Abstract
This paper introduces the concept of continuous chaotic printing, i.e. the use of chaotic flows for deterministic and continuous extrusion of fibers with internal multilayered micro- or nanostructures. Two free-flowing materials are coextruded through a printhead containing a miniaturized Kenics static mixer (KSM) composed of multiple helicoidal elements. This produces a fiber with a well-defined internal multilayer microarchitecture at high-throughput (>1.0 m min−1). The number of mixing elements and the printhead diameter determine the number and thickness of the internal lamellae, which are generated according to successive bifurcations that yield a vast amount of inter-material surface area (∼102 cm2 cm−3) at high resolution (∼10 µm). This creates structures with extremely high surface area to volume ratio (SAV). Comparison of experimental and computational results demonstrates that continuous chaotic 3D printing is a robust process with predictable output. In an exciting new development, we demonstrate a method for scaling down these microstructures by 3 orders of magnitude, to the nanoscale level (∼150 nm), by feeding the output of a continuous chaotic 3D printhead into an electrospinner. The simplicity and high resolution of continuous chaotic printing strongly supports its potential use in novel applications, including—but not limited to—bioprinting of multi-scale layered biological structures such as bacterial communities, living tissues composed of organized multiple mammalian cell types, and fabrication of smart multi-material and multilayered constructs for biomedical applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Multi-material and multi-layered architectures achieve functionality and/or performance that are not achievable with monolithic materials. Moreover, the functionality and performance of multilayered composites is frequently determined by the proximity, indeed the density, of the constituent layers. Multilayered materials with a high amount of internal surface area can yield higher capacitances in supercapacitors [1], elevated mechanical strength [2, 3] and fatigue resistance [4], better sensing capabilities [5], or improved energy-harvesting potential [6]. A multi-lamellar architecture that features highly accurate control of surface geometry and surface area is also desirable in applications related to the controlled release of pharmaceuticals [7].
Multilayered structures are particularly relevant in nature and in biological applications. Indeed, one of the most pressing challenges in biofabrication is the development of strategies for the facile and high-throughput creation of multilayered and multimaterial tissue-like constructs. Real tissues are composed of multiple micrometer-thickness layers of distinct cell types. Although appealing and enabling, the cost-effective fabrication of multi-material, and perhaps multi-cell type, lamellar microarchitectures has proven to be challenging, especially when adjacent thin, perhaps single cell layers, of multiple cell types are desired. The current bioprinting and bioassembly technologies are capable of fabricating relatively complex lamellar architectures, but have difficulty placing large surfaces of different types of cells next to each other in a cost-effective manner. For instance, current strategies for multi-material bioprinting or bioassembly of multiple inks in the same printing operation face severe limitations in resolution and speed [8, 9]. A combination of multiple channels, each one dispensing one material, has been demonstrated to fabricate multi-material constructs with resolutions in the range of 50–100 µm [10–15]. We recently demonstrated multi-material 3D printing of perfusable multi-layered cannulas by co-extruding multiple streams of inks through a set of concentric capillary tubes contained in a single nozzle [13, 16]. Similarly, Kang and coworkers presented an extrusion printing technique that produced multi-material tissue-like microstructures by co-extrusion of different materials through a head with a pre-set internal architecture [14]. These state-of-the-art approaches to 3D bioprinting produce structures at resolutions dictated (in the best scenario) by the smallest relevant length scale of the nozzle [8] (i.e. 300–500 µm) and exhibit only moderate speeds (i.e. BioX from Cellink prints at a maximum linear speed of the printhead of 40 mm s−1). To date, no study has demonstrated the robust, fast, and cost-effective fabrication of reproducible micro- and nanostructures in a multi-material construct through a single-nozzle printhead.
Here we introduce the concept of continuous chaotic printing: the use of a simple laminar chaotic flow induced by a static mixer for the continuous creation of fine and complex structures at the micrometer and submicrometer levels within polymer fibers. Chaotic flows are used to mix in the laminar regime, where the conditions of low speed and high viscosity preclude the use of turbulence to achieve homogeneity [17, 18]. In the context of 3D printing, they have been suggested as a tool to provide better homogenization of different materials [19, 20]. However, a much less exploited characteristic of chaotic flows is their potential to create defined multi-material and multi-lamellar structures [21–24]. A recent contribution from our group demonstrated, for the first time, the use of simple chaotic flows (i.e. Journal Bearing flow) to imprint fine microstructures within constructs in a controlled and predictable manner at an exponentially fast rate in a batch-wise fashion [25]. In the present study, we explore the utility of a chaotic printer, equipped with a Kenics static mixer (KSM)[26] as a key component of the printhead, for the printing of alginate-based fibers with massive amounts of lamellar microstructures in a continuous fashion (figure 1).
Figure 1. Experimental setup. Continuous chaotic printing is based on the ability of a static mixer to create structure within a fluid. The Kenics static mixer (KSM) induces a chaotic flow by a repeated process of reorientation and splitting of fluid as it passes through the mixing elements. (A) Schematic representation of a KSM with two inlets on the lid. The inks are fed at a constant rate through the inlets using syringe pumps. The inks flow across the static mixer to produce a lamellar structure at the outlet. The inks are crosslinked at the exit of the KSM to stabilize the structure. Our KSM design includes a cap with 2 inlet ports, a straight non-mixing section that keeps the ink injections independent, a mixing section containing one or more mixing elements, and a nozzle tip. The lid can be adapted to inject several inks simultaneously. (B) Two rotated views at 0° and 90°, of a single KSM element. (C) 3D design of a KSM with 6 elements and schematic representation of the flow splitting action, the increase in the number of striations, and the reduction in length scales, in a KSM-printhead. The resolution, namely the number of lamellae and the distance between them (δ), can be tuned using different numbers of KSM elements. (D) Actual continuous chaotic printing in operation. The inset (E) shows the inner lamellar structure formed at the cross-section of the printed fiber (the use of 4 KSM elements originates 16 striations). Scale bar: 250 µm. (F) Longitudinal or (G) cross-sectional microstructure of fiber obtained using different tip nozzle geometries. Images show CFD results of particle tracking experiments where two different inks containing red or green particles are coextruded through a printhead containing 4 KSM elements. The lamellar structure is preserved when the outlet diameter is reduced, from 4 mm (inner diameter of the pipe section) to 2 mm (inner diameter of the tip), through tips differing in their reduction slope.
Download figure:
Standard image High-resolution image2. Results and discussion
2.1. Continuous chaotic printing: a simple and effective microfabrication strategy
Our chaotic printer is composed of a flow distributor, a pipe, a static mixing section, and an outlet or nozzle tip (figures 1(A), (B)). The number of inks one can use is unrestricted, and different distributor geometries can be employed to accommodate the injection of multiple inks. However, in this communication we adhered to some of the simplest printing scenarios. To this end, we adopted a distributor configuration (figures 1(A), (C)) for dispensing two inks in a symmetrical fashion. The mixing section contains a KSM, a static mixer configuration widely adopted in the chemical industry, that consists of a serial arrangement of n number of helical elements contained in a tubular pipe, with each element rotated 90° with respect to the previous one (figures 1(B), (C)). In the laminar regime, the KSM (and other static mixers [27–29]) produces chaos by repeatedly splitting and reorienting materials as they flow through each element. With this simple mechanism, lamellar interfaces are effectively produced between fluids (i.e. printheads containing 1, 2, 3, 4, 5, or 6 KSM elements will produce 2, 4, 8, 16, 32, or 64 defined striations; figure 1 (C)). Our results show that multi-material lamellar structures with different degrees of inter-material surface can be printed using a single nozzle by simply co-extruding two different materials (i.e. inks) through a KSM.
In the experiments presented here, we used sodium alginate to formulate different inks consisting of pristine alginate or suspensions of particles (polymer microparticles, graphite microparticles, mammalian cells or bacteria). For instance, we conducted experiments in which one or two types of fluorescent microparticles (i.e. red and green bacteria or red and green polymer beads) were injected into the inlets of the mixer distributor (figures 1(D), (E)). The result is continuous composite fibers with complex lamellar microstructures (figure 1(E) and figure S1) that can be stabilized simply by crosslinking in a bath of calcium chloride solution. This preserved the internal microstructure of the fibers with high fidelity (figure S1). Fine and well-aligned microstructures with defined features can be robustly fabricated along the printed fibers at remarkably high extrusion speeds (1–5 m of fiber/min). As we will show later, a vast amount of contact area is developed within each linear meter of these fibers. This printing strategy is also robust across a wide range of operation settings. We conducted a series of printing experiments at different inlet flow rates to assess the stability of the printing process. As long as the flow regime is laminar and the fluid behaves in a Newtonian manner (figure S1), the quality of the printing process is not affected by the flow rate used in a wide range of flow conditions. For example, using a cone-shaped nozzle-tip with an outlet diameter of 1 mm, stable fibers were obtained in a window of flow rates from 0.003 to 5.0 ml min−1 (figure 1(D)). Having printheads with different geometries (different degrees of slope) did not disturb the lamellar structure generated by chaotic printing. Computational fluid dynamics (CFD) simulation results suggested that the angle of inclination of the conical tip of the printhead (nozzle tip) did not affect the microstructure within the fiber in the range of the tested flow rates and reduction slopes.
Figures 1(F), (G), and S2 show a computational analysis of the effect of the shape of the printhead tip (angle) on the conservation of the microstructure of printed fibers produced from a mixture of alginate inks containing red and green particles.
2.2. Multilayered and well-aligned microstructures
The fabrication of fibers with fine lamellar microstructures will enable the design of materials for relevant biological applications such as the development of high surface biosensors, or composite materials with tunable mechanical properties for cell culture or bio-actuation.
In figures 2 and 3, we present the results of an experiment in which a suspension of 0.5% graphite microparticles in pristine alginate ink (2%) was co-extruded with pristine alginate ink (2%). For this illustrative experiment, the printhead outlet had a diameter of 1 mm (figure 2(A)). Note that the features in the extruded structure were remarkably similar at different lengths of the fiber (figures 2(B)–(F)). For instance, we calculated the area (shadowed in yellow) and the perimeter (indicated with a green line) of each of the graphite striations in five cross-sectional cuts along a fiber segment (figure 2(C)). Figure 2(D) shows an overlap of the microstructure for three of these cross-sections. The standard deviation of the area (figure 2(E)) and perimeter (figure 2(F)) for each of the striations is relatively small (the variance coefficient smaller than 10%). This illustrates the robustness of this printing strategy with small nozzle diameters, as well as the reproducibility of the microstructure obtained at different lengths of the fiber.
Figure 2. Analysis of the reproducibility of the lamellar microstructure produced by continuous chaotic printing. (A), (B) Cross-sectional cuts along an alginate/graphite fiber printed using 3 KSM elements. Scale bars: 1000 µm and 500 µm for the fiber and cross-sectional cuts, respectively. (C) The area (shaded in yellow) and perimeter (indicated in green) of graphite striations were determined using image analysis at different cross-sectional cuts. Scale bar: 500 µm. (D) The contours of three different cross-sectional cuts along a fiber segment are shown as different colors (green, red, and blue). The striation pattern is remarkably similar. Statistical analysis of the (E) area (shadowed in yellow), and (F) perimeter (indicated in green) of each of the graphite striations (indicated with numbers from 1 to 4) among 5 different cross-sectional cuts along a fiber segment chaotically printed using a printhead containing 3 KSM elements.
Download figure:
Standard image High-resolution imageFigure 3. Evaluation of the striation profiles and mechanical properties of chaotically printed alginate/graphite fibers. (A) Lamellar microstructure of fibers produced with printheads containing 2, 3, 4, 5, or 6 KSM elements. The thickness of each lamella, along the red line, was determined by image analysis using Image J (shown below each cross-sectional cut). Scale bar (red): 2 mm. (B) The microstructure at each cross-section was reproduced by CFD simulations, and the thickness and position of each lamella was calculated. (C) Striation Thickness Distribution (STD) and (D) cumulative STD for constructs printed using 4, 5, 6, and 7 KSM elements. (E) Comparison of stress-strain curves of fibers fabricated by extrusion of pristine alginate and graphite without chaotic mixing (marked as hand-mixed) or with chaotic printing using 2, 4, or 6 KSM elements (marked as 2, 4, or 6 ke). (F) Comparison of the standard deviation of tensile properties (i.e. maximum stress, maximum strain, and Young's modulus for the same set of fibers; 5 fibers per treatment).
Download figure:
Standard image High-resolution imageNotably, the resolution of this technique is controlled by both the diameter of the nozzle and the number of mixing elements. As the number of elements used to print increased, the number of lamellae observed in any given cross-sectional plane of the fiber also increased, while the thickness of each lamella decreased (figure 3(A)). Therefore, users of continuous chaotic printing will have more degrees of freedom to determine the multi-scale resolution of a construct, as this is no longer mainly restricted by the diameter of the nozzle (or the smallest length-scale of the nozzle at cross-section). For instance, for our two-stream system (figure 1(C)), the number of lamellae increases exponentially according to the simple model s= 2n, where s is the number of lamellae or striations within the construct and n is the number of KSM elements within the extrusion tube.
Two streams of inks co-injected into the printhead will generate 4, 8, 16, 32, and 64 distinctive streams of fluid when passing through a series of 2, 3, 4, 5, and 6 KSM elements, respectively (figure 3(A)). The average resolution of the structure will then be governed by the average striation thickness of the construct (δ), given by δ = D/s, were D is the nozzle inner diameter (figure 1(C)). Since stretching is exponential in chaotic flows [21, 23, 25], the reduction in the length scale is also exponential, as is the increase in resolution (i.e. more closely packed lines). In the experiment portrayed in figure 3, the cross-sectional diameter of the fibers was 2 mm. We observed defined average striations with resolutions of ∼500, 250, 125, 62.5, and 31.75 μm by continuously printing using 2, 3, 4, 5, and 6 KSM elements, respectively. Even when 6 KSM elements were used, distinctive lamellae could be discriminated in the array of 64 aligned striations (figure 3(A)). The resolution values obtained through 6 elements already exceeded those achievable by state-of-the-art commercial 3D extrusion printers (∼100–75 μm) [30, 31] that use hydrogel-based inks (i.e. commercial bioprinters) [30, 32].
Another remarkable characteristic of continuous chaotic printing is that the structure obtained is fully predictable, since chaotic flows are deterministic systems (as any chaotic system) [23, 33]. Simulation results, obtained by solving the Navier-Stoke equations of fluid motion using CFD [34, 35], closely reproduced the cross-sectional lamellar microarchitecture within the fibers (figures 1(F), (G); figure 3(B)).
Moreover, we used optical microscopy and image analysis techniques to characterize the fine array of lamellae experimentally produced by continuous chaotic printing. We calculated the striation thickness distribution (STD) on the cross-sections of the graphite/alginate fibers. We did this by drawing several center lines of representative cross-sections and then calculating the distance between striations along those lines (Figure S3). The frequency distribution and the cumulative STD were then measured. Figures 3(C) and (D), respectively, show the STD and the cumulative STD for constructs printed using 4, 5, 6, and 7 KSM elements. Remarkably, this family of distributions exhibits self-similarity, one of the distinctive features of chaotic processes [22, 23, 25]. As discussed, for any of these particular cases, the average striation thickness could be calculated as the fiber diameter/number of striations (D/s). However, due to the highly skewed shape of the distribution toward smaller striation thicknesses (figure 3(D)), the median striation thickness is lower than the average striation value. For example, for the case where 4 KSM elements were used, the average striation thickness can be calculated as 2 mm/16 = 125 µm. Indeed, 50% of the striations measured less than 125 µm (figures 3(C), (D)), but the corresponding STD showed that most of the striations had a median value of about 75 µm. This has profound implications for crucial processes such as cell attachment, cell signaling, local reaction kinetics, and mass and heat transfer. For instance, the diffusional distances (δ) in these constructs decreased rapidly with an increase in the number of elements used to print (i.e. following the model δ/(2n)). The diffusional length scales are then reduced by half each time that a KSM element is added to a chaotic printhead. Since diffusion time increases with the square of the diffusion distance (and is only inversely proportional to the diffusion coefficient), the diffusion time decreases 4-fold per element added. This implies that the time relevant to cell signaling decreases 8-fold (almost an order of magnitude) if one KSM element is added to the printhead. As we will demonstrate later, the intermaterial area per unit of volume, which is key for surface-catalyzed reactions and cell attachment, increases exponentially as the number of elements is increased (figure 4).
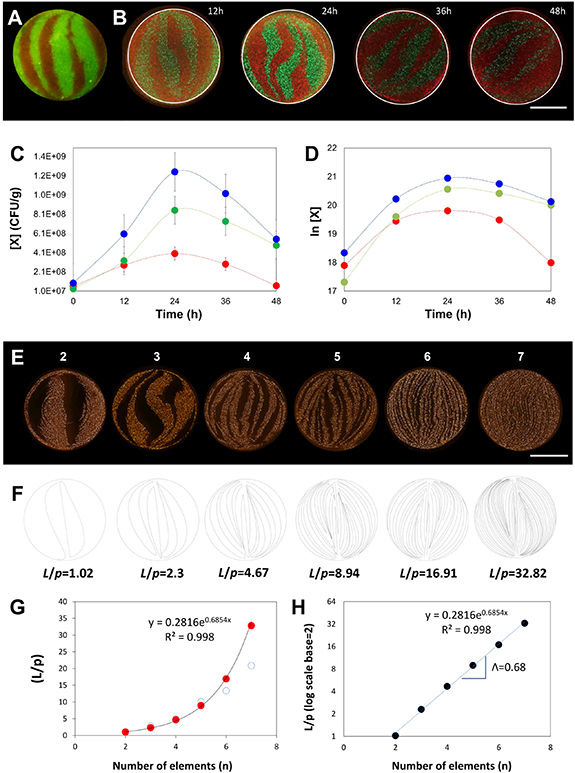
Figure 4. Chaotic bioprinting of bacteria. (A) Cross-section of a fiber where GFP- and RFP-bacteria shared an inter-material interface. The micrograph was obtained after chaotic printing at a high initial cell concentration and using 3 KSM elements. Scale bar: 500 µm. (B) The evolution of the concentration of living bacteria in the cross section of a fiber, initially printed with a low bacterial concentration, shown by micrographs taken at 12, 24, 36, and 48 h; Scale bar: 500 µm. (C) Growth curves showing the increasing concentration of viable cells over time, as determined by standard plate culture microbiological methods. Red and green symbols indicate the evolution of red and green fluorescent bacteria, respectively. Blue symbols show the total numbers of viable cells. (D) Plot of the natural log of bacterial populations over time. (E) Cross-sections of alginate fibers containing fine and aligned striations of RFP-E. coli. These fibers of 1 mm thickness were produced by chaotic bioprinting using printheads containing 2 to 7 KSM elements. Scale bar: 500 µm. (F) Determination of the shared interface from computational simulations. (G) Estimation of the total amount of interface shared between regions with and without bacteria (L), normalized by the perimeter of the fiber (p); red dots ( ) indicate approximations based on a simple geometric model, and empty blue circles (○) show determinations based in image analysis of experimentally obtained micrographs. (H) Natural log of the L/p ratio as a function of the number of elements used to print. The Lyapunov exponent (Λ) of the chaotic flow is calculated from the slope of the resulting straight line.
) indicate approximations based on a simple geometric model, and empty blue circles (○) show determinations based in image analysis of experimentally obtained micrographs. (H) Natural log of the L/p ratio as a function of the number of elements used to print. The Lyapunov exponent (Λ) of the chaotic flow is calculated from the slope of the resulting straight line.
Download figure:
Standard image High-resolution imageFiber and particle alignment is key in many applications in materials technology [36]. We next show that the fabrication of well-aligned microstructures achievable through chaotic printing can influence relevant characteristics of composites, such as the robustness of their mechanical performance. We characterized the mechanical properties of alginate-based fibers 2.5 cm in length produced by chaotic printing (and therefore having different internal structures) or hand-mixing and extrusion through an empty pipe (figure 3; figure S4). Specifically, we conducted tensile testing using a universal testing machine on fibers produced from a mixture of 0.5% graphite microparticles in alginate, generated either by hand-mixing (a control without lamellar structures) or by continuous chaotic printing using 2, 4, or 6 KSM elements. Figure 3(E) shows the stress-strain curves associated with the resulting fibers. We did not find significant differences in the Young's modulus, ultimate stress, or maximum elongation at break in these sets of fibers (figure S4). However, the fibers exhibited less variability when produced by continuous chaotic printing than those by extrusion of hand-mixed inks. Among the fibers produced by chaotic printing, the fibers were more homogeneous when co-extruded through printheads containing 4 or 6 elements than only two elements. Figure 3(F) shows an analysis of the standard deviation of relevant mechanical performance indicators associated with different microstructures. These results suggest that effective alignment of the microstructures within the fibers resulted in a more reproducible mechanical performance in structurally complex materials such as alginate hydrogels.
2.3. Bioprinting applications
Bioprinting (i.e. the printing of living cells and biomaterials in a predefined fashion) is presently even more limited in resolution and speed than additive manufacturing techniques in general. We further illustrate a biological application of continuous chaotic printing by fabricating constructs with specific microarchitectures containing living cells.
Tightly controlling the degree of intimacy (i.e. the density of interfaces between bacterial populations) may enable the fabrication of 3D multi-material constructs with novel functionalities [37] and is of paramount importance in modern microbiology [38], for example on the design of physiologically relevant gut-microbiota models [39]. The spatial arrangement and distribution of bacteria, recently described as 'microbiogeography' [38], is an important determinant of bacterial community dynamics. Different species of bacteria interact with other micro-organisms through chemical signals [38, 40, 41]. For example, quorum sensing, a well-studied phenomenon, depends on the vicinity and the amount of surface area shared among bacterial communities [42]. In general, the dynamics of competition or mutualism in mixed microbial communities is strongly influenced by spatial distribution [43–46]. However, relatively few studies have addressed the relationship between spatial distribution, distance, and cell density in bacterial systems [43–49]. This is partially due to the fact that conventional microbiology techniques offer only a limited degree of control over the spatial organization of mixed cultures [50].
Continuous chaotic printing enables precise control of the spatial distribution of bacterial communities, aligned in a lamellar microstructure, and allows meticulous and unprecedented design and regulation of the amount of interface between bands of bacteria.
In figures 4(A)–(E), we used two recombinant E. coli strains, one producing red fluorescent protein (RFP) and the other producing green fluorescent protein (GFP) to fabricate cell-laden fibers. As anticipated, well-defined bacterial striations could be printed using our technique (figure 4(A)).
Remarkably, the bacteria could be cultured in these fibers for extended time periods. We followed the kinetic behavior of both bacterial populations (i.e. GFP- and RFP-bacteria) in the fibers initially seeded at low concentrations. During the first 24 h of culture (from t = 0 to 24 h), the intensity of the fluorescence produced by the bacterial colonies increased, while the bacteria continued to respect the original patterns in which they had been printed (figure 4(B)). We corroborated the increase in the number of live bacteria by conventional colony-forming units (CFU) microbiological assays (figures 4(C), (D). To do this, we sampled multiple sections of fibers. We consistently observed that the bacterial populations grew in both areas for the first 24 h, exhibited a short plateau, and later decayed. Interestingly, we observed a statistically significant difference in the number of viable green and red bacteria (P < 0.05) at 24 and 48 h after printing. These differences suggest that these two populations of bacteria, although practically identical in their genetic makeup, establish a competition for resources along shared interfaces (see also [51, 52]). These results demonstrated that chaotic printing can be used for the fabrication of dynamic living systems that are capable of evolving in time from very well-defined initial conditions. In addition, massive amounts of interface between green and red bacterial regions could be developed if more KSM elements were used during printing. For example, the boundary between the green and red bacterial regions can be effectively tuned from ∼1 mm down to 15 µm by varying the number of KSM elements used to print (from 2 to 7, figure 4(E)). Since the maximum length of these bacteria is ∼2 µm, we were able to imprint lamellae of bacteria in the resolution range of tens of micrometers. The diameter of these fibers was 1 mm. Therefore, printing using 7 KSM elements yielded lamellae with average striation thicknesses of less than 10 µm (median lower than 7 µm). This means that each lamella might accommodate a few bacterial cells across its width. While a characteristic standard deviation occurs with chaotically printed constructs (figure 3(E)), the structures obtained by chaotic printing are repeatable. This will translate into the fact that the 'overall' functionality of the construct (i.e. the bacterial community or tissue construct to be fabricated) will be dictated by the architecture. Please note that, in figure 3(C), the STDs of constructs printed using four and six (or five and seven) elements are distinguishable (i.e. their overlapping is minimal).
In chaotic printing, the amount of inter-material area fabricated increases exponentially as a function of the number of elements used. We used image analysis techniques to quantify, at high magnification, the shared perimeters between red and black lamellae in figure 4(E) (cross-sectional cuts). Indeed, the amount of black-red perimeter at cross-sections grew exponentially with the increasing number of elements (figures 4(E), (G), (H)).
In addition, using computational strategies, we simulated the amount of surface area generated by the printing process in constructs printed using different KSM elements (figure 4(F); figure S5), and confirmed the data obtained experimentally (figures 4(E), (G)). For instance, when six elements were used to print, approximately 5 cm of shared linear interface were developed between the two materials (inks) at each cross-sectional plane (D = 0.1 cm); the ratio between the total amount of developed interface and the fiber perimeter was 16.91. This created a remarkably high density of shared interface (6.76 cm mm−2). Since the fiber exhibits the very same microstructure along its entire length (figure 3(B)), the inter-material surface density generated inside the fiber could be determined as ∼0.067 m2 cm−3.
In tissue engineering scenarios, multi-material and multilayer structures are required to mimic the architecture and functionality of real tissues [15]. We also conducted chaotic bioprinting experiments in which we fabricated bands of C2C12 murine skeletal myoblasts within alginate fibers added with gelatin methacryloyl (GelMA) [53]. We present the cross-sectional (figure 5(A)) and longitudinal view (figure 5(B)) of a cell-laden alginate fiber lightly enriched with protein (GelMA) to favor cell attachment and eventual proliferation.
Figure 5. Bioprinting of living micro-tissues: (A) Optical and (B) SEM micrographs of the cross-sectional view of a construct in which C2C12 cells are chaotically bioprinted in an alginate/GelMA hydrogel using a 3-KSM printhead; Scale bars: 500 µm and 50 µm, respectively. (C) Longitudinal view of a chaotically bioprinted construct; a high cell viability is observed at the initial time, as revealed by a live/dead staining and fluorescence microscopy. Scale bar: 500 µm. Inset shows a cross-sectional cut. Scale bar: 500 µm. (D) Cells spread along the chaotically printed striations, preserving their original positions after 13 d of culture. Scale bar: 200 µm. (E) Optical microscopy view of a segment of fiber containing C2C12 cells 18 d after printing. Scale bar: 500 µm. (F) Close-up of a region stained to reveal F-actin/nuclei, showing the cell spreading and the formation of interacting cell clusters. Cell nuclei can be identified as blue dots. Actin filaments appear in red. Scale bar: 200 µm.
Download figure:
Standard image High-resolution imageWell-defined bands of C2C12 cells are distinguished along the hydrogel fibers. Mammalian cells are shear-sensitive; however, the low shear laminar conditions prevalent at the printhead tip enabled high initial cell viabilities (higher than 90%; figure 5(C)). Cells survived and proliferated within these fibers. Most cells remained within the striations corresponding to the cell-laden ink. After 7 d of culture, the cells began to spread and interact with each other, and some clusters of cells appeared. After 2 weeks of culture (i.e. day 13 and 18), the proliferating cells elongated while maintaining their initial striation patterns (figures 5(D), (E)). The fibers were stained to reveal the position of the cell nuclei and their cytoskeletons. Note that in some cell clusters, multinucleated cells, a signature of myotubule development, started to be evident (figure 5(F)). This illustrative experiment suggests the potential of chaotic bioprinting to produce living fibers that closely resemble the multilayered structure observed in mammalian tissues and display massive amount of material interface. As demonstrated before (previous subsection), using a chaotic printhead containing 6 KSM elements, 0.067 m2 of material interface could be accommodated per 1 cm3 of cell-laden fiber. Therefore, ∼67 m2 l−1 of well-aligned inter-material surfaces could be fabricated within these living constructs. For comparison, human kidneys have an approximate volume of 150 cm3, and the total area of the capillaries of all the glomeruli within them is 0.6 m2 (4 m2 l−1) [54]. Printing at the flow rate of 1 ml min−1, which is a typical printing flow rate used in our system, could generate this amount of area per unit of volume every minute.
This massive amount of interface cannot be fabricated at this speed, precision, or resolution by any of the currently available micro-fabrication or printing platforms. It should be noted that flow rates of up to 3 ml min−1 could be conveniently achieved using our printing method.
2.4. Coupling of continuous chaotic printing with other fabrication techniques
The combination of continuous chaotic printing with other fabrication technologies (e.g. molding, electrospinning, or robotic assembly) will lead to the development of complex multi-scale architectures with high degrees of predictable external shapes and internal microstructure. Indeed, during printing, these fibers can be rearranged either into macrostructures or the individual fibers can be further reduced in diameter while preserving their lamellar architecture (figures 1(F), (G): figures 6(A)–(C)). We illustrate this by printing a long fiber of alginate containing multiple lamellae and then rearranging it into a block of several layers of fiber segments (figures 6(A)–(C)). The integration of this multi-material printhead into a 3D printer may thus enable rapid fabrication of multi-material (and/or multi-cellular) constructs that exhibit a great amount of material interface with a complex and tunable hierarchical architecture.
Figure 6. Development of multi-scale architectures based on 3D continuous chaotic printing: (A)–(C) 3D printing of hydrogel constructs using a KSM-printhead integrated to a commercial cartesian 3D printer. (A) Schematic comparison of the lack (prepared using conventional extrusion techniques) and presence of internal lamellar microstructures (developed using continuous chaotic printing). (B) Printing of a long fiber arranged into a macro-scale hydrogel construct (3 cm × 3 cm × 4 mm). Scale bar: 5 mm. (C) Transverse cut of the macro-construct showing the internal microstructures. Scale bar: 1 mm. (D)–(G) Chaotic printing of fibers coupled with electrospinning. (D) Schematic representation of the coupling between continuous chaotic printing and an electrospinning platform; an ink composed of a pristine alginate ink (4% sodium alginate in water) and an ink composed of a polyethylene oxide blend (7% polyethylene oxide in water), were coextruded through a chaotic printhead and electrospun into a nanomesh. (E) AFM image showing the diameter of three individual nanofibers ((1) 0.82 µm, (2) 1.05 µm, and (3) 0.437 µm) within the electrospun mesh. Scale bar: 5 µm. (F), (G) photo-induced force microscopy (PiFM) reveals the lamellar nature of the nanostructure within a nanofiber (white arrows) originated using (F) a 2-element KSM printhead, and (G) a 3-element KSM printhead. Scale bar: 1 µm.
Download figure:
Standard image High-resolution imageAlso, chaotic printing may be coupled with other techniques for the production of nanofibers that contain finely controlled structures at the submicron scale (figures 6(D)–(G)). This may enable, for example, the fabrication of microsensors or microactuators with enormous surface area, for biological applications. As an example, we coupled a 2-element KSM printhead with an electrospinning device (figure 6(D)) to produce a mesh of nanofibers containing well-defined nanostructures composed of a pristine alginate ink (4% sodium alginate in water) and polyethylene oxide (7% PEO in water). Fibers produced by 3D chaotic printing were continuously solidified as they were generated by direct feeding into an electrospinning apparatus, further reducing the fiber mean diameter to <300 nm (figure 6(E)). In this hybrid fabrication strategy, fiber solidification occurs by rapid evaporation during electrospinning, instead of crosslinking by immersion in calcium chloride.
Remarkably, the structure produced by chaotic printing is preserved during electrospinning. Based on estimates of the shape of the Taylor cone (∼0.18 µl) and the rate of injection of the materials (2–5 µl min−1), the average residence time in the Taylor cone is ∼2–5 s. The diffusion time may be roughly calculated as δ2/Dc, where δ is the average striation thickness (δ = 0.0128 when 3 KSM elements are used), and Dc is the diffusion coefficient. The diffusion coefficient of relatively small organic molecules in PEO has been reported as ∼108 [55]. Therefore, the actual diffusion coefficient of PEO in alginate should be much lower, at ∼109. The diffusion time for this process should then be in the range of ∼1000 s, or much higher than the residence time. Since the residence time at the Taylor cone is about 3 orders of magnitude shorter than the diffusion time, electrospinning is expected to have a negligible effect on the structure obtained by 3D chaotic printing.
A close inspection using photo-induced force microscopy (PiFM) [56] revealed multilayered nanostructures with average striation thicknesses in the range of 75–100 nm (figures 6(F), (G); figure S6). These results demonstrated that the microstructure created by 3D chaotic printing can be further scaled down by 3 orders of magnitude using electrospinning.
3. Conclusion
In this study, we have presented continuous chaotic printing as a strategy that enables delicate control of the spatial microstructures (i.e. number of layers and average spacing between them) within a single 3D printed fiber. The key element of this technological platform is the use of an on-line static mixer in the printhead to provide a partial mixing of different materials as they are coextruded through the nozzle tip. In particular, we have adopted the KSM as the first model and have used it to fabricate, in a simple fashion, highly convoluted 3D structures within polymer composites in a continuous stream at high speeds (>1.0 meters of fiber/min). The diameter of the printing head and the number of mixing elements determine the number and thickness of internal lamellae produced according to a process of successive bifurcations that yields an exponential generation of inter-material area. Illustratively, by using 6 internal elements, 64 lamellae of average widths of 15 µm can be generated in a 1 mm cross-section fiber, and an inter-material area of ∼67 m2 l−1 can be achieved. These values for microstructure resolution, internal surface area density, and fabrication speed all exceed the capabilities of any of the currently available commercial microfabrication techniques (i.e. commercial 3D printers) for the creation of microstructure.
Our results demonstrate the unrivaled ability of chaotic printing to deploy cells within high SAV fibers. As available bioprinting and bioassembly technologies approach the resolution and SAV of chaotic printing, they also tend to require long fabrication times and mechatronically coordinated control systems [57, 58]. In addition to multicellular, high SAV constructs, chaotic printing offers other breakthroughs in regards to currently available multi-material printing technologies that, typically, require optimized inks that must be deployed under a specific and narrow range of conditions.
The fundamentals underlying chaotic printing are solid, as this type of printing relies on the use of chaotic flows to develop microstructure at an exponential rate in a deterministic manner. Indeed, we have shown that the microstructure resulting from the use of different numbers of KSM elements is amenable to rigorous modeling using CFD simulations, and the resemblance between our experimental and simulation results is remarkable. This precise predictability of the microstructures within a printed construct will greatly expand the application of 3D printing and the complexity of printed composites. A wide spectrum of microstructures can be designed and obtained using this technique. The adoption of different types of static mixer elements (i.e. SMX-Sultzer, and novel ad hoc designs), the use of more than two inks (or materials), the manipulation of the injection location, and the dynamic changes in the speed of each one of the injections, can open up possibilities for obtaining structures with various degrees of complexity in a wide range of scales. For instance, we showed that chaotic printing is a simple and versatile micro- and nano-fabrication platform and that, when coupled with other fabrication resources, can generate macro-structures with an enormous amount of interface between their constituent materials.
The fabrication method introduced here produces non-uniform, but reproducible and well-defined, lamellar patterns. We strongly believe that chaotic printing adds to the existing arsenal of tools currently available to the biofabrication community. Reproducible non-uniformity is precisely one of the strengths of this printing method (and a signature of Nature). We were bioinspired by some of the highly complex (and ordered) patterns that are produced in Nature, which are mostly non-uniform (i.e. vasculature, marble and other multilayered geological formations, multi-layered tissues, and real bacterial communities, among many others).
Remarkably, our variability is statistically robust. As we have shown (figures 3(E), (F)), the striation thickness distribution (STD) of the microstructure generated through chaotic printing is known, reproducible, can be calculated by simulations, and is even self-similar (meaning that the overall shape of the STDs obtained by printing with different numbers of elements closely resemble each other). All these properties are rooted in the fundamental physics of chaotic advection. Chaotic flows generate microstructure with a reproducible distribution of length scales [22, 23].
In an exciting further development, we demonstrated that the output of a continuous 3D chaotic printhead can be fed into an electrospinning nozzle to create fiber meshes with lamellar nanostructures. By doing so, we have shown that the microstructure created by 3D chaotic printing can be further scaled down by three orders of magnitude. We envision numerous applications of continuous chaotic printing in biomedicine (i.e. bacterial and mammalian cell bioprinting), electronics (i.e. fabrication of high-sensitivity multi-branch electrodes and supercapacitors), and materials science in general.
4. Experimental section
4.1. Experimental set-up
Our continuous chaotic printer consisted of a syringe pump loaded with two 10 ml disposable syringes, a cylindrical printhead containing from 2 to 7 KSM elements, and a flask containing 550 ml of 2% calcium chloride (Fermont, Productos Químicos Monterrey, Monterrey, NL, Mexico) (figure 1(A)). Syringes were loaded with different inks (i.e. particle suspensions in pristine 2% alginate) and connected to one of the two inlet ports located in the lid of the printhead. Details of the geometry of the printer head and the internal KSM elements are shown in figures 1(B), (C). The fabrication of printer heads is described in a following subsection (KSM printheads). The syringe pump was set to operate at a flow rate of 0.8 to 1.5 ml min−1. We conducted experiments using printheads with different internal diameters, in the range from 5.8 to 2 mm. The tube containing the KSM could be connected to a tip to further reduce the diameter of the final fiber. Tip reducers with an outlet diameter of 4, 2, and 1 mm were used in the experiments presented here (figures 1 (D), (F), (G)). The outlet of the tip was submerged in 2% calcium chloride to crosslink the extruded fibers at the outlet of the tube (figure 1(D)).
4.2. KSM printheads
We fabricated our KSM printheads in house. KSM elements were designed using SolidWorks based on the optimum proportions reported in literature [59]. The sets of KSM elements were printed on a P3 Mini Multi Lens 3D printer (EnvisionTEC, Detroit, MI, USA) from the ABS Flex White material. We used a length-to-radius ratio of L:3R (figure 1(B)). For example, for printheads with an internal diameter of 5.8 mm, the length and diameter of each separate KSM element were 8.7 mm and 5.8 mm, respectively. Sets of 2, 3, 4, 5, 6, and 7 KSM elements, attached to a tube cap, were fabricated to ensure a correct orientation of the ink inlet ports on the cap with respect to the first KSM (figure 1(C)). The cap was designed so that each ink inlet was positioned on a different side of the first KSM element to maintain similar initial conditions in all experiments (figures 1(A), (C)).
4.3. Printing experiments and ink formulations
We used several different ink formulations for the experiments presented here. Inks consisted of particles suspended in 1% alginate or pristine alginate (CAS 9005-38-3, Sigma-Aldrich, St. Louis, MO, USA) solutions.
In a first set of experiments, we fabricated fibers loaded with either red or green fluorescent particles. Red and green fluorescent inks were prepared by suspending 1 part of commercial fluorescent particles (Fluor Green 5404 or Fluor Hot Pink 5407; Createx Colors; East Granby, CT, USA) in 9 parts of a 2% aqueous solution of sodium alginate (Sigma-Aldrich, St. Louis, MO, USA). The fluorescent particles were previously subjected to three cycles of washing, centrifugation, and decantation to remove surfactants present in the commercial preparation.
We also used chaotic printing to fabricate fibers containing an overall concentration of 0.5% graphite by co-extruding a suspension of 1.0% graphite in alginate solution (2%) and pristine alginate solution (2%) through printheads containing KSM elements. In addition, we produced control fibers by extruding pristine alginate (without graphite microparticles) through an empty tube, or by co-extruding two streams of ink containing 0.5% graphite microparticles hand-mixed in alginate.
In a third set of experiments, we used fluorescent inks based on suspensions of fluorescent E. coli bacteria. These fluorescent bacteria were engineered to produce either GFP or RFP. Bacterial inks were prepared by mixing either GFP- or RFP-expressing E. coli in 2% alginate solution supplemented with 2% Luria-Bertani (LB) broth (Sigma-Aldrich, St. Louis, MO, USA). For ink preparation, bacterial strains were cultivated for 48 h at 37 °C in LB media. Bacterial pellets, recovered by centrifugation, were washed and re-suspended twice in alginate-LB medium. The optical density of the re-suspended pellets was adjusted to 0.1 absorbance units before printing (approximately 5 × 108 CFU ml−1). Fibers were printed at a flow rate of 1.5 ml min−1 and cultured by immersion in LB media for 72 h. The number of viable cells present in the fibers at different times was determined by conventional plate-counting methods. Briefly, fiber samples of 0.1 g were cultured in tubes containing LB media. The number of viable cells was determined by washing the 0.1 g samples in 1X phosphate-buffered saline (PBS) at pH 7.4 (Gibco, Carlsbad, CA, USA) to remove the bacteria accumulated in the LB media. Each sample was disaggregated and homogenized in 0.9 ml of PBS. The resultant bacterial suspensions were decimally diluted, seeded onto 1.5% LB-Agar (Sigma-Aldrich, St. Louis, MO, USA) plates, and incubated at 37 °C for 36 h.
We also bioprinted muscular murine cells (C2C12 cell line, ATCC CRL 1772) in 1% alginate inks supplemented with 3% GelMA added with a photoinitiator (0.067% LAP). To this purpose, a first ink contained only alginate and GelMA, while the second was cell-laden with C2C12 cells at a concentration of 3 × 106 cell ml−1. Cell laden fibers were obtained by immersion in alginate and then further crosslinked by exposure to UV light at λ = 400 nm for 30 s. The bioprinted and cell-laden fibers were immersed in DMEM culture medium (Gibco, Carlsbad, CA, USA) and incubated for 20 d at 37 °C in an 5% CO2 atmosphere. Culture medium was renewed every 4th day during the culture period.
In a fifth set of experiments, we produced electrospun nanofiber mats by combining 3D chaotic printing in-line with electrospinning. First, we chaotically printed fibers by coextrusion of a pristine alginate ink (4% sodium alginate in water) and PEO (7% PEO in water) at a rate of 2–5 µl min−1. The resulting PEO-alginate fibers were then electrospun (in-line) to produce nanofiber mats.
4.4. Microscopy characterizations
The microstructure of the fibers produced by chaotic printing was analyzed by optical microscopy using an Axio Imager M2 microscope (Zeiss, Oberkochen, Germany) equipped with Colibri.2 led illumination and an Apotome.2 system (Zeiss, Oberkochen, Germany). Bright-field and fluorescence micrographs were used to document the lamellar structures within the longitudinal segments and cross-sections of the fibers. Wide-field images (up to 20 cm2) were created using a stitching algorithm included as part of the microscope software (Axio Imager Software, Zeiss, Oberkochen, Germany). Fibers were frozen by sudden immersion in liquid nitrogen to facilitate sectioning while preserving the microstructure. The microstructure of the nanofibers produced by chaotic printing coupled with electrospinning was analyzed by atomic force microscopy (AFM) and PiFM, a nano-IR technique (figure S6).
4.5. Mechanical testing of graphite-alginate fibers
We used a universal test bench machine (Tinius Olsen h10kn, Horsham, PA, USA), with a load cell of 50 N at a rate of 35 mm min−1, to evaluate the mechanical properties of alginate fibers containing 0.5% graphite particles and produced by different printing strategies. Specifically, we conducted tensile testing on fibers produced from a mixture of 0.5% graphite microparticles in alginate, generated either by hand-mixing and extrusion through an empty pipe (a control without lamellar structures) or by continuous chaotic printing using 2, 4, or 6 KSM elements. In these experiments, the gauge length between clamps was set to 25 mm. Stress-strain curves were obtained for each of the five different formulations. We determined the maximum tensile strength, strain at break, and Young modulus of the fibers from stress-strain data.
4.6. Computational simulations
The system was simulated using a finite element model (FEM) strategy in COMSOL Multiphysics 5. First, a 3D model was designed and solved, using laminar flow equations and a stationary solver, to determine the velocity field in the system for the various experimental scenarios explored. A fluid viscosity value of 1P and a density of 1000 kg m−3 were used. A time dependent solver was then used to track up to 105 massless particles using particle tracking for fluid flow physics in the previously solved stationary velocity field. The simulation was discretized with a reasonable fine mesh composed of free triangular elements. Mesh sensitivity studies were conducted to ensure the consistency of results. No-slip boundary conditions were imposed in the fluid flow simulation, while a freeze boundary condition was employed for the particle tracing module. The interface length was determined by importing the output results from the cross-section of the fibers (a set of points describing the interface position) into CorelDraw software X5 (Corel Corporation, Ottawa, Canada), drawing Bezier curves over the striations, and establishing the length of the curves using the software (figure S5).
Acknowledgments
CCM and MDdLD contributed equally to this work. GTdS acknowledges the funding received from CONACyT (Consejo Nacional de Ciencia y Tecnología, México), L'Oréal-UNESCO-CONACyT-AMC (National Fellowship for Women in Science, Mexico) and UC-MEXUS. MMA and GTdS acknowledge funding provided from CONACyT, Fronteras de la Ciencia No. 2442. Yu Shrike Zhang acknowledges the funding by the Brigham Research Institute. AK would like to acknowledge funding from the National Institutes of Health (HL137193, 5R01AR057837, 1R01EB021857). This research has been partially funded by the Tecnológico de Monterrey and the Massachusetts Institute of Technology (MIT) Nanotechnology Program. HKW and SOMC greatly acknowledge funding provided by Cátedra Federico Baur, Tecnológico de Monterrey. We gratefully acknowledge the experimental assistance of Gyan Prakash, Everardo González-González, Aimé Alexandra Cuellar-Monterrubio, Alan Roberto Márquez-Ipiña, Sara Cristina Pedroza, Felipe López-Pacheco, Matías Lobo-Zegers, Zamantha Escobedo-Avellaneda, and Esther Pérez-Carrillo. We acknowledge the valuable assistance of Centro de Investigación en Química Aplicada (CIQA) in Saltillo, Coahuila, México.
Author contributions
GTdS, MMA, designed the study. CChM and MDdLD developed the experimental set-up and conducted most of the printing experiments. MS performed all the computational simulations. CCMB and JFYdL fabricated the printheads by stereolitographic 3D printing. CFCG, JEBM and CChM. conducted most of the bioprinting experiments. SH, NAGF, and CChM performed the electrospinning experiments. MAA conducted PiMF characterization experiments. IGG and CChM conducted the mechanical testing experiments. GTdS and MMA produced the first complete draft of the manuscript. GTdS, MMA, MM, YSZ, CChM, SOMCh, CAR, HKW, DD, and AK significantly edited the manuscript. CChM, GTdS, MMA, MDdLD, MS, and MAA prepared illustrations. All authors read, commented, and approved the manuscript.
Conflicts of interest
There are no conflicts to declare.