Abstract
Two types of graphene oxide (GO) hybrids were synthesised for the administration of doxorubicin (DOX, named GO-CO-DOX) and interfering RNA - siRNA (named GO-PEG-PEI/siRNA). These nanomaterials were used for intervention on vascular endothelial growth factor (VEGF) that represents an important strategy to block the formation of new vessels to inhibit cancer progression. For the delivery of DOX, it was incorporated into GO, while for the delivery of siRNA, GO was covalently bonded with the cationic polyethyleneimine (PEI) and then complexed with siRNA. The nanostructures were characterised by attenuated total reflection ATR-FTIR, zeta potential, x-ray photoelectron spectroscopy (XPS), x-ray diffraction (XRD), and transmission electron microscopy (TEM). The cytotoxicity studies with GO-PEG-PEI and GO-PEG-PEI/siRNA systems showed that both formulations have IC50 values of around 100 μg ml−1. The systems were administered in vivo to investigate their antitumor effects against non-muscle-invasive bladder cancer (NMIBC) and showed to be promising for the treatment of NMIBC.
Export citation and abstract BibTeX RIS
1. Introduction
Bladder cancer (BC) is one of the most common forms of malignancy in the United States [1]. The highest mortality rates were found in Europe, USA, and Egypt. These mortality rates can be explained by differences in risk factors, diagnostic techniques, and treatments available in each country [2]. Little progress has been made for the treatment of BC. Intravesical instillation of Bacillus Calmette-Guerin (BCG) for the treatment of non-muscle-invasive bladder cancer (NMIBC) has been used for more than 30 years as a standard treatment [3]. Despite the relative success of intravesical administration of BCG, a non-effective biological response is obtained, and, in many cases, NMIBC persists. It is important to point out that approximately 40% of patients undergoing BCG treatment may experience progression to the invasive form of BC [4]. As a consequence, it is imperative to develop new therapeutic alternatives for the treatment of BC, such as drug delivery systems that allow the intravesical administration of molecules, since the bladder is not a highly vascularised tissue, which may limit the efficacy of the treatment based on systemic administration of drugs. At the same time, these drug delivery platforms should enhance the absorption of drugs by the bladder tissues, providing an improvement in the therapeutic response and avoiding the progression of NMIBC to muscular invasive bladder cancer (MIBC) [5]. Angiogenesis is essential for the growth, development, and invasion of tumour cells and plays an important role in the metastatic process. The growth of new vessels is regulated by mediators that bind to the receptors on the membranes of vascular endothelial cells. Among the central mediators, the vascular endothelial growth factor (VEGF) can be highlighted [6]. As VEGF represents an important target for antitumor therapy, new therapeutic strategies are emerging with the objective of preventing tumour progression, by the blockade of VEGF. One of the methods that have been investigated for this purpose is the use of interfering RNA (siRNA) for VEGF, which acts on the cleavage of the messenger RNA that expresses VEGF [7, 8]. Therefore, the use of platforms for delivering siRNA targeting VEGF represents a powerful strategy to block the formation of new vessels, inhibiting cancer progress. For many years, the focus and interest in bladder cancer were the main topics in our researches [9–12]. In this context, graphene oxide (GO) exhibits a sp2–sp3 hybridised carbon honeycomb network, with a spread-out broad range of oxygenated functional groups interrupting the π-conjugation structure. Instead, graphene is composed exclusively of sp2-bonded carbon atoms organised in a hexagonal lattice. The attendance of the oxygenated moieties on the GO's surface yields different aspects in its properties. As an example, GO is an electrically insulating material, but graphene is not. Studies have been performed to explore the graphene oxide properties that permit it to give up more than one activity at the same time and to associate multidrug systems with different therapies, indicating that graphene oxide is a grateful tool to overcome impediment in cancer therapies [13–15]. Lerra et al [16] described a nanohybrid site-specific doxorubicin (DOX) delivery in neuroblastoma SH-SY5Y cells, using GO and magnetic iron oxide nanoparticles (MIONPs), and a curcumin–human serum albumin conjugate as functional coating in order to avoid DOX toxicity. Cell internalisation studies demonstrated the presence of nanohybrid inside cell cytoplasm. MIONPs allowed remote actuation on the nanostructure by a magnetic field, increasing the effectivity at the target site. Farani et al [17] described a pegylated and functionalised magnetic graphene oxide (MG–NH2–PEG) complex loaded with DOX. In vitro cytotoxicity tests on MCF-7 cells line suggested a satisfactory outcome on the non-toxicity of the nanocomposite. Besides this, the nanostructure exhibited strong optical absorbance from the visible to near infrared regions, and the authors suggested that this nanostructure could be important for orientated photothermal ablation of cancer cells guided by the magnetic field. Cao et al [18] developed a GO scaffold for drug delivery where the GO was functionalised with folic acid conjugated to chitosan acting on MCF-7 human breast cancer cells. Doxorubicin (DOX) and siRNA were further added to the platform. The siRNA aimed to silence cancer-resistant genes, thus improving the response to doxorubicin treatment. This research aimed to develop GO systems for the transport of DOX (GO-CO-DOX), a conventional drug widely used in the treatment of many types of cancer, as well as siRNA associated to GO (GO-PEG-PEI/siRNA) for VEGF. The designed systems were tested alone or in combination, and their effects on the progression of NMIBC were preliminary evaluated in vivo through histopathological and immunohistochemical analysis.
2. Materials and methods
2.1. Materials
Graphene oxide (GO) (single-layer) purity 99% was purchased from Cheap Tubes Inc., Brattleboro, USA. The layer thickness was about 0.7–1.2 nm, and the lateral dimensions were 1–20 μm. Six-armed PEG (ARM-PEG-Amine) (PEG [6 ARM-Poly (ethylene glycol] amine) (15kD) was obtained from JenKem Technology. The following chemicals were brought from Sigma-Aldrich and used as received: hydrochloride 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, >97.0%); N-hydroxysuccinimide (NHS, 98%); sodium hydroxide >98%; chloroacetic acid 99% and doxorubicin (DOX, >98%) and PEI (Polyethylenimine, branched, MW around 2 KDa) (Sigma-408727). Milli-Q water (18.2 MΩ cm) was used throughout all experiments.
2.2. Synthesis of GO-PEG-PEI-siRNA and GO-CO-DOX
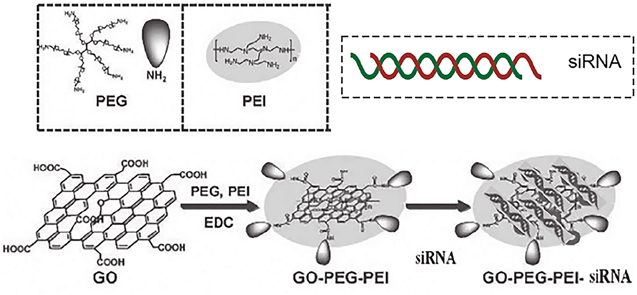
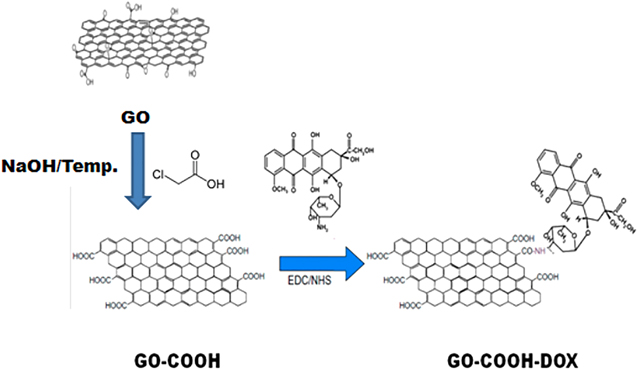
In order to explain the synthesis pathways, the schemes for graphene oxide derivatives (GO-CO-DOX and GO-PEG-PEI-siRNA) are shown in figures 1 and 2, respectively.
Figure 1. Scheme of the synthesis of GO-PEG-PEI and GO-PEG-PEI-siRNA (Modified from Durán and Fávaro, 2018).
Download figure:
Standard image High-resolution imageFigure 2. Schematic aspects of graphene oxide and doxorubicin complexation (Modified from Durán and Fávaro, 2018).
Download figure:
Standard image High-resolution image2.2.1. Synthesis of GO-PEG-PEI
The reaction was performed according to the methods described in the literature, with some modifications [19, 20]. 10 mg of GO was dispersed in 18 ml of deionised water. The dispersion was kept in an ultrasonic bath (42 kHz and 72 W) for 30 min. Next, 10 mg of previously dispersed ARM-PEG-amine in 1 ml of deionised water was added to the GO dispersion. The mixture was kept in an ultrasonic bath for another 5 min 10 mg of hydrochloride 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) dissolved in 1 ml of deionised water was added to the mixture, with subsequent stirring for 15 min. In the next step, 50 mg of PEI dispersed in 5 ml of deionised water was added, and the mixture was kept in the ultrasonic bath for another 5 min. Finally, 20 mg of EDC dissolved in 10 ml of deionised water was added. The mixture was kept under constant stirring overnight. After 24 h, the mixture was centrifuged at 14,000 rpm for 15 min. Thus, the conjugate was suspended in deionised water and centrifuged under the same conditions described above. The resuspension procedure in deionised water to remove unreacted PEG and PEI with GO was performed three times to ensure complete removal of unbound polymers. To verify the presence of free PEI in the final dispersion, the ninhydrin test was performed according to Huang et al (2016) [21]. The successive washing of the conjugate led to the complete removal of the polymers since it was not possible to observe the purple coloration after the second washing step in deionised water.
GO-PEG-PEI concentration was calculated by absorbance at 230 nm recorded by a UV–vis spectrometer (AJMicronal AJX-6100PC) with a mass extinction coefficient measured to be 65 g l−1 cm−1. PEI content in GO-PEG-PEI was measured by elementary analysis to be around the previous report (∼40%). In a similar procedure, PEG content in GO-PEG-PEI was quantified by thermogravimetric analysis (TGA), also similar to those reported by Feng et al [22].
2.2.2. Synthesis of GO-CO-DOX
Prior to the preparation of GO-COOH-DOX, the graphene oxide was further oxidised. The method followed the procedure previously reported [23, 24], with some modifications. To obtain the carboxylate graphene oxide, 10 mg of GO was dispersed in deionised water. The dispersion was stirred for homogenisation and maintained in an ultrasonic bath for 30 min. Then, 1.0 g of NaOH (Sigma-Aldrich) and 1.0 g of chloroacetic acid (Sigma-Aldrich) previously dissolved in 5.0 ml of deionised water were added successively to a final volume of 20 ml. Next, the volumetric flask containing the mixture was transferred to an ultrasonic bath with heating, where the temperature was maintained between 65 °C–68 °C for 3 h to convert the –OH groups to –COOH via conjugation of acetic acid moieties giving GO–COOH. The resulting GO–COOH solution was neutralised and purified by repeated rinsing and filtrations. After this step, the suspension was centrifuged at 14,000 rpm and 10 °C for 20 min for the removal of NaOH and chloroacetic acid. Subsequently, GO-COOH was resuspended in deionised water and centrifuged under the same conditions described above. This procedure was performed three times to promote the complete removal of NaOH and chloroacetic acid. The procedure for obtaining the GO-COOH-DOX system was carried out as described in the literature, with few modifications [23]. To obtain the GO-CO-DOX- conjugate, 10 mg of GO-COOH was dispersed in 18 ml of deionised water. The dispersion was stirred for homogenisation and maintained in an ultrasonic bath for 30 min. Then, EDC and NHS (at a final concentration of 20 mM of in the suspension) were added in that order. After stirring, the mixture was kept in an ultrasonic bath for 15 min and after that 5.0 mg of DOX was added. The mixture was kept in an ultrasonic bath for 30 min and under constant overnight stirring. Next day, the reaction medium was centrifuged at 14,000 rpm for 20 min. Finally, the conjugate was resuspended in deionised water and centrifuged under the same conditions described above for three times.
2.2.3. Characterisation of functionalised GOs
Transmission electron microscopy (TEM) images of GO, GO-PEG-PEI, GO-COOH, and GO-CO-DOX were obtained in a LEO 906 microscope at accelerating voltage of 80 kV. The samples were prepared by depositing 3 μl of sample suspension on copper grids (Ted Pella). Diffraction patterns of the different materials were carried out using an x-ray diffractometer—Shimadzu XDR7000. To investigate the chemical profile of the synthesised systems, attenuated total reflectance spectroscopy analyses were performed. The infrared spectra were obtained in an Agilent Cary 630 FTIR Fourier transform spectrometer, in the region of 4000–400 cm−1, and the solid sample was placed directly on the equipment crystal without the need of potassium bromide pellet formation. Chemical surface analyses of the graphene oxide samples were measured by x-ray photoelectron spectroscopy (XPS) by Thermo Fisher Scientific, UK, using a K-Alpha x-ray photoelectron spectrometer with a hemispherical electron analyser and an AlKα micro-focused monochromatic source: the resolution of 0.1 eV; x-ray spot size was about 400 μm; pass energy was fixed at 130 eV with a step of 1 eV for surveys and 40 eV with a step of 0.1 eV for core levels. The data were worked out using the Thermo Advantage Software (Version 5.921).
The size and zeta potential of GO and its derivatives were determined by dynamic scattering (DLS) and electrophoretic mobility, respectively, using the Zetasizer Nano ZS 90 (Malvern Instruments, UK) equipped with a He-Ne laser of 633 nm wavelength, detector angle of 90° and 1 ml sample volume (Malvern Instruments, UK). The samples were dispersed in deionised water (∼pH 7) at a concentration of 50 μg ml−1.
2.2.4. Evaluation of siRNA complexation by GO-PEG-PEI and DOX by GO–COOH
To evaluate the ability of GO-PEG-PEI to complex the siRNA for VGFA, the Ambion® In Vivo siRNAs (Thermo Fisher Scientific, Waltham, MA, USA) were used for this purpose. Thus, the electrophoretic mobility of the siRNA was evaluated against different conditions, by varying the incubation medium and the concentration of GO-PEG-PEI in relation to the siRNA concentration. The GO-PEG-PEI/siRNA complex was formed by incubating 0.4 μg of siRNA with 1.2 μg and 2.4 μg of GO-PEG-PEI, giving mass proportions of 3:1 and 6:1, respectively, of GO-PEG-PEI in relation to the siRNA. Complexation was performed in RPMI 1640 cell culture medium for 20 min at room temperature. Finally, the complex was subjected to a 2% agarose gel electrophoresis assay, with a run time of 30 min and voltage of 100 V. The test was performed by dissolving the ethidium bromide (EB) in a buffer solution of Tris-acetate-EDTA (TAE) containing 2% agarose prior to polymerisation. Then, the concentration of EB was 0.5 μg ml−1. Complexation was tested in the RPMI medium with and without fetal bovine serum (FBS).
In order to measure the functionalisation process of DOX-loaded nanocarriers was carried out through a UV–Vis spectrophotometer and 501 nm to 508 nm when covalently conjugated on GO. Fluorescence spectra of GO-CO-DOX had the similar fluorescent characteristic to that of DOX, but a suppression of the intensity occurred probably due to the quenching effect of GO. These results were precisely observed by Zhou et al [23] who suggested that DOX had been loaded on the GO-based nanocarrier (results not shown).
2.3. In vitro cytotoxicity studies using the MTT assay
The cell viability against DOX, GO-CO-DOX, GO-PEG-PEI, and GO-PEG-PEI/siRNA systems was evaluated using MTT (3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazoline bromide) method [25]. To perform the cytotoxicity, human bladder carcinoma 5637 cells (5637 ATCC/HTB-9) were seeded in 96-well microplates at the density of 1.5 × 104 cells per well. Thereafter, DOX, GO–COOH, GO-CO-DOX, GO-PEG-PEI, and GO-PEG-PEI/siRNA were added to the wells at different concentrations in serum-free RPMI medium. For DOX, GO–COOH, GO-CO-DOX GO-PEG-PEI, and GO-PEG-PEI/siRNA, the concentrations evaluated were 1; 2.5; 5.0; 10.0; 25.0; 50.0; 75.0 and 100 μg ml−1 (in the case of GO-CO-DOX, the concentration was based on the amount of DOX in the system). Subsequently, the plates were incubated at 37 °C for 24 h in order to determine cell viability as a function of different concentrations of the nanostructures. After exposure of nanostructures to the cells, the wells were washed several times with PBS (Phosphate Buffered Saline, pH 7.4), and a 0.5 mg ml−1 MTT solution (Sigma-Aldrich, USA) previously diluted in serum-free RPMI (Roswell Park Memorial Institute medium) was added. Therefore, the cells were incubated for 2 h at 37 °C. Then, the medium was replaced by 100 μl of dimethyl sulphoxide (DMSO) to dissolve the formazan crystals. Next, the plates were shaken for 10 min and the absorbance was measured on a microplate reader (Cytation 5, BioTek Instruments, Inc., USA) at λ = 570 nm. The final values were represented as percentages of reduction of MTT in relation to the control.
2.4. In vivo preliminary evaluation of graphene oxide derivatives on the non-muscle invasive bladder cancer (NMIBC) treatment
Seven weeks old Fischer 344 rats were supplied by CEMIB (Multidisciplinary Center for Biological Investigation) at University of Campinas (UNICAMP). The Institutional Committee for Ethics in Animal Use approved all experimental procedures (CEUA/UNICAMP, protocol no. 3795–1), and they follow the ethical standards of the Brazilian Society of Laboratory Animal Science guidelines. The cancer induction with N-methyl-N-nitrosourea (MNU), treatment protocol and histopathology were carried out following previous reports [26, 27].
In these preliminary experiments, the induced cancer rats were treated intravesically with DOX and GO-CO-DOX (1 mg kg−1) once a week for 6 consecutive weeks and GO-PEG-PEI/siRNA (140 μg K−1g−1, considering the amount of siRNA) each 14 days for the same period.
3. Results and discussion
3.1. Chemical and morphological characterisation of functionalised GOs
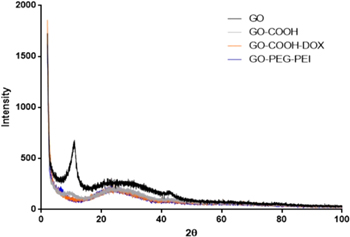
The schemes for synthesis of graphene oxide derivatives (GO-CO-DOX and GO-PEG-PEI-siRNA) are shown in figures 1 and 2, respectively. All the intermediate structures were analysed by TEM (figure 3), which showed that chemical modifications did not influence the GO structure. The graphene nanosheets with high-density x-ray diffraction patterns of the obtained nanosystems were investigated to verify if changes occurred in the intercalation distance among GO sheets. The diffraction peak of the GO was evidenced around 11.12° (2θ), corresponding to a distance between the sheets of 0.79 nm (figure 4) (http://www.rsc.org/suppdata/tb/c2/ c2tb00123c/c2tb00123c.pdf). This distance is significantly higher than that presented by graphite, which shows a diffraction peak at 26° (2θ). The XRD patterns of the GO-PEG-PEI did not exhibit the band at 11.22° (2θ), which can be indicative of the loss of the original lamellar structure and display of sheets in disordered arrays [28, 29]. However, the interim distance in the GO-PEG-PEI decreased to 0.360 nm, approaching the value obtained for graphite, which is in accordance with the literature [30]. Such structural changes could be explained by the reaction among OH groups and primary amines of PEG and PEI, making the structure better dispersed. The GO-COOH and GO-CO-DOX XRD spectra also showed the disappearance of the band at 11.12° (2θ) and the appearance of a broadband and low intensity between 15°–35° (2θ), also suggesting a disordered arrangement of the sheets. It is worth mentioning that during the reaction process, a slight reduction of the GO could have occurred, promoting a possible recovery of the electronic conjugation observed in the graphite, but to a small extent, given the relatively low intensity of the band.
Figure 3. Bright-field images obtained by TEM of GO(A), GO-COOH (B), GO-CO-DOX (C) and GO-PEG-PEI (D).
Download figure:
Standard image High-resolution imageFigure 4. X-ray diffractograms of GO, GO-COOH, GO-CO-DOX and GO-PEG-PEI.
Download figure:
Standard image High-resolution imageThe chemical modifications of GO were confirmed by FTIR and XPS in figures 5 and 6, respectively. According to the infrared spectrum of the GO, it is possible to observe a characteristic R–OH absorption peak in the region of 3000–3500 cm−1, and other C–O functions, such as –COOH (1721 cm−1) and C–O–C/C–OH (1400–1044 cm−1) [31, 32]. The presence of different groups with oxygen functions in graphene oxide is confirmed at 3400 cm−1 (OH groups), 1740 cm−1 (C=O groups), 1620 cm−1 (vibration related to non-oxidised graphite domains and water absorbed), 1220 cm−1 (stretching of C–OH) and 1060 cm−1 (stretching of C–O) [33].
Figure 5. FTIR spectra of (a) GO; (b) GO-COOH; (c) GO-PEG-PEI and (d) GO-CO-DOX.
Download figure:
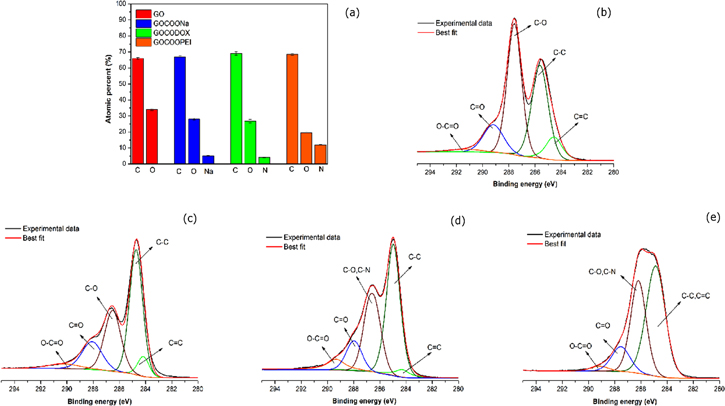
Standard image High-resolution imageFigure 6. X-ray photoelectron spectroscopy results. (a) atomic % acquired by survey spectra; C1s high-resolution for (b) GO, (c) GO-COOH, (d) GO-CO-DOX and (e) GO- PEG-PEI.
Download figure:
Standard image High-resolution imageThe conjugation of PEG and PEI to GO surface can be confirmed by the presence of characteristic bands of the polymers after conjugation. According to the spectra shown in figure 5, the spectrum of the GO-PEG-PEI conjugate exhibits bands related to GO, PEG, and PEI. The band between 2940–2830 cm−1 corresponds to the C–H stretching of PEI. The bands at 1581 cm−1 and 1462 cm−1 correspond, respectively, to the –N–H bending and C–H bending [30]. Finally, the 1070 cm−1 band is characteristic of O–H and C–O–H groups of PEG.
Considering the spectrum of GO-COOH, when compared to the GO spectrum, the band characteristic of C–O–C vibrations (around 1056.7 cm−1) decreased. The absorption peak at 1366.1 cm−1, corresponding to the angular deformation of the –OH, appeared in the carboxylate GO spectrum. In addition, the absorption band characteristic of the C=O groups in GO (around 1620 cm−1) was broadened and exhibited an increase of intensity, showing overlap with the peak at 1720 cm−1 (–COOH). This fact can be attributed to the vibrations of the oxidised carbon domains, suggesting an additional carboxylation of the hydroxyl groups of GO. In addition, the OH absorption band at 3000–3500 cm−1 became more intense and slightly shifted to lower wavelengths, which can be explained by the high density of carboxyl groups on the graphene oxide surface [30].
The surface composition of GO, GO–COOH, GO-CO-DOX and GO-PEG-PEI was assessed through XPS by survey spectra. Figure 6(a) depicts the atomic percentage of each element on the GO surface. After the oxidation step, the presence of sodium was detected on the sample surface (GO–COOH), confirming the addition of new carboxylate groups. The presence of nitrogen in the GO-CO-DOX and GO-PEG-PEI samples proves the functionalisation of GO surface with doxorubicin and PEG-PEI, respectively. High-resolution carbon spectra (C1s) of GO, shown in figures 6(b)–(e), display a considerable degree of oxidation, due to carbon atoms in different chemical environments (C–O, C=O, O=C–O). For the sample GO-PEG-PEI (figure 6(d)), the carbon spectrum has a distinct shape, probably due to the presence of a thick polymer layer.
The zeta potential and hydrodynamic size of the samples were measured, and the results are shown in table 1. GO particles showed zeta potential lower than −30 mV or higher than +30 mV and formed stable dispersions due to inter-particle electrostatic repulsion. No aggregation was observed, and the hydrodynamic size of the GO sheets presented similar values (table 1). For most biological applications, the stability of nanoparticle dispersion in water is required as one of the most important properties.
Table 1. Hydrodynamic size and zeta potential of GO, GO–COOH, GO-CO-DOX and GO-PEG-PEI.
| Sample | Hydrodynamic size (nm) | Zeta potential (mV) |
|---|---|---|
| GO | 133 ± 41 | −31 ± 7 |
| GO-COOH | 126 ± 29 | −28 ± 7 |
| GO-CO-DOX | 153 ± 10 | −36 ± 1 |
| GO-PEG-PEI | 149 ± 10 | +37 ± 1 |
GO, GO–COOH, and GO-CO-DOX present negative potential probably to the ionisation of the carboxylate/carboxylic as detected by FTIR and XPS. These groups are responsible for the build-up of negative charges (–COO−). On the other hand, GO-PEG-PEI conjugate showed a zeta potential of +37.2 mV in water, demonstrating, together with the FTIR and XPS data, the conjugation of the branched cationic polymer (PEI) to the nanostructure.
3.2. Evaluation of siRNA complexation by GO-PEG-PEI
Figure 7 discloses the complexation of siRNA by GO-PEG-PEI at different mass ratios.
Figure 7. Complexation of siRNA by GO-PEG-PEI with different mass ratios.
Download figure:
Standard image High-resolution imageAccording to the results, GO-PEG-PEI was able to complex siRNA in the proportions of 3 to 1 and 6 to 1, considering the mass of GO-PEG-PEI in relation to the mass of siRNA. In addition, GO-PEG-PEI complexed the siRNA in the presence and absence of fetal bovine serum (FBS).
In a similar research on MDA-MB-231 (an invasive breast cancer cell line), it was transfected with PEI-GO in complex with siRNAs against CXCR4 (siCXCR4), and a suppression of the mRNA and protein expression of CXCR4 by PEI-GO/siCXCR4 complex were observed. Therefore, these results point out that using an adequate proportion, GO derivatives can promote gene silencing efficiently and at low concentrations [21].
3.3. Cytotoxicity studies of GO derivatives towards bladder cancer cells
To evaluate the toxicity of DOX and GO-CO-DOX, cytotoxic studies were performed using bladder carcinoma 5637 cells. The study represents cell viability as a function of different concentrations of free DOX, GO-COOH, and GO-CO-DOX. In the case of DOX, it showed a potentiated toxicity against bladder cancer cells, with an IC50 of 3.64 μg ml−1 (3.05–4.34 μg ml−1). GO-COOH showed lower toxicity than GO-CO-DOX system, at the maximum concentration of GO-COOH tested (100 μg ml−1), since the obtained cell viability was 71.96 ± 5.65%. However, the GO-CO-DOX system presented an IC50 of 20.42 μg ml−1 (16.64–25.06 μg ml−1). Therefore, the results were indicative of the presence of DOX in the system. In addition, considering that the mass percentage of DOX present in the GO-CO-DOX was 33.3%, and taking into account the absolute concentration of DOX, it was found that the IC50 of the drug when incorporated into GO-CO-DOX was about 6.80 μg ml−1, similar to results found in free DOX. GO-PEG-PEI and GO-PEG-PEI-siRNA showed similar values of cytotoxicity to GO-COOH.
Considering the cytotoxicity studies with GO-PEG-PEI and GO-PEG-PEI/siRNA (data not shown), both systems showed an IC50 of around 100 μg ml−1. It is important to point out that during in vitro experiments, the cells remained in contact with the systems for 24 h. On the other hand, during in vivo experiments, the drug delivery systems were under the action of urine renewal, which causes the dilution and elimination of the drug in a short period, decreasing the time of exposure for the drug. Therefore, maybe the inherent toxicity of the GO-PEG-PEI and GO-PEG-PEI/siRNA platforms played a role over the biological effects observed in in vivo experiments, but it is suggested that the results may be more related to the silencing activity of the GO-PEG-PEI/siRNA.
3.4. Histopathological analyses of MNU induced cancer
Urinary bladder from the control group did not show any lesions through the ultrasound and microscopic analysis (table 2). Nevertheless, the urinary bladders previously treated with MNU (MNU group) demonstrated histopathological changes (not shown), such as high-grade pTa, low-grade pTa, and pTis in 60%, 20% and 20% of the animals, respectively (table 2). According to data, for the DOX group, 100% of the animals presented lesions classified as high-grade pTa, a diagnosis even worse than that presented by the MNU (cancer) group. Probably this observed effect was due to a high solubility of the drug in water, producing a rapid elimination of the drug from the urine. Ultrasonography revealed the presence of an infiltrating mass in 100% of the animals (not shown).
Table 2. Percentage of histopathological changes of the urinary bladder of rats from different experimental groups.
| Histopathology | Control (n = 5) | MNU (Cancer, n = 5) | DOX (n = 5) | GO-PEG-PEI /siRNA + GO-CO-DOX (n = 5) |
|---|---|---|---|---|
| Normal | 5 (100%)a | — | — | 3 (60%) |
| High-grade intraurothelial neoplasia–flat carcinoma in situ (pTis) | — | 1 (20%) | — | 1 (20%) |
| Low-grade papillary carcinoma in situ (pTa) | — | 1 (20%)a | — | — |
| High-grade papillary carcinoma in situ (pTa) | — | 3 (60%) | 5 (100%)a | 1 (20%) |
Then, the animals exhibited a normal bladder diagnosis for the association between GO-CO-DOX and GO-PEG-PEI/siRNA (60%). Nevertheless, for the same group, some animals had pTis lesions (20%), and the rest presented high-grade pTa associated with squamous metaplasia (20%) (table 2). Considering the ultrasonography analysis (not shown), 100% of the animals had no infiltrating mass. It is worth mentioning that these results were more promising than those related to the use of GO-CO-DOX and GO-PEG-PEI/siRNA alone (not shown). This fact suggests that the association between the two systems was entirely novel for the treatment of NMIBC. In other words, in addition to the action through VEGF blockade, maybe there is an additional therapeutic effect as a function of the cytotoxic activity of DOX, even at low concentrations.
4. Conclusions
Our findings demonstrated that GO systems were effective for the treatment of NMIBC. The GO-PEG-PEI system showed high dispersibility in aqueous medium and high stability that allowed its resuspension for application in vivo. The nanostructure systems promoted the complexation with siRNA even at low concentrations. The association between GO-CO-DOX and GO-PEG-PEI/siRNA showed to be the most promising strategy for NMIBC treatment and provided the absence of lesions in 60% of the animals, important data in the field of urology. Another critical point of this research was the use of ultrasonography to evaluate the progression of NMIBC. To some extent, the obtained results corroborated with the observed system effects can be seen in histopathological analyses. Therefore, graphene oxide hybrid systems can be up-and-coming agents for the treatment of NMIBC.
Acknowledgments
The authors would like to thank the São Paulo Research Council (FAPESP grant 2014/11154–1), and the Brazilian National Council for Scientific and Technological Development (CNPq grant 552120/2011–1), Brazil.