Abstract
Four-dimensional (4D) bioprinting of cell-laden constructs with programmable shape-morphing structures has gained increasing attention in the field of biofabrication and tissue engineering. Currently, most of the widely used materials for 4D printing, including N-isopropylacrylamide-based polymers, are not commonly used in bioinks for cell-laden bioprinting. Herein, we propose a facile approach to create cell-laden constructs with near-infrared (NIR)-triggered shape morphing using bioinks based on alginate (the most widely used bioink for cell-laden bioprinting). Three-dimensional (3D) printed bilayered scaffolds with orthogonal structures using concentrated alginate/polydopamine (PDA) inks (14–18 wt%) showed a change in folded shape during NIR-induced dehydration. The deformation angle of the scaffold could be controlled by laser power, irradiation time and the designed patterns of the printed alginate/PDA struts in scaffolds. Then, 3D printed biphasic scaffolds consisting of alginate/PDA and cell-laden hydrogels exhibited programmable shape change under NIR stimulation. Scaffolds were able to maintain their deformed structures, and the printed cells in hydrogels retained high viability during culture in medium for at least 14 days. The biocompatible and commonly used hydrogel bioinks, NIR-triggered shape-morphing structures and maintenance of the deformed shape in the medium give this facile approach great potential for application in the field of 4D bioprinting and 4D biofabrication of artificial tissues and organs.
Export citation and abstract BibTeX RIS
1. Introduction
Cell-laden three-dimensional (3D) bioprinting is recognized as an important approach for fabricating cellular constructs with designed geometries in the fields of tissue engineering, biofabrication and drug screening [1–5]. Indeed, cell-laden bioprinting has some apparent advantages, such as homogeneously programmable deposition of different types of cells in constructs [6, 7]. However, constructs with complex shapes such as overhanging structures are difficult to achieve directly by 3D printing, especially in cell-laden bioprinting, because the bioinks (hydrogels) for encapsulation of living cells generally possess low mechanical properties [8]. Sacrificial materials (fugitive inks) have generally been applied for the temporary support of overhanging structures during 3D bioprinting [9–11]. For example, water-soluble and cell-friendly biopolymers such as gelatin have been used in this approach as the sacrificial materials to create vascular channels by 3D bioprinting [12]. After the constructs were fabricated and crosslinked, the sacrificial materials were removed to create the overhanging structures (such as vascular channels). However, this strategy is generally time-consuming and wasteful of materials, and is challenging for fabrication of external constructs with designed structures and macropores [13]. Direct printing of sacrificial materials into cell-laden hydrogels is another approach to building overhanging structures, especially the channel networks mimicking vascular structures [14–16]. However, in this approach, sacrificial materials not only require good cytocompatibility but also maintain the printed morphology without mutual penetration with the cell-laden hydrogels [17]. In addition, the buoyant force from solution has been applied to support the overhanging structures during 3D printing. For instance, alginate inks were printed into crosslinking solutions (CaCl2) directly and hydrogels were printed into hydrophobic liquid at a high density [18, 19]. Buoyancy was able to overcome the gravitational force on the printed overhanging structures and no other sacrificial materials were needed for support. However, the printing precision of this strategy is generally low due to the floating motion of the printed struts in solution. In addition, this approach is difficult for cell-laden bioprinting because high densities of hydrophobic liquid might be harmful for the encapsulated cells.
Four-dimensional printing with shape change in an additional dimension (time) has been developing rapidly and is attracting great interest for applications in biomedical devices, autonomous robotics and tissue engineering [20–23]. It also has the potential to create constructs with complex geometries, including overhanging and hollow tubular structures, in an efficient manner. In this approach, shape-changing materials sensitive to different stimuli such as light, temperature and pH are generally required [24]. However, most shape-morphing materials are not suitable for encapsulating living cells for cell-laden 4D bioprinting. Therefore, cells are generally seeded on a shape-morphing polymer film to create vascular tube-like structures [25, 26], but this compromises the homogeneous distribution of different types of cells in a programmable manner. Self-folding hollow tubes mimicking blood vessels based on cell-laden hydrogel films after dehydration have also been reported [27], although it seems that this approach is only possible for preparation of thin films and tubes.
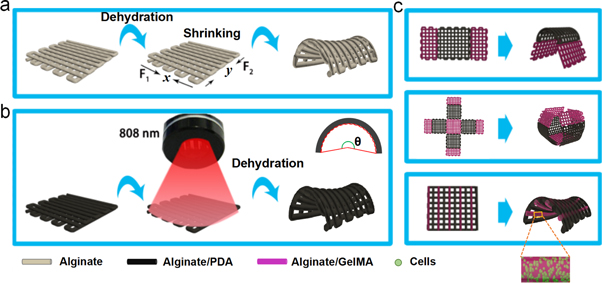
In this study we present a facile approach for achieving cell-laden 4D bioprinting of constructs with programmable shape change. Alginate hydrogels, the most widely used bioinks for cell-laden 3D bioprinting, were used not only because of their good biocompatibility and easy gelation [28–30] but also their shape-morphing ability during dehydration [31]. Three-dimensionally printed bilayered alginate scaffolds were able to achieve folding shape change by optimizing various parameters including the concentration of alginate bioinks, the crosslinking agent, macropore size (number of struts) and printing nozzle, etc. Moreover, we were able to introduce polydopamine (PDA), which has excellent biocompatibility and photothermal effects, into the alginate inks, endowing the printed scaffolds with a near-infrared (NIR) laser-triggered shape-morphing ability. This shape-morphing behavior (such as the deformation angle) could be controlled by the laser power and irradiation time. Alginate/PDA inks and cell-laden alginate/gelatin methacryloyl (GelMA) bioinks were printed into biphasic scaffolds exhibiting NIR-triggered shape change (see scheme
Scheme 1. (a) A three-dimensionally printed bilayered alginate scaffold transformed its original structure to a saddle-like structure due to dehydration-induced shrinkage in the x and y directions. (b) NIR-driven (808 nm laser) shape morphing of the bilayered alginate/polydopamine (PDA) scaffold was able to transform its structure in several minutes. (c) Biphasic scaffolds consisting of alginate/PDA struts and cell-laden alginate/gelatin methacryloyl (GelMA) struts with designed patterns were fabricated, and alginate/PDA struts endowed these scaffolds with NIR-driven shape-morphing behavior.
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Fabrication and shape-changing study of alginate scaffolds
Alginate inks for 3D printing were prepared by dissolving alginate (sodium alginate, Sigma-Aldrich, USA) in deionized water (DW) and stirring to achieve a homogeneous mixture. Then, bilayered scaffolds with designed structures were prepared by extruding alginate inks via 3D printing (Gesim, BS31, Germany). After crosslinking by divalent cations, the fabricated scaffolds were kept at room temperature for 24 h for dehydration-induced shape change (deformation). The deformation angles (θ) of scaffolds were calculated by 3D Studio Max. The effects of several parameters on the shape-changing behavior were studied, including: the concentration of alginate inks (4, 10, 14, 16, 18, 20 and 22 wt%); the concentration of crosslinking solution (CaCl2; 0.1, 0.25, 0.5 and 1.0 mol l−1); different crosslinking agents (four different divalent cations were selected in this study according to the decreasing binding affinity to alginate : Cu2+ > Ca2+ > Zn2+ > Mn2+ [32, 33]); number of struts (4, 6, 8, 10, 12 and 14) and cross-angle between layers (0, 15, 30, 45, 60, 75 and 90°) in 10 mm × 10 mm scaffolds; the size of nozzles (18, 20 and 22 G); composite inks (alginate/PVA, alginate/GelMA and alginate/PEG in ratio of 95:5); number of layers (2, 4, 6 and 8) and the width–length ratio (1:1, 1:2, 1:3, 1:4 and 1:5) of the scaffolds. Unless otherwise stated, bilayered scaffolds were prepared in square and orthogonal structures with 12 struts in each layer (10 mm × 10 mm) by extruding alginate inks with a concentration of 18 wt% using 20 G nozzles, and then the scaffolds were crosslinked by a 1 mol l−1 CaCl2 solution.
2.2. Fabrication and shape changing of alginate/PDA scaffolds
First, PDA solution was prepared by dissolving 10 mg of dopamine (dopamine hydrochloride, J&K, Beijing, China) in 2 ml of Tris (hydroxymethyl) aminomethane buffer (TRIS, J&K, Beijing, China) (10 mmol l−1, pH = 8.5). Then, alginate/PDA inks were prepared by mixing 1 g of alginate powders with 4.5 g of the prepared PDA solution with an alginate concentration of around 18 wt%. The mixture was stirred vigorously until a homogeneous paste was obtained. The alginate/PDA scaffolds were printed and crosslinked according to the above description. The shape-changing (deformation) behavior of alginate/PDA scaffolds was studied by calculating the deformation angles of the scaffolds after irradiation with a 808 nm laser. The temperature and water content of the scaffolds during laser irradiation, as well as the effect of laser power (0.3, 0.5, 0.7, 1.0, 1.5 W cm−2) on the deformation of scaffolds, were studied.
2.3. Cell-laden 4D bioprinting and cell culture
First, GelMA was synthesized according to the general method reported in the literature [34]. Briefly, gelatin (Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS) (pH 7.4) at 50 °C. Then, methacrylic anhydride (Sigma-Aldrich) was added to the solution during vigorous stirring, and the reaction was maintained for 2 h. Afterwards, the reaction was stopped by diluting the mixture with PBS (5×), followed by dialyzing the mixture against DW using 12–14 kDa cutoff dialysis tubing for 7 days. Then, the solution was freeze dried and stored under refrigeration for further use. Before bioprinting, the materials (alginate and GelMA) and containers were autoclaved, and CaCl2 solutions were sterilized through a 220 nm filter. Bioinks (4 wt%) consisting of alginate and GelMA (with 0.1% Irgacure 2959) in mass ratio of 1:1 were prepared by dissolving materials in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Then, 293T (human embryonic kidney cells, purchased from CTCC obtained from the ATCC) cell suspensions containing 1 × 106 cells were added to 1 ml of composite bioink. After mixing to homogeneity, the cell-laden bioinks were loaded into a printing tube. Then biphasic scaffolds with designed structures consisting of cell-laden alginate/GelMA and alginate/PDA struts were fabricated by printing cell-laden alginate/GelMA bioinks and alginate/PDA inks, alternately, using 20 G nozzles. The printing pressure and speed were 20 kPa and 10 mm s−1, respectively, for printing cell-laden bioinks, and 400 kPa and 5 mm s−1 for printing alginate/PDA inks. After the scaffolds were fabricated, they were photo-crosslinked by UV light (365 nm, 150 mW, 180 s) and then 0.5 mol l−1 CaCl2 solution (200–800 μl) was dropped on the surface of the scaffolds for crosslinking of alginate. Afterwards, the remaining CaCl2 solution was removed and the scaffolds were irradiated with a 808 nm laser. Finally, the deformed scaffolds were washed with PBS, followed by incubation in DMEM under 37 °C and 5% CO2.
Cell viability was measured using 3-(4,5-dimethyl-2-thiazolyl)−2,5-diphenyl-2-H-tetrazolium bromide (MTT). In brief, 1 ml of MTT solution (0.5 mg ml−1 in DMEM) was added to each scaffold and incubated for 4 h. Then the solution was removed carefully and 700 μl of dimethyl sulfoxide (DMSO) was added to each scaffold to solubilize the formazan product. The absorbance at 590 nm was measured using a microplate reader (BioTek, USA). In addition, calcein-AM and propidium iodide (Sigma-Aldrich) solutions were added to samples for live/dead cell staining, and the results were observed with a fluorescence microscope (Nikon Canada, Mississauga, Canada). Furthermore, cells in the hydrogels were fixed and stained with TRITC Phalloidin (Solarbio Life Science) and 4',6-diamidino-2-phenylindole (DAPI; Solarbio Life Science) and then imaged by confocal laser scanning microscopy (CLSM; TCS SP5II, Leica, Germany).
3. Results and discussion
3.1. Shape-changing study of 3D printed alginate scaffolds
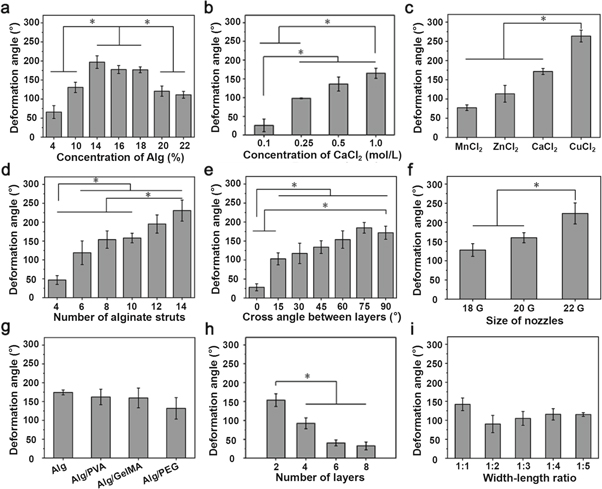
Alginate hydrogels are widely used bioinks for cell-laden 3D bioprinting because of their apparent advantages such as excellent biocompatibility and easy gelation using divalent cations in cell-friendly conditions [28–30]. Additionally, in a previous study we showed that 3D printed cubic alginate scaffolds suffered regular shrinkage in three dimensions after drying [31]. Although inspired by biological linear contractile tissues, 3D printed bilayered scaffolds with anisotropic alginate struts presented a saddle-like shape change during drying, since the shrinkage mainly happened in the x and y directions [35]. The shape-changing behavior was related to several factors including the concentration of alginate, the crosslinking agents with different binding affinities to alginate and the structures of the scaffolds (macropores etc) (figure 1 and figure S1 available online at stacks.iop.org/BF/11/045019/mmedia). The results indicated that 3D printed orthogonally growing bilayered scaffolds using alginate inks with concentrations in the range of 14–18 wt% and strong ionic crosslinking presented significant shape change. The data also indicated that a smaller gap between alginate struts in scaffolds generated a greater folding shape change. In addition, it seems that the folding shape-changing behavior mainly occurred on the printed scaffolds with bilayered structures [35], while scaffolds with a monolayer structure showed nearly no shape deformation after dehydration (figure S1(h)). The width–length ratio of the scaffolds showed no significant influence on folding ability. Moreover, the printing inks mixing alginate with other hydrogels (such as 5 wt% PVA, GelMA or PEG) also showed no significant influence on the folding ability of the printed bilayered scaffolds.
Figure 1. The influence of different parameters on the deformation of the 3D printed alginate scaffolds, including: (a) the concentration of alginate inks; (b) the concentration of CaCl2 solution for crosslinking; (c) different divalent cations (Mn2+, Zn2+, Ca2+ and Cu2+) for crosslinking; (d) the number of alginate struts; (e) the cross-angle of alginate struts; (f) the size of the printing nozzle; (g) alginate inks mixed with different hydrogels (PVA, GelMA or PEG); (h) the number of layers of printed scaffolds; and (i) the width–length ratio of the scaffolds (bilayer).
Download figure:
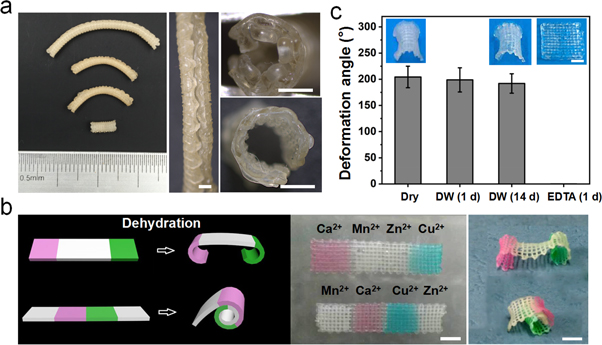
Standard image High-resolution imageVascular-like tube structures of different sizes were obtained by printing alginate scaffolds with optimal parameters (18 wt% of alginate ink, orthogonally growing bilayered structures, crosslinked by 1 M CaCl2, and using 20 G and 22 G nozzles) after dehydration-triggered shape change (figure 2(a)). In addition, programmed morphing structures were also achieved just by simply adjusting the added position of different crosslinking agents in the scaffolds. For instance, dumbbell-like and rolled paper-like structures were obtained by alternately using Ca2+/Cu2+ and Mn2+/Zn2+ to crosslink the sides and middle, respectively, of an alginate patch (figure 2(b)). Importantly, the shape-morphing scaffolds were able to maintain their deformed structures after incubation in solution (such as deionized water) for at least 14 days, indicating that the deformed scaffolds were suitable for cell culture in the following steps of cell-laden biofabrication. The deformed scaffolds were able to recover to the original structures after removal of the crosslinking agents (Ca2+) from the alginate scaffolds by incubation in EDTA solution (figure 2(c)). These results indicate that the crosslinking agents (Ca2+) could be preserved in the highly concentrated alginate matrix, which would be the main contributor to structural maintenance of the scaffold during the 14 days of incubation.
Figure 2. (a) Photographs of 3D printed alginate tubes of different sizes after dehydration-induced deformation. (b) The programmed deformation of alginate constructs using different divalent cations for crosslinking. (c) Shape recovery of deformed alginate scaffolds after incubation in deionized water (37 °C) for 1 and 14 days, and in EDTA for 1 day, respectively. Scale bar = 1 mm (a), 5 mm (b) and 2 mm (c).
Download figure:
Standard image High-resolution image3.2. NIR-triggered shape change of 3D printed alginate/PDA scaffolds
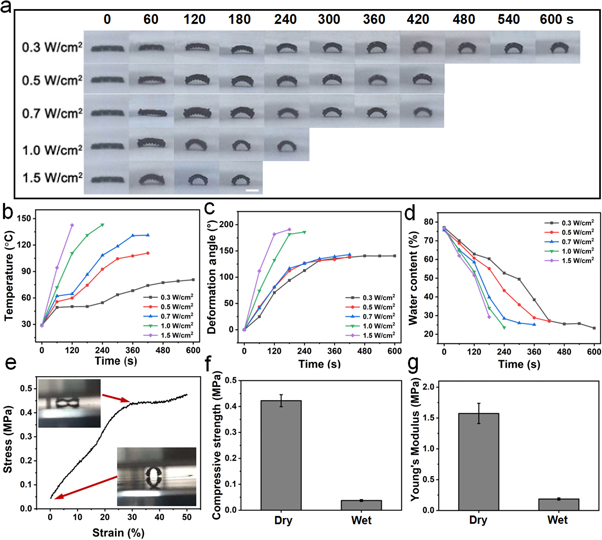
Although 3D printed bilayered alginate scaffolds with lattice structures showed the ability to change their folding shape during dehydration, the process is time-consuming because the dehydration of alginate scaffolds at room temperature required more than 1 day [31]. Certainly, this time could be shortened by incubating alginate scaffolds in an oven at high temperature to accelerate the rate of dehydration. However, whole scaffolds suffering high temperature are inappropriate for cell-laden biofabrication. Therefore, NIR-induced shape-changing scaffolds were prepared by printing hydrogel mixtures of alginate and PDA. PDA has been widely used in biomedical engineering due to its excellent biocompatibility and photothermal effect [36–38]. As shown in figure 3(a), 3D printed alginate/PDA scaffolds underwent folding shape change within several minutes under the irradiation of a 808 nm laser. Since the rate of shape change is not very fast (as in several seconds), the shape-change process is easy to control precisely. The speed and degree of shape changing could be tuned by adjusting the laser power and irradiation time. A higher laser power caused faster shape changing since higher laser powers generate a higher temperature and induce a faster dehydration rate (figures 3(b)–(d)). It seems that the deformation angle of scaffolds reaches a plateau without significant increase after 300 s of irradiation using 0.3, 0.5 and 0.7 W cm−2 laser powers. While the temperature of scaffolds irradiated by a NIR laser with powers higher than 1.0 W cm−2 was rapidly increased to 120 °C, the deformation angle of scaffolds showed no significant increase after 120 s of irradiation. Additionally, other materials with a photothermal effect also could be applied in this system. For example, copper ions as crosslinking agents of alginate also exhibited an excellent photothermal effect [39], endowing the scaffolds with NIR-triggered folding deformation in 300 s (figures S2(a)–(c)). However, due to the uncontrolled release of copper ions from the alginate matrix, which might be potentially cytotoxic, calcium ions were generally applied for the crosslinking of alginate in the subsequent experiments. Furthermore, the NIR laser was also able to penetrate skin (pig skin) to induce dehydration of scaffolds, which gradually transformed their original shape to a saddle-like structure under NIR irradiation for 480 s with the temperature being maintained around 37 °C (figures S2(d)–(f)). Moreover, the mechanical properties of alginate/PDA scaffolds after NIR-induced folding shape change were also measured. The data showed that the Young's moduli of the deformed scaffolds were 1.57 ± 0.19 and 0.19 ± 0.02 MPa for dry and wet scaffolds, respectively (figures 3(e)–(g)), indicating the applicability of the deformed scaffolds in tissue engineering.
Figure 3. The photographs of the shape changes (a), temperature (b), deformation angle (c) and water content (d) of 3D printed alginate/PDA scaffolds under laser irradiation with different powers for different times. The scale bar in (a) = 5 mm. (e) The strain–stress curves of the deformed alginate/PDA scaffolds (dry) (the inset images showing the scaffolds before and after compression), and (f) the compressive strength and (g) Young's moduli of alginate/PDA scaffolds in dry and wet states.
Download figure:
Standard image High-resolution image3.3. Shape-morphing study of 3D printed biphasic scaffolds
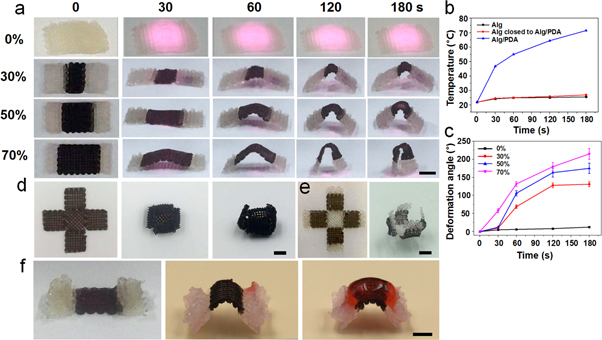
Another advantage of an NIR-triggered shape-changing scaffold is that the shape-changing behavior of the scaffold can also be controlled by controlling the area and position of alginate/PDA struts in the printed scaffolds. For instance, biphasic scaffolds with different contents (30%, 50% and 70%) of alginate/PDA in the alginate scaffolds were fabricated using 3D printing. This showed that the speed and degree of shape changing were increased with increasing content of printed alginate/PDA struts in the scaffold. The 70% alginate/PDA scaffold showed a fully folded shape change under 180 s of NIR irradiation (figures 4(a)–(c) and video in the supporting information). As a control, the pure alginate scaffold without PDA showed nearly no temperature rise or shape change under 180 s of NIR irradiation. In addition, the shape-changing behavior was also related to the direction of the alginate/PDA struts in a biphasic scaffold. For example, a biphasic scaffold with twisting shape change could be achieved by printing alginate/PDA struts at a certain angle to the length of the scaffold (figure S3). The temperature of alginate/PDA struts in a biphasic scaffold increased rapidly during laser irradiation, while the alginate struts close to alginate/PDA in the biphasic scaffolds showed very limited temperature rise because of the existence of gaps between the alginate and alginate/PDA struts in the scaffolds (figure 4(b)). This is important for cell-laden bioprinting, with the following steps to minimize heat damage to the embedded cells. According to this strategy, a 3D printed cross-shaped scaffold using alginate/PDA composite inks was deformed to a full cage structure, while a biphasic scaffold using alginate and alginate/PDA inks was deformed to an unclosed cage structure under NIR irradiation (figures 4(d), (e)). In addition, this strategy was also suitable for creating large-size scaffolds (i.e. more than two layers). For instance, ten layers (5 mm) of alginate struts and two layers (1 mm) of alginate/PDA struts were printed in the ends and middle of the biphasic scaffold, respectively. After shape changing using NIR irradiation in the alginate/PDA area, additional alginate bioinks (or alginate mixed with other hydrogels) were printed on the shape-changing area (alginate/PDA) to create a larger-size scaffold with overhanging structures (figure 4(f)). Furthermore, multiple cells could probably be encapsulated in the bioinks to fabricate human-scale cell-laden constructs because of the cell-friendly processing conditions involved in this strategy.
Figure 4. The shape changes (a), temperature (b) and deformation angle (c) of 3D printed biphasic scaffolds consisting of alginate and alginate/PDA struts with different area ratios under laser irradiation (0.5 W cm−2, 808 nm) for different times. Photographs show the shape change of 3D printed alginate/PDA (d) and biphasic (e) scaffolds in cross structures before and after laser irradiation (0.5 W cm−2, 120 s). (f) A 3D printed biphasic scaffold with different heights in the alginate (ten layers) and alginate/PDA (two layers) parts undergoing NIR-induced shape change followed by printing alginate (staining with red ink) on the shape-changing area (alginate/PDA) (f). Scale bar = 5 mm.
Download figure:
Standard image High-resolution image3.4. Cell-laden 4D bioprinting of biphasic scaffolds
Since biphasic scaffolds possess the ability to control local shape change using NIR irradiation, the non-deforming parts (areas that do not increase in temperature) were able to be used for encapsulation of cells. Herein, cell-laden alginate/GelMA bioinks were printed on the ends of the scaffold, and alginate/PDA inks were printed in the middle of scaffold. After irradiation (0.5 W cm−2) for 180 s, the cell-laden biphasic scaffold was deformed to a saddle-like structure with high cell viability (figures 5(a)–(c), S4(c), (d)). Furthermore, the cells still maintained high viability after 14 days in culture (figures 5(d), (e)). These results indicate that this biphasic scaffold holds the possibility for cell-laden 4D bioprinting by NIR-triggered shape changing. However, a deficiency of this type of biphasic scaffold is that cells are missing in the deformation areas, resulting in an inhomogeneous cell distribution in the scaffold.
Figure 5. Fluorescent images of 3D printed alginate/GelMA (containing cells) and alginate/PDA biphasic scaffolds in top and side view (a), and images of the scaffolds before (b) and after (c) shape change under laser irradiation (0.5 W cm−2 ,180 s). Live/dead staining (d) and cell viability (e) of the printed cell-laden scaffolds after culture over 14 days. Scale bar = 300 μm in (a) and (d) and 2 mm in (b).
Download figure:
Standard image High-resolution imageAnother type of biphasic scaffold was therefore designed and fabricated by printing cell-laden alginate/GelMA bioinks and alginate/PDA inks alternately. Gaps between cell-laden alginate/GelMA struts and alginate/PDA struts were designed to prevent heat transfer and water penetration under NIR irradiation (figures 6(a)–(c), S4(a)). In addition, the gradual dehydration of alginate/PDA struts could be realized by using a low laser power and long irradiation time to further minimize the heat damage to cells. The cell-laden bilayered scaffold was deformed to a saddle-like shape using 0.5 W cm−2 laser irradiation for 240 s. Cells survived well in the scaffold before and after NIR-induced shape morphing. Furthermore, after suffering NIR irradiation, the cells retained 80% viability in the hydrogel scaffolds over 14 days of culture. The laser irradiation inflicted no significant damage to the encapsulated cells (figures 6(d)–(h), S4(b)). Many studies have reported that low-level laser irradiation caused no significant cytotoxic effects on various normal cells, and even had positive effects on cell proliferation in some situations [40–42]. For example, murine bone marrow cells were irradiated by an 800 mW continuous laser with a wavelength of 808 nm and an energy density of 4 J cm−2, and showed no difference from the control samples in proliferation and osteoclast or osteoblast differentiation [43].
Figure 6. (a) Schematic illustrating the printed biphasic scaffolds consisting alginate/PDA struts and cell-laden alginate/GelMA struts, and the shape change after NIR irradiation. Images of the scaffolds before (b) and after (c) shape changing. Fluorescent images of 3D printed alginate/GelMA (containing cells) and alginate/PDA biphasic scaffolds before (d), (e) and after (f) shape change under laser irradiation (0.5 W cm−2, 240 s). (g) Cell viability in the printed scaffolds for 1, 7 and 14 days of culture after NIR irradiation, and samples without NIR irradiation as a control. (h) Confocal images of cells in the printed hydrogel scaffolds over 14 days of culture after laser irradiation. The stains are nuclear DAPI (blue) and F-actin (red). Scale bar = 1 mm (b)–(f), 20 μm (h).
Download figure:
Standard image High-resolution imageThus, the proposed 4D bioprinting in this study could fabricate cell-laden bilayered constructs with controlled curved structures in an effective manner. These curved bilayered constructs could be directly used in the field of tissue engineering and regenerative medicine, such as cardiac patches for myocardial repair and scaffolds for cartilage and skin tissue repair in curved bending areas.
4. Conclusion
In this study we report a facile cell-laden 4D bioprinting system with dehydration-driven shape-morphing behavior. In this system, a 3D printed bilayered scaffold of concentrated alginate hydrogels (14∼18 wt%) transformed its structure from lamellar to a saddle-like morphology during gradual dehydration. The shape-changing behavior could be tuned by several parameters such as the concentration and type of crosslinking agent, the space between two struts and the cross-angle between layers etc. Long hollow tubes mimicking blood vessels and programmed morphing structures were achieved by adjusting these parameters. In addition, PDA with excellent biocompatibility and photothermal effect was introduced into this system to accelerate the dehydration-induced deformation, as well as programmable shape change. Therefore, cell-laden 4D bioprinting was realized by fabricating biphasic scaffolds with cell-laden alginate/GelMA and alginate/PDA inks. The programmable shape-morphing behavior was controlled by the laser power, irradiation time and the area/position of the alginate/PDA struts in the scaffold. Cells retained high viability in the scaffolds after NIR-induced shape morphing. Therefore, this facile system might be useful for fabricating large-scale cell-laden constructs with programmable shape-morphing ability, and may pave the way for the development of 4D bioprinting and biofabrication of artificial tissues.
Acknowledgments
This research was funded by the Basic Research Program of Shenzhen (grant no. JCYJ20170817094407954). The authors also acknowledge support from the Instrumental Analysis Center of Shenzhen University (Xili Campus).