Abstract
The effects of antimony (Sb) on the deformation behaviour of high-grade non-oriented silicon steel at a low temperature were studied by using a Gleeble-3800 thermal simulator and the field-emission scanning-electron microscope. The tensile strength of the high-grade non-oriented silicon steel without Sb was significantly lower than that of the high-grade non-oriented silicon steel with Sb within the tensile temperature range of 25 °C–240 °C. It is possible for Sb atoms near the grain boundary to diffuse at hundreds of atoms through dislocations as a rapid diffusion channel, which is driven by thermal activation with an increase in temperature. Atomic diffusion can weaken the damage to bonding forces on the grain boundary and crack propagation along the grain boundary under load action. The segregation of Sb atoms at the grain boundary decreased gradually, which weakened the cleavage characteristics of the fracture morphology and enhanced its dimple characteristics. For a stretching temperature of 80 °C, the fracture dimple of high-grade non-oriented silicon steel without Sb was significantly more than the high-grade non-oriented silicon steel with Sb. Because of the weakening effect of Sb segregation at the grain boundary, the suitable low-temperature deformation range for high-grade non-oriented silicon steel with Sb was 120 °C–160 °C.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Non-oriented silicon steel is an important magnetic material for preparing high-efficiency motor cores, which require a low iron loss and a high magnetic induction. The chemical composition, crystal texture and grain size are the main factors that affect the electromagnetic properties of non-oriented silicon steel sheets. An improvement of the favourable texture by composition and process adjustment is one of the methods to prepare non-oriented silicon steel with a high magnetic induction [1, 2]. In recent years, many studies have shown that the development of a recrystallization texture in the <001> direction of easy magnetization could be promoted and {111} γ fibres that are difficult to magnetize could be suppressed by controlling the non-oriented silicon steel texture by adding elements, such as tin (Sn), antimony (Sb) and rhenium (Re), which yields good magnetic properties [3, 4].
In general, high-grade non-oriented silicon steel contains ∼2 wt%–3.2 wt% silicon (Si), and the solid–solution strengthening of silicon could increase the low-temperature deformation resistance of steel plates significantly and decrease the cold workability [5]. Although high-grade non-oriented silicon steel with some elemental Sb and Sn has a high magnetic susceptibility, these elements are segregated easily at the grain boundary, and the grain-boundary strength can be weakened. Edge cracks and other processing defects occur easily during the cold rolling of high-grade non-oriented silicon steel, and an understanding of the material brittleness is of significance to improve the cold workability of high-grade non-oriented silicon steel and to formulate the cold-rolling process, while considering the adverse effects of solid–solution strengthening and element weakening in the cold-rolling plasticity. Previous studies have focused on the effects of elements, such as Sb and Sn, on the texture and magnetic properties of non-oriented silicon steel. From first-principles, Yamaguchi Masatake and Chandan Pandey calculated the segregation energy of some solute elements like boron (B), carbon (C), phosphorous (P), and sulfur (S) in bcc Fe Sigma 3 (111) symmetrical tilt grain boundary and on (111) fracture surface with varying the segregation density [6]. Very little work has been done on Sb elements.However, limited systematic research has been conducted on the mechanical behaviour, the fracture morphology and the brittleness principle of high-grade non-oriented silicon steel with Sb and Sn at a low temperature [7, 8]. In this paper, the effect of low temperature deformation behavior and micro Sb on grain boundary binding force at different temperatures was studied comparatively for high grade non-oriented silicon steel with Sb.
2. Experimental details
High-grade non-oriented silicon steel (50 kg) used in this experiment was smelted in a vacuum induction furnace. The chemical compositions of the steel ingot are shown in table 1. Steel A was not added with bismuth, and 0.036% bismuth was added to steel B for comparison. A cubic ingot (150 mm × 150 mm × 210 mm) was cast, forged at 1100 °C and annealed at 930 °C for 20 min. The forged blank was processed into cylindrical tensile specimen of 4-mm diameter with a gauge distance of 50 mm (figure 1).
Table 1. Chemical composition of experimental steel (mass fraction/%).
| C | Si | Mn | Al | S | P | Sb | |
|---|---|---|---|---|---|---|---|
| A | 0.0023 | 2.56 | 0.27 | 0.32 | 0.004 | 0.008 | 0 |
| B | 0.0025 | 2.60 | 0.23 | 0.30 | 0.003 | 0.008 | 0.036 |
Figure 1. Schematic of tensile test specimen for experimental steel.
Download figure:
Standard image High-resolution imageLow-temperature tensile tests were performed by using a Gleeble 3800 thermal simulator. Two sample ends were packed in the working chamber of a thermal simulator. After a vacuum had been applied to the working chamber, the sample was heated to 50 °C, 80 °C, 120 °C, 160 °C, 200 °C and 240 °C at room temperature (25 °C) at 5 °C s−1, which simulated the range of temperature increase caused by cold deformation of steel plate during continuous cold rolling. After heat preservation for 480 s, the sample was stretched to fracture with a deformation rate of 0.01 s−1. The section shrinkage rate at each temperature was calculated according to the fracture diameter and initial sample diameter. The mechanical properties in different condition of heat treatment as discussed are shown in table 2.The fracture morphology was observed by using an Ultra 55 field-emission scanning-electron microscope.
Table 2. Mechanical properties of experimental steel.
| Tensile strength /MPa | Reduction of area /% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental steel | 25 °C | 50 °C | 80 °C | 120 °C | 160 °C | 200 °C | 240 °C | 25 °C | 50 °C | 80 °C | 120 °C | 160 °C | 200 °C | 240 °C |
| A | 747 | 714 | 691 | 681 | 675 | 653 | 626 | 19.00 | 26.24 | 35.75 | 39.75 | 41.02 | 42.75 | 46.26 |
| B | 767 | 725 | 715 | 711 | 707 | 678 | 646 | 9.75 | 13.25 | 24.51 | 28.50 | 29.63 | 32.27 | 34.23 |
3. Results
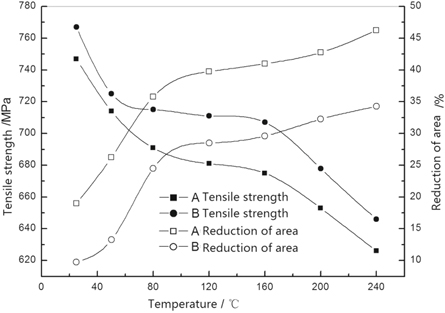
The tensile strength and section shrinkage rate of high-grade non-oriented silicon steel used in the experiment are shown in figure 2. The tensile strength of the experimental steel decreased continuously and the section shrinkage rate increased gradually with an increase in the stretching temperature. For the same stretching temperature, the tensile strength of steel A was lower than that of steel B, but the section shrinkage of steel B was significantly lower than that of steel A. As the stretching temperature increased, some disorders of dislocation motion will be weakened that they completely lose their ability to block motion dislocations, so the blocking ability of dislocation weakened gradually, and the ability of dislocations to overcome obstacles was strengthened gradually. Therefore, the tensile strength of experimental steel decreased and its plasticity increased during the tensile-deformation process. Sb atoms were dissolved in the iron base solid solution, which causes small distortion of the Fe atoms crystal lattice, so that the strength of steel B containing Sb is slightly higher than that of steel A.
Figure 2. Effect of temperature on the tensile properties of experimental steels.
Download figure:
Standard image High-resolution imageFor steel A, the tensile strength at room temperature was ∼747 MPa, and the section shrinkage rate was 19%. For steel B, the tensile strength at room temperature was 767 MPa, and the section shrinkage rate was 9.7%. As the stretching temperature increased to 80 °C, the section shrinkage rate of steel A increased to 36%, and that of steel B increased to 24%. Experimental steels A and B underwent a brittle–ductile transformation. When the temperature exceeded 50 °C, steel appeared and had a good plasticity. For a stretching temperature between 80 °C and 160 °C, the tensile strength and section shrinkage rate of steels A and B decreased significantly. When the tensile temperature exceeded 160 °C, the tensile strength of steels A and B decreased significantly, but their corresponding section shrinkage rate increased only slightly. At a tensile temperature of 240 °C, the section shrinkage rate of steel A was as high as 46%, whereas that of steel B steel was only 34%. Steel A without bismuth was significantly more plastic than steel B with 0.036% bismuth at a low temperature.
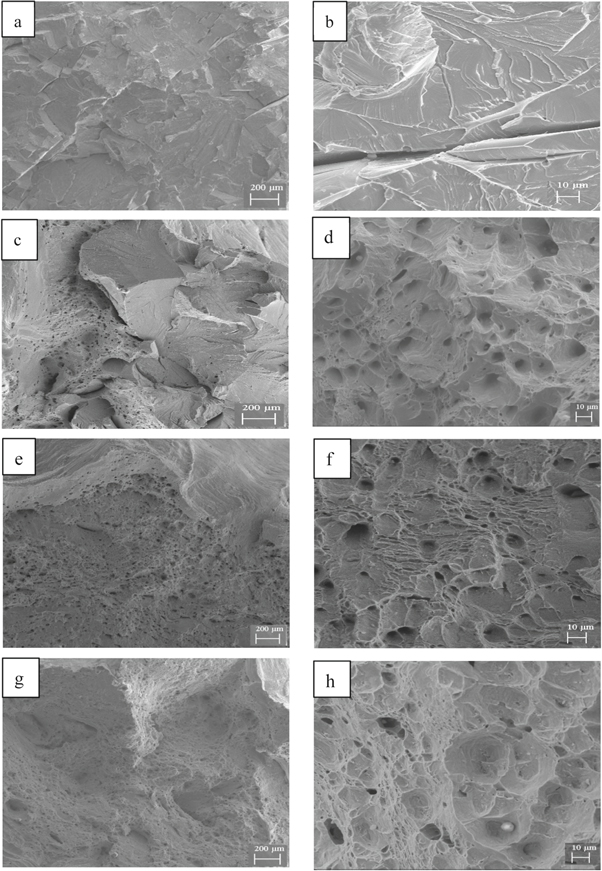
Figure 3 shows the scanning-electron micrograph of the fracture of steel A at different stretching temperatures. At room temperature (25 °C), the brittle fracture was mainly a river-like cleavage fracture, and the river-like pattern originated from the grain boundary (figures 3(a), (b)). For a stretching temperature of 80 °C, a mixed fracturing of cleavage cracks and dimples existed, but the cleavage crack area decreased compared with that at 25 °C (figures 3(c), (d)). As the stretching temperature increased to 160 °C, the fracture morphology was mainly dimple-like with a much smaller number of river-like cleavage cracks (figures 3(e), (f)). When the stretching temperature reached 240 °C, no brittle fracture was observed. The entire fracture was dimple-like (figures 3(g), (h)), and the corresponding equiaxed dimple was larger than that at a stretching temperature of 160 °C.
Figure 3. Fractography of experimental steels A at different temperatures. Macro-fractography: (a) 25 °C; (c) 80 °C; (e) 160 °C;(g) 240 °C; Micro-fractography: (b) 25 °C; (d) 80 °C; (f) 160 °C; (h) 240 °C.
Download figure:
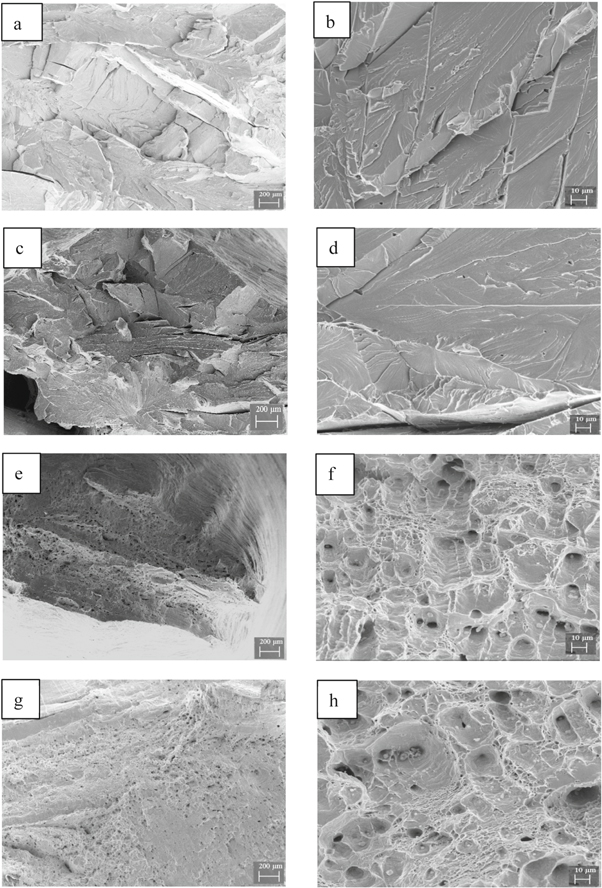
Standard image High-resolution imageFigure 4 shows a scanning-electron micrograph of the fracture for steel B at different stretching temperatures. The brittle fracture is obvious for a stretching temperature of 25 °C, and is mainly river-like cleavage fracture (figure 4(a)). According to figure 4(b), the tensile fracture propagates along different cleavage planes, which presents a stepped crack. For a stretching temperature of 80 °C, a mixed fracture with cleavage cracks and a small number of dimples existed, but the dimple area was smaller than that of steel A (figures 3(c) and 4(c)). As the stretching temperature increased to 160 °C, the fracture morphology was mainly dimple-like with a small number of river-like cleavage cracks (figures 4(e), (f)). The cleavage crack area was slightly larger than that of steel at this temperature. As the stretching temperature reached 240 °C, the entire fracture was dimple-like (figures 4(g), (h)). In the fiber zone, both deep and equiaxed fine dimples are observed that confirms the dimple rupture leads to the ductile fracture.
Figure 4. Fractography of experimental steels B at different temperatures. Macro-fractography: (a) 25 °C; (c) 80 °C; (e) 160 °C; (g) 240 °C; Micro-fractography: (b) 25 °C; (d) 80 °C; (f) 160 °C; (h) 240 °C.
Download figure:
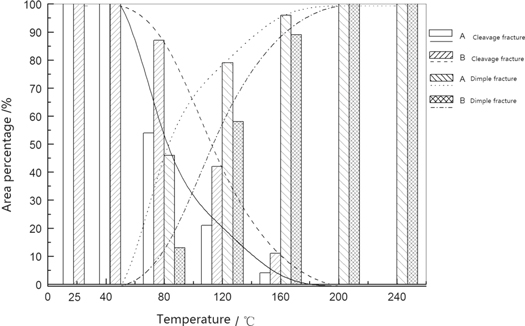
Standard image High-resolution imageThe fracture was magnified 100 times by using the field-emission scanning elec-tron microscope (FE-SEM), the area percentages that were occupied by cleavage and dimple cracks in tensile fractures at different experimental steel temperatures were analysed statistically, as shown in figure 5. The fractures of steels A and B were cleavage cracks with a stretching temperature below 80 °C. All fractures were dimple cracks for a stretching temperature above 160 °C. For a stretching temperature of 80 °C–160 °C, mainly mixed cleavage and dimple fractures resulted. The cleavage cracks decreased and the dimple cracks increased with an increase in stretching temperature. For the same stretching temperature, the dimple fracture area of steel B was less than that of steel A.
Figure 5. Area distribution of various types fractures at different tensile temperatures.
Download figure:
Standard image High-resolution image4. Discussion
The theoretical basis of cleavage-crack initiation was dislocation and twin deformation. Because the material plastic deformation was hindered, stress concentration occurred in an area of strong deformation, and stress was released by initiating microcracks. Because dislocation movement on the slip surface was hindered by barriers, such as grain boundaries and two-phase particles in the tensile process, microcracks appeared as the stress that was produced by dislocation accumulated in front of the barriers, which reached the theoretical material fracture strength.
For the tensile stress, the fracture-propagation path tended to propagate through the crystal, as the bonding force between atoms at the grain boundary was higher than that in the crystal [9]. As shown in figure 4, the experimental steel presented almost complete brittle fracture at room temperature and 50 °C. Because the tensile temperature was higher than 80 °C, the cleavage fracture characteristics decreased gradually with an increase in the tensile temperature, and the ductile fracture characteristics became increasingly obvious. A low temperature promoted the occurrence of rational fractures. The cleavage facet is a low energy fracture that propagates along the well-defined low-index crystallographic plane known as cleavage plane. A cleavage fracture has perfectively matching faces and it is completely flat and featureless [10, 11]. According to the composition of steels A and B, the Sb content was significantly different. The electronegativity of Sb was 2.05, and the outer Sb layer formed the electronic structure of a 533 covalent bond easily [12]. The ability of the restraining electron was much stronger, which reduced the number of free electrons that were adjacent to the metal bond and decreased the grain-boundary cohesion. Therefore, Sb could embrittle the grain boundary extensively. The segregation of Sb atoms at the grain boundary was easy, so the reduction in bonding force between the atoms at a low temperature and embrittlement of the grain boundary should be related to the Sb atom segregation at the grain boundary [12–14].
The equilibrium segregation of the grain boundary is a thermodynamic equilibrium site. According to the thermodynamic equilibrium equation of the McLean solute atomic grain-boundary segregation [15], the concentration Xsb of Sb atoms at the grain-boundary equilibrium segregation can be expressed as:
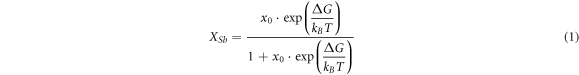
where x0 = 0.00036 is the concentration of Sb atoms in steel B, ΔG is the difference in free energy that is caused by Sb atom segregation at the grain boundary, kB is the Boltzmann constant and t is the thermodynamic temperature.
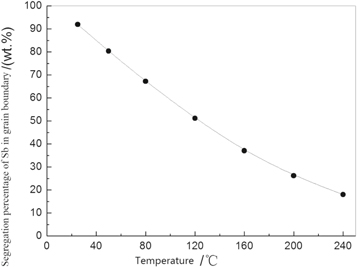
The equilibrium free-energy difference (ΔG) of Sb is –96.4 kJ mol−1, and the equilibrium segregation concentration Xsb of Sb at the grain boundary for steel B was calculated according to equation (1) (figure 5). According to figure 5, the equilibrium segregation concentration of Sb at the ferrite grain boundary was reduced significantly with an increase in temperature. Seah M P studied the effect of grain-boundary segregation on the bonding force in an iron matrix for approximately 70 solute atom types. It is suggested that partial element convergence can affect the bonding length of the nearest neighbour at the grain boundary. Elemental bismuth (Bi), phosphorus (P), sulfur (S), Sb, Sn and selenium (Se) can weaken the grain-boundary bonding force significantly and increase the apparent matrix brittleness [16]. The grain boundary embrittlement of iron by solute segregation is studied by first-principles calculations. The reduction of grain boundary cohesive energy by solute (P, Sn, Sb) segregation in iron grain boundary is calculated. It is found that the grain-boundary cohesive energy decreases by increasing the solute segregation [6].
As the tensile deformation temperature increases, Sb atoms tend to diffuse through channels, such as dislocations and grain boundaries. In this study, the holding time t before stretching at different temperatures was 480 s on the Gleeble test machine, and the diffusion coefficient D of Sb in α-Fe was 0.101 × 10–2.76/KT m2s-1, which is substituted into d = (6Dt)1/2 to obtain the estimated diffusion distance d for Sb atoms in a crystal. K is the Boltzmann constant (K = 1.38 × 10–23 J K−1), thus the diffusion range of Sb in the grain boundary can be observed, as shown in figure 6.
Figure 6. Equilibrium segregation percentage of Sb in grain boundary at different temperatures.
Download figure:
Standard image High-resolution imageAccording to figure 7, the diffusion distance of Sb atoms from 25 °C to 240 °C is lower than 104 nm for the holding time of 48 s. Also, Sb atoms cannot diffuse in crystals, and the activation energy of atoms that diffuse on the grain boundary is approximately 0.4 to 0.6 times [17] the grain interior. The dislocation is a rapid channel for atomic diffusion. The diffusion coefficient of Sb atoms on the grain boundaries and dislocations can be considered approximate. The dislocation density of annealed metal is ∼1010–1014/m2 [17], and the average dislocation spacing is 0.1–10 μm. Because of the dislocation proliferation during tensile deformation, the average dislocation spacing was 0.1 μm = 100 nm, which is much lower than the original grain size of the thermal-simulation sample. For steel B, the diffusion distances of Sb on the grain boundary were compared for a holding temperature of 80 °C for 480 s and 120 °C for 120 s, as shown in figure 7. Although Sb atoms cannot diffuse through dislocations at a large scale in 480 s, it is possible for Sb atoms near the grain boundary to diffuse at hundreds of atoms through dislocations as a rapid diffusion channel, which is driven by thermal activation with an increase in temperature. Atomic diffusion can weaken the damage to bonding forces on the grain boundary and crack propagation along the grain boundary under load action. The segregation concentration of Sb on the grain boundary decreases significantly as the tensile deformation temperature increases to 120 °C (figure 6). The bonding strength of the atom at the grain boundary can exceed the cleavage strength in the grain gradually with a decrease in Sb segregation, so the fracture can present cleavage fracture characteristics (figure 5). As the temperature continues to increase, grain-boundary segregation of atomic Sb continues to decrease, and the tensile fractures present dimple fracture characteristics. The increase in temperature is also the reason why more dimples occur at the fracture surface of steel A without Sb compared with steel B with Sb at a tensile temperature of 80 °C.
Figure 7. Diffusion distance of Sb atoms in α-Fe.
Download figure:
Standard image High-resolution imageDuring high-grade non-oriented silicon steel production, normalizing annealing is conducted after hot rolling at 800 °C–900 °C. After continuous cooling to a certain temperature, cooling can be carried out rapidly to a lower temperature during the continuous cooling stage. Thus, most Sb cannot diffuse to the grain boundary on time, which can weaken Sb segregation on the grain boundary to weaken the grain boundary, and the steel plate cannot crack easily during continuous cold rolling.
In summary, high-grade non-oriented silicon steel with Sb is deformed at a low temperature. A temperature of 120 °C to 160 °C is a suitable deformation temperature. Below this temperature range, Sb segregation on the grain boundary can lead to an increase in brittleness of the high-grade non-oriented silicon steel, so cold rolling can cause easy cracking.
5. Conclusions
The high-grade non-oriented silicon steel presents an obvious brittleness at a low temperature. From room temperature to 80 °C, Sb can segregate near the grain boundary, which can lead to brittleness that is caused by cleavage fracture during stretching. The analyses show that the Sb content on the grain boundary decreases, the bonding strength of the grain boundary improves, the tensile fracture characteristics are enhanced, and the cleavage fracture is weakened as the stretching temperature increases. Based on the weakening effect of Sb segregation on the grain boundary, the suitable temperature range of the high-grade non-oriented silicon steel with Sb is 120 °C–160 °C.
Acknowledgments
The present work was supported by the Inner Mongolia Natural Science Foundation. Influence of trace elements on Microstructure and properties of high grade non-oriented electrical steels (2017MS0518).We thank Laura Kuhar, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.