularization volumes in the 0.1 mg simvastatin group were increased dramatically by 158.06% and 66.67% compared with the 0 and 0.5 mg simvastatin groups (p < 0.001, respectively). Moreover, the volume of neovascularization in the 0.5 mg simvastatin group was significantly increased by 54.84% compared to the 0 mg simvastatin group (p < 0.01). In addition, the volumes of neovascularization surrounding the pTi scaffolds in the 0.1 and 0.5 mg simvastatin groups were significantly increased by 20.31% and 27.09% compared with the 0 mg simvastatin group (p < 0.001, respectively).
At 8 weeks, the volume of neovascularization in the pTi scaffolds was greater than that at 4 weeks. However, the volume of neovascularization around the pTi scaffolds was unchanged compared to 4 weeks. In detail, the volume of neovascularization in the 0.5 mg simvastatin group was increased significantly by 96.08% and 40.35% compared with the 0 and 0.1 mg simvastatin groups (p < 0.001) and by 39.71% in the 0.1 mg simvastatin group compared with the 0 mg simvastatin group (p < 0.01). The volume of neovascularization around the pTi scaffolds in the 0.5 mg simvastatin group was also significantly increased by 78.66% and 17.38% compared with those in the 0 and 0.1 mg simvastatin groups (p < 0.001, p < 0.05). In addition, the neovascularization volume in the 0.1 mg simvastatin group was significantly increased by 52.20% compared with the 0 mg simvastatin group (p < 0.001).
The histology of the undecalcified tissues with pTi scaffolds further verified that the incorporated simvastatin promoted neovascularization in and around the pTi scaffolds (figure 6).
Figure 6. Representative images of infused vessels in and around the pTi scaffolds. The infused vessels in and around the pTi scaffolds were evaluated ex vivo by slicing undecalcified tissues at 4 and 8 weeks (brownish-red represents vessels, and lavender represents cortical bone; the zone below the cortical bone represents bone marrow).
Download figure:
Standard image High-resolution image3.9. Correlations between new bone and neovascularization in and around the pTi scaffolds
In general, there were significant correlations between the BV/TV in the new bone and the volume of neovascularization in and around the pTi scaffolds at 4 and 8 weeks (figure S2). In detail, significant correlations were found between the new bone volume and the volume of neovascularization in the pTi scaffolds at 4 weeks (r = 0.648, p < 0.01) (figure S2(a)) and 8 weeks (r = 0.899, p < 0.001) (figure S2(c)). In addition, there were also significant correlations between the new bone volume and the volume of neovascularization around the pTi scaffolds at 4 weeks (r = 0.669, p < 0.01) (figure S2(b)) and 8 weeks (r = 0.878, p < 0.001) (figure S2(d)).
4. Discussion and conclusion
Manufactured porous biomaterials with additives are among the most promising potential candidates for synthetic bone substitutes [26]. However, the application of porous titanium alloys alone has a weak effect on bone ingrowth and osseointegration. One of the major obstacles to the application of porous bone graft substitutes is their poor neovascularization after implantation [27]. A functional microvascular network provides oxygen and nutrients to facilitate growth, differentiation, and tissue functionality [28]. The development of functional vasculature is particularly critical with respect to bone defects to improve bone ingrowth and osseointegration.
Accumulating evidence indicates that simvastatin promotes bone vascularization in vitro and in vivo [29]. Kureishi et al have shown that simvastatin promotes angiogenesis by activating the protein kinase Akt in normocholesterolemic animals [30]. Moreover, Du et al demonstrated that simvastatin attenuates TNF-α-induced apoptosis in endothelial progenitor cells via the upregulation of silent information regulator type-1 (SIRT1) [31]. We previously demonstrated that a single local injection of simvastatin/poloxamer 407 induces autogenous BMP-2 and VEGF expression and augments bone formation [12]. However, the injectable thermosensitive poloxamer 407 hydrogel has poor mechanical properties, hindering its application as a bone substitute. Porous titanium alloys have good mechanical properties and internally interconnected porous structures, which provide ideal spaces for filling with biodegradable poloxamer 407 hydrogel and bone ingrowth. Furthermore, the autogenous osteogenic factors that are induced by simvastatin release, such as BMP-2 and VEGF, might make the pTi scaffolds osteoconductive and osteoinductive.
Pullisaar et al reported that simvastatin coating of pTi scaffolds induces osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells and enhances osteoblast differentiation of primary human osteoblasts in vitro [32, 33]. Tai et al have concluded that simvastatin promotes the osteogenic differentiation of mesenchymal stem cells by enhancing the Rho/actin/cell rigidity pathway [34]. Some studies also support the hypothesis that statins enhance osteoblastic differentiation and bone matrix mineralization by inhibiting FPP and GGPP synthesis [35–37]. Athanasios et al determined that statins inhibit osteoblastic apoptosis via the TGFβ/Smad3 signaling pathway and further promote osseointegration and bone ingrowth [38]. Here, we also observed that filling the pTi scaffolds with simvastatin/hydrogel promoted osseointegration and bone ingrowth. As expected, the bone mass from pTi scaffolds was highest in the 0.5 mg simvastatin group among the 3 groups at 8 weeks. However, the 0.1 mg simvastatin group had the highest bone mass among the 3 groups at 4 weeks. Moreover, both the bone mass and the volume of neovascularization were lower in the 0.5 mg simvastatin group than in the 0.1 mg simvastatin group, particularly in the pTi scaffolds, which was inconsistent with the results at 8 weeks. Simvastatin has a biphasic dose-dependent effect on angiogenesis characterized by a proangiogenic effect at low doses (nM range) and antiangiogenic effects at high doses (μM range or higher) [39]. Thus, the local simvastatin concentration in the microenvironment of the pTi scaffold may be higher than the optimal concentration for the stimulation of neovascularization in the 0.5 mg simvastatin group, which might be antiangiogenic at early stages. Therefore, the bone anabolic effect was not greater in the 0.5 mg simvastatin group than in the 0.1 mg simvastatin group. As the hydrogel degraded with the slow release of simvastatin at 8 weeks, the local simvastatin concentration in the pTi scaffold decreased to an ideal concentration and better stimulated bone formation. Consequently, the bone mass with the pTi scaffolds was highest in the 0.5 mg simvastatin group among the 3 groups at 8 weeks. The mechanisms underlying the different bone masses at different times require further investigation.
In this study, we prepared a bone defect hole in tibiae without trabecular bone to avoid interference from other autogenous osteogenic factors in the osteogenesis induced by simvastatin. There was no ectopic osteogenesis in the simvastatin-treated groups, that is, neither bone ingrowth in the pTi scaffold outside the cortical bone nor bone ingrowth in the porous titanium alloy in the bone medullary compartment were observed, in contrast to BMP-induced bone formation [40, 41]. Additionally, osseointegration was promoted by filling with simvastatin/hydrogel. There was considerable new bone formation around the pTi scaffolds. Therefore, our research demonstrates that simvastatin would promote proper osteogenesis rather than chaotic osteogenesis.
Furthermore, cortical bone was significantly increased in the 0.5 mg simvastatin group and was nearly identical to that of normal cortical bone at 8 weeks (figure 3). Oxlund et al determined that simvastatin treatment increases cortical bone formation to partially prevent bone loss in ovariectomized rats, similar to our results [42].
The results of the push-in tests demonstrated that the maximum force at 8 weeks was dramatically increased by 4-to 6-fold compared with that at 4 weeks; this increase may be associated with the bone ingrowth and maturity of the cortical bone [43]. Both the cortical and trabecular compartments contribute to the fixation of the implant. We previously demonstrated that local injection of simvastatin promotes trabecular bone formation [10]. It would be interesting to explore the effects of implanting pTi scaffolds filled with simvastatin/hydrogel in areas with both cortical and trabecular bones, such as vertebrae.
Vascularization of tissue-engineered constructs is one of the most important challenges in regenerative medicine. Without sufficient blood supply, oxygen and metabolic needs are not met, which can lead to the central necrosis of constructs [44]. We previously demonstrated that the local application of simvastatin can recruit autogenous EPCs to calvarial defects implanted with simvastatin [45], and a single local injection of simvastatin significantly promotes osseointegration, increases implant fixation and enhances angiogenesis of the target bone in ovariectomized rats [12].
In this study, we used Pb3O4 contrast agent to infuse the blood vessels of rabbits. Neovascularization was precisely localized in and around the pTi scaffolds based on analysis of micro-CT and slices of undecalcified tissue. Roche et al determined that blood vessel infusion with barium sulfate provided the best vessel images and was compatible with staining procedures used in bone histomorphometry [46]. We determined that Pb3O4 infusion could provide good micro-CT images. Furthermore, the blood vessels infused by the brownish-red Pb3O4 contrasting agent were distinguished from the black pTi scaffolds when undecalcified tissue sections were examined using a stereoscope. Based on these improved methods, our results quantitatively exhibited the changes in the volume of neovascularization and observed that the volume of neovascularization was dramatically increased at 8 weeks compared with 4 weeks. The bone mass exhibited a similar trend, particularly in the pTi scaffolds. Furthermore, the results also supported the hypothesis that the local administration of simvastatin enhances angiogenesis during the bone repair process, which promotes bone formation [47]. Bivariate linear regression analyses demonstrated that there was a significant correlation between the new bone mass and the volume of neovascularization in and around the pTi scaffolds, particularly at 8 weeks, consistent with previous research [48, 49].
There are some limitations of this study. We mainly focused on the combination of a 3D printed titanium scaffold and simvastatin/poloxamer 407 on neovascularization and bone ingrowth in a rabbit model, and we did not evaluate how different pore sizes impact angiogenesis, osseointegration and bone ingrowth of the composites. Second, the injected thermosensitive biodegradable hydrogel filled the porous 3D printed titanium scaffold and blocked the pores. The effects of these changes on osseointegration and angiogenesis and alternative method such as coating techniques that preserve pore size and porosity remain to be examined further. We speculate that after the porous titanium scaffold was implanted, blood fills the pores of the implant; this coagulated blood might block the pores of the 3D printed titanium scaffold. The organization and absorption of hematomas are lengthy processes that might influence angiogenesis and bone ingrowth. However, the rapid degradation of the injected thermosensitive hydrogel might be beneficial for angiogenesis and bone ingrowth. This interesting topic merits further study.
In conclusion, the 3D printed porous Ti6Al4V scaffold composites filled with simvastatin/poloxamer 407 hydrogel significantly improved osseointegration, bone ingrowth and neovascularization in the proximal tibiae of rabbits. This study may provide an effective strategy to promote osseointegration, bone ingrowth and neovascularization of bone defects.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant number 81672133, 81641079) and the National High Technology Research and Development Program of China (863 Program, grant number SS2015AA020304). We thank Beijing AKEC Medical Co., Ltd, for their excellent technical assistance in the fabrication of the porous Ti6Al4V scaffolds.
Disclosure
The authors declare no conflicts of interest.