Abstract
The reactivity of reduced graphene sheets (graphenide), obtained by dissolution of graphite intercalation compounds, in particular KC8, was systemically studied by covalent functionalization with 4-fluorobenzenediazonium tetrafluoroborate and 4-iodoaniline in different solvents (5 pure and 7 solvents with addition of K+ chelating agents). Interestingly, proper solvation of the potassium cations was beneficial to the covalent functionalization, while excessive solvation led to destabilization and flocculation, with decreased reactivity of graphenide. The results were confirmed by scanning Raman spectroscopy (SRS) and x-ray photoelectron spectroscopy (XPS).
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
The unique and diverse physical and chemical properties of graphene lead graphene-based nanomaterials to be promising candidates for many practical applications [1–3], not only in electronics and photonics [4] and energy conversion and storage [3], but also in biomedical and clinical treatments [5], such as drug/gene delivery, tissue engineering, diagnostic imaging, imaging-mediated therapies and bioelectrodes [6–8]. In order to improve selectivity and functionality, the covalent modification of graphene represents an attractive approach of opening/adjusting the band gap, in a combination of a large variety of functional groups for the creation of novel architectures, while improving dispersibility and processability [9–11]. Graphite intercalation compounds (GICs), can be dispersed to negatively charged graphene sheets (graphenides) in suitable solvents [12–14], providing one of the most efficient methods for the covalent functionalization, compared to the lower reactivity of neutral graphite/graphene [15], since negative charges on the surface of graphenides introduce Columbic repulsion, facilitates exfoliation and increase reactivity. In addition, among the different generated species, monolayer graphenide sheets are predominant because of strong solvation. Regarding the low solubility of graphene derivatives, the solubility of the graphenides can be improved with the assistance of a cation complexing agent such as 18-crown-6 ether [16], which binds potassium cations, avoiding aggregation. However, until now, studies about the reactivity of the graphenides in bulk solvents are very limited. For instance, excess of KC8 (GIC) as reducing agent was used to reduce alkyl and aryl halides in different solvents to yield hydrocarbons, but without discussion of relationship between solvents and covalent functionalization of graphene [17]. In the reaction of KC8 with porphyrin diazonium salts, dimethyl formamide (DMF) was found to be a more inert solvent compared to tetrahydrofuran (THF) [18]. Additionally, 1,2-dimethoxyethane (DME) and THF are considered similarly inert solvents for the functionalization of graphenide with electrophiles [19]. Besides, after ultrasonication, the D bands (related to defects) of the resulting graphenides in Raman spectra would vary according to solvents [18], probably dependent on physical properties (viscosity, surface tension, etc). All these results indicate that the solvent is one of the most important parameters in the generation of graphenides and, consequently, in its reactivity. Therefore, the systematic exploration of GICs reactivity dependent on the nature of the solvent is highly desirable, as it will shine light on the covalent functionalization of GICs in bulk solvents.
Herein, we report a systematic study on the effects of solvation and flocculation on the reactivity of graphenides, generated by the dispersion of GIC (scheme 1) in different solvents. The results indicate that solvation of potassium cations in KC8 can improve its reactivity, which was confirmed by an increased degree of covalent functionalization. However, the strong solvation of K cation can cause ion condensation and lead to flocculation, which inhibits the reactivity of graphenide, resulting in a lower degree of functionalization. The functionalization of graphene was investigated by scanning Raman spectroscopy (SRS) and x-ray photoelectron spectroscopy (XPS). In the functionalization process, the possible solvent residues were also considered for evaluation of the degree of functionalization by thermogravimetric analysis (TGA).
Scheme 1. Reaction of graphenides generated by GIC (KC8) in different solvents with 1 and 2. Potassium cations are solvated by organic solvents.
Download figure:
Standard image High-resolution imageTo set up the experimental conditions, spherical graphite was used as graphite source because of its comparatively good dispersibilty [19]. KC8 was selected as the GIC, since it leads to the highest loading of potassium graphene layers and is the most reactive species among KCn (n = 4, 8, 10, 24, 48) [20].
In order to study the influence of solvents in the functionalization of graphenide, the following five solvents were used: DMF, NMP (N-Methyl-2-pyrrolidone), THF, DME, diglyme. Moreover, the addition of 18-crown-6 or diglyme as potassium binding agent was addressed, and 18-crown-6 (1 mmol) or diglyme (3 mmol) as additives in 20 ml of DME, were employed. Alternatively, 18-crown-6 (0.02, 0.05, 0.08, 0.11, 0.60, 1 mmol) or DME (4 mmol) as additives were used in DMF (20 ml). Regarding covalent functionalization of graphenide, reactions with 4-iodoaniline (1) and 4-fluorobenzenediazonium tetrafluoroborate (2) were used to afford the functionalized graphene derivatives with para-aniline (GA) and para-fluorophenyl substituents (GF), respectively, via radical mechanism as previous report [20]. In addition, benzonitrile was used to quench any possible remaining negative charges after the reaction [21]. The procedures in the control experiments (CEs) were performed similar to those for preparation of GA or GF in the corresponding solvent, but without addition of 1 or 2, respectively (see the ESI for details).
A reliable characterization of covalently functionalized graphene derivatives by the reductive exfoliation/functionalization of GICs can be obtained by Raman spectroscopy, particularly, by SRS [22, 23]. An increase of the D-band (1340 cm−1) with respect to the G-band (1579 cm−1) is generally attributed to an increase of the defects associated with covalent functionalization. Therefore, the intensity ratio ID/IG between the D- and the G-band can be considered a semiquantitative estimate of the functionalization degree. However, it is worth noting that edge defects produced during the exfoliation process can also contribute to the ID/IG intensity ratio relative to pristine graphite.
The covalent functionalization of graphenide was carried out with 1 and 2 using the above-mentioned solvents. The Raman spectra of pristine graphite and samples (GA and GF) obtained with the different solvents (diglyme, DME, THF, NMP and DMF), as well as the spectra of the respective reference samples (CEs) are displayed in figures 1 and S3 (ESI).
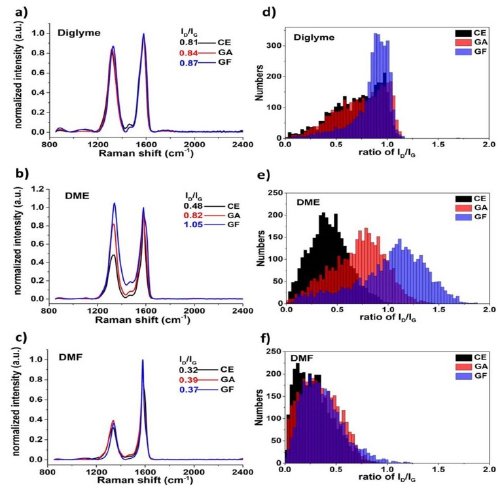
Figure 1. (a)–(c) Averaged Raman spectra (~3000 single-point spectra, λexc = 532 nm), (d)–(f) Raman histograms (ID/IG ratio distribution) of graphene samples (CE, GA and GF) obtained in diglyme, DME, and DMF, respectively.
Download figure:
Standard image High-resolution imageFrom the average Raman spectra (figures 1(a)–(c) and S3(a)–(c) ESI) we reach the following conclusions: (i) after ultrasonication, all the CEs show different degrees of increased of ID/IG ratios in comparison with pristine graphite. This experimental evidence was in agreement with previous reports [18]. This indicates that GICs are partially decomposed with the formation of smaller sheets (with more edge defects) after sonication, even without undergoing any covalent reaction in the basal plane (CEs). Therefore, the CE was a more adequate reference than pristine graphite to evaluate the degree of functionalization. (ii) Compound 2 is more reactive than 1 due to its lower reduction potential (−0.16 V/SCE for benzenediazonium tetrafluoroborate versus −2.24 V/SCE for Iodobenzene) [24–26], which is consistent with the previous study about carbon nanotubides [27]. (iii) More importantly, the solvation ability of potassium cations in the various solvents (free energy of solvation of K+ (ΔGs): DME > THF > NMP > DMF) [28, 29] contributes to an increase of the reactivity of graphenide (for GA: ΔID/IG(DME) = 0.34 > ΔID/IG(THF) = 0.12 > ΔID/IG(NMP) = 0.11 > ΔID/IG(DMF) = 0.07; for GF: ΔID/IG(DME) = 0.57 > ΔID/IG(THF) = 0.34 > ΔID/IG(NMP) = 0.28 > ΔID/IG(DMF) = 0.05). Another model for interpretation of Raman spectra is distance between 0D point like defects (LD), indicating the amount of disorder [30]. The LD analysis (table S1) results in the same outcomes obtained from ID/IG ratio analysis. A better potassium solvation facilitates the dispersibility and increase the reactivity due to the weakness of the K+-graphenide interaction that makes the negative charge on graphenide sheets more accessible. Surprisingly, diglyme with higher binding energy of Li+ than that of DME is expected [31], in principle, to cause stronger solvation of K+ and a higher increase of functional degree than DME. Nevertheless, the reaction in diglyme displayed a very low degree of functionalization (ΔID/IG(diglyme) = 0.03 and 0.06 for GA and GF, respectively) similar to the poor solvent DMF. This result suggests another crucial factor(s) that resulted of greater impact in the reactivity of graphenide. A very recent report showed that the destabilization and flocculation of the graphenide polyelectrolyte in solution can be introduced by high effective ionic concentration because of addition of a large amount of the cation complexing agent 18-crown-6 ether [16]. In our case, diglyme acts as solvent, as well as a similar role of 18-crown-6 ether with strong solvation of K+, leading to destabilization and subsequently flocculation (colloids come out of suspension in the form of floc or flake). Consequently, the fast aggregation of graphenide flakes can lead to lower degree of functionalization (detailed discussion vide infra) [19].
The ID/IG distribution with respect to the degree of functionalization is displayed in figures 1(d)–(f) and S3(d)–(f) (at least 3 × 31 μm × 31 μm areas were analyzed, with more than 3000 single-point spectra). Firstly, after ultrasonication, CEs showed broader distributions and shifts to higher values of ID/IG ratio compared to pristine graphite. This confirms that graphenide under reaction conditions but in absence of reagents 1 or 2 shows changes related to the ID/IG ratio such as small layer dimensions, resulted in an increment of edge defects. Thus, the CEs were considered better references than pure graphite. Secondly, the successful covalent functionalization of graphene and degree of reactivity can be clearly observed by the overlapped areas with CEs: the less overlap is present, the higher is the functionalization degree and reactivity.
In principle, the overlap between CE and GA or GF was similar to the bimodal distribution reported previously for similar functionalization of graphenide [19]. This overlap indicates the presence of remaining unfunctionalized areas of graphite after radical additions. This is because not all the carbon surface of graphenide is available for trapping molecules due to incomplete exfoliation, lattice energy and charge condensation and screening [19, 32]. DME showed the lowest overlapping between its corresponding CE with GA and, in particular, GF. However, as already mentioned above, diglyme and DMF were not good solvents for the reaction of graphenide, as shown by the pronounced overlaps of ID/IG ratio distribution between functionalized graphene samples and the corresponding CE (figures 1(e) and (f), respectively). It should be noted that flocculation in diglyme is the crucial issue for the low functionalization degree.
In order to better understand the effect of flocculation, additives were used during the functionalization of bulk graphenide solutions. All the averaged Raman spectra obtained in these mixed solvents are displayed in figures S4–S12. It is well-known that 18-crown-6 is a good cation ligand and can coordinate K+ in solution to avoid aggregation on the surface of the graphenide sheets therefore improving its solubility [16]. Thus, we firstly used different amounts of 18-crown-6 (0, 0.02, 0.05, 0.08, 0.11, 0.60, 1 mmol) in DMF (20 ml) to screen the minimum concentration for flocculation. As shown in figure 2(a) (black points), the increase of functionalization degree when 0.02 mmol of 18-crown-6 was used as additive in
Figure 2. (a) Increase of ID/IG intensity ratio in DMF (20 ml) with 0, 0.02, 0.05, 0.08, 0.11, 0.60, 1 mmol of 18-crown-6 (black points) and DME (4 mmol, red point), respectively, (b) pure DME, diglyme (3 mmol), and 18-crown-6 ether (1 mmol) in DME (20 ml).
Download figure:
Standard image High-resolution imageDMF (ΔID/IG = 0.09) is almost twice that of pure DMF (ΔID/IG = 0.05). However, a further increase of crown ether in DMF caused a decrease of functionalization degree due to lower dispersibility of potassium graphenide induced by flocculation. This experimental evidence is in agreement with the previously reported dispersibility changes of graphenide in NMP [16]. Such flocculation was not detected with DME (4 mmol) as additive (figure 2(a) red point), where the functionalization degree resulted three times that of pure DMF (ΔID/IG 0.16 versus 0.05). This increase indicates that the solvation of K+ in DME is sufficient to improve the dispersibility and the reactivity of graphenide, without causing flocculation. Diglyme (3 mmol) and 18-crown-6 (1 mmol) were explored as additives also in DME (figure 2(b)). Both showed lower increase of ID/IG ratio compared to pure DME (figure 2(b)). This suggests a strong solvation of K+ by diglyme and 18-crown-6 ether leading to flocculation.
Therefore, solvation of K+ and flocculation are important parameters for graphenide in solution, whose balance governs the reactivity: appropriate solvation of K+ is beneficial for improving reactivity, while excessive solvation will lead to destabilization and flocculation, consequently decreasing the reactivity.
To complement the Raman data, XPS was performed on pristine graphite, CE obtained in DME, and GFs obtained in different pure solvents. Survey XPS spectra and quantification of C, O and F atoms are displayed in figure S13 and table 1, respectively. As expected, F atoms were not detected in both pristine graphite and CE from DME as solvent (high resolution XPS spectra of F atom are shown in figure S15), while only GFs showed a strong peak at ~587.2 eV. The appearance of this peak suggests that the F atoms in GFs were introduced by compound 2 during the reaction. In addition, the amount of F atom (table 1) in relation to the solvent was the following: DME (3.1%) > THF (2.8%) > NMP (2.7%) > Diglyme (2.6%) > DMF (2.5%), in good agreement with the results obtained by scanning Raman spectra; although, flocculation and subsequent encapsulation of reagents for GFs is also considered, because may lead to the relatively high amount of F atom in XPS of the poor solvents compared to DME. The amount of oxygen in CE in DME (3.8%) was nearly the same as that of pristine graphite (2.9%), indicating that no H2O/O2 was introduced during the water bath ultrasonication in our conditions (experimental details in ESI). The total oxygen content in GFs (7%–13%) was higher than those of pristine graphite and CE of DME in XPS, probably due to solvent residues (even after drying under vacuum overnight) [19], which was also observed in air exposed graphenide (from 8.9% to 15%) [33]. Interestingly, GF obtained in diglyme showed the lowest increase of oxygen. This further confirmed that flocculation inhibits trapping of more solvent molecules between graphene layers. In addition, no non-covalent modification, such as nanoparticles or polymers, is found on the surface of graphene materials by transmission electron microscopy (TEM) (S16–S18).
Table 1. Atomic percentage (At) of samples (pristine graphite and graphene samples obtained in each solvent) based on the areas of the corresponding regions in XPS analysis
| At (%) | PG |
Diglyme | DME | THF | NMP | DMF | CE |
|---|---|---|---|---|---|---|---|
| C | 97.1 | 90.7 | 87.6 | 89.4 | 85.8 | 84.6 | 96.2 |
| O | 2.9 | 6.7 |
9.3 |
7.8 |
11.5 |
12.9 |
3.8 |
| F | — | 2.6 | 3.1 | 2.8 | 2.7 | 2.5 | — |
aThe high-resolution spectra of elements in DME in figure S13 as a model. bPristine graphite. cIncrease of oxygen in each sample probably due to solvents residue. dCE obtained in DME.
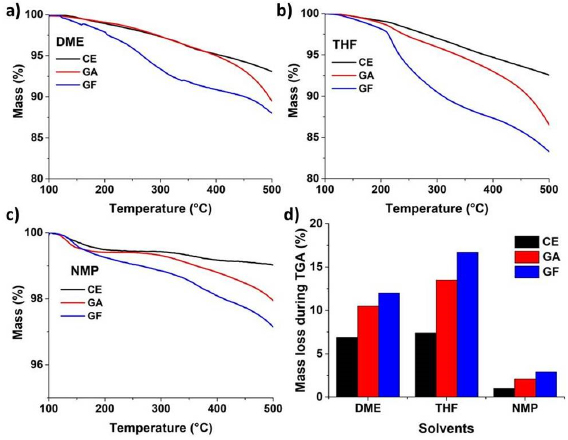
TGA of the samples obtained in DME, THF and NMP were performed between 100 °C and 700 °C (figure S19–S21). Because it is difficult to find a plateau for each sample, and organic functional group cannot survive above 500 °C, we used here the weight differences between 100 °C and 500 °C (figure 3) to determine the degrees of functionalization. The TGA of each sample was measured three times: the averaged mass loss is shown in figure 3(d). The result of TGA is in agreement with the previous Raman and XPS data: GFs show higher degree of functionalization than the corresponding GAs. The mass loss of products obtained in THF was more than in DME, but solvents residue must be taken into account, as shown the increase of total oxygen contents in each sample of XPS analysis and in previous reports [18, 19].
In summary, we have reported a systematic study on the reactivity of graphenide dependent on the balance between solvation of K+ and flocculation in solvents: appropriate solvation of potassium cations can improve the reactivity of graphenide (DME > THF > NMP > DMF). However, excessive solvation (diglyme) leads to flocculation and lower reactivities of the corresponding graphenides. Control experiments, performed under reaction conditions but in absence of reagent 1 and 2, are better references than pristine graphite for evaluation of degrees of functionalized graphene. The higher degree of covalent functionalization of graphene provides the possibility to combine bio-, photo- and/or electroactive compounds with graphene and improve practical applications, such as solar energy conversion and biological applications [5, 15]. This work provides a better understanding of fundamental principle of reactivity of reduced graphene dependent on solvation of K cation of solvents and will play as a guide for improving functionalization of GICs for further applications.
Figure 3. (a)–(c) Averaged TGA curves of CE, GA and GF obtained in DME, THF and NMP, respectively. (d) Weight loss (%) during TGA process from 100 to 700 °C under N2 atmosphere.
Download figure:
Standard image High-resolution imageAcknowledgments
M Prato is the recipient of the AXA Chair (2016–2023). A Criado thanks MINECO for his researcher grant (Juan de la Cierva—Incorporación). This work was supported by the EU H2020-SGA-FET-GRAPHENE-2017 (no. 785219) and EU H2020-MSCA-RISE-2016 (no. 734381).