Abstract
This paper presents tables of key thermoelectric properties, which define thermoelectric conversion efficiency, for a wide range of inorganic materials. The twelve families of materials included in these tables are primarily selected on the basis of well established, internationally-recognized performance and promise for current and future applications: tellurides, skutterudites, half Heuslers, Zintls, Mg–Sb antimonides, clathrates, FeGa3-type materials, actinides and lanthanides, oxides, sulfides, selenides, silicides, borides and carbides. As thermoelectric properties vary with temperature, data are presented at room temperature to enable ready comparison, and also at a higher temperature appropriate to peak performance. An individual table of data and commentary are provided for each family of materials plus source references for all the data.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Scope and organization
This compilation is concerned with the properties of inorganic thermoelectric materials which are being explored to provide fundamental understanding and for a wide variety of thermoelectric applications. We begin with a brief introduction defining the thermoelectric figure of merit, which specifies conversion efficiency, then define the thermoelectric-related parameters in the data tables and provide an overview of the twelve families of materials forming the basis of the compilation. This is followed by individual sections which comprise, for each family of materials, a table of data plus a commentary on the data for that section, with source references for the data in the table and any additional references cited in the commentary. In the final section we summarize challenges and future perspectives for inorganic thermoelectric materials.
The rationale for the selection of materials is outlined in section 1.3. The twelve sections and their authors are:
- 1.Tellurides (Tanmoy Ghosh and Kanishka Biswas)
- 2.Skutterudites (Pengfei Qiu, Shun Wan and Lidong Chen)
- 3.Half Heuslers (Shen Han, Chenguang Fu, Tiejun Zhu)
- 4.Zintls (A K M Ashiquzzaman Shawon and Alexandra Zevalkink)
- 5.Antimonides (Mg3Sb2) (Kazuki Imasato and G Jeffrey Snyder)
- 6.Clathrates (Melis Ozen, Kivanc Saglik and Umut Aydemir)
- 7.FeGa3-type materials (Raúl Cardoso-Gil)
- 8.Actinides and lanthanides (Eteri Svanidze)
- 9.Oxides (Dursun Ekren, Robert Freer and Ryoji Funahashi)
- 10.Sulfides and selenides (Anthony V Powell, Shriparna Mukherjee, Sahil Tippireddy and Paz Vaqueiro)
- 11.Silicides (Franck Gascoin and Theodora Kyratsi)
- 12.Borides and carbides (Philipp Sauerschnig and Takao Mori)
Thermoelectric figure of merit
Thermoelectrics can be used to generate power, when the material is located in a temperature gradient, or enable cooling when a current is passed through the material. The thermoelectric performance (for either mode of operation) depends on the efficiency of the material for converting heat into electricity. The efficiency of a thermoelectric material depends primarily on the thermoelectric materials figure-of-merit, known as zT or ZT [1, 2]. Whilst both versions are found in the literature, we will employ zT when referring to the figure of merit of a material, and ZT for a device or module. In its simplest form zT is described by:
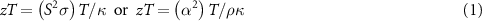
where the voltage generated is defined by the Seebeck coefficient (denoted by S or α). In order to maximize efficiency at a particular temperature (T), a high electrical conductivity σ (or low electrical resistivity ρ) is required along with low thermal conductivity κ. The latter parameter (κ) is made up of two components, lattice thermal conductivity (κL) and electronic thermal conductivity (κe). As the electrical transport and the electronic contribution to thermal transport are directly linked through the Wiedemann–Franz law [3], there have been considerable efforts to modify σ and κ independently in order to maximize zT [1, 2, 4].
To achieve sufficient power, a thermoelectric generator must be used efficiently across a large temperature difference ΔT = Th − Tc and so the material zT must be high across this temperature range. The Device ZT is a weighted average of the thermoelectric material zT that gives the maximum efficiency η across this finite ΔT, where the maximum efficiency is given by:

Thermoelectric materials and the tables
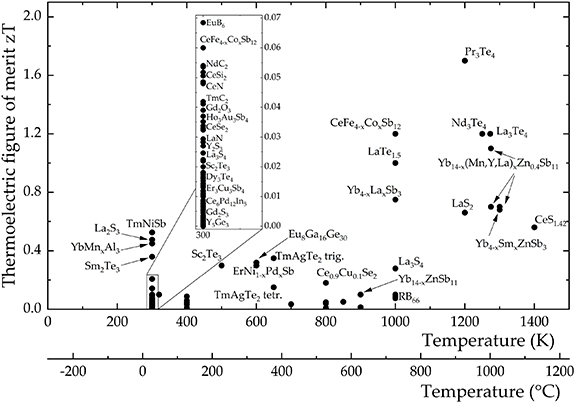
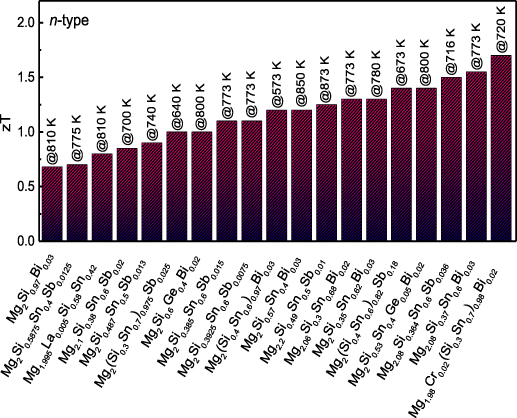
The thermoelectric performance of most materials varies widely with temperature, thereby defining an effective temperature range of operation or 'thermal window' (see section 3 and figure 1). Thus, it is important that peak zT occurs in the range of temperatures appropriate to a specific application, and indeed the average zT over that range may be more important than maximum zT. For convenience, thermoelectric materials are broadly divided into families suitable for low temperature (273–500 K), medium temperature (500–900 K) and high temperature (900–1300 K) applications [2, 4], although some materials, or combination of materials, can be exploited in more than one range. Traditionally, telluride materials (such as Bi2Te3) were established as the first commercial thermoelectric materials and are still employed widely today. However, because of their limited thermal window, restricting operation to low temperatures (peak performance ∼380 K), and increasing environmental and sustainability concerns, there is active interest in a wide range of alternative materials. This compilation presents data for 12 families of inorganic thermoelectrics (listed in section 1.1) which are candidates for a wide range of applications. These inorganic materials have been selected primarily on the basis of well established, internationally-recognized performance (some for up to 60 years) and their promise for current and future applications. This applies to the tellurides, skutterudites, half Heuslers, Zintls, Mg–Sb antimonides, clathrates, oxides, sulfides, selenides, and silicides. With growing interest in ultra-high temperature applications, above 1300 K, we include data for carbides and borides as these represent some of the most promising materials for such demanding environments. Finally, we include three families of 'exotic' materials with relatively modest properties, namely FeGa3 materials, actinides and lanthanides. For these materials, the structures and chemistry offer alternative atomic interactions and bonding scenarios for the regulation of charge carrier and transport properties. Developing a better understanding of the relationships between crystal chemistry, chemical bonding and electronic structure should allow the tailoring and enhancement of their thermoelectric properties. The approaches may be relevant and transferable to other families of materials. Indeed, we hope this review encourages the scientific community to investigate the full range of available materials using the spectrum of modern tools.
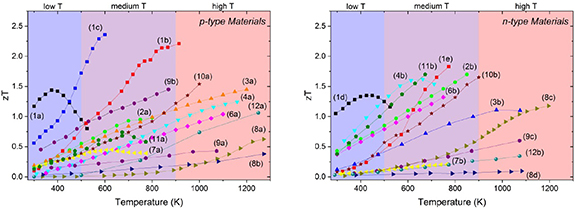
Figure 1. Thermoelectric figure of merit (zT) as a function of temperature for the families of materials. Details: (1) tellurides: p-type—(1a) Bi0.5Sb1.5Te3, (1b) Pb0.98Na0.02Te—4%SrTe, (1c) Ge0.86Pb0.1Bi0.04Te, n-type—(1d) Bi1.8Sb0.2Te2.7Se0.3, (1e) PbTe—4%InSb; (2) skutterudites: p-type—(2a) CeFe3.85Mn0.15Sb12, n-type—(2b) Ba0.08La0.05Yb0.04 Co4Sb12; (3) half Heuslers: p-type—(3a) Nb0.88Hf0.12FeSb, n-type—(3b) Zr0.2Hf0.8NiSn0.985Sb0.015; (4) Zintls (including Mg3Sb2): p-type—(4a) Yb14Mn0.2Al0.8Sb11, n-type—(4b) Mg3Sb1.5Bi0.5; (6) clathrates: p-type—(6a) Ba8Ga15.8Cu0.033Sn30.1, n-type—(6b) Ba8Ga16.6Ge28.7; (7) FeGa3-type materials: p-type—(7a) RuGa2.95Zn0.05, n-type—(7b) FeGa2.80Ge0.20; (8) actinides and lanthanides: p-type—(8a) Yb3.8Sm0.2Sb3, (8b) USi3, n-type—(8c) La3Te4, (8d) URu2Si2; (9) oxides: p-type—(9a) Ca2.8Bi0.2 Co4O9, (9b) Bi0.94Pb0.06Cu0.99Fe0.01SeO, n-type—(9c) Sr0.95(Ti0.8Nb0.2)0.95Ni0.05O3; (10) sulfides and selenides: p-type—(10a) Cu2Se, n-type—(10b) Pb0.93Sb0.05S0.5Se0.5; (11) silicides: p-type—(11a) Mg2Li0.25Si0.4Sn0.6, n-type—(11b) Mg1.98Cr0.02(Si0.3Sn0.7)0.98Bi0.02; (12) Borides and Carbides: p-type—(12a) Boron carbide (13.3 at.% C), n-type—(12b) Ca0.5Sr0.5B6.
Download figure:
Standard image High-resolution imageAs all the material families have different temperature dependencies, we include data relevant to temperatures for peak performance (on the basis of zT or power factor), and also properties close to room temperature, to enable comparison between the families of materials. Whilst the room temperature properties provide a useful baseline, it is accepted that such thermoelectric parameters will be modest for the high temperature materials, and the documented high temperature performance will be more relevant and representative.
Thermoelectric parameters and relationships
At a particular temperature, T (K), data for up to 12 thermoelectric performance-related parameters are reported, depending on the data available. We first present abbreviations (and units) for these parameters, and then outline important inter-relationships.
Abbreviations and units.
- Weighted mobility, µw (cm2 V−1 s−1)
- Hall mobility, μH (cm2 V−1 s−1)
- Intrinsic mobility, µo (cm2 V−1 s−1)
- Lattice thermal conductivity, κL (W m−1 K−1)
- Seebeck coefficient, S (µV K−1)
- Electrical conductivity, σ (Ω−1 cm−1)
- Thermoelectric quality factor, zT
- Bandgap, Eg (eV)
- Effective mass, ms* (me)
- Static dielectric constant/relative permittivity,
 r
r
- Thermal conductivity, κ (W m−1 K−1)
- Carrier concentration, n (cm−3)
Inter-relationships
All the parameters contributing to zT (equation (1)), i.e. Seebeck coefficient, electrical resistivity and thermal conductivity vary significantly with charge carrier concentration in contrasting ways. Achieving high zT in a material typically requires optimization of the charge-carrier concentration. As the charge-carrier concentration can be controlled by intrinsic defects (such as vacancies and interstitials) as well as extrinsic dopants (impurities), then the search for (or comparison between) good thermoelectric materials is really a search for a material with the highest potential for high zT assuming it can be optimally doped. This potential high zT is determined by the thermoelectric quality factor B [5, 6], defined in equation (3), which at a particular temperature is directly proportional to zT [7]:

Here kB, me, e and h are the Boltzmann constant, electron rest mass, electron charge and Planck's constant respectively; thus except for temperature, the quality factor is proportional to μw/κL. This indicates the quality of a thermoelectric material can be divided into the quality of its electronic properties, given by the weighted mobility μw, and the quality of its thermal properties, given by the lattice thermal conductivity κL [8]. Hence, improvements in 'electronic properties' can be defined as a higher μw , while improved 'thermal properties' means a lower κL for all material changes other than doping.
Several types of mobility are reported in the literature, most commonly the Hall mobility, intrinsic mobility, and weighted mobility. The Hall mobility, μH, is directly obtained from measurements of the Hall coefficient and resistivity. The intrinsic mobility, μo, is usually calculated using a single parabolic band (SPB) model, and can be viewed as an estimate of μH at the limit of very low carrier concentration (i.e. intrinsic behavior). Thus, for a given material and temperature, μo > μH. Hall mobility (μH) is reported for over half the material families in the compilation and intrinsic mobility (μo) for most of the remainder; this reflects the available data in the source publications.
Finally, the weighted mobility, μW, is generally described as the drift mobility, μ, weighted by the density-of-states effective mass ( ). Equation (4) further relates μw to the effective valley degeneracy (NV) and inertial effective mass,
). Equation (4) further relates μw to the effective valley degeneracy (NV) and inertial effective mass,  [9]:
[9]:

High weighted mobility is achieved in materials with lighter inertial effective mass mI (which is equal to the single band effective mass  for an isotropic band) and/or higher effective valley degeneracy. The advantage of comparing weighted mobility values among various materials is that it does not require Hall measurements (and is therefore widely accessible) and it combines two different parameters (
for an isotropic band) and/or higher effective valley degeneracy. The advantage of comparing weighted mobility values among various materials is that it does not require Hall measurements (and is therefore widely accessible) and it combines two different parameters ( and μ) that should be maximized to increase thermoelectric performance.
and μ) that should be maximized to increase thermoelectric performance.
The weighted mobility has been calculated for all material families in this paper using equation (5), where S and ρ are the experimental values of Seebeck coefficient and electrical resistivity, respectively, at temperature T [7]:
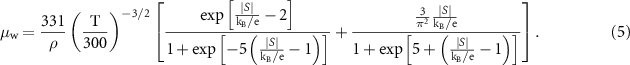
2. New entries
Since this is the first release of the Key Properties of Inorganic Thermoelectric Materials, all the entries in the tables can be treated as 'new'. Therefore, we present and discuss here the most important materials and the trends observed in the tables. The reader is referred to the original publications for further details.
3. Data tables and commentaries
The data tables are presented in the following sequence as sections 3.1–3.12:
(3.1) Tellurides, (3.2) Skutterudites, (3.3) Half Heuslers, (3.4) Zintls, (3.5) Antimonides (Mg3Sb2), (3.6) Clathrates, (3.7) FeGa3-type materials, (3.8) Actinides and Lanthanides, (3.9) Oxides, (3.10) Sulfides and Selenides, (3.11) Silicides, (3.12) Borides and Carbides.
To set the scene and highlight the relationships between the different families of materials we show in figure 1, typical zT values as a function of temperature for each of the families. This provides a very limited representation of the available data, but highlights the similarities and differences between current materials. Whilst the highest zT values (above 1.5) are available in the low and medium temperature ranges, there are many high-temperature materials with peak zT values well above 1.0. A clear feature across all the materials is the temperature dependencies; most medium and high temperature materials only really reach peak zT at the highest temperatures, whilst some of the low temperature tellurides (e.g. 1a p-type; 1d n-type) soon reach a very clear peak after which zT decreases rapidly with increasing temperature. Such behavior defines the range of temperatures (or operating window) for which the material will be most suitable as a thermoelectric. In this way the average zT (over a range of temperatures) can be more important, in determining performance, than the peak zT at one temperature.
A common, though not universal, feature across many materials is that the n-type materials exhibit higher zT values than their p-type counterparts. There are clear exceptions to this trend; notably among the sulfides, where a high-performance n-type material continues to be elusive. Consequently, there is considerable effort to develop related p-type and n-type materials of comparable performance to maximize the efficiency of thermoelectric modules. Looking at examples of material families, it is evident that skutterudites have their peak zT values at medium temperatures; the n-type skutterudites exhibit much higher zT values than the p-type skutterudites due to superior electrical transport performance. However, there can also be stark contrasts within individual families; the Sn clathrates display peak zT values at low-to-medium temperatures, whilst the Ge clathrates are best suited for medium-to-high temperature applications. The n-type Mg3Sb2–Mg3Bi2 alloys have only a relatively short history as thermoelectrics, but show promising performance from room temperature to around 700 K. The peak zT temperature can be easily adjusted by just changing the Sb:Bi ratio. Finally, carbides, such as SiC, are ideally suited to 'ultra high temperature' thermoelectric applications. Whilst their performance is average to good at 1200 K (curve 12a: p-type; curve 12b: n-type data) their zT values are still increasing and will not reach their peak until much higher temperatures, still within their stability range.
3.1. Tellurides
Thermoelectrics based on metal tellurides
Tanmoy Ghosh1 and Kanishka Biswas1,2,3
1 New Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Jakkur PO, Bangalore 560064, India
2 School of Advanced Materials, Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Jakkur PO, Bangalore 560064, India
3 International Centre for Materials Science, Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Jakkur PO, Bangalore 560 064, India
E-mail: kanishka@jncasr.ac.in
Introduction
Metal tellurides are among the most extensively studied families of thermoelectric materials for near room temperature to mid-temperature thermoelectric power generation. Particularly, the IV–VI semiconductors like GeTe, SnTe, and PbTe and tetradymites Bi2Te3-based thermoelectric materials have attracted wide attention in the community. Many of the modern-day approaches of improving thermoelectric performance, based on either electronic structure modulation or phonon scattering manipulation strategies, were first demonstrated on these materials. These families of materials, such as (GeTe)x (AgSbTe2)100−x -based TAGS-x and Bi2Te3-based materials are some of the most widely used thermoelectrics for commercial applications. Figure 2 exhibits the maximum thermoelectric figure of merit, zT for a range of current metal telluride thermoelectric materials. Here, we outline the status of these metal telluride-based thermoelectric materials, the challenges, and recent progress. Typical thermoelectric performance-related parameters and zT values for various Te-based thermoelectric materials are listed in table 1.
Figure 2. Maximum thermoelectric figure of merit, zT for various metal tellurides: AgInTe2 [57], AgGaTe2 [58], CuInTe2 [61], CuGaTe2 [63], AgSbTe2 [64], BiTe [66], n-type GeTe [36], p-type GeTe [10], n-type PbTe [40], p-type PbTe [11], SnTe [12], n-type Bi2Te3 [46], p-type Bi2Te3 [45], BiCuOTe [67], La3−x Te4 [68], Pr3−x Te4 [65], MnTe [50], Ag2Te [56], AgCuTe [52], and Cu2Te [54].
Download figure:
Standard image High-resolution imageTable 1. Tellurides thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κL (W m−1 K−1) a | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µ0 (cm2 V−1 s−1) | ms a (me) |
 r or r or  ( ( 0) 0) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bix Sb2−x Te3 | 300 | 479.5 | 0.6 | 186 | 1242 | 1.2 | — | — | — | — | [42] |
| Bix Sb2−x Te3 | 373 | 318.4 | 0.44 | 212 | 845 | 1.4 | — | — | — | — | [42] |
| Bi0.5Sb1.5Te3 | 320 | 429.4 | 0.33 | 241 | 647 | 1.86 | — | — | — | — | [45] |
| Bi0.3Sb1.625In0.075Te3 | 300 | 339.6 | 0.61 | 185 | 890 | 0.75 | 0.12 | — | 1.6 | — | [69] |
| Bi0.3Sb1.625In0.075Te3 | 500 | 164.6 | 1.09 (kT) | 220 | 618 | 1.4 | — | — | — | — | [69] |
| Bi0.5Sb1.495Cu0.005Te3 | 300 | 363.5 | 0.34 | 154 | 1371 | 0.97 | — | — | — | — | [70] |
| Bi0.5Sb1.495Cu0.005Te3 | 450 | 152.6 | 0.31 | 201 | 610 | 1.4 | — | — | — | — | [70] |
| Bi0.5Sb1.5Te3 | 350 | 332.4 | 0.65 | 237 | 600 | 1.24 | — | — | — | — | [71] |
| Zn0.015Bi0.46Sb1.54Te3.015 | 300 | 465.6 | 0.52 | 185 | 1220 | 1.14 | — | — | 1.03 | — | [43] |
| Zn0.015Bi0.46Sb1.54Te3.015 | 373 | 305.7 | 0.5 | 208 | 850 | 1.4 | — | — | — | — | [43] |
| Bi0.4Sb1.6Te3 | 323 | 458.7 | 1.03 (kT) | 232 | 778 | 1.38 | 0.25 | 320 | 1.4 | — | [72] |
| Bi2Te2.7Se0.3 | 300 | 389.5 | 0.7 | −190 | 963 | 0.9 | — | — | — | — | [73] |
| Bi2Te2.7Se0.3 | 398 | 221.2 | 1.14 (kT) | −209 | 670 | 1.04 | — | — | — | — | [73] |
| Bi2Te2S | 300 | 159.9 | 1.01 (kT) | −148 | 648 | 0.4 | 0.2 | — | — | — | [74] |
| Bi2Te2S | 573 | 52.8 | 0.93(kT) | −171 | 430 | 0.8 | — | — | — | — | [74] |
| K0.06Bi2Te3.18 | 300 | 455.4 | 0.66 | −180 | 1265 | 0.98 | — | — | — | — | [48] |
| K0.06Bi2Te3.18 | 350 | 359.4 | 0.66 | −197 | 1032 | 1.1 | — | — | — | — | [48] |
| Bi2Te2.7Se0.3 | 300 | 182.7 | 0.44 | −167 | 591 | 0.68 | 0.25 | — | 0.76 | — | [47] |
| Bi2Te2.7Se0.3 | 480 | 105.3 | 0.36 | −198 | 480 | 1.23 | — | — | — | — | [47] |
| Bi1.8Sb0.2Te2.7Se0.3+15%Te | 300 | 398.8 | 0.41 | −171 | 1231 | 1 | — | 100 | 1.7 | — | [46] |
| Bi1.8Sb0.2Te2.7Se0.3+15%Te | 425 | 215.6 | 0.38 | −198 | 819 | 1.4 | — | — | — | — | [46] |
| GeTe | 300 | 310.2 | 2.62 | 32 | 8069 | 0.03 | 0.2 | — | 1.43 | — | [75] |
| GeTe | 673 | 148.4 | 0.74 | 137 | 2307 | 0.93 | — | — | — | — | [75] |
| Ge0.87Pb0.13Te | 723 | 115.9 | 1.2 (kT) | 196 | 1000 | 2.27 | — | — | — | — | [76] |
| Ge0.87Pb0.13Te-5%Bi2Te3 | 373 | 155.5 | 1.05 (kT) | 130 | 1087 | 0.66 | — | 75 | — | — | [77] |
| Ge0.87Pb0.13Te-5%Bi2Te3 | 690 | 86.5 | 0.78 (kT) | 243 | 403 | 2.1 | — | — | — | — | [77] |
| (CoGe2)0.2(GeTe)17Sb2Te3 | 723 | 135.9 | 0.64 | 258 | 571 | 1.9 | — | — | — | — | [78] |
| Ge0.9Sb0.1Te | 300 | 222.2 | 1.42 | 107 | 1503 | 0.22 | 0.08 | — | — | — | [75] |
| Ge0.9Sb0.1Te | 725 | 179 | 1.15 | 256 | 773 | 1.85 | — | — | — | — | [75] |
| Ge0.94Bi0.06Te | 300 | 236.9 | 1.13 | 71 | 2680 | 0.14 | 0.08 | — | — | — | [79] |
| Ge0.94Bi0.06Te | 725 | 130.5 | 1.2 | 217 | 886 | 1.3 | — | — | — | — | [79] |
| Ge0.85Bi0.05Sb0.1Te | 300 | 159.7 | 0.48 | 130 | 805 | 0.47 | 0.08 | — | 2.44 | — | [18] |
| Ge0.85Bi0.05Sb0.1Te | 725 | 128.3 | 0.65 | 232 | 732 | 1.8 | — | — | — | — | [18] |
| Ge0.9Sb0.1Te0.9Se0.05S0.05 | 300 | 172.5 | 0.96 | 148 | 699 | 0.29 | — | — | — | — | [80] |
| Ge0.9Sb0.1Te0.9Se0.05S0.05 | 630 | 191 | 0.65 | 260 | 638 | 2.1 | — | — | — | — | [80] |
| Ge0.9Cd0.05Bi0.05Te | 300 | 205.6 | 1.06 | 105 | 1428 | 0.32 | — | 43 | 1.7 | — | [21] |
| Ge0.9Cd0.05Bi0.05Te | 650 | 149.7 | 0.48 | 232 | 725 | 2.23 | — | — | — | — | [21] |
| Ge0.89Sb0.1In0.01Te | 300 | 167.7 | 0.73 | 192 | 405 | 0.4 | — | — | 3.8 | — | [32] |
| Ge0.89Sb0.1In0.01Te | 780 | 99.9 | 0.52 | 245 | 547 | 2.3 | — | — | 6.2 | — | [32] |
| Ge0.86Pb0.1Bi0.04Te | 300 | 243.1 | 0.63 | 138 | 1111 | 0.55 | — | 69 | 1.94 | — | [34] |
| Ge0.86Pb0.1Bi0.04Te | 600 | 151.7 | 0.49 | 282 | 365 | 2.4 | — | 7 | 5.92 | — | [34] |
| Ge0.86Sb0.1Zn0.04Te | 300 | 183.5 | 0.74 | 157 | 668 | 0.46 | — | 24 | 2.8 | — | [20] |
| Ge0.86Sb0.1Zn0.04Te | 780 | 109.3 | 0.55 | 235 | 672 | 2.2 | — | — | — | — | [20] |
| Ge0.93In0.01Bi0.06Te | 300 | 233.8 | 0.46 | 87 | 2082 | 0.33 | 0.09 | — | 1.9 | — | [29] |
| Ge0.93In0.01Bi0.06Te | 723 | 212.5 | 0.72 | 246 | 1026 | 2.1 | — | — | — | — | [29] |
| (GeTe)0.8(AgBiSe2)0.2 | 300 | 91.7 | 0.28 | 242 | 124 | 0.63 | — | — | — | — | [36] |
| (GeTe)0.8(AgBiSe2)0.2 | 467 | 87.7 | 0.32 | 279 | 150 | 1.3 | — | — | — | — | [36] |
| (Ge0.9Sb0.1Te)0.95(SnSe)0.025(SnS)0.025 | 300 | 129.4 | 0.27 | 86 | 1169 | 0.26 | — | — | — | — | [81] |
| (Ge0.9Sb0.1Te)0.95(SnSe)0.025(SnS)0.025 | 710 | 97.1 | 0.32 | 206 | 726 | 1.9 | — | — | — | — | [81] |
| Ge0.87Sn0.05Sb0.08Te | 300 | 173.4 | 0.92 | 89 | 1501 | 0.25 | — | 62 | 1.57 | — | [35] |
| Ge0.87Sn0.05Sb0.08Te | 735 | 125.3 | 0.53 | 205 | 998 | 2.2 | — | — | 3.73 | — | [35] |
| (GeTe)0.95(Sb2Te3)0.05 | 323 | 204.8 | 1.44 (kT) | 115 | 1394 | 0.4 | — | — | — | — | [10] |
| (GeTe)0.95(Sb2Te3)0.05 | 720 | 136.5 | 1.19 (kT) | 217 | 917 | 2.7 | — | — | — | — | [10] |
| (GeTe)0.5(AgBiSe1.995Br0.005)0.5 | 300 | 15.7 | 0.21 | −149 | 63 | 0.15 | 0.36 | — | — | — | [36] |
| (GeTe)0.5(AgBiSe1.995Br0.005)0.5 | 500 | 16.2 | 0.19 | −167 | 113 | 0.6 | — | — | — | — | [36] |
| SnTe | 300 | 188.5 | 2.88 | 19 | 8261 | — | — | — | — | — | [82] |
| SnTe | 710 | 58.1 | 1.06 | 91 | 1779 | 0.29 | — | — | — | — | [82] |
| Sn0.9975In0.0025Te | 300 | 260.8 | 1.61 | 50 | 4300 | 0.09 | 0.18 | — | — | — | [16] |
| Sn0.9975In0.0025Te | 873 | 45 | 0.88 | 162 | 767 | 1.1 | — | — | — | — | [16] |
| SnCd0.03Te-2%CdS | 300 | 130.8 | 1.31 | 47 | 2300 | 0.06 | — | — | — | — | [24] |
| SnCd0.03Te-2%CdS | 873 | 40.6 | 0.63 | 205 | 419 | 1.3 | — | — | — | — | [24] |
| Sn0.985In0.015Te0.85Se0.15 | 300 | 136.4 | 1.28 | 66 | 1674 | 0.09 | — | — | — | — | [83] |
| Sn0.985In0.015Te0.85Se0.15 | 860 | 43.2 | 1.26 | 172 | 639 | 0.8 | — | — | — | — | [83] |
| Sn0.94Mg0.09Te | 300 | 131.6 | 2.72 | 35 | 3126 | — | — | — | 0.69 | — | [23] |
| Sn0.94Mg0.09Te | 860 | 70.6 | 0.79 | 174 | 1021 | 1.2 | — | — | — | — | [23] |
| Sn0.98Bi0.02Te-3%HgTe | 300 | 229.8 | 1.13 | 67 | 2774 | 0.13 | — | — | — | — | [25] |
| Sn0.98Bi0.02Te-3%HgTe | 910 | 59.1 | 0.66 | 182 | 847 | 1.35 | — | — | — | — | [25] |
| Sn0.97In0.015Cd0.015Te-3%CdS | 300 | 132.7 | 1.64 | 92 | 1101 | 0.13 | — | — | — | — | [26] |
| Sn0.97In0.015Cd0.015Te-3%CdS | 923 | 44 | 0.59 | 196 | 548 | 1.4 | — | — | — | — | [26] |
| Sn0.94Ca0.09Te | 325 | 257.6 | 1 | 44 | 5466 | 0.2 | — | — | 0.35 | — | [84] |
| Sn0.94Ca0.09Te | 873 | 56.9 | 0.79 | 185 | 740 | 1.35 | — | — | — | — | [84] |
| Sn0.97Bi0.03Te-3%SrTe | 300 | 289.4 | 1.56 | 82 | 2770 | 0.17 | — | — | — | — | [85] |
| Sn0.97Bi0.03Te-3%SrTe | 823 | 47.4 | 0.86 | 173 | 649 | 1.2 | — | — | — | — | [85] |
| Sn0.85Sb0.15Te | 300 | 150.9 | 0.67 | 33 | 3806 | — | — | — | — | — | [82] |
| Sn0.85Sb0.15Te | 800 | 56.2 | 151 | 956 | 1 | — | — | — | — | [82] | |
| SnAg0.025In0.025Te1.05 | 300 | 239 | 2.39 | 98 | 1825 | 0.08 | — | — | — | — | [27] |
| SnAg0.025In0.025Te1.05 | 856 | 69.7 | 1.17 | 167 | 1087 | 1 | — | — | — | — | [27] |
| Sn0.97Bi0.03Te-3%PbTe | 300 | 157.1 | 1.15 | 27 | 4847 | — | — | — | — | — | [86] |
| Sn0.97Bi0.03Te-3%PbTe | 900 | 54.2 | 1.1 | 197 | 642 | 1.1 | — | — | — | — | [86] |
| (Sn0.89Mn0.14Te)(Cu2Te)0.05 | 300 | 102.6 | 1.72 | 45 | 1886 | 0.06 | — | — | — | — | [12] |
| (Sn0.89Mn0.14Te)(Cu2Te)0.05 | 920 | 34.4 | 0.51 | 200 | 407 | 1.6 | — | — | — | — | [12] |
| Sn0.915Mn0.11In0.005Te | 300 | 106.8 | 1.64 | 117 | 634 | 0.13 | — | — | — | — | [87] |
| Sn0.915Mn0.11In0.005Te | 823 | 47.6 | 0.92 | 237 | 310 | 1.15 | — | — | — | — | [87] |
| (Sn0.91Mg0.12Te)(Cu2Te)0.05 | 300 | 138.9 | 1.23 | 28 | 4132 | — | — | — | — | — | [88] |
| (Sn0.91Mg0.12Te)(Cu2Te)0.05 | 900 | 32.7 | 0.55 | 198 | 383 | 1.4 | — | — | — | — | [88] |
| Sn0.57Sb0.13Ge0.3Te | 300 | 166 | 0.48 | 67 | 2004 | 0.16 | — | — | — | — | [13] |
| Sn0.57Sb0.13Ge0.3Te | 721 | 77.8 | 0.3 | 174 | 864 | 1.6 | — | — | — | — | [13] |
| Sn0.83Ag0.03Mn0.17Te | 300 | 148 | 1.64 | 46 | 2669 | 0.05 | — | — | — | — | [30] |
| Sn0.83Ag0.03Mn0.17Te | 865 | 55 | 0.3 | 164 | 908 | 1.45 | — | — | — | — | [30] |
| Sb2Te3(Sn0.996Re0.004Te)8 | 325 | 190.8 | 0.72 | 84 | 2000 | 0.2 | — | — | — | — | [89] |
| Sb2Te3(Sn0.996Re0.004Te)8 | 773 | 77.1 | 0.48 | 183 | 855 | 1.4 | — | — | — | — | [89] |
| Sn1.03Te0.85Se0.075S0.075-2%Ag-2%In | 300 | 162.6 | 1.21 | 90 | 1387 | 0.16 | — | — | — | — | [90] |
| Sn1.03Te0.85Se0.075S0.075-2%Ag-2%In | 854 | 62 | 0.6 | 182 | 808 | 1.3 | — | — | — | — | [90] |
| Na0.95Pb20SbTe22 | 300 | 190.6 | 0.74 | 94 | 1538 | 0.25 | — | [91] | |||
| Na0.95Pb20SbTe22 | 650 | 126 | 0.55 | 330 | 196 | 1.7 | — | — | — | — | [91] |
| Pb0.98Tl0.02Te | 300 | 93.2 | 2.17 (kT) | 137 | 431 | 0.1 | — | — | 0.93 | — | [14] |
| Pb0.98Tl0.02Te | 773 | 87.3 | 0.95 (kT) | 332 | 172 | 1.5 | — | — | — | — | [14] |
| PbTe-12%PbS-2%Na | 315 | 136 | 1.61 | 69 | 1710 | 0.09 | — | — | — | — | [92] |
| PbTe-12%PbS-2%Na | 800 | 75.6 | 0.73 | 263 | 349 | 1.8 | — | — | — | — | [92] |
| PbTe-1%Na2Te-6%CaTe | 300 | 179.4 | 1.32 | 65 | 2239 | 0.09 | 0.26 | — | — | — | [93] |
| PbTe-1%Na2Te-6%CaTe | 765 | 66.6 | 0.48 | 259 | 301 | 1.5 | — | — | — | — | [93] |
| PbTe0.85Se0.15-2%Na | 300 | 147.2 | 1.41 | 50 | 2427 | 0.06 | — | — | — | — | [15] |
| PbTe0.85Se0.15-2%Na | 850 | 59.7 | 0.52 | 222 | 485 | 1.8 | — | — | — | — | [15] |
| MgxPb1−x Te:Na(9E19) b | 300 | 197.1 | 1.73 | 105 | 1369 | 0.19 | — | — | — | — | [94] |
| MgxPb1−x Te:Na(9E19) b | 725 | 92.1 | 0.64 | 269 | 342 | 1.7 | — | — | — | — | [94] |
| PbTe:Na(9E19) b | 300 | 216.2 | 2.05 | 62 | 2840 | 0.1 | — | — | — | — | [95] |
| PbTe:Na(9E19) b | 750 | 87.5 | 0.8 | 270 | 338 | 1.4 | — | — | — | — | [95] |
| Pb0.96Mn0.04Te:Na | 300 | 190.4 | 1.33 | 101 | 1396 | 0.2 | 0.38 | — | — | — | [96] |
| Pb0.96Mn0.04Te:Na | 700 | 86 | 0.64 | 263 | 325 | 1.6 | — | — | — | — | [96] |
| PbTe-4%SrTe-2%Na | 300 | 145.1 | 1.96 | 79 | 1452 | 0.09 | — | — | — | — | [28] |
| PbTe-4%SrTe-2%Na | 915 | 64.8 | 0.52 | 281 | 297 | 2.2 | — | — | — | — | [28] |
| K0.02Pb0.98Te0.75Se0.25 | 300 | 98.4 | 1.49 | 53 | 1526 | 0.06 | — | — | — | — | [97] |
| K0.02Pb0.98Te0.75Se0.25 | 773 | 78.5 | 0.8 | 312 | 195 | 1.6 | — | — | — | — | [97] |
| PbTe-2%MgTe-2%Na2Te | 300 | 197.9 | 2.05 | 67 | 2388 | 0.1 | — | — | — | — | [98] |
| PbTe-2%MgTe-2%Na2Te | 780 | 76.8 | 0.74 | 250 | 397 | 1.6 | — | — | — | — | [98] |
| Pb0.98Na0.02Te-6%MgTe | 300 | 175.3 | 1.73 | 110 | 1140 | 0.16 | 0.39 | — | 1.18 | — | [99] |
| Pb0.98Na0.02Te-6%MgTe | 823 | 74.6 | 0.53 | 295 | 248 | 2 | — | — | — | — | [99] |
| PbTe0.7S0.3-2.5%K | 300 | 127 | 0.64 | 70 | 1460 | 0.14 | — | — | — | — | [100] |
| PbTe0.7S0.3-2.5%K | 923 | 42.7 | 0.37 | 299 | 161 | 2.2 | — | — | — | — | [100] |
| (PbTe)0.86(PbSe)0.07(PbS)0.07-2% Na | 300 | 163.3 | 69 | 1908 | 0.1 | 0.28 | — | — | — | [101] | |
| (PbTe)0.86(PbSe)0.07(PbS)0.07-2% Na | 823 | 82.7 | 0.61 | 265 | 389 | 2 | — | — | — | — | [101] |
| Pb0.98Na0.02Te-8%SrTe | 300 | 242.6 | 1.7 | 91 | 2041 | 0.18 | 0.34 | — | 1.37 | — | [102] |
| Pb0.98Na0.02Te-8%SrTe | 923 | 80.9 | 0.57 | 294 | 323 | 2.5 | — | — | — | [102] | |
| Pb0.953Na0.04Ge0.007Te | 300 | 251.7 | 2.15 | 69 | 2941 | 0.1 | 0.4 | — | 0.8 | — | [103] |
| Pb0.953Na0.04Ge0.007Te | 805 | 89.5 | 0.67 | 263 | 417 | 1.9 | — | — | — | — | [103] |
| Na0.03Eu0.03Sn0.02Pb0.92Te | 300 | 88.2 | 0.87 | 104 | 621 | 0.14 | — | — | 1.6 | — | [11] |
| Na0.03Eu0.03Sn0.02Pb0.92Te | 850 | 62.9 | 0.42 | 273 | 283 | 2.57 | — | — | — | — | [11] |
| PbTe-1%CdTe-0.055%PbI2 | 300 | 330.5 | 0.89 | −88 | 2901 | 0.21 | 0.3 | — | — | [104] | |
| PbTe-1%CdTe-0.055%PbI2 | 720 | 52.6 | 0.5 | −225 | 322 | 1.2 | — | — | — | — | [104] |
| PbTe0.9988I0.0012 | 300 | 364.2 | 1.53 | −83 | 3436 | 0.27 | — | — | 0.25 | — | [105] |
| PbTe0.9988I0.0012 | 723 | 74.4 | 0.78 | −206 | 571 | 1.4 | — | — | — | — | [105] |
| PbTe:I(1.8E19) b | 300 | 392.8 | 3.32 (kT) | −81 | 3816 | 0.22 | — | 1120 | 0.25 | — | [106] |
| PbTe:I(1.8E19) b | 725 | −212 | 1.39 | — | — | — | — | [106] | |||
| PbTe-4%InSb | 323 | 78 | 2.25 | −132 | 428 | 0.09 | — | — | — | — | [40] |
| PbTe-4%InSb | 773 | 56 | 0.25 | −205 | 484 | 1.83 | — | — | — | — | [40] |
| PbTe-4%MnTe | 300 | 171 | 1.15 | −88 | 1500 | 0.15 | 0.34 | — | 0.4 | — | [39] |
| PbTe-4%MnTe | 773 | 60 | 0.53 | −238 | 353 | 1.6 | — | — | — | [39] | |
| Pb0.9965In0.0035Te0.996I0.004 | 300 | 301.5 | 1.33 | −140 | 1345 | 0.4 | — | — | 0.4 | — | [37] |
| Pb0.9965In0.0035Te0.996I0.004 | 773 | 56.9 | 0.7 | −224 | 392 | 1.4 | — | — | — | — | [37] |
| (Pb0.93Sn0.07)(Te0.93Se0.07) | 300 | 152.8 | 1.35 | −54 | 2324 | 0.08 | — | — | 0.31 | — | [107] |
| (Pb0.93Sn0.07)(Te0.93Se0.07) | 773 | 47.5 | 0.6 | −199 | 437 | 1.4 | — | — | — | — | [107] |
| Pb0.98Ga0.02Te-5%GeTe | 300 | 330.2 | 1.13 | −222 | 563 | 0.59 | — | — | 0.4 | — | [38] |
| Pb0.98Ga0.02Te-5%GeTe | 673 | 86.4 | 0.66 | −287 | 233 | 1.47 | — | — | — | — | [38] |
| AgSbTe2 | 300 | 137.4 | 0.5 | 279 | 121 | 0.55 | — | — | — | — | [64] |
| AgSbTe2 | 573 | 58.5 | 0.5 | 328 | 77 | 0.89 | — | — | — | — | [64] |
| AgSb0.96Zn0.04Te2 | 300 | 107.9 | 0.52 | 234 | 160 | 0.56 | — | — | 2.65 | — | [108] |
| AgSb0.96Zn0.04Te2 | 585 | 96.5 | 0.35 | 289 | 206 | 1.9 | — | — | — | — | [108] |
| AgSbTe1.85Se0.15 | 300 | 84.2 | 0.37 | 203 | 179 | 0.53 | — | 20 | 2.32 | — | [109] |
| AgSbTe1.85Se0.15 | 573 | 92 | 0.29 | 309 | 151 | 2.1 | — | 4 | 3.38 | — | [109] |
| AgSb0.94Cd0.06Te2 | 300 | 174.4 | 0.15 | 248 | 220 | 1.5 | — | — | — | — | [64] |
| AgSb0.94Cd0.06Te2 | 573 | 92.6 | 0.17 | 265 | 253 | 2.6 | — | — | — | — | [64] |
| MnTe | 300 | 14.1 | 1.18 | 463 | 1.47 | — | 0.82 | 7 | — | [110] | |
| MnTe | 900 | 17.7 | 0.67 | 302 | 62 | 0.67 | — | — | — | — | [110] |
| Mn0.98Na0.02Te | 300 | 51.8 | 0.82 | 192 | 125 | 0.15 | — | — | 7.7 | — | [110] |
| Mn0.98Na0.02Te | 900 | 19.7 | 0.58 | 270 | 100 | 0.89 | — | — | — | — | [110] |
| MnTe+0.5%Na2S | 300 | 79.4 | 1.56 | 185 | 208 | 0.1 | — | — | — | — | [49] |
| MnTe+0.5%Na2S | 873 | 25.9 | 0.56 | 267 | 130 | 1.09 | — | — | — | — | [49] |
| MnTe+3%Li | 923 | 20.5 | 0.9 (kT) | 208 | 222 | 1 | — | — | 1.05 | — | [51] |
| Mn1.06Te+2%SnTe | 323 | — | 1.48 | 522 | — | — | 0.84 | — | 2.69 | — | [50] |
| Mn1.06Te+2%SnTe | 873 | 64.9 | 0.66 | 379 | 89 | 1.4 | — | — | — | — | [50] |
| Ag2Te | 300 | 213.1 | 0.25 | −90 | 1818 | 0.45 | 0.04 | — | <0.1 | — | [55] |
| Ag2Te | 550 | 32.2 | 0.23 | −119 | 463 | 0.62 | 0.2 | — | — | — | [55] |
| (Ag1.9996Te)0.9(PbTe)0.1 | 300 | 177.2 | 0.38 | −101 | 1299 | 0.38 | — | — | — | [55] | |
| (Ag1.9996Te)0.9(PbTe)0.1 | 550 | 34.1 | 0.23 | −178 | 241 | 1 | 0.28 | — | — | — | [55] |
| Ag2Sb0.02Te0.98 | 300 | 99.2 | 0.36 | −105 | 689 | 0.35 | 0.08 | — | — | — | [56] |
| Ag2Sb0.02Te0.98 | 410 | 80.6 | — | −106 | 883 | 1.4 | — | — | — | [56] | |
| Cu2Te | 320 | 79.9 | 6.1 (kT) | 15 | 4878 | — | 0.26 | — | 1.5 | — | [53] |
| Cu2Te | 1000 | 20.2 | 2.36 (kT) | 93 | 1008 | 0.37 | — | — | — | — | [53] |
| Cu1.98Ag0.2Te | 300 | 128.3 | 1.98 (kT) | 47 | 2256 | 0.08 | — | — | — | — | [111] |
| Cu1.98Ag0.2Te | 900 | 30.8 | 1.3 (kT) | 158 | 575 | 1 | — | — | — | — | [111] |
| Cu2S0.52Te0.48 | 300 | 20.2 | 0.49 (kT) | 62 | 265 | — | — | — | 4.5 | — | [54] |
| Cu2S0.52Te0.48 | 1000 | 15.5 | 0.38 (kT) | 218 | 168 | 2.09 | — | — | — | — | [54] |
| Cu1.9Sn0.1Te | 320 | 109.6 | 1.22 (kT) | 38 | 2638 | 0.1 | 0.25 | — | 2.56 | — | [53] |
| Cu1.9Sn0.1Te | 1000 | 27.7 | 0.97 (kT) | 133 | 819 | 1.5 | — | — | — | — | [53] |
| Cu2Te+50%Ag2Te | 300 | 67.5 | 1.32 (kT) | 54 | 1027 | 0.07 | — | — | — | — | [112] |
| Cu2Te+50%Ag2Te | 1000 | 20.1 | 0.64 (kT) | 178 | 348 | 1.8 | — | — | — | — | [112] |
| AgCuTe | 300 | 39.3 | 0.35 | 31 | 1055 | 0.15 | — | — | — | — | [52] |
| AgCuTe | 660 | 29.9 | 0.23 | 228 | 155 | 1.3 | — | — | — | — | [52] |
| AgCuTe0.9Se0.1 | 300 | 31.2 | 0.15 | 70 | 359 | 0.36 | — | — | — | — | [52] |
| AgCuTe0.9Se0.1 | 670 | 38.4 | 0.25 | 223 | 216 | 1.6 | — | — | — | — | [52] |
| AgCuTe0.9I0.1 | 300 | 39 | 0.21 | 143 | 168 | 0.35 | — | — | 1.21 | — | [113] |
| AgCuTe0.9I0.1 | 463 | 33.8 | 0.17 | 287 | 52 | 0.9 | — | — | — | — | [113] |
| AgGaTe2 | 300 | 1.42 | — | 1.06 | — | — | — | [57] | |||
| AgGaTe2 | 750 | 16.5 | 0.33 | 414 | 12 | 0.48 | — | — | — | — | [57] |
| Ag0.95GaTe2 | 300 | 26.2 | 1.26 (kT) | 673 | 0.24 | — | — | — | — | [114] | |
| Ag0.95GaTe2 | 850 | 14.1 | 0.2 (kT) | 382 | 18 | 0.77 | — | — | — | — | [114] |
| AgGa0.93Te2 | 300 | — | 1.16 (kT) | — | — | — | — | — | — | — | [58] |
| AgGa0.93Te2 | 873 | 11.9 | 0.18 (kT) | 396 | 13.4 | 1.02 | — | — | — | — | [58] |
| AgInTe2 | 300 | 1.42 | — | 0.87 | — | — | — | [57] | |||
| AgInTe2 | 750 | 14.2 | 0.33 | 570 | 1.7 | 0.18 | — | — | — | — | [57] |
| CuGaTe2 | 300 | 76.9 | 6.7 | 380 | 21 | — | 1.2 | — | — | — | [115] |
| CuGaTe2 | 950 | 30.5 | 0.51 | 244 | 227 | 1.4 | — | — | — | — | [115] |
| CuGaTe2 | 300 | 117.6 | 6.7 | 395 | 27 | — | 1.18 | — | — | — | [57] |
| CuGaTe2 | 875 | 38 | 0.95 | 281 | 163 | 1 | — | — | — | — | [57] |
| CuGa0.36In0.64Te2 | 320 | 36.6 | 1.48 | 390 | 9.8 | — | 0.85 | — | — | — | [116] |
| CuGa0.36In0.64Te2 | 701 | 39.2 | 0.56 | 285 | 115 | 0.91 | — | — | — | — | [116] |
| Cu0.98GaSb0.02Te2 | 310 | 14.4 | 3.1 | 176 | 44 | — | 0.95 | — | — | — | [117] |
| Cu0.98GaSb0.02Te2 | 721 | 40.6 | 0.48 | 262 | 162 | 1.07 | — | — | — | — | [117] |
| Cu0.7Ag0.3Ga0.4In0.6Te2 | 300 | 252.7 | — | 588 | 6.2 | — | — | — | — | — | [63] |
| Cu0.7Ag0.3Ga0.4In0.6Te2 | 873 | 32.9 | 0.25 | 406 | 33 | 1.64 | — | — | — | — | [63] |
| CuInTe2 | 300 | 112.7 | 6 (kT) | 247 | 144 | — | 1.02 | — | 0.52 | — | [118] |
| CuInTe2 | 850 | 35.8 | 1.05 (kT) | 270 | 167 | 1.18 | — | — | — | — | [118] |
| CuInTe2 | 300 | 155 | 5.9 | 464 | 16 | — | 0.92 | — | — | — | [57] |
| CuInTe2 | 875 | 28 | 0.81 | 290 | 108 | 0.9 | — | — | — | — | [57] |
| Cu0.9InTe2 | 300 | 78.8 | 3 (kT) | 170 | 246 | 0.11 | — | — | 0.6–0.8 | — | [119] |
| Cu0.9InTe2 | 710 | 35.5 | 1.33 (kT) | 214 | 242 | 0.54 | — | — | — | — | [119] |
| Cu0.75Ag0.2InTe2 | 300 | 46.5 | 1.86 (kT) | 234 | 69 | 0.07 | — | — | — | — | [120] |
| Cu0.75Ag0.2InTe2 | 850 | 27.6 | 0.52(kT) | 320 | 72 | 1.25 | — | — | — | — | [120] |
| CuInTe1.99Sb0.01+1%ZnO | 300 | 86.6 | 3.6 | 104 | 610 | — | — | — | — | — | [61] |
| CuInTe1.99Sb0.01+1%ZnO | 823 | 47.7 | 0.45 | 284 | 180 | 1.61 | — | — | — | — | [61] |
| CuInTe2+6%ZnS | 300 | 85.7 | 3.54 | 157 | 312 | — | — | — | — | [60] | |
| CuInTe2+6%ZnS | 823 | 37.5 | 0.44 | 282 | 145 | 1.52 | — | — | — | — | [60] |
| Cu0.89Ag0.2In0.91Te2 | 300 | 149.4 | 2.68 (kT) | 446 | 19 | — | 0.9 | — | — | — | [62] |
| Cu0.89Ag0.2In0.91Te2 | 850 | 31.8 | 0.47 (kT) | 315 | 88 | 1.6 | — | — | — | — | [62] |
| BiTe | 300 | 149.3 | 0.72 | −37 | 3352 | 0.05 | 0.1 | — | — | — | [66] |
| BiTe | 500 | 78.8 | 0.76 | −61 | 2267 | 0.13 | — | — | — | — | [66] |
| BiTe0.5Se0.5 | 300 | 170.6 | 1.8 (kT) | −49 | 2873 | 0.12 | — | — | — | — | [66] |
| BiTe0.5Se0.5 | 500 | 93.5 | 2.1(kT) | −79 | 2014 | 0.32 | — | — | — | — | [66] |
| BiCuOTe | 300 | 62.7 | 0.68(kT) | 173 | 189 | 0.4 | 0.21 | — | — | — | [121] |
| BiCuOTe | 673 | 24.2 | 0.73(kT) | 183 | 218 | 0.66 | — | — | — | — | [121] |
| BiCuO0.88Te | 323 | 114.8 | 0.59 | 185 | 336 | 0.48 | — | — | — | — | [67] |
| BiCuO0.88Te | 673 | 29.6 | 0.33 | 207 | 202 | 1.06 | — | — | — | — | [67] |
| La3−x Te4 (1.2E21) b | 1275 | 9.3 | 0.5 (kT) | −300 | 56 | 1.13 | — | — | — | — | [122] |
| La3−x Te4 (1.2E21) b | 300 | — | — | — | — | — | 0.83 | 2.75 | — | [122] | |
| La2.6Yb0.4Te4 (3E20) b | 1273 | 11.1 | 0.66 (kT) | −289 | 76 | 1.2 | — | — | — | — | [68] |
| Pr2.74Te4 | 300 | 58.1 | 0.846 | −48 | 1000 | 0.03 | — | — | 3.5 | — | [65] |
| Pr2.74Te4 | 1200 | 18.1 | 0.427 | −240 | 200 | 1.7 | — | — | — | — | [65] |
a kT—indicates total thermal conductivity. b (1.2E21)—numbers of this type after the material name indicates the carrier concentration (cm−3).
IV–VI tellurides
While both SnTe and PbTe crystallize in the cubic rocksalt structure at ambient conditions, GeTe has a rhombohedral crystal structure at room temperature. However, GeTe undergoes a rhombohedral to cubic phase transition at ∼720 K. All three are narrow band gap semiconductors with band gap in the range ∼0.18–0.32 eV. As a result of this favorable band gap, highly-symmetric crystal structure leading to degenerate electronic bands, easy tuneability of electronic structure via chemical doping and alloying, and heavy constituent elements, they are ideal for exploring high thermoelectric performance. At present, a thermoelectric figure of merit, zT as high as ∼2.7 at 720 and ∼2.57 at 850 K can be achieved in GeTe [10] and PbTe [11] based p-type thermoelectric materials, respectively. While SnTe is much less toxic than PbTe, the maximum zT obtained so far is only 1.6 at 720 K [12, 13].
PbTe is a very important thermoelectric material; both band convergence and resonant level formation strategies were first demonstrated in PbTe. Resonant level formation in PbTe upon Tl doping [14] and valence band convergence in PbTe1−x Sex :Na [15] resulted in maximum zT ∼ 1.5 and 1.8 in 2008 and 2011, respectively. Since then, these electronic structure modulation strategies have been followed up for numerous materials, and many 2nd (1 ⩽ zT ⩽ 2) and 3rd (zT > 2) generation thermoelectric materials have been achieved, including in GeTe and SnTe. For example, In doping has been very effective in inducing resonant levels in both SnTe [16] and GeTe [17], significantly improving their thermoelectric performance. Similarly, doping or alloying of Sb [18], Mn [19], Zn [20], Cd [21] in GeTe is also highly effective in inducing valence band convergence. While PbTe and SnTe have the same rocksalt crystal structure, the larger energy gap (∼0.35 eV) between the primary and secondary valence bands in SnTe results in low Seebeck coefficients and consequently poor thermoelectric performance. Doping and alloying of many elements like Mn [22], Mg [23], Cd [24], Hg [25] etc are highly effective in reducing the gap between the primary and secondary valence bands. It has also been demonstrated that synergistic effects of band convergence and resonant level can further improve the thermoelectric performance. This has been achieved in SnTe via co-doping of In and Cd [26], and In and Ag [27]. Another highly successful strategy, based on phonon scattering manipulation, namely, all-scale hierarchical architecture, was first demonstrated in PbTe, resulting in maximum zT ∼ 2.2 at 915 K [28]. Recently, lattice strain in Na–Eu–Sn doped PbTe has been shown to be very effective in reducing lattice thermal conductivity without compromising the carrier mobility; it resulted in zT ∼ 2.57 at 850 K [11]. Many synergistic effects of electronic structure modulation and thermal transport optimization have also been achieved via co-doping in GeTe and SnTe. For example, complementary effects of resonant state formation via In doping, and reduced thermal conductivity due to solid solution point defects with Bi doping, results in high thermoelectric performance with zT ∼ 2.1 at 723 K in In and Bi co-doped GeTe [29]. Similarly, synergistic effects have also been achieved in GeTe by co-doping Sb and Bi [18], and in SnTe via co-doping of Ag and Mn [30].
One major problem in SnTe and GeTe is their high p-type carrier concentration due to cation vacancies. Doping, such as Bi in GeTe [21, 31] and self-compensation in SnTe [30] have been effective in reducing the hole concentration. The rhombohedral to cubic phase transition in GeTe has been used as an added control parameter to achieve high thermoelectric performance. The high temperature cubic phase of GeTe possesses a four-fold degenerate light L band at higher energy and 12-fold degenerate heavy Σ band at lower energy. The polar distortion along the [1 1 1] crystallographic direction in the rhombohedral phase, however, splits the 4 L pockets into 3 L and 1 Z pockets, and the 12 Σ pockets into 6 Σ and 6 η pockets. Moreover, in the rhombohedral phase, the Σ band becomes the principal valance band with higher energy. This reduction of band degeneracy, and practical problems arising from the high-temperature phase transition, led to much effort on reducing the phase transition temperature and achieving higher thermoelectric performance in the cubic phase at lower temperature. For example, In and Sb co-doping [32], Bi and Mn co-doping [33] and MnTe alloying [19] have been successful. In recent years, however, it has been shown that precise control of the rhombohedral distortion can be used to achieve a higher degree of effective band degeneracy due to band orbital overlap and reduced lattice thermal conductivity using Bi doping [34]. This resulted in a maximum zT ∼ 2.4 at 600 K. Very recently, it was shown that Rashba spin splitting can be used to achieve effective band convergence in Sn doped GeTe [35].
As can be seen from figure 2, high thermoelectric performance has been achieved in these materials with p-type thermoelectric transport. In fact, because of intrinsic Sn and Ge vacancies, it is very difficult to achieve n-type thermoelectric transport. Only recently n-type thermoelectric transport has been reported in GeTe through AgBiSe2 alloying [36]. However, the zT ∼ 0.6 at 500 K is much lower than that of the high zT of p-type, GeTe-based thermoelectric materials. Indeed, n-type thermoelectric transport is far more explored in PbTe. Still, the presence of a single conduction band at the L point, compared to the multivalley degenerate valence band structure at L and Σ points, makes it challenging to achieve high n-type thermoelectric transport in PbTe. Introduction of mid-gap states through In [37] and Ga [38] doping in PbTe was highly effective in enhancing the n-type thermoelectric performance. The n-type thermoelectric performance of PbTe has also benefited from enhanced effective mass by conduction band flattening through MnTe alloying [39]. In a recent study, introduction of energy filtering through multiphase nano-structuring in a PbTe–InSb composite greatly improved the n-type thermoelectric performance of PbTe, with zT of 1.83 at 773 K [40].
Bi2Te3
Bi2Te3 and its solid solution alloys with Sb2Te3 and Bi2Se3 have been used in practical applications since the 1950s [41], and today they are still the most widely used thermoelectric materials for near room temperature applications. These are layered materials and have hexagonal close packed arrangement of anions. The structure comprises quintuple atomic layers of Te(1)–Bi–Te(2)–Bi–Te(1) along the crystallographic c-direction and two successive quintuple layers are held together by weak van der Waals' (vdW) interactions between two Te(1) layers. Such a layered structure causes anisotropic electrical and thermal transport properties when measured parallel and perpendicular to the layered plane. The highly polarizable Bi–Te bonds, the presence of weak vdW bonds within the layered structure and the constituent heavy elements result in strong lattice anharmonicity and consequently a low lattice thermal conductivity. Bi2Te3 is an indirect narrow band gap semiconductor with Eg ∼ 0.15 eV. A low band effective mass and a highly degenerate electronic band structure also results in high Seebeck coefficient with high charge carrier mobility. Phonon scattering from point defects due to Bi and Sb disorder in Bi0.5Sb1.5Te3 also markedly reduces lattice thermal conductivity. These combinations of low lattice thermal conductivity, favorable electronic band structure and high charge carrier mobility makes Bi2Te3 based materials good candidate thermoelectric materials. The thermoelectric properties of these materials have been greatly improved recently by microstructural engineering and nanostructuring, which result in lower lattice thermal conductivity while retaining relatively high carrier mobility with optimized μ/κL; p-type zT ∼ 1.4 at 373 K was obtained in Bix Sb2−x Te3 alloys by hot pressing ball-milled nanopowders [42]. Recently, nanostructuring with secondary phase nanoprecipitates has also been achieved in BiSbTe–Zn alloys [43]. Melt-centrifugation has been very effective in controlling the microstructure in (Bi,Sb)2Te3, with microscale dislocation arrays and a porous network, giving superior thermoelectric performance than zone-melted and hot pressed ingots [44]. While point defects scatter high frequency phonons, boundary scattering targets the low frequency phonons. Recently, liquid phase sintering was adopted to produce low energy grain boundaries with dense dislocation arrays which include scattering of mid-frequency phonons without simultaneously decreasing the charge carrier mobility. This resulted in a record high thermoelectric p-type figure of merit zT ∼ 1.86 at 320 K in (Bi,Sb)2Te3 alloy [45]. Such liquid phase sintering has also been applied in Sb doped Bi2Te2.7Se0.3 to obtain a high zT n-type material and a large density of dislocation arrays has been observed in the sample. The consequent decrease of lattice thermal conductivity while retaining high charge carrier mobility resulted in n-type zT ∼ 1.4 at 425 K [46]. Bi2Te2.7Se0.3 nanoplates have also been synthesized in a microwave-assisted synthesis route, which have zT ∼ 1.23 at 480 K [47]. While many studies have focused on microstructure engineering to optimize the thermal transport, recently K doping has been used to modulate the electrical transport in Bi2Te3; for n-type material, zT ∼ 1.1 was obtained at 350 K [48].
Transition metal (TM) tellurides
In this section, we discuss the TM based tellurides including MnTe, Ag2Te, Cu2Te and AgCuTe. MnTe crystallizes in a hexagonal structure and is an indirect band gap semiconductor with Eg ∼ 0.8 eV. While MnTe exhibits high Seebeck coefficient, its low carrier concentration (1018 cm−3) results in poor thermoelectric performance. Many dopants, such as Cu, Ag, Na have been introduced to improve the carrier concentration, which resulted in a maximum zT ∼ 1.09 at 873 K [49]. Recently, the incorporation of SnTe nanocrystals was very effective in improving zT ∼ 1.4 at 873 K [50]. Another novel concept based on paramagnon drag enhancement of the Seebeck coefficient has been used to improve thermoelectric performance [51]. This resulted in maximum zT ∼ 1 at 923 K in Li doped MnTe. On the other hand, Cu2Te, Ag2Te and AgCuTe are superionic conductors. At room temperature, they have a complex crystal structure: for example, the ambient structure of AgCuTe and Ag2Te are hexagonal and monoclinic, respectively. However, at high temperature, these materials have a cubic structure and exhibit superionic conduction. In the superionic phase, the anions form a rigid framework which supports high electrical conduction while the cations become superionic and impede phonon propagation. Such a resemblance to the phonon-glass electron-crystal (PGEC) scenario drives thermoelectric interest in these materials. However, high hole concentrations due to cation vacancies causes metallic conduction and low Seebeck coefficients. Recently, Se alloying in AgCuTe was very effective in suppressing cation vacancies due to stronger Ag–Se/Cu–Se bonds compared to Ag–Te/CuTe bonds. Additionally, dynamic cation disorder decreases lattice thermal conductivity, and an impressive zT ∼ 1.6 at 670 K was obtained in Se doped AgCuTe [52]. Similarly, Sn doping has been used to tune the high hole concentration of Cu2Te and zT ∼ 1.5 has been achieved at 1000 K [53]. A mosaic crystal of Cu2(Te,S) has also been reported with zT ∼ 2.09 at 1000 K [54]. In contrast to p-type electronic transport of Cu2Te and AgCuTe, Ag2Te exhibits n-type conduction. In Ag2Te, increased band conduction in PbTe alloyed Ag2Te resulted in zT ∼ 1 at 550 K in the high temperature superionic phase [55]. In contrast, Sb doping has been shown to be effective in increasing the carrier concentration and electrical conductivity in the low temperature monoclinic phase of Ag2Te and zT ∼ 1.4 has been achieved at 410 K [56].
Chalcopyrites
The I–III–VI2 (I = Ag, Cu; III = Ga, In; VI = Te) semiconductors, with unique electronic and thermal transport properties, are potential high performance thermoelectric materials. These materials have diamond like structures formed by the interconnected (I–VI)4 and (III–VI)4 tetrahedra. Compared to IV–VI semiconductors, these chalcopyrites are wide band gap semiconductors with Eg ∼ 0.8–1.2 eV. Despite having similar crystal structures, all the I–III–VI2 (I = Ag, Cu; III = Ga, In; VI = Te) semiconductors have distinctly different electrical and thermal transport properties [57]. AgGaTe2 and AgInTe2 possess low thermal conductivity; however, their low electrical conductivity renders them unsuitable as high-performance thermoelectric materials. The presence of Ga vacancies in AgGaTe2 greatly improves thermoelectric performance, with zT ∼ 1.02 at 873 K [58]. The related tellurides CuGaTe2 and CuInTe2 possess both high electrical conductivity and high thermal conductivity. Therefore, much effort has been devoted to lowering the lattice thermal conductivity of CuGaTe2 and CuInTe2 via a range of strategies based on inclusions and point defects. For example, the inclusion of nanophase Cu2Se in CuGaTe2 significantly reduces lattice thermal conductivity [59]. Similarly, ZnS nanoscale heterostructures [60] and In2O3 nanoinclusions [61] have been incorporated in CuInTe2 to lower its lattice thermal conductivity. Solid solution alloying of Ag into CuInTe2 lowers lattice thermal conductivity by forming weak Ag–Te bonds, which results in a high zT ∼ 1.6 at 850 K [62]. Multicomponent alloying of Ag and In in CuGaTe2 has also been very successful in lowering the lattice thermal conductivity, yielding zT ∼ 1.64 at 873 K [63].
Concluding remarks
Many metal tellurides materials are used in practical applications because of their high thermoelectric performance. Recent advances in understanding of electronic structure, electrical and thermal transport properties, and sophisticated material processing techniques have led to important improvements in their thermoelectric performance in recent years. Furthermore, a range of novel strategies have been developed, including the precise control of the rhombohedral distortion [34] and Rashba spin splitting in GeTe [35], engineering ferroelectric instability in SnTe [13], and greatly improved material processing techniques for Bi2Te3 based materials [45] for microstructure and nanostructure engineering. Recently, a very high zT ∼ 2.6 at 573 K was achieved in I–IV–VI2 compound AgSbTe2 by inducing nanoscale ordering [64]. Similarly, the novel concept of paramagnon drag in MnTe was employed to improve thermoelectric performance [51]. In recent years, rare earth tellurides like Pr3−x Te4 have also been introduced with high zT ∼ 1.7 at 1200 K [65]. In addition to developing new high performance thermoelectric materials, unique strategies of electronic structure and phonon transport manipulation are necessary to achieve higher thermoelectric performance. Currently, the inferior performance of the counterpart n-type thermoelectric materials is major drawback, which needs to be addressed. Similarly, Te-based oxide thermoelectric materials, which have great potential in practical applications for high resistance against corrosion and thermal degradation, are worthy of attention.
3.2. Skutterudites
Skutterudite thermoelectrics
Pengfei Qiu1,4, Shun Wan2,4 and Lidong Chen1,3
1 State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, People's Republic of China
2 Center for High Pressure Science and Technology Advanced Research (HPSTAR), Shanghai 201203, People's Republic of China
3 Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
4Equally contributed to this work.
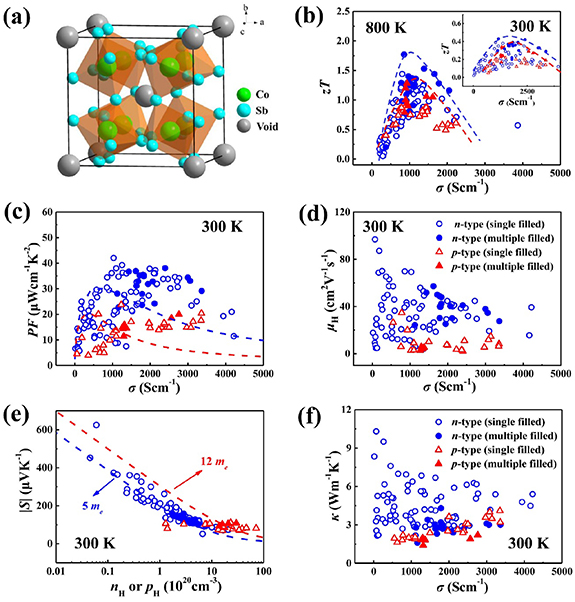
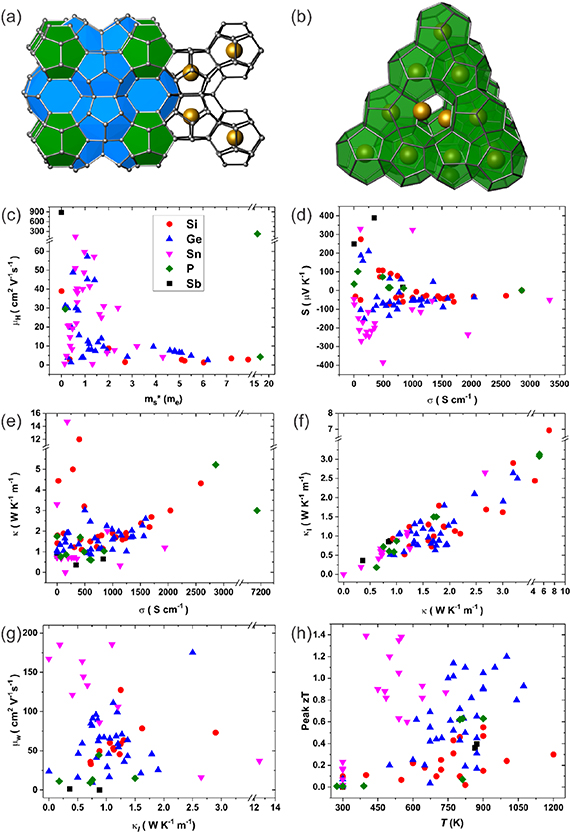
Skutterudites (table 2) are among the best thermoelectric materials for applications in intermediate temperatures [123–125]. Binary skutterudites, with the chemical formula of MX3 (M = Co, Rh, or Ir, X = P, As, or Sb), crystalize in a body-centered cubic structure (Im-3), with the structure shown in figure 3(a). There are two large icosahedral voids per unit (M8X24), which can be filled with guest atoms (e.g. alkalis, alkaline earths, rare earths, and others) forming filled skutterudites. The chemical formula of filled skutterudites can be written as GyM4 X12, where G represents the fillers and y is the filling fraction. The fillers G are weakly bonded with the surrounding atoms with large atomic displacement parameters, which can strongly interrupt the normal transport of phonons by introducing additional localized vibrational modes and therefore significantly lower the lattice thermal conductivity (κL). By the combination of significantly reduced thermal conductivity and (maintaining) good electrical transport, the filled skutterudites well satisfy the PGEC concept proposed by Slack [126, 127].
Figure 3. (a) Crystal structure of binary skutterudites. (b) Electrical conductivity (σ) dependence of dimensionless figure-of-merit (zT) for (filled) skutterudites at 300 K and 800 K. σ dependences of (c) power factor (PF) and (d) Hall mobility (μH) at 300 K. (e) The absolute Seebeck coefficients (|S|) as a function of Hall carrier concentration (nH or pH) at 300 K. (f) σ dependence of thermal conductivity (κ) at 300 K. The dashed lines in (b) are guides to the eyes. The dashed lines in (c) and (e) represent the fitted curves based on the single parabolic model, in which the used μw values are 335 cm2V−1s−1 and 225 cm2V−1s−1, and the used m* values are 5 me and 12 me, for n-type and p-type skutterudites, respectively.
Download figure:
Standard image High-resolution imageTable 2. Skutterudite thermoelectrics properties.
| Material/actual composition | µw (cm2 V−1 s−1) | κ (W m−1 K−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | n or p (1020 cm−3) | Eg (eV) | µH (cm2 V−1 s−1) | ms * (me) |
 r or r or  ( ( 0) 0) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K0.09±0.01 Co4Sb12 (300 K) | 339.5 | 7.0 | 6.6 | −212 | 650 | 0.1 | — | — | — | — | — | [128] |
| K0.09±0.01 Co4Sb12 (800 K) | 39.4 | 4.4 | 3.8 | −193 | 410 | 0.3 | — | — | — | — | — | [128] |
| K0.22±0.01 Co4Sb12 (300 K) | 286.9 | 6.7 | 5.7 | −120 | 1640 | 0.1 | — | — | — | — | — | [128] |
| K0.22±0.01 Co4Sb12 (800 K) | 53.7 | 4.3 | 3.3 | −180 | 650 | 0.4 | — | — | — | — | — | [128] |
| K0.38±0.02 Co4Sb12 (300 K) | 430.9 | 6.3 | 4.7 | −114 | 2660 | 0.2 | — | — | — | — | — | [128] |
| K0.38±0.02 Co4Sb12 (800 K) | 125.8 | 3.9 | 2.0 | −199 | 1220 | 1.0 | — | — | — | — | — | [128] |
| K0.45±0.02 Co4Sb12 (300 K) | 398.2 | 6.2 | 4.5 | −106 | 2730 | 0.2 | — | — | — | — | — | [128] |
| K0.45±0.02 Co4Sb12 (800 K) | 113.7 | 4.1 | 1.9 | −181 | 1360 | 0.9 | — | — | — | — | — | [128] |
| CoSb3 (300 K) | 164.3 | 8.5 | 8.1 | 145 | 690 | 0.1 | — | — | — | — | — | [129] |
| CoSb3 (600 K, peak) | 51.2 | 3.9 | 3.5 | 197 | 330 | 0.2 | — | — | — | — | — | [129] |
| CoSb3 (850 K) | 2.2 | 3.8 | 3.3 | 28 | 310 | — | — | — | — | — | [129] | |
| Na0.13 Co4Sb12 (300 K) | 390.8 | 6.9 | 6.4 | −200 | 860 | 0.2 | 0.8 | — | 69.7 | 2.4 | — | [129] |
| Na0.13 Co4Sb12 (700 K, peak) | 103.6 | 3.8 | 3.2 | −259 | 410 | 0.5 | 0.8 | — | 33.2 | 1.7 | — | [129] |
| Na0.13 Co4Sb12 (850 K) | 37.2 | 3.3 | 2.6 | −200 | 390 | 0.4 | 0.8 | — | 31.6 | 0.9 | — | [129] |
| Na0.23 Co4Sb12 (300 K) | 401.9 | 6.8 | 6.1 | −169 | 1270 | 0.2 | 1.4 | — | 55.8 | 2.8 | — | [129] |
| Na0.23 Co4Sb12 (700 K, peak) | 119.5 | 3.7 | 2.8 | −237 | 610 | 0.7 | 1.4 | — | 26.8 | 2.1 | — | [129] |
| Na0.23 Co4Sb12 (850 K) | 53.4 | 3.7 | 2.7 | −200 | 560 | 0.5 | 1.4 | — | 24.6 | 1.3 | — | [129] |
| Na0.36 Co4Sb12 (300 K) | 415.9 | 6.1 | 4.7 | −117 | 2470 | 0.2 | 4.0 | — | 38.7 | 3.3 | — | [129] |
| Na0.36 Co4Sb12 (850 K) | 99.2 | 3.8 | 1.8 | −190 | 1170 | 1.0 | 4.0 | — | 18.4 | 2.4 | — | [129] |
| Na0.48 Co4Sb12 (300 K) | 431.6 | 5.4 | 3.8 | −111 | 2770 | 0.2 | 5.9 | — | 29.5 | 4.0 | — | [129] |
| Na0.48 Co4Sb12 (850 K) | 119.4 | 3.4 | 1.3 | −203 | 1210 | 1.3 | 5.9 | — | 12.9 | 1.4 | — | [129] |
| Ba0.07 Co4Sb11.88 (300 K) | 228.5 | 6.4 | 5.8 | −139 | 1032 | 0.1 | — | — | — | — | — | [130] |
| Ba0.07 Co4Sb11.88 (850 K) | 49.2 | 4.4 | 3.5 | −192 | 567 | 0.4 | — | — | — | — | — | [130] |
| Ba0.16 Co4Sb11.85 (300 K) | 434.6 | 5.9 | 4.6 | −132 | 2138 | 0.2 | — | — | — | — | — | [130] |
| Ba0.16 Co4Sb11.85 (850 K) | 95.8 | 4.4 | 2.5 | −190 | 1130 | 0.8 | — | — | — | — | — | [130] |
| Ba0.24 Co4Sb11.87 (300 K) | 368.4 | 5.4 | 3.4 | −88 | 3234 | 0.1 | — | — | — | — | — | [130] |
| Ba0.24 Co4Sb11.87 (850 K) | 126 | 4.2 | 1.3 | −178 | 1709 | 1.1 | — | — | — | — | — | [130] |
| Ba0.38 Co4Sb11.74 (300 K) | 342.6 | 4.8 | 2.4 | −70 | 3939 | 0.1 | — | — | — | — | — | [130] |
| Ba0.38 Co4Sb11.74 (850 K) | 92.6 | 3.9 | 0.5 | −137 | 2044 | 0.8 | — | — | — | — | — | [130] |
| Ba0.44 Co4Sb11.90 (300 K) | 537.2 | 6.6 | 2.8 | −68 | 6378 | 0.1 | — | — | — | — | — | [130] |
| Ba0.44 Co4Sb11.90 (850 K) | 115.6 | 5.9 | 0.1 | −114 | 3404 | 0.6 | — | — | — | — | — | [130] |
| Sr0.12 Co4Sb12.46 (300 K) | 195.3 | 5.6 | 5.1 | −137 | 903.6 | 0.1 | 1.3 | — | 43.4 | 1.9 | — | [131] |
| Sr0.12 Co4Sb12.46 (850 K) | 47.2 | 3.8 | 2.7 | −180 | 625 | 0.5 | 1.3 | — | 30 | 1.0 | — | [131] |
| Sr0.17 Co4Sb12.88 (300 K) | 265.4 | 5.4 | 4.6 | −129 | 1355 | 0.1 | 1.6 | — | 52.9 | 2.1 | — | [131] |
| Sr0.17 Co4Sb12.88 (850 K) | 67.9 | 3.3 | 2.0 | −195 | 755 | 0.7 | 1.6 | — | 29.5 | 1.3 | — | [131] |
| Sr0.22 Co4Sb12.72 (300 K) | 309.4 | 5.2 | 4.1 | −119 | 1791 | 0.2 | 2.3 | — | 48.6 | 2.4 | — | [131] |
| Sr0.22 Co4Sb12.72 (850 K) | 73.9 | 3.3 | 1.7 | −179 | 990 | 0.8 | 2.3 | — | 26.9 | 1.5 | — | [131] |
| Sr0.28 Co4Sb12.88 (300 K) | 417.8 | 5.1 | 3.4 | −110 | 2717 | 0.2 | 3.8 | — | 44.6 | 3.0 | — | [131] |
| Sr0.28 Co4Sb12.88 (850 K) | 80 | 3.3 | 1.4 | −176 | 1110 | 0.9 | 3.8 | — | 18.2 | 2.0 | — | [131] |
| Sr0.40 Co4Sb12.54 (300 K) | 266.6 | 5.4 | 2.9 | −52 | 4220 | 0.1 | 6.7 | — | 39.3 | 2.0 | — | [131] |
| Sr0.40 Co4Sb12.54 (850 K) | 78.5 | 4.1 | 0.6 | −123 | 2060 | 0.7 | 6.7 | — | 19.2 | 1.8 | — | [131] |
| Ga0.03 Co4Sb11.985Ga0.015 (300 K) | 137.7 | 3.5 | 3.5 | −286.6 | 111 | 0.1 | 0.2 | — | 29.6 | 2.2 | — | [132] |
| Ga0.03 Co4Sb11.985Ga0.015 (600 K) | 78.2 | 2.4 | 2.3 | −323 | 117 | 0.3 | 0.2 | — | 31.8 | 1.5 | — | [132] |
| Ga0.03 Co4Sb11.985Ga0.015 (650 K) | 37 | 2.6 | 2.4 | −268 | 118 | 0.2 | 0.2 | — | 32 | 0.9 | — | [132] |
| Ga0.06 Co4Sb11.97Ga0.03 (300 K) | 249.9 | 3.4 | 3.3 | −272.6 | 237 | 0.2 | 0.4 | — | 41.1 | 2.7 | — | [132] |
| Ga0.06 Co4Sb11.97Ga0.03 (600 K) | 114.6 | 2.4 | 2.2 | −315 | 188 | 0.5 | 0.4 | — | 32.6 | 1.9 | — | [132] |
| Ga0.06 Co4Sb11.97Ga0.03 (650 K) | 77.2 | 2.6 | 2.3 | −289 | 193 | 0.4 | 0.4 | — | 33.5 | 1.4 | — | [132] |
| Ga0.10 Co4Sb11.95Ga0.05 (300 K) | 281.2 | 3.4 | 3.2 | −228.6 | 444 | 0.2 | 0.4 | — | 63 | 2.1 | — | [132] |
| Ga0.10 Co4Sb11.95Ga0.05 (600 K) | 158 | 2.6 | 2.1 | −285 | 367 | 0.7 | 0.4 | — | 52.1 | 1.7 | — | [132] |
| Ga0.10 Co4Sb11.95Ga0.05 (650 K) | 127.2 | 2.7 | 2.2 | −281 | 349 | 0.7 | 0.4 | — | 49.5 | 1.5 | — | [132] |
| Ga0.15 Co4Sb11.925Ga0.075 (300 K) | 241.3 | 3.2 | 3.0 | −246.4 | 310 | 0.2 | 0.4 | — | 47.2 | 2.4 | — | [132] |
| Ga0.15 Co4Sb11.925Ga0.075 (600 K) | 117.7 | 2.4 | 2.1 | −291 | 255 | 0.5 | 0.4 | — | 38.8 | 1.7 | — | [132] |
| Ga0.15 Co4Sb11.925Ga0.075 (650 K) | 94.7 | 2.5 | 2.2 | −285 | 248 | 0.5 | 0.4 | — | 37.8 | 1.5 | — | [132] |
| In0.075 Co4Sb11.975 (300 K) | 307.1 | 3.7 | 3.5 | −237 | 440 | 0.2 | 0.5 | — | 57.2 | 2.4 | — | [133] |
| In0.075 Co4Sb11.975 (600 K, peak) | 157.8 | 2.5 | 2.1 | −289 | 350 | 0.7 | 0.5 | — | 45.5 | 1.9 | — | [133] |
| In0.075 Co4Sb11.975 (800 K) | 45.3 | 3.4 | 2.9 | −221 | 340 | 0.4 | 0.5 | — | 44.2 | 0.8 | — | [133] |
| In0.15 Co4Sb11.95 (300 K) | 479 | 3.2 | 2.6 | −202 | 1030 | 0.4 | 0.9 | — | 69.1 | 2.8 | — | [133] |
| In0.15 Co4Sb11.95 (700 K, peak) | 144.1 | 2.5 | 1.7 | −259 | 570 | 1.1 | 0.9 | — | 38.3 | 1.9 | — | [133] |
| In0.15 Co4Sb11.95 (800 K) | 96.2 | 2.8 | 1.9 | −243 | 560 | 1.0 | 0.9 | — | 37.6 | 1.5 | — | [133] |
| In0.225 Co4Sb11.925 (300 K) | 384.8 | 3.0 | 2.3 | −173 | 1160 | 0.4 | 2.4 | — | 30.8 | 4.0 | — | [133] |
| In0.225 Co4Sb11.925 (700 K, peak) | 139.5 | 2.5 | 1.6 | −241 | 680 | 1.1 | 2.4 | — | 18.1 | 3.1 | — | [133] |
| In0.225 Co4Sb11.925 (800 K) | 99.1 | 2.8 | 1.8 | −234 | 640 | 1.0 | 2.4 | — | 17 | 2.6 | — | [133] |
| In0.30 Co4Sb11.90 (300 K) | 399.3 | 2.9 | 2.0 | −162 | 1370 | 0.4 | 1.7 | — | 49.1 | 3.0 | — | [133] |
| In0.30 Co4Sb11.90 (750 K, peak) | 136.2 | 2.7 | 1.6 | −236 | 780 | 1.2 | 1.7 | — | 28 | 2.3 | — | [133] |
| In0.30 Co4Sb11.90 (800 K) | 117.8 | 2.8 | 1.6 | −233 | 770 | 1.2 | 1.7 | — | 27.6 | 2.1 | — | [133] |
| p-type CoSb3 (300 K)-121OB22 | 66.2 | — | — | 280 | 57.6 | — | — | From resistivity: 0.75 eV From Hall: 0.63 eV | 2996.3 | 0.1 | — | [134] |
| p-type CoSb3-121OB22 | 0.9 | — | — | 10 (890 K) | 400 (930 K) | — | — | — | — | — | [134] | |
| p-type CoSb3 (300 K)-10OB22 | 136.3 | — | — | 240 | 188.6 | — | — | 2675.6 | 0.1 | — | [134] | |
| p-type CoSb3-10OB22 | 0.9 | — | — | 10 (890 K) | 400 (930 K) | — | — | — | — | — | [134] | |
| p-type CoSb3 (300 K)-23NB12 | 222.9 | — | — | 179 | 626.6 | — | — | 2590.3 | 0.2 | — | [134] | |
| p-type CoSb3-23NB12 | — | — | 400 (930 K) | — | — | — | — | — | [134] | |||
| p-type CoSb3 (300 K)-2NB13 | 272.7 | — | — | 139 | 1231.5 | — | — | 1976.2 | 0.2 | — | [134] | |
| p-type CoSb3-2NB13 | 8.5 | — | — | 85 (890 K) | 400 (930 K) | — | — | — | — | — | [134] | |
| p-type CoSb3 (300 K)-2NB9 | 234.8 | — | — | 80 | 2314.8 | — | 0.1 | 1529 | 0.2 | — | [134] | |
| p-type CoSb3-2NB9 | 1.1 | — | — | 10 (890 K) | 476 (850 K) | — | 0.1 | — | — | — | [134] | |
| n-type CoSb3 (300 K)-1CS10-0.08 at.% Te | 593.7 | 10.3 | 10.3 | −452 | 70.42 | 0.04 | 0.05 | 0.55 eV | 96.8 | 2.8 | — | [134] |
| n-type CoSb3-1CS10-0.08 at.% Te | 4.6 | 5.3 (760 K) | — | 46 (800 K) | 358 (800 K) | — | 0.05 | — | — | — | [134] | |
| n-type CoSb3 (300 K)-1CS11-0.15 at.% Te | 606 | — | — | −373 | 179.53 | — | 0.1 | 87.1 | 3.0 | — | [134] | |
| n-type CoSb3-1CS11-0.15 at.% Te | 4.1 | — | — | 44 (800 K) | 334.45 (800 K) | — | 0.1 | — | — | — | [134] | |
| n-type CoSb3 (300 K)-2CS9-0.12 at.%Te | 507.7 | — | — | −364 | 166.94 | — | 0.2 | 68.6 | 3.1 | — | [134] | |
| n-type CoSb3-2CS9-0.12 at.%Te | 4.1 | — | — | 38 (800 K) | 393.7 (800 K) | — | 0.2 | — | — | — | [134] | |
| n-type CoSb3 (300 K)-4OB25-0.6 at.% Pd | 302.4 | 9.5 | 9.3 | −280 | 263.16 | 0.1 | 0.4 | 37.2 | 3.3 | — | [134] | |
| n-type CoSb3-4OB25-0.6 at.% Pd | 11 | 4.9 (770 K) | — | −103 (780 K) | 330 (800 K) | — | 0.4 | — | — | — | [134] | |
| n-type CoSb3 (300 K)-OB26 ∼1 at.% Pd | 327.3 | 6.1 | 5.6 | −180 | 909.09 | 0.1 | 1.4 | 41.1 | 3.0 | — | [134] | |
| n-type CoSb3-OB26 ∼1 at.% Pd | 69.6 | 4.1 (770 K) | — | −207 (770 K) | 581 (800 K) | — | 1.4 | — | — | — | [134] | |
| CoSb3 (300 K) | 1064 | 8.3 | 8.3 | −625 | 17 | 0.02 | 0.1 | — | 17.7 | 12.8 | — | [135] |
| CoSb3 (600 K, peak) | 31 | 4.2 | 4.0 | 205 | 182 | 0.1 | 0.1 | — | 189.3 | 0.2 | — | [135] |
| CoSb3 (850 K) | 4.1 | 4.7 | 4.2 | 56 | 284 | 0.02 | 0.1 | — | 295.5 | 0.0 | — | [135] |
| Co4Sb11.95Te0.05 (300 K) | 191.1 | 7.6 | 7.3 | −194 | 451 | 0.1 | 0.9 | — | 30.9 | 2.6 | — | [135] |
| Co4Sb11.95Te0.05 (600 K, peak) | 159.3 | 4.0 | 3.7 | −310 | 277 | 0.4 | 0.9 | — | 19 | 3.4 | — | [135] |
| Co4Sb11.95Te0.05 (850 K) | 26.1 | 5.0 | 4.6 | −218 | 222 | 0.2 | 0.9 | — | 15.2 | 1.1 | — | [135] |
| Co4Sb11.7Te0.3 (300 K) | 129.1 | 5.1 | 4.8 | −148 | 523 | 0.1 | 1.8 | — | 18 | 2.7 | — | [135] |
| Co4Sb11.7Te0.3 (850 K) | 45.1 | 3.9 | 3.3 | −231 | 330 | 0.4 | 1.8 | — | 11.4 | 2.0 | — | [135] |
| Co4Sb11.5Te0.5 (300 K) | 182.5 | 3.9 | 3.3 | −123 | 1004 | 0.1 | 5.7 | — | 11 | 4.5 | — | [135] |
| Co4Sb11.5Te0.5 (850 K) | 62.7 | 3.1 | 2.0 | −200 | 658 | 0.7 | 5.7 | — | 7.2 | 3.3 | — | [135] |
| Co0.98Ni0.02Sb3 (300 K) | 184.8 | — | — | −243 | 247 | — | 0.7 | — | 22 | 3.3 | — | [136] |
| Co0.98Ni0.02Sb3 (800 K) | 39.2 | — | — | −203 | 363 | — | 0.7 | — | 32.4 | 0.9 | — | [136] |
| Co0.97Ni0.03Sb3 (300 K) | 176 | — | — | −207 | 357 | — | 1.5 | — | 14.9 | 4.0 | — | [136] |
| Co0.97Ni0.03Sb3 (800 K) | 58.9 | — | — | −217 | 463 | — | 1.5 | — | 19.3 | 1.6 | — | [136] |
| Co0.955Ni0.045Sb3 (300 K) | 122.6 | — | — | −144 | 521 | — | 2.5 | — | 13 | 3.2 | — | [136] |
| Co0.955Ni0.045Sb3 (800 K) | 81.6 | — | — | −225 | 585 | — | 2.5 | — | 14.6 | 2.5 | — | [136] |
| Co0.94Ni0.06Sb3 (300 K) | 137.5 | — | — | −133 | 668 | — | 3.4 | — | 12.3 | 3.5 | — | [136] |
| Co0.94Ni0.06Sb3 (800 K) | 85.6 | — | — | −210 (700 K) | 730 | — | 3.4 | — | 13.4 | — | [136] | |
| Co0.925Ni0.075Sb3 (300 K) | 135.8 | — | — | −113 | 849 | — | 4.4 | — | 12 | 3.4 | — | [136] |
| Co0.925Ni0.075Sb3 (800 K) | 64.7 | — | — | −180 | 783 | — | 4.4 | — | 11.1 | 2.4 | — | [136] |
| Co0.91Ni0.09Sb3 (300 K) | 135.6 | — | — | −101 | 994 | — | 5.5 | — | 11.3 | 3.5 | — | [136] |
| Co0.91Ni0.09Sb3 (800 K) | 82.7 | — | — | −180 (700 K) | 1000 | — | 5.5 | — | 11.3 | — | [136] | |
| Co0.88Ni0.12Sb3 (300 K) | 129.4 | — | — | −88 | 1136 | — | 12.0 | — | 5.9 | 5.0 | — | [136] |
| Co0.88Ni0.12Sb3 (800 K) | 78.9 | — | — | −167 | 1111 | — | 12.0 | — | 5.8 | 4.2 | — | [136] |
| Co0.85Ni0.15Sb3 (300 K) | 126.3 | — | — | −74 | 1364 | — | 20.9 | — | 4.1 | 6.0 | — | [136] |
| Co0.85Ni0.15Sb3 (800 K) | 75.3 | — | — | −151 | 1282 | — | 20.9 | — | 3.8 | 5.3 | — | [136] |
| Yb0.066 Co4Sb12 (300 K) | 185.3 | 5.1 | 4.8 | −186 | 480 | 0.1 | — | — | — | — | — | [137] |
| Yb0.066 Co4Sb12 | 148.1 | — | — | −259 (600 K) | 465 (700 K) | 0.43 (600 K) | — | — | — | — | — | [137] |
| Yb0.19 Co4Sb12 (300 K) | 372.1 | 4.1 | 2.9 | −141 | 1640 | 0.3 | — | — | — | — | — | [137] |
| Yb0.19 Co4Sb12 (640 K) | 158 | — | — | −216 (640 K) | 900 (640 K) | 1.2 | — | — | — | — | — | [137] |
| Yb0.3 Co4Sb12 (300 K) | 434.5 | 3.0 | 1.9 | −138 | 1986 | 0.4 | 2.8 | — | 44.6 | 3.2 | — | [138] |
| Yb0.3 Co4Sb12 (823 K) | 109.7 | 3.3 | 1.7 | −199 | 1110 | 1.1 | 2.8 | — | 24.9 | 2.1 | — | [138] |
| Yb0.35 Co4Sb12 (300 K) | 422.6 | 2.9 | 1.8 | −130 | 2131 | 0.4 | 3.3 | — | 40.1 | 3.4 | — | [138] |
| Yb0.35 Co4Sb12 (823 K) | 118 | 3.3 | 1.4 | −193 | 1280 | 1.2 | 3.3 | — | 24.1 | 2.2 | — | [138] |
| Yb0.40 Co4Sb12 (300 K) | 410.6 | 2.9 | 1.6 | −120 | 2347 | 0.4 | 3.8 | — | 38.5 | 3.3 | — | [138] |
| Yb0.40 Co4Sb12 (823 K) | 115.7 | 3.5 | 1.4 | −183 | 1410 | 1.1 | 3.8 | — | 23.1 | 2.2 | — | [138] |
| Yb0.5 Co4Sb12 (300 K) | 385.5 | 2.9 | 1.6 | −109 | 2540 | 0.3 | 5.0 | — | 31.8 | 3.5 | — | [138] |
| Yb0.5 Co4Sb12 (823 K) | 106.4 | 3.7 | 1.4 | −170 | 1510 | 1.0 | 5.0 | — | 18.9 | 2.4 | — | [138] |
| Eu0.03 Co4Sb12 (300 K) | 197.1 | 6.2 | 6.0 | −235 | 289 | 0.1 | — | — | — | — | — | [139] |
| Eu0.03 Co4Sb12 (850 K) | 16.3 | 4.1 | 3.5 | −146 | 323 | 0.1 | — | — | — | — | — | [139] |
| Eu0.10 Co4Sb12 (300 K) | 167.2 | 4.1 | 3.8 | −171 | 516 | 0.1 | 1.4 | — | 23 | 2.8 | — | [139] |
| Eu0.10 Co4Sb12 (850 K) | 58 | 3.0 | 2.2 | −217 | 500 | 0.7 | 1.4 | — | 22.3 | 1.5 | — | [139] |
| Eu0.19 Co4Sb12 (300 K) | 260.1 | 4.2 | 3.3 | −118 | 1525 | 0.2 | 4.0 | — | 23.7 | 3.4 | — | [139] |
| Eu0.19 Co4Sb12 (850 K) | 76.6 | 3.4 | 1.9 | −191 | 893 | 0.8 | 4.0 | — | 13.9 | 2.4 | — | [139] |
| Eu0.27 Co4Sb12 (300 K) | 347.7 | 4.2 | 2.3 | −87 | 3096 | 0.2 | 10.1 | — | 19.1 | 4.4 | — | [139] |
| Eu0.27 Co4Sb12 (850 K) | 87.4 | 3.6 | 1.2 | −163 | 1414 | 0.9 | 10.1 | — | 8.7 | 3.4 | — | [139] |
| Eu0.34 Co4Sb12 (300 K) | 367.8 | 4.5 | 2.0 | −71 | 4162 | 0.1 | 16.7 | — | 15.6 | 4.9 | — | [139] |
| Eu0.34 Co4Sb12 (850 K) | 98.8 | 4.1 | 0.8 | −148 | 1910 | 0.9 | 16.7 | — | 7.1 | 4.2 | — | [139] |
| Ce0.14 Co4Sb12 (300 K) | 384.5 | 3.7 | 2.4 | −121 | 2170 | 0.3 | 3.3 | — | 41 | 3.1 | — | [140] |
| Ce0.14 Co4Sb12 (850 K) | 121.2 | 3.4 | 1.1 | −196 | 1333 | 1.3 | 3.3 | — | 25.2 | 2.2 | — | [140] |
| Ce0.14 Co4Sb12 (300 K) | 386.3 | 3.5 | 2.2 | −121 | 2180 | 0.3 | 4.8 | — | 28.3 | 3.9 | — | [140] |
| Ce0.14 Co4Sb12 (850 K) | 115.7 | 3.2 | 0.9 | −192 | 1333 | 1.3 | 4.8 | — | 17.3 | 2.7 | — | [140] |
| Ce0.16 Co4Sb12 (300 K) | 334.3 | 3.4 | 2.2 | −118 | 1960 | 0.2 | 4.2 | — | 29.1 | 3.5 | — | [140] |
| Ce0.16 Co4Sb12 (850 K) | 101.2 | 3.2 | 1.0 | −184 | 1280 | 1.2 | 4.2 | — | 19 | 2.3 | — | [140] |
| Ce0.15 Co4Sb12 (300 K) | 261.7 | 3.3 | 2.4 | −119 | 1515 | 0.2 | 4.6 | — | 20.5 | 3.7 | — | [140] |
| Ce0.15 Co4Sb12 (850 K) | 102.2 | 3.0 | 1.1 | −194 | 1150 | 1.2 | 4.6 | — | 15.6 | 2.7 | — | [140] |
| Yb0.20 Co4Sb12 (300 K) | 330 | 3.0 | 2.1 | −139 | 1490 | 0.3 | — | — | [140] | |||
| Yb0.20 Co4Sb12 (850 K) | 101.4 | 3.0 | 1.3 | −208 | 970 | 1.2 | — | — | [140] | |||
| Dy0.02 Co4Sb12 (300 K) | 257.9 | 5.3 | 5.2 | −268 | 258 | 0.1 | 0.2 | — | 70 | 1.9 | — | [141] |
| Dy0.02 Co4Sb12 (550 K, peak) | 136.6 | 3.7 | 3.4 | −303 | 226 | 0.3 | 0.2 | — | 61.3 | 1.4 | — | [141] |
| Dy0.02 Co4Sb12 (800 K) | 14.9 | 4.4 | 3.9 | −131 | 323 | 0.1 | 0.2 | — | 87.7 | 0.2 | — | [141] |
| Tb0.03 Co4Sb12 (300 K) | 270.7 | 4.8 | 4.6 | −242 | 366 | 0.1 | 0.4 | — | 65.3 | 2.1 | — | [141] |
| Tb0.03 Co4Sb12 (600 K, peak) | 122.3 | 3.3 | 2.9 | −280 | 301 | 0.4 | 0.4 | — | 53.7 | 1.4 | — | [141] |
| Tb0.03 Co4Sb12 (800 K) | 30.5 | 4.0 | 3.4 | −176 | 387 | 0.2 | 0.4 | — | 69 | 0.4 | — | [141] |
| Gd0.04 Co4Sb12 (300 K) | 336.7 | 4.4 | 4.1 | −226 | 548 | 0.2 | 0.6 | — | 57 | 2.6 | — | [141] |
| Gd0.04 Co4Sb12 (650 K, peak) | 133.5 | 3.3 | 2.7 | −265 | 441 | 0.6 | 0.6 | — | 45.9 | 1.6 | — | [141] |
| Gd0.04 Co4Sb12 (800 K) | 62.2 | 3.8 | 3.1 | −222 | 462 | 0.5 | 0.6 | — | 48.1 | 0.9 | — | [141] |
| Nd0.15 Co4Sb12 (300 K) | 334.2 | 3.0 | 2.1 | −133 | 1624 | 0.3 | 3.8 | — | 26.5 | 3.8 | — | [141] |
| Nd0.15 Co4Sb12 (800 K, peak) | 109.3 | 2.9 | 1.1 | −195 | 1110 | 1.2 | 3.8 | — | 18.1 | 2.5 | — | [141] |
| Sm0.15 Co4Sb12 (300 K) | 421.7 | 3.6 | 2.3 | −131 | 2100 | 0.3 | 3.1 | — | 41.7 | 3.3 | — | [141] |
| Sm0.15 Co4Sb12 (800 K, peak) | 121.3 | 2.9 | 1.1 | −204 | 1110 | 1.3 | 3.1 | — | 22.1 | 2.4 | — | [141] |
| Ba0.03 Co4Sb12.05 (300 K) | 228.7 | 5.4 | 5.1 | −220 | 399 | 0.1 | 0.5 | — | 49.8 | 2.2 | — | [142] |
| Ba0.03 Co4Sb12.05 (800 K) | 24.5 | 3.8 | 3.3 | −161 | 370 | 0.2 | 0.5 | — | 46.2 | 0.5 | — | [142] |
| Ba0.15Yb0.01 Co4Sb12.08 (300 K) | 333.4 | 4.3 | 3.0 | −125 | 1789 | 0.2 | 2.8 | — | 39.9 | 2.9 | — | [142] |
| Ba0.15Yb0.01 Co4Sb12.08 (800 K) | 92.9 | 3.6 | 2.0 | −190 | 1000 | 0.8 | 2.8 | — | 22.3 | 2.0 | — | [142] |
| Ba0.11Yb0.03 Co4Sb12.07 (300 K) | 293.3 | 3.2 | 1.9 | −115 | 1787 | 0.2 | 3.7 | — | 30.1 | 3.1 | — | [142] |
| Ba0.11Yb0.03 Co4Sb12.07 (800 K) | 89.9 | 3.1 | 1.3 | −179 | 1100 | 0.9 | 3.7 | — | 18.6 | 2.2 | — | [142] |
| Yb0.12 Co4Sb12.11 (300 K) | 184.3 | 2.7 | 2.2 | −146 | 765 | 0.2 | 2.3 | — | 20.8 | 3.1 | — | [142] |
| Yb0.12 Co4Sb12.11 (800 K) | 89.5 | 2.7 | 1.6 | −220 | 680 | 1.0 | 2.3 | — | 18.5 | 2.2 | — | [142] |
| Ba0.05Yb0.09 Co4Sb12.13 (300 K) | 313 | 2.8 | 2.0 | −158 | 1126 | 0.3 | 2.9 | — | 24.2 | 4.0 | — | [142] |
| Ba0.05Yb0.09 Co4Sb12.13 (800 K) | 119.4 | 2.6 | 1.4 | −233 | 780 | 1.3 | 2.9 | — | 16.8 | 2.9 | — | [142] |
| Ba0.08Yb0.09 Co4Sb12.12 (300 K) | 390.2 | 2.5 | 1.0 | −126 | 2068 | 0.4 | 3.2 | — | 40.3 | 3.2 | — | [142] |
| Ba0.08Yb0.09 Co4Sb12.12 (800 K) | 107.7 | 2.5 | 0.6 | −190 | 1160 | 1.4 | 3.2 | — | 22.6 | 2.2 | — | [142] |
| Ba0.11Yb0.08 Co4Sb12.08 (300 K) | 312.5 | 2.4 | 0.9 | −107 | 2114 | 0.3 | 4.4 | — | 30 | 3.2 | — | [142] |
| Ba0.11Yb0.08 Co4Sb12.08 (800 K) | 89.4 | 2.3 | 0.5 | −177 | 1120 | 1.2 | 4.4 | — | 15.9 | 2.4 | — | [142] |
| Ba0.25 Co4Sb11.91 (300 K) | 312.4 | 5.6 | 4.9 | −145 | 1312 | 0.2 | 2.0 | — | 40.3 | 2.8 | — | [143] |
| Ba0.25 Co4Sb11.91 (850 K) | 67.3 | 4.5 | 3.2 | −192 | 775 | 0.5 | 2.0 | — | 23.8 | 1.5 | — | [143] |
| Ba0.21In0.04 Co4Sb11.93 (300 K) | 408.2 | 3.4 | 2.5 | −160 | 1434 | 0.3 | 1.7 | — | 51.7 | 2.9 | — | [143] |
| Ba0.21In0.04 Co4Sb11.93 (850 K) | 83.8 | 2.9 | 1.6 | −209 | 792 | 1.0 | 1.7 | — | 28.6 | 1.6 | — | [143] |
| Ba0.19In0.07 Co4Sb11.85 (300 K) | 414.2 | 3.0 | 2.0 | −151 | 1619 | 0.4 | 1.8 | — | 57.1 | 2.7 | — | [143] |
| Ba0.19In0.07 Co4Sb11.85 (850 K) | 88.1 | 2.8 | 1.2 | −199 | 935 | 1.1 | 1.8 | — | 33 | 1.5 | — | [143] |
| Ba0.16In0.12 Co4Sb11.85 (300 K) | 400 | 2.8 | 1.8 | −143 | 1721 | 0.4 | 2.2 | — | 50 | 2.9 | — | [143] |
| Ba0.16In0.12 Co4Sb11.85 (850 K) | 86.5 | 2.6 | 1.0 | −194 | 973 | 1.2 | 2.2 | — | 28.2 | 1.6 | — | [143] |
| Ba0.15In0.16 Co4Sb11.83 (300 K) | 405 | 2.7 | 1.6 | −139 | 1829 | 0.4 | 2.7 | — | 42.3 | 3.2 | — | [143] |
| Ba0.15In0.16 Co4Sb11.83 (850 K) | 94.2 | 2.5 | 0.9 | −200 | 989 | 1.3 | 2.7 | — | 22.9 | 2.0 | — | [143] |
| Ba0.15In0.20 Co4Sb11.84 (300 K) | 405.7 | 2.8 | 1.8 | −140 | 1810 | 0.4 | 2.9 | — | 39.6 | 3.4 | — | [143] |
| Ba0.15In0.20 Co4Sb11.84 (850 K) | 90.9 | 2.8 | 1.1 | −198 | 977 | 1.2 | 2.9 | — | 21.4 | 2.0 | — | [143] |
| Ba0.14In0.23 Co4Sb11.84 (300 K) | 376.1 | 2.6 | 1.5 | −128 | 1944 | 0.4 | 4.8 | — | 25.2 | 4.2 | — | [143] |
| Ba0.14In0.23 Co4Sb11.84 (850 K) | 95 | 2.6 | 0.7 | −190 | 1120 | 1.3 | 4.8 | — | 14.5 | 2.7 | — | [143] |
| Ba0.06La0.05Yb0.06 Co4Sb12 (300 K) | 400.8 | 3.0 | 1.9 | −138 | 1832 | 0.4 | 2.4 | — | 47.8 | 2.9 | — | [144] |
| Ba0.06La0.05Yb0.06 Co4Sb12 (850 K) | 113.2 | 2.9 | 1.1 | −211 | 1046 | 1.4 | 2.4 | — | 27.3 | 2.0 | — | [144] |
| Ba0.08La0.05Yb0.08 Co4Sb12 (300 K) | 452.5 | 2.7 | 1.3 | −126 | 2398 | 0.4 | 3.7 | — | 40.9 | 3.5 | — | [144] |
| Ba0.08La0.05Yb0.08 Co4Sb12 (850 K) | 125.1 | 2.6 | 0.4 | −198 | 1344 | 1.7 | 3.7 | — | 22.9 | 2.4 | — | [144] |
| Ba0.10La0.05Yb0.10 Co4Sb12 (300 K) | 443.5 | 3.1 | 1.3 | −107 | 3000 | 0.3 | 5.0 | 37.8 | 3.4 | [144] | ||
| Ba0.10La0.05Yb0.10 Co4Sb12 (850 K) | 130.5 | 3.0 | 0.3 | −185 | 1631 | 1.6 | 5.0 | — | 20.6 | 2.6 | — | [144] |
| Ba0.10La0.05Yb0.15 Co4Sb12 (300 K) | 434.3 | 3.0 | 1.2 | −104 | 3058 | 0.3 | 5.5 | — | 34.6 | 3.6 | — | [144] |
| Ba0.10La0.05Yb0.15 Co4Sb12 (850 K) | 120.7 | 3.2 | 0.3 | −174 | 1715 | 1.4 | 5.5 | — | 19.4 | 2.5 | — | [144] |
| Ba0.10La0.05Yb0.20 Co4Sb12 (300 K) | 411.6 | 3.0 | 1.0 | −93 | 3367 | 0.3 | 7.6 | — | 27.5 | 3.9 | — | [144] |
| Ba0.10La0.05Yb0.20 Co4Sb12 (850 K) | 121.3 | 3.4 | 0.1 | −164 | 1938 | 1.3 | 7.6 | — | 15.8 | 2.9 | — | [144] |
| Co4Sb11.46Te0.43 (300 K) | 293.9 | 4.9 | 3.9 | −120 | 1680 | 0.2 | 5.6 | — | 18.5 | 4.3 | — | [145] |
| Co4Sb11.46Te0.43 (850 K) | 102.6 | 3.7 | 2.0 | −203 | 1040 | 1.0 | 5.6 | — | 11.5 | 3.3 | — | [145] |
| S0.26 Co4Sb11.11Te0.73 (300 K) | 239.9 | 2.2 | 1.5 | −137 | 1110 | 0.3 | 3.1 | — | 22.1 | 3.5 | — | [145] |
| S0.26 Co4Sb11.11Te0.73 (850 K) | 98.5 | 2.2 | 0.9 | −221 | 810 | 1.5 | 3.1 | — | 16.3 | 2.6 | — | [145] |
| Se0.17 Co4Sb11.31Te0.53 (300 K) | 243.4 | 3.0 | 2.4 | −142 | 1060 | 0.2 | 3.2 | — | 20.4 | 3.7 | — | [145] |
| Se0.17 Co4Sb11.31Te0.53 (850 K) | 91.3 | 2.5 | 1.3 | −220 | 760 | 1.2 | 3.2 | — | 14.7 | 2.6 | — | [145] |
| Br0.16 Co4Sb11.34Te0.52 (300 K) | 288.2 | 4.2 | 3.4 | −136 | 1350 | 0.2 | 3.7 | — | 22.5 | 3.8 | — | [145] |
| Br0.16 Co4Sb11.34Te0.52 (850 K) | 94 | 3.2 | 1.6 | −207 | 910 | 1.1 | 3.7 | — | 15.2 | 2.6 | — | [145] |
| S0.18 Co3.4Ni0.58Sb11.94 (300 K) | 126.6 | 2.9 | 2.2 | −90 | 1080 | 0.1 | 16.0 | — | 4.2 | 6.2 | — | [145] |
| S0.18 Co3.4Ni0.58Sb11.94 (850 K) | 67.3 | 3.4 | 1.7 | −171 | 990 | 0.7 | 16.0 | — | 3.8 | 5.0 | — | [145] |
| Se0.03 Co4Sb11.94Se0.06 (300 K) | 305.5 | 5.3 | 5.2 | −361 | 104 | 0.1 | 0.3 | 0.3 | 24.7 | 4.4 | — | [146] |
| Se0.03 Co4Sb11.94Se0.06 (600 K, peak) | 100.2 | 3.4 | 3.2 | −334 | 132 | 0.3 | 0.3 | — | 31.3 | 1.8 | — | [146] |
| Se0.03 Co4Sb11.94Se0.06 (800 K) | 12.8 | 4.1 | 3.7 | −152 | 215 | 0.1 | 0.3 | — | 51 | 0.3 | — | [146] |
| Se0.05 Co4Sb11.9Se0.1 (300 K) | 222.1 | 4.1 | 4.1 | −354 | 82 | 0.1 | 0.4 | 0.4 | 14.1 | 5.1 | — | [146] |
| Se0.05 Co4Sb11.9Se0.1 (600 K, peak) | 115.9 | 2.9 | 2.8 | −354 | 121 | 0.3 | 0.4 | — | 20.9 | 2.6 | — | [146] |
| Se0.05 Co4Sb11.9Se0.1 (800 K) | 16.5 | 3.5 | 3.1 | −172 | 219 | 0.2 | 0.4 | — | 37.8 | 0.4 | — | [146] |
| Se0.1 Co4Sb11.8Se0.2 (300 K) | 128.7 | 3.3 | 3.2 | −327 | 65 | 0.1 | 0.4 | 0.4 | 9.2 | 4.7 | — | [146] |
| Se0.1 Co4Sb11.8Se0.2 (600 K, peak) | 68 | 2.4 | 2.3 | −328 | 96 | 0.3 | 0.4 | — | 13.6 | 2.4 | — | [146] |
| Se0.1 Co4Sb11.8Se0.2 (800 K) | 15.8 | 3.0 | 2.7 | −175 | 202 | 0.2 | 0.4 | — | 28.7 | 0.5 | — | [146] |
| Se0.15 Co4Sb11.7Se0.3 (300 K) | 111.5 | 2.5 | 2.5 | −300 | 77 | 0.1 | 0.7 | 0.7 | 7.1 | 5.1 | — | [146] |
| Se0.15 Co4Sb11.7Se0.3 (600 K, peak) | 82.1 | 2.0 | 1.9 | −339 | 102 | 0.4 | 0.7 | — | 9.4 | 3.5 | — | [146] |
| Se0.15 Co4Sb11.7Se0.3 (800 K) | 24.9 | 2.6 | 2.2 | −214 | 203 | 0.3 | 0.7 | — | 18.8 | 0.9 | — | [146] |
| Se0.2 Co4Sb11.6Se0.4 (300 K) | 87.7 | 2.2 | 2.1 | −262 | 94 | 0.1 | 1.2 | 0.4 | 4.8 | 5.5 | — | [146] |
| Se0.2 Co4Sb11.6Se0.4 (600 K, peak) | 77.1 | 1.9 | 1.8 | −324 | 114 | 0.4 | 1.2 | — | 5.8 | 4.6 | — | [146] |
| Se0.2 Co4Sb11.6Se0.4 (800 K) | 27.6 | 2.6 | 2.2 | −218 | 215 | 0.3 | 1.2 | — | 11 | 1.4 | — | [146] |
| Se0.3 Co4Sb11.4Se0.6 (300 K) | 66.6 | 2.2 | 2.1 | −213 | 126 | 0.1 | 1.6 | 0.3 | 4.8 | 4.5 | — | [146] |
| Se0.3 Co4Sb11.4Se0.6 (750 K, peak) | 36.1 | 2.3 | 1.9 | −233 | 214 | 0.4 | 1.6 | — | 8.2 | 2.1 | — | [146] |
| Se0.3 Co4Sb11.4Se0.6 (800 K) | 30.7 | 2.5 | 2.1 | −212 | 256 | 0.4 | 1.6 | — | 9.8 | 1.7 | — | [146] |
| Pd0.20(±0.03) Co3.80Sb12.01(±0.07) (300 K) | 435.2 | 5.6 | 4.9 | −177 | 1252 | 0.2 | 2.1 | 0.3 | 38.1 | 3.8 | — | [147] |
| Pd0.20(±0.03) Co3.80Sb12.01(±0.07) (700 K) | 126.1 | 4.2 | 3.0 | −217 | 812 | 0.6 | 2.1 | — | 24.7 | 2.3 | — | [147] |
| Pd0.20(±0.03) Co3.80Sb12.01(±0.07) (850 K) | 77 | 4.6 | 3.3 | −203 | 781 | 0.6 | 2.1 | — | 23.8 | 1.7 | — | [147] |
| S0.02(±0.01)Pd0.20(±0.02) Co3.80Sb12.06(±0.07) (300 K) | 423.9 | 4.1 | 3.4 | −186 | 1098 | 0.3 | 1.6 | 0.3 | 43.4 | 3.5 | — | [147] |
| S0.02(±0.01)Pd0.20(±0.02) Co3.80Sb12.06(±0.07) (700 K) | 133.9 | 3.5 | 2.5 | −233 | 716 | 0.8 | 1.6 | — | 28.3 | 2.2 | — | [147] |
| S0.02(±0.01)Pd0.20(±0.02) Co3.80Sb12.06(±0.07) (850 K) | 78.9 | 4.0 | 2.8 | −214 | 704 | 0.7 | 1.6 | — | 27.8 | 1.6 | — | [147] |
| S0.04(±0.01)Pd0.20(±0.01) Co3.80Sb11.99(±0.08) (300 K) | 341 | 3.7 | 3.2 | −189 | 853 | 0.3 | 1.4 | 0.3 | 39.2 | 3.2 | — | [147] |
| S0.04(±0.01)Pd0.20(±0.01) Co3.80Sb11.99(±0.08) (700 K) | 123.2 | 3.3 | 2.4 | −244 | 580 | 0.7 | 1.4 | — | 26.6 | 2.2 | — | [147] |
| S0.04(±0.01)Pd0.20(±0.01) Co3.80Sb11.99(±0.08) (850 K) | 67.6 | 3.8 | 2.8 | −216 | 589 | 0.6 | 1.4 | — | 27 | 1.4 | — | [147] |
| S0.08(±0.01)Pd0.20(±0.02) Co3.80Sb12.04(±0.11) (300 K) | 310.3 | 3.3 | 2.9 | −212 | 594 | 0.2 | 1.0 | 0.3 | 36.4 | 3.3 | — | [147] |
| S0.08(±0.01)Pd0.20(±0.02) Co3.80Sb12.04(±0.11) (600 K) | 144.8 | 2.9 | 2.4 | −262 | 439 | 0.6 | 1.0 | — | 26.9 | 2.5 | — | [147] |
| S0.08(±0.01)Pd0.20(±0.02) Co3.80Sb12.04(±0.11) (850 K) | 52.7 | 3.6 | 2.8 | −213 | 476 | 0.5 | 1.0 | — | 29.1 | 1.2 | — | [147] |
| S0.05(±0.01)Pd0.15(±0.01) Co3.85Sb12.03(±0.14) (300 K) | 356.4 | 3.7 | 3.4 | −226 | 580 | 0.2 | 0.8 | 0.3 | 45.8 | 3.1 | — | [147] |
| S0.05(±0.01)Pd0.15(±0.01) Co3.85Sb12.03(±0.14) (600 K) | 162.5 | 3.1 | 2.6 | −276 | 419 | 0.6 | 0.8 | — | 33.1 | 2.3 | — | [147] |
| S0.05(±0.01)Pd0.15(±0.01) Co3.85Sb12.03(±0.14) (850 K) | 44.5 | 3.9 | 3.1 | −202 | 456 | 0.4 | 0.8 | — | 36 | 0.9 | — | [147] |
| S0.04(±0.01)Pd0.21(±0.02) Co3.75Sb11.92(±0.09) (300 K) | 405.3 | 3.4 | 2.8 | −192 | 979 | 0.3 | 2.1 | 0.3 | 29.5 | 4.4 | — | [147] |
| S0.04(±0.01)Pd0.21(±0.02) Co3.75Sb11.92(±0.09) (700 K) | 139.5 | 3.1 | 2.3 | −244 | 657 | 0.9 | 2.1 | 19.8 | 3.4 | — | [147] | |
| S0.04(±0.01)Pd0.21(±0.02) Co3.75Sb11.92(±0.09) (850 K) | 76.1 | 3.7 | 2.6 | −216 | 663 | 0.7 | 2.1 | 20 | 1.9 | — | [147] | |
| CeFe4As12 | — | — | — | — | — | — | — | 0.2 | — | — | 34.4 (static) | [159] |
| CeFe4Sb12 | — | — | — | — | — | — | — | 0.1 | — | — | 39.8 (static) | [159] |
| CoSb3 | — | — | — | — | — | — | — | — | — | — |
 ∞ = 31.67 ∞ = 31.67 | [160] |
| TlFeCo3Sb12 | — | — | — | — | — | — | — | — | — | — |
 ∞ = 38.57 ∞ = 38.57 | [160] |
| CoSb3 | — | — | — | — | — | — | — | — | — | — |
 ∞ = 25.6 (130 K), ∞ = 25.6 (130 K),  ∞ = 24.3 (623 K) ∞ = 24.3 (623 K) | [161] |
| UFe4P12 | — | — | — | — | — | — | — | — | — | — |
 ∞ = 17 ∞ = 17 | [162] |
| CeFe4P12 | — | — | — | — | — | — | — | — | — | — |
 ∞ = 31 ∞ = 31 | [162] |
| LaFe3 CoSb12 (300 K) | 87.6 | 1.6 | 1.3 | 103 | 625 | 0.1 | — | — | — | — | [148] | |
| LaFe3 CoSb12 | 55.3 | 1.6 (740 K) | 1.0 (740 K) | 203 (700 K) | 455 (700 K) | 0.9 (740 K) | — | — | — | — | [148] | |
| CeFe3 CoSb12 (300 K) | 74.9 | — | — | 87 | 667 | — | — | — | — | — | — | [148] |
| CeFe3 CoSb12 | 37.6 | — | — | 150 (680 K) | 508 (680 K) | 0.7 (800 K) | — | — | — | — | — | [148] |
| CeFe2.5 Co1.5Sb12 (300 K) | 51.8 | — | — | 105 | 360 | — | — | 0.43 | — | — | — | [148] |
| CeFe2.5 Co1.5Sb12 (680 K) | 25.7 | — | — | 161 | 304 | — | — | — | — | — | — | [148] |
| La0.89Fe4Sb12.02 (300 K) | 241.3 | 3.1 | 1.6 | 78.3 | 2440 | 0.2 | 13.8 | — | 11 | 4.8 | [151] | |
| La0.89Fe4Sb12.02 (800 K) | 81.3 | 3.4 | 1.1 | 150 | 1400 | 0.8 | 13.8 | — | 6.3 | 4.0 | [151] | |
| Ce0.91Fe4Sb11.97 (300 K) | 224.2 | 2.6 | 1.3 | 79.4 | 2230 | 0.2 | 38.6 | — | 3.6 | 9.7 | — | [151] |
| Ce0.91Fe4Sb11.97 (800 K) | 86.8 | 3.1 | 0.8 | 152 | 1460 | 0.9 | 38.6 | — | 2.4 | 8.0 | — | [151] |
| Pr0.90Fe4Sb12.00 (300 K) | 248.9 | 2.5 | 1.1 | 81.5 | 2400 | 0.2 | 71.9 | — | 2.1 | 15.1 | — | [151] |
| Pr0.90Fe4Sb12.00 (800 K) | 84.4 | 3.0 | 0.8 | 152 | 1420 | 0.9 | 71.9 | — | 1.2 | 12.2 | — | [151] |
| Nd0.85Fe4Sb12.00 (300 K) | 253.8 | 2.4 | 1.0 | 83.7 | 2370 | 0.2 | 46.6 | — | 3.2 | 11.6 | — | [151] |
| Nd0.85Fe4Sb12.00 (800 K) | 84.6 | 3.0 | 0.7 | 151 | 1440 | 0.9 | 46.6 | — | 1.9 | 9.0 | — | [151] |
| Eu0.96Fe4Sb11.94 (300 K) | 261.7 | 3.6 | 1.8 | 70.8 | 2970 | 0.1 | 34.3 | — | 5.4 | 7.9 | — | [151] |
| Eu0.96Fe4Sb11.94 (800 K) | 79 | 4.0 | 1.0 | 124 | 1870 | 0.6 | 34.3 | — | 3.4 | 5.6 | — | [151] |
| Yb0.94Fe4Sb12.07 (300 K) | 330.3 | 3.2 | 1.2 | 77.9 | 3360 | 0.2 | 25.4 | — | 8.3 | 7.2 | — | [151] |
| Yb0.94Fe4Sb12.07 (800 K) | 93.1 | 4.4 | 0.9 | 123 | 2230 | 0.6 | 25.4 | — | 5.5 | 4.6 | — | [151] |
| Ca0.98Fe4Sb12.08 (300 K) | 290.4 | 3.5 | 1.6 | 73.9 | 3140 | 0.2 | 13.4 | — | 14.6 | 4.4 | — | [151] |
| Ca0.98Fe4Sb12.08 (800 K) | 84.3 | 4.2 | 0.9 | 123 | 2020 | 0.6 | 13.4 | — | 9.4 | 3.0 | — | [151] |
| Sr0.94Fe4Sb12.08 (300 K) | 303.2 | 4.1 | 2.1 | 72.5 | 3350 | 0.1 | 33.6 | — | 6.2 | 8.0 | — | [151] |
| Sr0.94Fe4Sb12.08 (800 K) | 86.5 | 4.5 | 1.1 | 122 | 2100 | 0.6 | 33.6 | — | 3.9 | 5.4 | — | [151] |
| Ba0.92Fe4Sb12.04 (300 K) | 265.4 | 4.0 | 2.2 | 69.4 | 3080 | 0.1 | 16.1 | — | 11.9 | 4.7 | — | [151] |
| Ba0.92Fe4Sb12.04 (800 K) | 74.8 | 4.4 | 1.2 | 116 | 1960 | 0.5 | 16.1 | — | 7.6 | 3.1 | — | [151] |
| Ce0.2FeCo3Sb12 (300 K) | 80.2 | 2.0 | 1.7 | 106 | 550 | 0.1 | 1.3 | — | 26.4 | 1.4 | — | [152] |
| Ce0.2FeCo3Sb12 (700 K, peak) | 42 | 2.2 | 1.7 | 193 | 357 | 0.4 | 1.3 | — | 17.1 | 1.4 | — | [152] |
| Ce0.2FeCo3Sb12 (800 K) | 31.6 | 2.5 | 1.9 | 182 | 373 | 0.4 | 1.3 | — | 17.9 | 1.1 | — | [152] |
| Ce0.5Fe2 Co2Sb12 (300 K) | 105.1 | 1.8 | 1.4 | 102 | 760 | 0.1 | 8.0 | — | 5.9 | 4.5 | — | [152] |
| Ce0.5Fe2 Co2Sb12 (700 K, peak) | 64.8 | 2.0 | 1.3 | 190 | 571 | 0.7 | 8.0 | — | 4.5 | 4.5 | — | [152] |
| Ce0.5Fe2 Co2Sb12 (800 K) | 41.9 | 2.3 | 1.4 | 171 | 563 | 0.6 | 8.0 | — | 4.4 | 3.4 | — | [152] |
| Ce0.9Fe3 CoSb12 (300 K) | 161 | 1.9 | 1.3 | 112 | 1020 | 0.2 | 23.3 | — | 2.7 | 10.2 | — | [152] |
| Ce0.9Fe3 CoSb12 (700 K, peak) | 81.5 | 2.0 | 0.9 | 184 | 770 | 0.9 | 23.3 | — | 2.1 | 8.8 | — | [152] |
| Ce0.9Fe3 CoSb12 (800 K) | 64.7 | 2.4 | 1.2 | 185 | 738 | 0.9 | 23.3 | — | 2 | 7.8 | — | [152] |
| CeFe4Sb12 (300 K) | 222.9 | 2.6 | 1.3 | 79 | 2230 | 0.2 | — | — | [152] | |||
| CeFe4Sb12 (775 K, peak) | 86.9 | 3.1 | 0.8 | 147 | 1480 | 0.8 | — | — | [152] | |||
| CeFe4Sb12 (800 K) | 81.3 | 3.1 | 0.8 | 146 | 1470 | 0.8 | — | — | [152] | |||
| Yb0.3FeCo3Sb12 (300 K) | 78.9 | 2.2 | 1.7 | 80 | 778 | 0.1 | 1.4 | — | 34.7 | 1.1 | — | [152] |
| Yb0.3FeCo3Sb12 (700 K, peak) | 52.7 | 2.0 | 1.1 | 164 | 630 | 0.6 | 1.4 | — | 28.1 | 1.1 | — | [152] |
| Yb0.3FeCo3Sb12 (800 K) | 44 | 2.4 | 1.3 | 165 | 634 | 0.6 | 1.4 | — | 28.3 | 1.0 | — | [152] |
| Yb0.5Fe1.5 Co2.5Sb12 (300 K) | 130.3 | 2.0 | 1.4 | 103 | 930 | 0.2 | 2.9 | — | 20 | 2.3 | — | [152] |
| Yb0.5Fe1.5 Co2.5Sb12 (700 K, peak) | 67.3 | 1.9 | 0.9 | 171 | 740 | 0.8 | 2.9 | — | 15.9 | 2.0 | — | [152] |
| Yb0.5Fe1.5 Co2.5Sb12 (800 K) | 53.3 | 2.3 | 1.1 | 173 | 700 | 0.7 | 2.9 | — | 15.1 | 1.7 | — | [152] |
| Yb0.7Fe2 Co2Sb12 (300 K) | 152.1 | 1.9 | 1.2 | 102 | 1100 | 0.2 | 7.5 | — | 9.2 | 4.3 | — | [152] |
| Yb0.7Fe2 Co2Sb12 (700 K, peak) | 73.7 | 2.0 | 0.8 | 166 | 860 | 0.8 | 7.5 | — | 7.2 | 3.5 | — | [152] |
| Yb0.7Fe2 Co2Sb12 (800 K) | 60 | 2.3 | 1.0 | 168 | 835 | 0.8 | 7.5 | — | 6.9 | 3.1 | — | [152] |
| Yb0.9Fe2.5 Co1.5Sb12 (300 K) | 221 | 2.4 | 1.5 | 107 | 1495 | 0.2 | 9.2 | — | 10.1 | 5.2 | — | [152] |
| Yb0.9Fe2.5 Co1.5Sb12 (700 K, peak) | 88.3 | 2.5 | 1.0 | 162 | 1080 | 0.8 | 9.2 | — | 7.3 | 3.9 | — | [152] |
| Yb0.9Fe2.5 Co1.5Sb12 (800 K) | 71.6 | 3.0 | 1.4 | 166 | 1020 | 0.8 | 9.2 | — | 6.9 | 3.5 | — | [152] |
| YbFe3 CoSb12 (300 K) | 237.5 | 2.5 | 1.4 | 92 | 1970 | 0.2 | 16.4 | — | 7.5 | 6.4 | — | [152] |
| YbFe3 CoSb12 (700 K, peak) | 101.2 | 2.7 | 0.8 | 151 | 1410 | 0.8 | 16.4 | — | 5.4 | 5.1 | — | [152] |
| YbFe3 CoSb12 (800 K) | 80.6 | 3.1 | 0.9 | 153 | 1340 | 0.8 | 16.4 | — | 5.1 | 4.6 | — | [152] |
| YbFe3.5 Co0.5Sb12 (300 K) | 242.4 | 3.1 | 1.6 | 77 | 2500 | 0.2 | — | — | [152] | |||
| YbFe3.5 Co0.5Sb12 (700 K, peak) | 92 | 3.6 | 1.0 | 121 | 1850 | 0.5 | — | — | [152] | |||
| YbFe3.5 Co0.5Sb12 (800 K) | 71.8 | 4.0 | 1.3 | 123 | 1720 | 0.5 | — | — | [152] | |||
| BaFe3 CoSb12 (300 K) | 220.2 | 3.6 | 2.4 | 85.6 | 2000 | 0.1 | 17.8 | — | 7 | 6.3 | — | [153] |
| BaFe3 CoSb12 (800 K) | 76.7 | 3.3 | 1.3 | 154 | 1260 | 0.7 | 17.8 | — | 4.4 | 4.9 | — | [153] |
| Ce0.9Fe3 CoSb12 (300 K) | 172.5 | 2.0 | 1.4 | 111.5 | 1100 | 0.2 | 23.1 | — | 3 | 10.1 | — | [153] |
| Ce0.9Fe3 CoSb12 (750 K, peak) | 76.4 | 2.2 | 1.0 | 183 | 810 | 0.9 | 23.1 | — | 2.2 | 8.1 | — | [153] |
| Ce0.9Fe3 CoSb12 (800 K) | 68.3 | 2.4 | 1.2 | 184 | 788 | 0.9 | 23.1 | — | 2.1 | 7.6 | — | [153] |
| YbFe3 CoSb12 (300 K) | 235.5 | 2.5 | 1.3 | 91.4 | 1970 | 0.2 | 16.4 | — | 7.5 | 6.4 | — | [153] |
| YbFe3 CoSb12 (700 K, peak) | 99.5 | 2.7 | 0.7 | 149 | 1420 | 0.8 | 16.4 | — | 5.4 | 5.0 | — | [153] |
| YbFe3 CoSb12 (800 K) | 79.7 | 3.1 | 0.9 | 152 | 1340 | 0.8 | 16.4 | — | 5.1 | 4.5 | — | [153] |
| Ba0.4Ce0.6Fe3 CoSb12 (300 K) | 160.5 | 2.0 | 1.2 | 93.7 | 1300 | 0.2 | 13.9 | — | 5.8 | 5.9 | — | [153] |
| Ba0.4Ce0.6Fe3 CoSb12 (750 K, peak) | 63.9 | 2.2 | 0.8 | 160 | 888 | 0.8 | 13.9 | — | 4 | 4.7 | — | [153] |
| Ba0.4Ce0.6Fe3 CoSb12 (800 K) | 58.9 | 2.3 | 1.0 | 163 | 869 | 0.8 | 13.9 | — | 3.9 | 4.5 | — | [153] |
| Ce0.45Nd0.45Fe3 CoSb12 (300 K) | 191.7 | 1.8 | 1.1 | 108.6 | 1270 | 0.3 | 29.7 | — | 2.7 | 11.6 | — | [153] |
| Ce0.45Nd0.45Fe3 CoSb12 (750 K, peak) | 83.7 | 2.1 | 0.8 | 182 | 898 | 1.1 | 29.7 | — | 1.9 | 9.5 | — | [153] |
| Ce0.45Nd0.45Fe3 CoSb12 (800 K) | 72.2 | 2.3 | 0.8 | 178 | 894 | 1.0 | 29.7 | — | 1.9 | 8.6 | — | [153] |
| Ce0.6Yb0.4Fe3 CoSb12 (300 K) | 193.5 | 1.9 | 1.1 | 105.8 | 1330 | 0.2 | 20.8 | — | 4 | 8.9 | — | [153] |
| Ce0.6Yb0.4Fe3 CoSb12 (750 K, peak) | 79.7 | 2.1 | 0.7 | 173 | 950 | 1.0 | 20.8 | — | 2.9 | 6.9 | — | [153] |
| Ce0.6Yb0.4Fe3 CoSb12 (800 K) | 69.6 | 2.3 | 0.8 | 172 | 925 | 0.9 | 20.8 | — | 2.8 | 6.4 | — | [153] |
| Ce0.4Yb0.6Fe3 CoSb12 (300 K) | 192.3 | 1.8 | 1.0 | 103.1 | 1370 | 0.2 | 16.0 | — | 5.3 | 7.2 | — | [153] |
| Ce0.4Yb0.6Fe3 CoSb12 (750 K, peak) | 76.3 | 2.1 | 0.6 | 167 | 975 | 1.0 | 16.0 | — | 3.8 | 5.5 | — | [153] |
| Ce0.4Yb0.6Fe3 CoSb12 (800 K) | 66.3 | 2.3 | 0.8 | 165 | 956 | 0.9 | 16.0 | — | 3.7 | 5.0 | — | [153] |
| DD0.86Fe4Sb12 (300 K) | 301.4 | 2.2 | 0.5 | 85 | 2762 | 0.3 | — | — | — | [154] | ||
| DD0.86Fe4Sb12 (800 K) | 91.3 | 2.9 | 0.6 | 157 | 1447 | 1.1 | — | — | — | [154] | ||
| DD0.78Fe3.2 Co0.8Sb12 (300 K) | 300 | 2.0 | 0.3 | 83 | 2830 | 0.3 | 2.8 | — | 64 | 1.8 | — | [154] |
| DD0.78Fe3.2 Co0.8Sb12 | 97.7 | 3.1 (675 K) | — | 152 (800 K) | 1643 (800 K) | — | — | — | — | — | — | [154] |
| DD0.44Fe2.1 Co1.9Sb12 (300 K) | 185.1 | 1.9 | 1.2 | 103 | 1321 | 0.2 | 3.4 | — | 24.3 | 2.6 | — | [154] |
| DD0.44Fe2.1 Co1.9Sb12 | 53.6 | 2.8 (725 K) | — | 159 (800 K) | 830 (800 K) | 0.4 (800 K) | — | — | — | [154] | ||
| DD0.25Fe1.2 Co2.8Sb12 (300 K) | 153.2 | 2.7 | 2.0 | 98 | 1170 | 0.1 | 1.7 | — | 43.5 | 1.5 | — | [154] |
| DD0.25Fe1.2 Co2.8Sb12 | 65.2 | 3.4 (775 K) | — | 198 (800 K) | 640 (800 K) | 0.4 (800 K) | — | — | — | — | — | [154] |
| DD0.12FeCo3Sb12 (300 K) | 111.7 | 3.5 | 2.7 | 69 | 1305 | 0.1 | 1.5 | — | 52.9 | 1.0 | — | [154] |
| DD0.12FeCo3Sb12 | 85.1 | 4.4 (675 K) | — | 168 (800 K) | 1076 (750 K) | 0.5 (800 K) | — | — | — | — | — | [154] |
| DD0.08FeCo3Sb12 (300 K) | 166.6 | 3.4 | 3.3 | −228 | 265 | 0.1 | 1.0 | — | 16.5 | 3.7 | — | [154] |
| DD0.08FeCo3Sb12 | 49.3 | 4.88 (775 K) | — | −230 (750 K) | 303 (800 K) | 0.3 (800 K) | — | — | — | — | — | [154] |
| DD0.68Fe3.2Ni0.8Sb12 (300 K) | 304.9 | 2.3 | 1.4 | 132 | 1500 | 0.3 | 0.8 | — | 117 | 1.3 | — | [154] |
| DD0.68Fe3.2Ni0.8Sb12 (800 K) | 104.7 | 2.9 | 2.4 | 211 | 883 | 1.1 | 0.8 | — | 68.9 | 2.7 | — | [154] |
| DD0.76Fe3.4Ni0.6Sb12 (300 K) | 251.1 | 2.0 | 0.8 | 94 | 2026 | 0.3 | 1.4 | — | 89.7 | 1.3 | — | [154] |
| DD0.76Fe3.4Ni0.6Sb12 (800 K) | 81.6 | 2.8 | 2.0 | 155 | 1325 | 0.9 | 1.4 | — | 58.7 | 2.4 | — | [154] |
| DD0.40Fe2.8Ni1.2Sb12 (300 K) | 54.4 | 2.1 | 2.0 | 135 | 258 | 0.1 | 0.5 | — | 32.9 | 1.0 | — | [154] |
| DD0.40Fe2.8Ni1.2Sb12 (800 K) | 5.3 | 3.4 | 2.8 | 20 | 957 | 0.01 | 0.5 | — | 121.9 | 0.1 | — | [154] |
| DD0.08Fe2Ni2Sb12 (300 K) | 23.2 | 2.2 | 2.0 | −70 | 267 | 0.02 | 0.7 | — | 23.5 | 0.6 | — | [154] |
| DD0.08Fe2Ni2Sb12 (800 K) | 108.4 | 4.3 | 0.6 | −62 | 6200 | 0.4 | 0.7 | — | 545.1 | 0.5 | — | [154] |
| Sr0.03Ba0.03Yb0.1 Co4Sb12 (300 K) | 357.5 | 2.7 | 1.7 | −138 | 1634 | 0.4 | — | — | — | — | — | [149] |
| Sr0.03Ba0.03Yb0.1 Co4Sb12 (800 K) | 121 | 2.8 | 1.3 | −217 | 952 | 1.3 | — | — | — | — | — | [149] |
| Sr0.07Ba0.07Yb0.07 Co4Sb12 (300 K) | 376.8 | 2.3 | 1.3 | −140 | 1681 | 0.4 | — | — | — | — | — | [149] |
| Sr0.07Ba0.07Yb0.07 Co4Sb12 (800 K) | 111.1 | 2.4 | 0.9 | −208 | 970 | 1.4 | — | — | — | — | — | [149] |
| Sr0.07Ba0.07Yb0.07 Co4Sb12 (300 K), HPT | 260.7 | 1.6 | 0.9 | −140 | 1163 | 0.4 | — | — | — | — | — | [149] |
| Sr0.07Ba0.07Yb0.07 Co4Sb12 (800 K), HPT | 103.8 | 1.8 | 0.4 | −213 | 855 | 1.8 | — | — | — | — | — | [149] |
| DD0.88Fe4Sb12 (300 K) | 279.8 | 1.9 | 0.4 | 85 | 2564 | 0.3 | — | — | — | — | — | [155] |
| DD0.88Fe4Sb12 (800 K) | 105.4 | 3.2 | 0.6 | 160 | 1613 | 1.1 | — | — | — | — | — | [155] |
| Mm0.68Fe4Sb12 (300 K) | 202.4 | 1.4 | 0.6 | 110 | 1316 | 0.3 | — | — | — | — | — | [155] |
| Mm0.68Fe4Sb12 (800 K) | 81 | 2.1 | 0.7 | 188 | 893 | 1.2 | — | — | — | — | — | [155] |
| DD0.60Fe3 CoSb12 (300 K) | 187 | 1.8 | 1.1 | 106 | 1282 | 0.2 | — | — | — | — | — | [155] |
| DD0.60Fe3 CoSb12 (800 K) | 79.4 | 1.9 | 0.5 | 183 | 927 | 1.3 | — | — | — | — | — | [155] |
| DD0.88Fe4Sb12 (300 K) | 279.8 | 1.9 | 0.4 | 85 | 2564 | 0.3 | — | — | — | — | — | [155] |
For simplicity, the carrier concentration values at high temperature are taken as the same values at room temperature to calculate the m* in this table.
Cobalt antimonide (CoSb3) based filled skutterudites are reported to show excellent thermoelectric performance among the skutterudite family [128–149]. When rare earth, alkaline earths, or alkali metals are accommodated in the Sb-icosahedral voids without additional charge compensation, the resulting filled skutterudites Gy Co4Sb12 usually show n-type conductivity due to the extra electrons introduced into the [Co4Sb12] framework by the filler, while the fillers hardly modify the band structure near the conduction band minimum. The maximum filling fraction increases with the type of filler, roughly following the sequence of rare earths, alkaline earths, and alkali metals, although it is also affected by the electronegativity, radius, and valence of the filler ions. The optimal filling fraction for maximum power factors (PFs) roughly obeys a '0.5 electron per unit cell' rule, in which the carrier concentration reaches ∼1020 cm−3 [150]. Binary CoSb3 has an intrinsic high κL of about ∼10 Wm−1K−1 at 300 K. Introducing foreign elements into the Sb-icosahedral voids can greatly lower κL due to the filler-introduced resonant scattering of phonons. One important realization is that these resonant scattering centers are tuned to a particular spectrum of phonons. By filling the voids with different types of elements possessing different resonant frequencies, it enables phonons with a broad range of frequencies to be scattered, leading to a significant reduction of lattice thermal conductivity in the so-called multiple-filled skutterudites. With comprehensive strategies, combining the optimization of carrier concentration by optimizing the filling fraction, the reduction of κL through multiple-filling, as well as the introduction of magnetic nanocomposites, the maximum zT has been enhanced to a very high level, exceeding 1.7 in CoSb3-based n-type filled skutterudites [144, 149].
It is easy to obtain p-type filled skutterudites by alloying Fe at the Co-site or Ge/Sn at the Sb-site in Gy Co4Sb12. Gy Co4−x Fex Sb12 (where x is the Fe doping content) represents one of the most promising p-type filled skutterudites [149, 151–157]. The x in Gy Co4−x Fex Sb12 is usually in the range of 1.5–4. The maximum filling fraction y in Gy Co4−x Fex Sb12 is usually less than x/n, where n is the valence state of fillers. In Gy Fe4Sb12, the electrical transport is sensitively dependent on the valence states of the fillers, while the lattice thermal resistivity WL (=1/κL ) obeys the relationship of WL ∼ (rcage–rion)3, where rcage and rion are the radii of Sb-icosahedron void and filler, respectively [151]. It should be noted that the Sb in p-type filled skutterudites is easier to volatilize at elevated temperature than in n-type filled skutterudites, thus the maximum measurement temperature for p-type filled skutterudites is usually around 800 K, about 50–100 K lower than that for n-type filled skutterudites. The maximum zT is around unity for p-type Gy Co4−x Fex Sb12, which is much lower than for n-type Gy Co4Sb12 (figure 3(b)) [151–153].
Figures 3(b)–(f) present the collected physical properties (electrical conductivity, thermal conductivity, Seebeck coefficient, and carrier mobility) and thermoelectric parameters (PF and zT) of p—and n-type skutterudites at 300 K [128–149, 151–157]. These parameters are plotted as the dependencies of electrical conductivity or carrier concentration. All these dependencies show similar trends even at high temperature. As shown in figure 3(b), at 300 K, the zTs of p-type skutterudites are smaller than those for n-type materials across the whole range of electrical conductivity (σ), although the optimal electrical conductivity for peak zT takes occurs in the same range, around 1500–2500 S cm−1. The disparity between the optimal zT values of n- and p-type skutterudites is numerically determined by the different levels of PF as shown in figure 3(c). For example, the maximum PF for n-type skutterudites at 300 K is approximately 40 μW cm−1K−2, while that for p-type materials is only around 20 μW cm−1K−2.
Generally, the weighted mobility μw (=μ0(m*/m0)3/2, where μ0 is the drift mobility and m* is the density-of-state effective mass) is an important factor to discuss the PF. Since the m* varies with the variation of carrier concentration in skutterudites, it is difficult to achieve a satisfactory fit to all data by using fixed μw, but the general trend should be valuable and meaningful from the estimated μw [158]. In figure 3(c), the PF—σ lines for n- and p-type skutterudites are drawn using the estimated μw (335 cm2V−1s−1 and 225 cm2V−1s−1 for n- and p-type skutterudites, respectively, estimated from all collected data) and m* (5me and 12me, for n- and p-type, respectively, estimated from all collected data). As shown in figures 1(d) and (e), the much larger μH guarantees the larger PFs for n-type skutterudites although their m* values are smaller than in p-type ones. This scenario is consistent with analyses based on band structure. For n-type skutterudites, the conduction band edge is dominated by the Sb-dominated threefold degenerated bands. However, for p-type skutterudites, there is one Sb-dominated light band and one Fe-dominated heavy band in the valence band edge. Considering the heavily doped character of Gy Co4−x Fex Sb12, the Fermi level crosses the Fe-dominated heavy band. The localized nature of Fe 3d orbitals is responsible for the large m* but small μ0 observed in p-type skutterudites. This implies that, efforts to optimize the band structure through exploring new alloy systems (if possible) to enhance the carrier mobility may be worthwhile for the development of p-type filled skutterudites with higher thermoelectric performance.
Figure 3(f) shows that the p-type (filled) skutterudites generally exhibit lower total thermal conductivity (κ) and κL than the n-type (filled) skutterudites under the comparable range of σ. This is reasonable since the p-type skutterudites usually possess higher filling fractions, which can lead to stronger scattering of phonons. In addition, the coexistence of Fe and Co at the same atomic site introduces extra point defects to scatter phonons, which are also responsible for the lower κL values observed in p-type skutterudites. However, in figure 3(f), it can be seen that many n-type samples exhibit comparable κ to the p-type samples in the optimal σ range. It is noted that these low κ values are obtained in the multiple-filled skutterudites. The combination of the low κ and reduced influence on electrical transport by multiple-filling guarantees the realization of high zT in n-type filled skutterudites.
Although skutterudites have been widely studied in the aspects of both material optimization and device development, there are still great challenges for skutterudite thermoelectrics. Firstly, the current TE performance of p-type skutterudites is behind that of the n-type skutterudites, which limits the development of high efficiency devices. Considering that the localized 3d orbitals of Fe is the main reason for the low carrier mobilities and poor zTs of existing p-type skutterudites, alternative doping elements should be explored to develop new p-type skutterudites. Secondly, the κL of n-type skutterudites below 700 K still has much scope for reduction. Novel approaches, such as nanostructure engineering, are to be encouraged to further strengthen phonon scattering, which is beneficial for enhancing the average zT of n-type skutterudites over the entire temperature range and therefore effectively improving the conversion efficiency of the devices. Furthermore, at high temperatures, skutterudites face severe oxidation and Sb volatilization issues, leading to relatively poor reliability during service. Developing effective protective coating or sealing technology against oxidation and volatilization is also important for their practical applications.
Acknowledgments
The work of Lidong Chen and Pengfei Qiu is supported by the National Key Research and Development Program of China (2018YFB0703600) and the National Natural Science Foundation of China (91963208).
3.3. Half Heuslers
Half-Heusler thermoelectric materials
Shen Han, Chenguang Fu and Tiejun Zhu
State Key Laboratory of Silicon Materials, and School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, People's Republic of China
E-mail: zhutj@zju.edu.cn
Studies of thermoelectric properties of half-Heusler (HH) compounds have been carried out since the end of the 20th century, focusing on compositions with 18 valence electrons represented by three typical systems, i.e. MNiSn (M = Ti, Zr, Hf), MCoSb and XFeSb (X = V, Nb, Ta). In recent years, the 18-electron NbCoSn, ReNiSb (Re is a rare earth element), and nominal 19-electron XCoSb compounds have also attracted increasing attention. A summary of the peak zT values obtained for different HH compounds is shown in figure 4, and a detailed comiplation of representative data are given in table 3. These advances make HH compounds promising thermoelectric candidates for power generation applications with advantages of mechanical robustness, thermal stability, and relatively low-cost constituents.
Download figure:
Standard image High-resolution imageTable 3. Half Heusler thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µ0 (cm2 V−1 s−1) | ms* (me) |
 r or r or  ( ( 0) 0) | κ (W m−1 K−1) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNiSn (M = Ti, Zr, Hf) system | ||||||||||||
| ZrNiSn | 309 | 172.7 | — | −224.7 | 298.1 | 0.07 | 0.1–0.36 (Exp.) 0.5 (DFT) 0.66 (ARPES) | 37.1 | 2.8 | 20.6–26(Cal.) | 6.06 | [163, 188, 237, 241, 244, 248–250] |
| 874 | 89.6 | — | −222.6 | 754.4 | 0.60 | — | — | — | — | 5.22 | ||
| ZrNiSn0.99Sb0.01 | 309 | 301.4 | 7.35 | −129.2 | 1604.6 | 0.10 | — | 48.1 | 2.8 | — | 7.44 | |
| 874 | 122.9 | 4.48 | −205.7 | 1259.1 | 0.77 | — | — | — | — | 5.45 | ||
| Hf0.5Zr0.5Ni0.8Pd0.2Sn0.99Sb0.01 | 300 | 300.5 | — | −104.0 | 2115.5 | 0.15 | — | — | — | — | 4.52 | [192] |
| 800 | 71.3 | — | −153.2 | 1182.7 | 0.69 | — | — | — | — | 3.07 | ||
| Hf0.75Zr0.25NiSn0.975Sb0.025 | 312 | 316.5 | — | −73.1 | 3663.5 | 0.09 | — | — | — | — | 7.04 | [193] |
| 977 | 92.6 | — | −158.3 | 1950.2 | 0.81 | — | — | — | — | 5.83 | ||
| Hf0.6Zr0.4NiSn0.98Sb0.02 | 291 | 301.1 | — | −92.1 | 2377.3 | 0.12 | — | 45.0 | 2.8 | — | 5.00 | [233] |
| 1030 | 75.4 | — | −170.6 | 1485.4 | 1.01 | — | — | — | — | 4.28 | ||
| Ti0.5Zr0.25Hf0.25NiSn0.998Sb0.002 | 373 | 202.6 | 2.88 | −215.8 | 515.2 | 0.26 | — | — | — | — | 3.34 | [164] |
| 824 | 92.1 | 0.76 | −206.5 | 854.6 | 1.21 | — | — | — | — | 2.47 | ||
| MCoSb (M = Ti, Zr, Hf) system | ||||||||||||
| ZrCoSb | 334 | 0.8 | — | −121.4 | 5.4 | 1.06 (DFT) | — | 17.9–19(Cal.) | 16.36 | [165, 238, 249–251] | ||
| 991 | 2.4 | — | −149.1 | 56.7 | 0.02 | — | — | — | — | 6.56 | ||
| ZrCoSn0.1Sb0.9 | 328 | 241.4 | — | 161.2 | 956.0 | 0.08 | — | 10.5 | 9.2 | — | 9.87 | |
| 956 | 59.2 | — | 257.1 | 382.3 | 0.45 | — | — | — | — | 5.40 | ||
| Zr0.5Hf0.5 CoSb0.8Sn0.2 | 322 | 151.3 | — | 109.7 | 1100.0 | 0.11 | — | 10.9 | 5–9 | — | 4.04 | [166] |
| 965 | 35.6 | — | 173.8 | 613.1 | 0.49 | — | — | — | — | 3.61 | ||
| Zr0.5Hf0.5 CoSb0.8Sn0.2 | 301 | 214.1 | 2.85 | 146.3 | 887.6 | 0.17 | — | — | — | — | 3.40 | [167] |
| 974 | 53.4 | 2.19 | 216.7 | 565.8 | 0.80 | — | — | — | — | 3.24 | ||
| Ti0.25Hf0.75 CoSb0.85Sn0.15 | 391 | 192.4 | 2.98 | 196.1 | 659.2 | 0.29 | — | 4.9 | — | — | 3.43 | [168] |
| 981 | 71.2 | 1.99 | 266.3 | 429.5 | 1.15 | — | — | — | — | 2.68 | ||
| Zr0.5Hf0.5 Co0.9Ni0.1Sb | 300 | 107.7 | 5.07 | −112.7 | 677.6 | 0.05 | — | 6.3 | 6 | — | 5.44 | [169] |
| 1074 | 58.8 | 2.37 | −226.9 | 641.2 | 1.02 | — | — | — | — | 3.44 | ||
| (Zr0.4Hf0.6)0.88Nb0.12 CoSb | 300 | 184.4 | 3.86 | −98.9 | 1391.4 | 0.08 | — | 9.2 | 6.5 | — | 4.68 | [170] |
| 1174 | 40.1 | 2.04 | −212.7 | 588.9 | 0.99 | — | — | — | — | 3.15 | ||
| (Hf0.3Zr0.7)0.88Nb0.12 CoSb | 301 | 129.2 | 4.15 | −114.8 | 794.5 | 0.07 | — | 6.9 | 6.5 | — | 4.60 | [171] |
| 1123 | 39.3 | 2.22 | −228.2 | 451.0 | 0.85 | — | — | — | — | 3.04 | ||
| XFeSb (X = V, Nb, Ta) system | ||||||||||||
| NbFeSb | 300 | — | — | −2.7 | 7.2 | 0.54 (DFT) | — | — | 23(Cal.) | — | [172, 173, 242, 249, 250] | |
| V0.95Ti0.05FeSb | 300 | — | — | 57.6 | 2125.6 | — | — | — | — | — | — | |
| (V0.6Nb0.4)0.8Ti0.2FeSb | 300 | 267.2 | 3.09 | 143.5 | 1142.7 | 0.19 | — | 7.6 | 10 | — | 3.70 | [173] |
| 900 | 64.6 | 2.37 | 214.1 | 626.7 | 0.80 | — | — | — | — | 3.24 | ||
| Nb0.8Ti0.2FeSb | 300 | 431.6 | 4.54 | 71.6 | 4833.5 | 0.09 | — | 24.9 | 80(Exp.) | 7.47 | [174, 252] | |
| 1100 | 71.1 | 2.68 | 204.0 | 1048.4 | 1.09 | — | — | — | — | 4.55 | ||
| Nb0.88Hf0.12FeSb | 301 | 723.5 | 4.82 | 93.7 | 5893.6 | 0.19 | — | 32.5 | 6.9 | — | 8.31 | [175] |
| 1200 | 79.9 | 2.62 | 246.1 | 823.5 | 1.45 | — | — | — | — | 4.21 | ||
| Nb0.95Ti0.05FeSb | 304 | 1150.4 | — | 175.6 | 3427.8 | 0.25 | — | 35.8 | 7.5 | — | 13.09 | [176] |
| 975 | 144.4 | — | 306.3 | 542.6 | 0.74 | — | — | — | — | 6.57 | ||
| (Nb0.6Ta0.4)0.8Ti0.2FeSb | 300 | 508.3 | 1.76 | 74.7 | 5416.4 | 0.18 | — | 25.0 | 6.9 | — | 5.07 | [177] |
| 1200 | 63.9 | 1.43 | 218.2 | 910.1 | 1.60 | — | — | — | — | 3.19 | ||
| NbFe0.94Ir0.06Sb | 300 | 164.4 | 6.21 | −76.8 | 1700.2 | 0.04 | — | 81.0 | 1.6 | 20(Exp.) | 6.89 | [178] |
| 1101 | 24.0 | 3.46 | −205.9 | 346.3 | 0.50 | — | — | — | — | 4.11 | ||
| Ta0.74V0.1Ti0.16FeSb | 304 | 460.8 | 2.33 | 116.0 | 2832.2 | 0.29 | — | — | — | — | 3.93 | [179] |
| 969 | 93.8 | 1.56 | 227.7 | 868.8 | 1.52 | — | — | — | — | 2.91 | ||
| 19-electron HH materials | ||||||||||||
| NbCoSb | 303 | 170.7 | 3.98 | −59.2 | 2393.7 | 0.04 | — | 3.7 | — | — | 5.56 | [180] |
| 974 | 38.8 | 3.04 | −139.8 | 1013.8 | 0.40 | — | — | — | — | 4.80 | ||
| Nb0.8 CoSb | 299 | 68.7 | 4.17 | −174.2 | 203.3 | 0.04 | 1.0 (DFT) 0.5 (GSF) | 2.9 | — | — | 4.26 | [181, 236] |
| 1123 | 21.1 | 1.92 | −225.7 | 249.4 | 0.62 | — | — | — | — | 2.36 | ||
| Nb0.83 CoSb | 299 | 155.6 | 4.83 | −87.4 | 1372.9 | 0.06 | — | 7.0 | 7.7 | — | 5.64 | |
| 1123 | 36.8 | 1.93 | −204.0 | 558.8 | 0.90 | — | — | — | — | 2.93 | ||
| Nb0.8 Co0.92Ni0.08Sb | 299 | 118.0 | 3.85 | −106.3 | 801.0 | 0.07 | — | — | — | — | 4.31 | [182] |
| 1123 | 32.5 | 1.69 | −213.0 | 445.1 | 0.90 | — | — | — | — | 2.49 | ||
| V0.855Ti0.1 CoSb | 300 | 148.2 | 2.20 | −57.9 | 2093.7 | 0.06 | — | 3.3 | 10 | — | 3.56 | [183] |
| 972 | 50.0 | 1.73 | −147.5 | 1188.4 | 0.67 | — | — | — | — | 3.74 | ||
| Ti0.82PtSb | 301 | 131.8 | 2.53 | −78.1 | 1339.0 | 0.08 | 0.33 (GSF) | 2.0 | 14.5 | — | 3.34 | [184] |
| 1072 | 32.2 | 1.55 | −164.0 | 728.5 | 0.74 | — | — | — | — | 2.86 | ||
| Other HH materials | ||||||||||||
| ZrCoBi0.65Sb0.15Sn0.20 | 303 | 293.1 | 2.18 | 129.9 | 1501.0 | 0.26 | — | — | 12 | — | 2.98 | [185] |
| 973 | 83.2 | 1.59 | 232.2 | 735.8 | 1.42 | — | — | — | 2.73 | |||
| ZrCo0.9Ni0.1Bi0.85Sb0.15 | 302 | 147.4 | 3.25 | −111.4 | 951.0 | 0.09 | — | — | 6.8 | — | 3.78 | [186] |
| 972 | 61.7 | 1.86 | −212.0 | 687.9 | 1.04 | — | — | — | 2.92 | |||
| NbCoSn | 301 | — | 7.94 | −65.4 | — | — | 1 (DFT) | — | — | 22.6(Cal.) | 7.95 | [187, 249, 250] |
| 972 | 33.4 | 4.36 | −284.9 | 160.2 | 0.26 | — | — | — | — | 4.63 | ||
| NbCoSn0.9Sb0.1 | 302 | 301.1 | 7.64 | −85.6 | 2758.3 | 0.07 | — | 18.9 | 6 | — | 9.31 | |
| 973 | 60.9 | 3.53 | −179.8 | 989.8 | 0.61 | — | — | — | — | 5.15 | ||
| NbCo0.95Pt0.05Sn | 319 | 226.4 | 4.65 | −123.2 | 1358.7 | 0.13 | — | 14.3 | 6.5 | — | 5.45 | [189] |
| 770 | 92.1 | 3.26 | −198.7 | 845.6 | 0.59 | — | — | — | — | 4.34 | ||
| ErPdSb | 333 | 119.1 | — | 241.2 | 189.7 | 0.07 | 0.28 (AF) | — | — | — | 5.55 | [190] |
| 698 | 45.8 | — | 141.6 | 710.8 | 0.16 | — | — | — | — | 6.37 | ||
| ErPdBi | 332 | 126.9 | — | 82.8 | 1398.9 | 0.06 | 0.05 (AF) | — | — | — | 5.30 | |
| 495 | 75.3 | — | 71.0 | 1806.9 | 0.08 | — | — | — | — | 6.50 | ||
| ScNiSb | 341 | 54.3 | — | 221.4 | 113.0 | 0.02 | 0.38 (AF) 0.21 (GSF) | 30.6 | 1.52 | — | 9.74 | [191] |
| 807 | 26.0 | — | 122.5 | 634.1 | 0.10 | — | — | — | — | 7.55 | ||
| DyNiSb | 329 | 30.2 | — | 46.9 | 612.1 | 0.01 | 0.13 (AF) 0.09 (GSF) | 53.5 | 0.56 | — | 3.67 | |
| 717 | 35.6 | — | 72.5 | 1452.6 | 0.11 | — | — | — | — | 4.90 | ||
| ErNiSb | 343 | 28.7 | — | 80.6 | 342.5 | 0.01 | 0.17 (AF) 0.13 (GSF) | 10.9 | 10.9 | — | 4.13 | |
| 688 | 47.3 | — | 106.7 | 1115.6 | 0.17 | — | — | — | — | 5.31 | ||
| TmNiSb | 318 | 96.7 | — | 205.1 | 218.7 | 0.07 | 0.21 (AF) 0.15 (GSF) | 17.1 | 17.1 | — | 3.82 | |
| 698 | 55.3 | — | 130.0 | 989.0 | 0.26 | — | — | — | — | 4.53 | ||
| LuNiSb | 323 | 59.9 | — | 113.3 | 416.2 | 0.03 | 0.19 (AF) 0.12 (GSF) | 29.1 | 29.1 | — | 5.06 | |
| 699 | 56.6 | — | 113.6 | 1247.8 | 0.18 | — | — | — | — | 5.88 |
Notes:AF—Arrhenius formula.APERS—angle-resolved photoemission spectroscopy.Cal.—calculation.DFT—density functional theory.Exp.—experiment.GSF—Goldsmid–Sharp formula.
The intermetallic MNiSn, found to exhibit semiconducting behavior around 1988 [231], was the first HH system to seriously arouse the interest of the thermoelectric community before the end of the 20th century [232]. With the efforts in the past two decades, MNiSn-based HH compounds have now been developed into the best n-type HH thermoelectric materials with peak zT above unity [164, 188, 192, 193, 233]. MCoSb has attracted research attention since 2000 and rapidly developed as a representative p-type thermoelectric system with a zT of about unity [168, 214]. It is worth noting that n-type MCoSb has recently been found to show a similar high zT value as its p-type counterpart [169–171], making it the first HH system with both good n-type and p-type thermoelectric performance. The studies on the thermoelectric properties of XFeSb started as early as those on MNiSn and MCoSb [172], but it did not attract much attention at that time, owing to the poor thermoelectric properties. Since 2014, with guidance of the band engineering concept and the selection of rational dopants, the heavy-band XFeSb-based HH system has been developed as high-performance p-type thermoelectric materials with a peak zT value of about 1.5 through rational compositional design and optimal doping [173–177, 179]. Very recently, prototype eight-pair HH thermoelectric modules using n-type MNiSn and p-type XFeSb were assembled [234, 235]; they show a maximum conversion efficiency of 10.5% and power density of 3.1 W cm−2 for a temperature difference of 680 K, demonstrating the encouraging prospect of HH compounds for power generation.
The nominal 19-electron HH system was usually thought to show metallic behavior and thus, it was unexpected that NbCoSb exhibited a respectable zT value of 0.4 in 2015 [180]. Subsequently, with the knowledge of defect chemistry [236], XCoSb was identified to be a defective HH compound with a considerable fraction of cation vacancies (up to ∼20%). Through tuning the content of cation vacancies that lead to suppressed lattice thermal conductivity and optimized electrical properties, a peak zT ∼ 0.9 was achieved in Nb1−x CoSb [181], demonstrating that the nominal 19-electron HH system provides a new class of material for the exploration of high-performance thermoelectrics and the understanding of the relationship between vacancies and transport properties.
In addition, some other HH compounds have also attracted some attention, including ZrCoBi, which was reported to show a peak zT of ∼1.4 for p-type and ∼1.0 for n-type [185, 186]. The thermoelectric properties of ReNiSb, a family of HH compounds with rare-earth elements, were also studied [191]. Further performance improvement is expected if the optimization strategies, generally used for MNiSn, MCoSb, and XFeSb, are successfully applied to ReNiSb. Similarly, NbCoSn, another 18-electron system with the predicted high PF for both p-type and n-type [237], has also been investigated. A peak zT of ∼0.6 was reported when it was doped as n-type [187, 189], whereas optimal p-type doping for NbCoSn is still not successful.
Different from many other good thermoelectric materials, HH compounds are characterized by their high PF (S2
σ), which directly contributes to their high zT value. The high crystal symmetry, from their cubic structure, leads to multiple carrier pockets and high band degeneracy NV near the band edge, such as the NV of 8 for p-type NbFeSb and ZrCoSb [238, 173]. Thus, a large density of states (DOS) effective mass is obtained, resulting in a large Seebeck coefficient even at a high carrier concentration. In addition, the low deformation potential guarantees weak carrier scattering by phonons and thus the relatively high carrier mobility in the heavy-band HH system [175, 188]. Another distinct feature of the heavy-band HH system is the high optimal carrier concentration nopt, defined as the carrier concentration where the peak zT occurs. In a single-band system, the nopt is approximately proportional to  under the classical statistics approximation [239], where
under the classical statistics approximation [239], where  is the DOS effective mass. For the HH system, nopt increases from ∼4 × 1020 cm−3 for n-type ZrNiSn, ∼2.6 × 1021 cm−3 for p-type NbFeSb, to ∼4 × 1021 cm−3 for n-type TiPtSb, whilst the
is the DOS effective mass. For the HH system, nopt increases from ∼4 × 1020 cm−3 for n-type ZrNiSn, ∼2.6 × 1021 cm−3 for p-type NbFeSb, to ∼4 × 1021 cm−3 for n-type TiPtSb, whilst the  increases from 2.8 me, 6.4 me, to 14.5 me, respectively [174, 184, 188]. In comparison, the nopt of PbTe is about 3 × 1019 cm−3 [240], one or two orders of magnitude lower than that of the HH system. High nopt indicates that a high level of chemical doping is required for optimizing the electrical performance, which could also bring additional point-defect scattering of phonons. Thus, the selection of a rational doping element is important for the simultaneous optimization of PF and strong suppression of lattice thermal conductivity in the heavy-band HH system [175, 179].
increases from 2.8 me, 6.4 me, to 14.5 me, respectively [174, 184, 188]. In comparison, the nopt of PbTe is about 3 × 1019 cm−3 [240], one or two orders of magnitude lower than that of the HH system. High nopt indicates that a high level of chemical doping is required for optimizing the electrical performance, which could also bring additional point-defect scattering of phonons. Thus, the selection of a rational doping element is important for the simultaneous optimization of PF and strong suppression of lattice thermal conductivity in the heavy-band HH system [175, 179].
Knowledge of the intrinsic electronic structure of thermoelectric materials is of vital importance for the selection of optimization strategies. The bandgap Eg is considered to be the foremost parameter for a semiconductor. The calculated Eg for MNiSn, MCoSb, and XFeSb by density functional theory (DFT) is 0.4–0.5 eV, 0.95–1.13 eV, and 0.34–0.86 eV, respectively [163, 172, 173, 237, 238, 241–243]. Experimentally, polycrystalline MNiSn samples, synthesized using high-temperature techniques and probably having excess Ni-induced in-gap states, show Eg values of 0.1–0.36 eV by different experimental methods. Recently, using high-quality ZrNiSn single crystals, a combined study involving resistivity and optical measurements together with angle-resolved photoemission spectroscopy (ARPES) gave experimental Eg values of 0.5–0.66 eV [244]. These results demonstrate the effect of defects on the electronic structure and thermoelectric properties of HH compounds. Experimental studies on the interplay between defects and electronic structure for the other HH compounds are required.
Most HH compounds with high thermoelectric performance are generally heavily doped narrow-bandgap semiconductors. The dominant scattering mechanism is acoustic phonon scattering (APS) and the electrical transport properties can be explained using the SPB model. Under the assumption of SPB and APS, the weighted mobility μW performs as a good descriptor characterizing the electrical performance for thermoelectric materials [245]. The μW values of MNiSn and XFeSb are above 300 cm2V−1s−1 at room temperature and above 60 cm2V−1s−1 at temperatures higher than 900 K. In contrast, the MCoSb system shows values of 100–250 cm2V−1s−1 at room temperature and less than 60 cm2V−1s−1 at high-temperature, corresponding to the lower zT compared with that of MNiSn and XFeSb. It is worth noting that ZrCoBi and NbCoSn also have a μW above 300 cm2V−1s−1 at room temperature, implying their potential as good TEs.
High lattice thermal conductivity, κL, is the main disadvantage of HH compounds that prevents high thermoelectric figures of merit. By introducing multiple phonon scattering mechanisms through alloying, nanostructuring, the formation of nanocomposites, and phase separation, the κL, at the temperature where the peak zT occurs, can be largely suppressed to values of 2–3 W m−1K−1, which, however, is still higher than its minimum value (∼1 W m−1K−1 above 300 K) estimated using the Cahill model [246]. In comparison, the nominal 19-electron HH compounds, such as unalloyed XCoSb and TiPtSb, show significantly lower κL below 2 W m−1K−1 in the high-temperature region [182–184], which can be ascribed to strong point defect scattering resulting from the existence of substantial intrinsic cation vacancies. However, the net lower carrier mobility, compared to the routine 18-electron HH systems, limits their thermoelectric performance. This highlights the dilemma in developing high-performance HH thermoelectric materials, specifically, how to maximally suppress lattice thermal conductivity while maintaining high carrier mobility [124].
In summary, the past two decades have witnessed significant development of HH thermoelectric materials with the establishment of several low-cost, high-performance material systems. Targeting the future optimization of thermoelectric performance and practical application of HH compounds, several future directions are suggested:
- (a)The interplay of point defects, electronic structures and transport properties is an appealing theme, including intrinsic defects in the 18-electron HH system and short-range order in the defective 19-electron HH system.
- (b)Further reduction in thermal conductivity, especially near room-temperature, is highly desirable, with the aim of improving the average zT.
- (c)The development of devices using the current best HH thermoelectric compounds is progressing but the related interfacial issues need to be solved. In addition, active Peltier coolers, which requires materials with high PF and high κ also brings new potential applications for HH compounds [247].
- (d)HH is a large compound family with many members; the exploration of new thermoelectric candidates in the HH system is always attractive. To aid exploration and development, guidance from accurate, rapid electronic and phonon calculations is important.
Acknowledgments
Tiejun Zhu and Chenguang Fu acknowledge the support from the National Key Research and Development Program of China (2019YFA0704902) and the National Science Fund for Distinguished Young Scholars (No. 51725102).
3.4. Zintls
Thermoelectric performance in Zintl phases: a bird's-eye view
A K M Ashiquzzaman Shawon and Alexandra Zevalkink
Chemical Engineering and Materials Science Department, Michigan State University, East Lansing, MI 48824, United States of America
E-mail: alexzev@msu.edu
One of the most recent additions to the thermoelectric material realm, Zintl phases are a class of intermetallic compounds following the Zintl-Klemm concept, with structures consisting of both covalent and ionic bonding. The cations (typically alkali, alkaline-earth or rare-earth metals) are treated as fully ionized, providing valence electrons to main group metalloids, which in turn form covalent bonds to achieve a closed-shell configuration. Although united by a common bonding scheme, the crystal structures of Zintl phases vary widely. Enthusiasm for Zintl thermoelectrics stems primarily from their structural complexity and diversity, with novel structures and compounds being reported regularly [253]. Further, many Zintls exhibit salt-like behavior with high melting points and brittle mechanical properties.
Zintl compounds are often structurally complex, which leads to very low thermal conductivities, comparable in most cases with the amorphous limit. This, along with the prospect of tuning carrier transport properties through doping, makes Zintl phases ideal thermoelectric materials. Of the Zintl phases studied in the last two decades, the A14MX11 (A = Ca, Sr, Ba, Yb, Eu; M = Zn, Cd, Mn, Mg; X = As, Sb, Bi) and AM2X2 (A = Ca, Sr, Ba, Yb, Eu, Mg; M = Zn, Cd, Mn, Mg, Ga; X = As, Sb, Bi) compounds have been most widely investigated. Other important classes of Zintl phases include those with compositions A5M2X6, A11M6X12, A3MX3 and A9M4.5X9. These materials have significant promise, as depicted by both theoretical modeling and experimental results. With the exception of the AM2X2 family, the compounds above exhibit extremely low inherent lattice thermal conductivities. Their optimized thermoelectric properties are found at various temperatures depending on the compositions. This opens up the possibility of applications at low, mid and high temperatures. Yb14MnSb11 and Yb14MgSb11 are two of the most promising p-type thermoelectric materials available for high-temperature applications. At intermediate temperatures, p-type AM2X2 compounds have zT values as high as 1.3, and compounds like Ca9Zn4.5+x Sb9 have already reached a zT value of 1.1.
Most Zintl thermoelectrics are p-type, as they have a tendency to form intrinsic acceptor type defects (e.g. cation vacancies). However, recent progress has uncovered strategies to achieve n-type behavior. The most prominent and successful examples are Mg3Sb2-based compounds, which are now important enough to deserve their own section 3.5. The only other n-type Zintl phases with promising performance, to date, are the AMX4 (A = Na, K, Rb, Cs; M = Al, Ga, In; X = As, Sb, Bi) compounds. While their properties are impressive, reaching zT values >1, there is still much need for newer n-type compound discovery.
Two sets of data are presented in table 4 for each composition, for room temperature and for the temperature with the maximum thermoelectric figure of merit. To ensure a consistent approach to calculating κL, we used the reported total thermal conductivity, and estimated the electronic contribution (κE) using the Wiedemann–Franz law [3]. The Lorenz number was calculated using the equation of Kim et al [254]. The values of κL reported in the table are then given by κ − κE.
Table 4. Zintl thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κ (W m−1 K−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | n (1020 cm−3) | µ0 (cm2 V−1 s−1) | µH (cm2 V−1 s−1) | ms* (me) |
 r or r or  ( ( 0) 0) | L (10−8 W Ω K−2) | κE (W m−1 K−1) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca14 MgSb10.80 Sn0.20 | 300 | 5.1 | 0.52 | 0.50 | 115.3 | 31.25 | 0.02 | 0.6 | — | — | — | — | — | 1.87 | 0.02 | [256] |
| Ca14 MgSb10.80 Sn0.20 | 1075 | 4.9 | 0.76 | 0.61 | 193 | 80 | 0.49 | — | — | — | — | — | — | 1.69 | 0.15 | [256] |
| Sr14 MgSb11 | 300 | 1.6 | 0.55 | 0.55 | 230 | 2.45 | 0.007 | 1.18 | — | — | — | — | — | — | — | [257] |
| Sr14 MgSb11 | 773 | 1.9 | 0.8 | 0.69 | 110 | 50 | 0.067 | — | — | — | — | — | — | — | — | [257] |
| Eu14 MgSb11 | 300 | 3.6 | 0.775 | 0.58 | 12.5 | 241.4 | 0.002 | — | — | — | — | — | — | — | — | [257] |
| Eu14 MgSb11 | 823 | 3.1 | 0.82 | 0.45 | 55 | 210.5 | 0.08 | — | — | — | — | — | — | — | — | [257] |
| Yb14 Mn0.2 Al0.8 Sb11 | 300 | 14.4 | 0.75 (500 K) | 0.42 (500 K) | 50 | 238.1 | 0.08 | 0.55 | 4.35 | — | 5.5 | 2.75 | — | — | — | [253] |
| Yb14 Mn0.2 Al0.8 Sb11 | 1223 | 9.7 | 0.5 | 0.25 | 240 | 109.9 | 1.3 | — | — | — | — | — | — | — | — | [253] |
| Yb14 MgSb11 | 300 | 24.3 | 0.67 | 0.41 | 50 | 400 | 0.005 | 0.56 | 5.3 | — | 4.66 | — | — | 2.15 | 0.26 | [258, 259] |
| Yb14 MgSb11 | 1200 | 10.7 | 0.73 | 0.46 | 228 | 136.4 | 1.26 | — | 11.2 | — | 0.7 | — | — | 1.64 | 0.27 | [258] |
| Yb14 MgBi11 | 300 | 22.2 | 1.56 | 0.97 | 25 | 740.7 | 0.015 | — | 6.9 | — | 8 | — | — | — | — | [260] |
| Yb14 MgBi11 | 1073 | 12.3 | 1.6 | 0.56 | 110 | 540.5 | 0.42 | — | 7.25 | — | 6.1 (573 K) | — | — | — | — | [260] |
| Eu14 MgBi11 | 300 | 18.5 | 1.55 | 1.07 | 27 | 571.4 | 0.015 | — | 7.25 | — | 5.2 | — | — | — | — | [260] |
| Eu14 MgBi11 | 1073 | 12.2 | 1.6 | 0.70 | 121 | 465.1 | 0.46 | — | 7.8 | — | 4.15 (573 K) | — | — | — | — | [260] |
| Ca14 MgBi11 | 300 | 13.7 | 0.75 | 0.61 | 56 | 200 | 0.046 | 1.06 | 5.9 | — | 2.2 | — | — | — | — | [260] |
| Ca14 MgBi11 | 1073 | 6.4 | 1.6 | 0.92 | 97.5 | 333.33 | 0.21 | — | 6.75 | — | 2.3 (573 K) | — | — | — | — | [260] |
| Sr14 MgBi11 | 300 | 24.0 | 1.26 | 0.78 | 32 | 625 | 0.03 | — | 4.75 | — | 9.8 | — | — | — | — | [260] |
| Sr14 MgBi11 | 1073 | 16.6 | 1.48 | 0.54 | 140 | 500 | 0.72 | — | 5.3 | — | 7.05 (573 K) | — | — | — | — | [260] |
| CaZn2 Sb2 | 300 | 73.8 | 2.6 | 2.37 | 120 | 421.9 | 0.075 | 0.33 | 1.9 | — | 45 | 0.6 | 1.86 | 0.23 | [255, 261, 262] | |
| CaZn2 Sb2 | 773 | 30.8 | 1.42 | 0.95 | 178.9 | 358.42 | 0.56 | — | — | — | — | — | — | 1.71 | 0.47 | [255, 261, 262] |
| 300 | 123.6 | 1.225 | 0.95 | 157 | 450 | 0.3 | 0.44 | 0.26 | — | 106.67 | 0.82 | — | — | — | ||
| Yb0.9Mg0.1Mg0.8Zn1.198Ag0.002 Sb2 | 773 | 46.3 | 0.76 | 0.47 | 256 | 220 | 1.5 | — | — | — | 35 | — | — | — | — | [255, 263] |
| Mg3 Sb1.8 Pb0.2 | 300 | 6.3 | 0.69 | 0.68 | 183 | 17 | 0.025 | 0.8 | 5.8 | — | 12.1 | 1.21 | 32.04 | — | — | [255, 264, 265] |
| Mg3 Sb1.8 Pb0.2 | 773 | 10.1 | 0.30 | 0.25 | 280 | 36.25 | 0.84 | — | — | — | — | 1.21 | — | — | [255, 264, 265] | |
| Ca0.99 Na0.01 MgZnSb2 | 300 | 70.5 | 1.39 | 1.27 | 167.5 | 226.67 | 0.16 | 1.12 | 0.32 | 60 | — | 1.1 | — | — | — | [262, 266] |
| Ca0.99 Na0.01 MgZnSb2 | 850 | 54.6 | 0.82 | 0.35 | 245 | 340 | 0.87 | 0.5 | 22 | — | — | — | — | — | [262, 266] | |
| EuCd1.4 Zn0.6 Sb2 | 300 | 128.0 | 1.76 | 1.43 | 130 | 645.16 | 0.18 | 0.5 | 0.21 | — | 182.4 | 0.56 | — | — | — | [255, 267] |
| EuCd1.4 Zn0.6 Sb2 | 700 | 45.4 | 1 | 0.72 | 219 | 285.71 | 0.96 | 0.5 | 0.23 | — | 80 | 0.62 | — | — | — | [255, 267] |
| Ba0.7975 Yb0.2 Na0.0025 Cd2 Sb2 | 300 | 81.6 | 1.65 | 1.30 | 100 | 606 | 0.1 | 0.7 | 0.4 | — | 12 | 0.315 | 0.29 | — | — | [268] |
| Ba0.7975 Yb0.2 Na0.0025 Cd2 Sb2 | 700 | 34.1 | 0.83 | 0.55 | 208 | 244 | 0.92 | 0.42 | — | 13 | 0.37 | — | — | — | [268] | |
| YbCd1.6 Zn0.4 Sb2 | 300 | 159.0 | 1.85 | 1.19 | 102 | 1150 | 0.2 | 0.63 | 0.6 | — | 118 | 0.74 | — | — | — | [255, 269] |
| YbCd1.6 Zn0.4 Sb2 | 700 | 58.2 | 1.1 | 0.44 | 184 | 550 | 1.2 | — | — | — | — | — | — | 1.70 | 0.66 | [255, 269] |
| Eu0.2 Yb0.2 Ca0.6 Mg2 Bi2 | 300 | 137.2 | 1.5 | 1.25 | 165 | 454.5 | 0.25 | 0.46 | 0.177 | — | 171 | 0.77 | — | — | — | [255, 270] |
| Eu0.2 Yb0.2 Ca0.6 Mg2 Bi2 | 873 | 33.0 | 0.925 | 0.52 | 217.5 | 294.1 | 1.3 | — | — | — | — | — | [255, 270] | |||
| Ca0.5 Yb0.5 Mg2 Bi2 | 300 | 68.3 | 1.25 | 1.20 | 260 | 75 | 0.12 | 0.5 | 0.033 | — | 138 | 0.61 | — | — | — | [255, 271] |
| Ca0.5 Yb0.5 Mg2 Bi2 | 873 | 28.7 | 1.115 | 0.55 | 188 | 360 | 0.98 | — | — | — | — | — | — | — | — | [255, 271] |
| Ba1.975 Na0.025 Ga2 Sb2 | 300 | 53.4 | 1.46 | 1.27 | 140 | 238.1 | 0.1 | 0.4 | 1.02 | — | 15.2 | 1.7 | — | — | — | [272] |
| Ba1.975 Na0.025 Ga2 Sb2 | 750 | 22.5 | 0.98 | 0.72 | 200 | 196.1 | 0.65 | — | 1.6 | — | 7.1 | — | — | — | [272] | |
| Yb9 Mn4.2 Sb9 | 300 | 9.5 | 0.675 | 0.46 | 60 | 307.7 | 0.033 | 0.4 | 2.1 | — | 10 | 1.2 | — | — | — | [273] |
| Yb9 Mn4.2 Sb9 | 973 | 20.7 | 0.59 | 0.39 | 186 | 129 | 0.75 | — | 2 | 5 | 4 | — | — | — | [273] | |
| Eu9 Cd3.76 Ag1.43 Sb9 | 300 | 56.6 | 1.44 | 0.84 | 39 | 1204.8 | 0.03 | — | 1.6 | — | 36 | 0.44 | — | — | — | [274] |
| Eu9 Cd3.76 Ag1.43 Sb9 | 773 | 13.6 | 0.92 | 0.40 | 85 | 515.5 | 0.29 | — | 2 | — | 15 | — | — | — | [274] | |
| Ca9 Zn4.6 Sb9 | 300 | 36.4 | 0.67 | 0.65 | 170 | 113.6 | 0.1 | 0.35 | 0.22 | — | 30.5 | 0.56 (400 K) | — | — | — | [275] |
| Ca9 Zn4.6 Sb9 | 875 | 18.2 | 0.47 | 0.36 | 268 | 90.9 | 1.1 | — | 0.46 | — | 11 | — | — | — | [275] | |
| Eu11 Cd6 Sb12 | 300 | 24.4 | 0.82 | 0.71 | 118 | 142.9 | 0.06 | — | 0.35 | — | 33.5 | — | — | — | [276] | |
| Eu11 Cd6 Sb12 | 773 | 5.2 | 0.68 | 0.54 | 132 | 105.3 | 0.23 | — | 1.2 | — | 7 | — | — | — | [276] | |
| Eu11 Cd4.5 Zn1.5 Sb12 | 300 | 46.2 | 0.88 | 0.70 | 132 | 227.2 | 0.11 | 0.1 | 0.25 | — | 53 | 0.6 | — | — | — | [277] |
| Eu11 Cd4.5 Zn1.5 Sb12 | 800 | 12.6 | 0.65 | 0.42 | 188 | 138.9 | 0.51 | — | 0.75 | — | 10 | — | — | — | [277] | |
| Ca5 Al0.95 In0.95 Zn0.1 Sb6 | 300 | 28.7 | 1.12 | 1.05 | 140 (423 K) | 128.2 (323 K) | 0.02 | 0.65 | 2 | — | 4.9 | 1.92 | — | 1.80 | 0.07 | [278, 279] |
| Ca5 Al0.95 In0.95 Zn0.1 Sb6 | 900 | 16.4 | 0.81 | 0.56 | 210 | 166.67 | 0.7 | — | 4 | — | 2 | — | 1.66 | 0.25 | [278, 279] | |
| Ca5 Ga1.9 Zn0.1 Sb6 | 300 | 24.5 | 1.65 | 1.50 | 60 | 333.33 | 0.04 | 0.43 | 5 | — | 8 | 1.46 | — | — | — | [279, 280] |
| Ca5 Ga1.9 Zn0.1 Sb6 | 773 | 15.1 | 1.1 | 0.70 | 150 | 246.9 | 0.37 | — | 6.5 | — | 3.5 | — | — | — | [279, 280] | |
| Ca5 In1.9 Zn0.1 Sb6 | 300 | 26.9 | 1.25 (323 K) | 1.13 (323 K) | 100 (323 K) | 200 | 0.025 | 0.64 | 2 | — | 5.5 | 1.8 | — | — | — | [279, 281] |
| Ca5 In1.9 Zn0.1 Sb6 | 973 | 11.5 | 0.75 | 0.53 | 190 | 166.67 | 0.72 (943 K) | — | 3.8 | — | 2.2 | — | — | — | — | [279, 281] |
| Sr5 In1.9 Zn0.1 Sb6 | 300 | 3.8 | 1.14 | 1.10 | 155 (325 K) | 14.3 | 0.01 | 0.44 | 0.7 | — | 2.1 (350 K) | — | — | — | — | [282] |
| Sr5 In1.9 Zn0.1 Sb6 | 750 | 15.3 | 0.78 | 0.70 | 275 | 55.6 | 0.4 | — | 1.6 | — | 2.5 | — | — | — | — | [282] |
| Eu5 In1.9 Zn0.1 Sb6 | 300 | 9.3 | 1.16 | 1.12 | 92 | 76.9 | 0.018 | 0.29 | — | — | 5 | 1.5 | — | — | — | [283] |
| Eu5 In1.9 Zn0.1 Sb6 | 673 | 18.0 | 0.84 | 0.80 | 200 | 133.33 | 0.39 | — | — | — | 8.5 | — | — | — | [283] | |
| Ca2.97 Na0.03 AlSb3 | 300 | 1.8 | 1.65 | 1.63 | 120 | 10 | ∼ | 0.5 | 0.46 | — | 1.25 | 0.8 | — | — | — | [284] |
| Ca2.97 Na0.03 AlSb3 | 1050 | 12.4 | 0.67 | 0.58 | 285 | 66.67 | 0.8 | — | 0.65 | — | 5.74 | — | — | — | — | [284] |
| Sr3 AlSb3 | 300 | 1.8 | 1.1 | 1.10 | 325 | 0.95 | ∼ | 0.6 | 0.8 | — | 1.25 | — | — | 1.56 | 0.00 | [285] |
| Sr3 AlSb3 | 800 | 14.6 | 0.575 | 0.57 | 450 | 7.69 | 0.15 | — | 4 | — | 1.5 | — | — | 1.52 | 0.01 | [285] |
| Sr3 Ga0.93 Zn0.07 Sb3 | 300 | 22.0 | 1.1 | 1.03 | 110 | 142.9 | 0.015 | 0.75 | 1.01 | — | 13 | 0.9 | — | — | — | [286] |
| Sr3 Ga0.93 Zn0.07 Sb3 | 1000 | 11.1 | 0.65 | 0.43 | 215 | 125 | 0.9 | — | 0.7 | — | 7 | — | — | — | — | [286] |
| K0.99 Ba0.01 AlSb4 | 298 | 11.6 | 1.16 | 1.05 | −150 | 45.5 | 0.025 | 0.5 | 0.1 | — | 18 | 0.5 | — | — | — | [287] |
| K0.99 Ba0.01 AlSb4 | 643 | 29.0 | 0.645 | 0.52 | −260 | 100 | 0.72 | — | 0.1 | — | 52 | — | — | — | — | [287] |
| K0.985 Ba0.015 GaSb4 | 323 | 53.3 | 1.25 | 1.14 | −145 | 250 | 0.143 | 0.39 | 0.021 | — | 70 | 0.6 | — | — | — | [288] |
| K0.985 Ba0.015 GaSb4 | 673 | 30.0 | 0.6 | 0.45 | −238 | 142.9 | 0.94 | — | 0.019 | — | 50 | — | — | — | [288] | |
| Yb20.6 Na0.4 Mn4 Sb18 | 300 | 20.7 | 0.6 | 0.54 | 125 | 111.11 | 0.07 | 0.4 | 2.2 | — | 2.75 | 5.7 | — | — | — | [289] |
| Yb20.6 Na0.4 Mn4 Sb18 | 800 | 16.5 | 0.45 | 0.38 | 290 | 55.56 | 0.78 | — | 3.2 | — | 0.75 | — | — | — | — | [289] |
| LiZnSb | 300 | 160.0 | 5.5 | 3.50 | 45 | 2941 | 0.034 | — | 3.5 | — | 52 | 0.7 | — | — | — | [290] |
| LiZnSb | 525 | 58.9 | 4.5 | 2.20 | 58 | 1923 | 0.075 | — | 5 | — | 24 | — | — | — | — | [290] |
| Ca0.84 Ce0.16 Ag0.87 Sb | 300 | 79.3 | 1.75 | — | 33 | 2000 | 0.05 | 0.18 | — | — | — | 0.82 | — | — | — | [291, 292] |
| Ca0.84 Ce0.16 Ag0.87 Sb | 1073 | 20.9 | 1.55 | — | 100 | 1052.6 | 0.66 | — | — | — | — | — | — | — | — | [291, 292] |
| Mg0.97 Zn0.03 Ag0.9 Sb0.95 | 323 | 177.9 | 0.62 | 0.54 | 284 | 165.5 | 1.11 | — | 0.25 | — | 49.1 | — | 1.54 | 0.08 | — | [293] |
| Mg0.97 Zn0.03 Ag0.9 Sb0.95 | 423 | 142.8 | 0.70 | 0.52 | 246 | 307.21 | 1.4 | — | 0.54 | — | 33.9 | — | 1.55 | 0.18 | — | [293] |
| (Zn3.98 Pb0.02 Sb3 )0.97 (Cu3 SbSe4 )0.03 | 300 | 109.6 | 0.95 | 0.59 | 125 | 588.2 | 0.28 | 0.26 | 2.24 | — | 16.8 | 2.14 | — | — | — | [294] |
| (Zn3.98 Pb0.02 Sb3 )0.97 (Cu3 SbSe4 )0.03 | 650 | 61.1 | 0.7 | 0.20 | 195 | 454.5 | 1.25 | — | — | — | — | — | — | — | — | [294] |
L = Lorenz number.
Figure 5 shows the weighted mobility vs lattice thermal conductivity for selected Zintl compounds at 300 K, including both n-type (open symbols) and p-type (filled symbols). The contour lines represent slopes, denoted by m. The ratio of weighted mobility to lattice thermal conductivity is directly proportional to the thermoelectric quality factor, B, which indicates the potential zT that could be achieved under optimal doping concentrations [245]. The relationship is given in equation (3). Therefore, as a general rule, compounds closer to the top left corner of the plot are expected to have better thermoelectric performance when optimizally doped. Indeed, the n-type Mg3Sb2−x
Bix
compounds show the most promising ratio of  at room temperature.
at room temperature.
Figure 5. Weighted mobility vs lattice thermal conductivity plot for several promising members of the Zintl family. Filled markers indicate p-type while hollow markers show n-type materials. The abbreviations MA, AM, SPS and VHP refer to mechanical alloying, arc melting, spark plasma sintering and vacuum hot pressing respectively.
Download figure:
Standard image High-resolution imageThe introduction of Zintl phases for thermoelectric applications has provided the option to find inherently low lattice thermal conductivity materials made of earth-abundant, non-toxic elements. While many p-type Zintl compounds have been discovered with respectable zT values, tuning Zintl compounds n-type is particularly challenging. This is primarily the case due to the low band degeneracy in conduction bands, which are largely dominated by the s-orbitals of the cation [255]. The discovery of n-type Mg3Sb2 phases are attributed largely to the low formation energy of Mg vacancies, but this is not a common phenomenon. Therefore, new Zintl phases new phases are being actively pursued computationally and experimentally. Furthermore, the elements generally used to make Zintl compounds are highly reactive. Though most Zintls studied for thermoelectrics are stable in air, such is not the case for the entire family. This is a hindrance when it comes to the pursuit of new n-type Zintl compounds.
Acknowledgment
A K M Ashiquzzaman Shawon has received funding from the National Science Foundation (Award No. 2045122).
3.5. Mg3Sb2
High thermoelectric performance of n-type Mg3Sb2-Mg3Bi2 alloys
Kazuki Imasato1,2 and G Jeffrey Snyder1
1Department of Materials Science and Engineering, Northwestern University, Evanston, IL, United States of America
2Global Zero Emission Research Center, National Institute of Advanced Industrial Science and Technology, Tsukuba (AIST), Japan
The n-type Mg3Sb2-Mg3Bi2 alloys (Mg3
X2 where X is a group 15 element, often a mixture of Sb and Bi) are receiving heightened attention as one of the most prominent thermoelectric materials with high performance in the range of room temperature (∼300 K) to mid-temperature (∼700 K) [295–298]. Owing to their highly degenerate conduction band structure (Valley degeneracy Nv = 6) [295, 296, 299] and extremely low phonon thermal conductivity ( ∼ 0.5 W m−1 K−1), Peng et al [300] reported zT values are higher than 1.5 at 700 K and reaching 1.0 around room temperature. Since the discovery of n-type Mg3Sb1.5Bi0.5 [295], extensive research has been conducted to optimize their thermoelectric performance by engineering the electronic band structure [301–303], chemical doping [304–310], and the optimization of microstructure [311–314]. The crystal structure of Mg3Sb2 and Mg3Bi2 is identical to that of a relatively large class of AM2
X2 Zintl phases. The space group is P-3m1 (No. 164). Mg3Sb2 and Mg3Bi2 can be treated as the special cases of AM2
X2 in which Mg atoms occupy both the A and M sites. Twelve different reports for the most studied composition Mg3Sb1.5Bi0.5 and some other compositions with different Sb:Bi ratios are summarized in table 5.
∼ 0.5 W m−1 K−1), Peng et al [300] reported zT values are higher than 1.5 at 700 K and reaching 1.0 around room temperature. Since the discovery of n-type Mg3Sb1.5Bi0.5 [295], extensive research has been conducted to optimize their thermoelectric performance by engineering the electronic band structure [301–303], chemical doping [304–310], and the optimization of microstructure [311–314]. The crystal structure of Mg3Sb2 and Mg3Bi2 is identical to that of a relatively large class of AM2
X2 Zintl phases. The space group is P-3m1 (No. 164). Mg3Sb2 and Mg3Bi2 can be treated as the special cases of AM2
X2 in which Mg atoms occupy both the A and M sites. Twelve different reports for the most studied composition Mg3Sb1.5Bi0.5 and some other compositions with different Sb:Bi ratios are summarized in table 5.
Table 5. Mg3Sb2 thermoelectric properties.
| Composition (dopant/additive) | Bicontent | Dopant/additive | T (K) | µw (cm2 V−1 s−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µ0 (cm2 V−1 s−1) | ms* (me) |
 r or r or  ( ( 0) 0) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg3.2Sb1.5Bi0.49Te0.01 | 25% | Te | 350 | 47.1 | 0.85 | −214.8 | 110.1 | 0.19 | — | 41.7 | 1.08 | — | [295] |
| — | — | 550 | 81.0 | 0.64 | −254.4 | 235.4 | 0.98 | — | 76.1 | 1.04 | — | ||
| — | — | 700 | 67.6 | 0.56 | −281.6 | 206.0 | 1.44 | — | 53.9 | 1.16 | — | ||
| Mg3.2Sb1.5Bi0.49Te0.01 | 25% | Te | 350 | 192.0 | 0.74 | −203.0 | 514.6 | 0.71 | — | 148.6 | 1.19 | — | [298] |
| — | — | 550 | 99.7 | 0.64 | −247.7 | 313.2 | 1.15 | — | 79.3 | 1.17 | — | ||
| — | — | 700 | 66.3 | 0.59 | −275.4 | 216.9 | 1.38 | — | 48.3 | 1.23 | — | ||
| Mg3.2Sb1.5Bi0.49Te0.01 | 25% | Te | 350 | 162.9 | 0.68 | −210.4 | 400.4 | 0.68 | — | 129.8 | 1.16 | — | [333] |
| — | — | 550 | 107.2 | 0.47 | −255.0 | 309.7 | 1.48 | — | 82.0 | 1.20 | — | ||
| — | — | 700 | 69.7 | 0.49 | −280.7 | 214.6 | 1.63 | — | 49.4 | 1.26 | — | ||
| Mg3Sb1.48Bi0.48Te0.04 | 25% | Te | 350 | 129.3 | 0.53 | −213.9 | 305.0 | 0.69 | 0.6 eV (DFT) | 106.7 | 1.14 | — | [318] |
| — | — | 550 | 77.7 | 0.41 | −252.1 | 232.1 | 1.32 | — | 55.4 | 1.25 | — | ||
| — | — | 700 | 50.0 | 0.35 | −264.3 | 185.9 | 1.63 | — | 30.6 | 1.39 | — | ||
| Mg3.2Sb1.5Bi0.49Te0.01 | 25% | Te | 350 | 51.7 | 0.95 | −209.1 | 129.0 | 0.19 | 0.433 eV | 29.5 | 1.45 | — | [297] |
| — | — | 550 | 88.0 | 0.67 | −251.9 | 263.2 | 1.02 | — | 55.7 | 1.36 | — | ||
| — | — | 700 | 63.5 | 0.60 | −274.5 | 209.9 | 1.33 | — | 42.3 | 1.31 | — | ||
| Mg3+δ Sb1.5Bi0.49Te0.01 | 25% | Te | 350 | 219.6 | 0.67 | −200.0 | 609.3 | 0.83 | — | — | [313] | ||
| — | — | 550 | 91.2 | 0.59 | −240.2 | 312.3 | 1.15 | — | — | ||||
| Mg3.15Nb0.05Sb1.5Bi0.49Te0.01 | 25% | Te, Nb | 350 | 153.1 | 0.76 | −204.3 | 404.1 | 0.59 | — | 105.4 | 1.28 | — | [317] |
| — | — | 550 | 112.2 | 0.71 | −249.2 | 346.2 | 1.32 | — | 85.9 | 1.20 | — | ||
| — | — | 700 | 65.9 | 0.46 | −274.5 | 217.7 | 1.36 | — | 53.0 | 1.16 | — | ||
| Mg3.175Mn0.025Sb1.5Bi0.49Te0.01 | 25% | Te, Mn | 350 | 165.7 | 0.77 | −232.0 | 316.9 | 0.63 | — | 98.4 | 1.42 | — | [334] |
| — | — | 550 | 132.7 | 0.60 | −271.9 | 314.9 | 1.46 | — | 95.9 | 1.24 | — | ||
| — | — | 700 | 90.7 | 0.55 | −297.5 | 229.7 | 1.78 | — | 65.0 | 1.25 | — | ||
| Mg3.17B0.03Sb1.5Bi0.49Te0.01 | 25% | Te, B | 350 | 212.0 | 0.62 | −205.7 | 550.2 | 0.87 | — | 196.0 | 1.05 | — | [343] |
| — | — | 550 | 117.3 | 0.51 | −260.7 | 317.1 | 1.50 | — | 80.1 | 1.29 | — | ||
| — | — | 700 | 75.3 | 0.49 | −287.7 | 213.7 | 1.71 | — | 45.6 | 1.40 | — | ||
| Mg3.2Nd0.03Sb1.5Bi0.5 | 25% | Nd | 350 | 78.6 | 0.82 | −152.8 | 384.6 | 0.30 | — | 48.5 | 1.38 | — | [341] |
| — | — | 550 | 111.5 | 0.53 | −222.7 | 468.1 | 1.34 | — | 57.5 | 1.56 | — | ||
| Mg3.05La0.005Sb1.5Bi0.5 | 25% | La | 350 | 95.9 | 0.86 | −200.9 | 263.3 | 0.37 | — | 79.2 | 1.14 | — | [306] |
| — | — | 550 | 80.8 | 0.59 | −261.0 | 217.6 | 1.05 | — | 67.8 | 1.12 | — | ||
| Mg3+δ Sb1.5Bi0.49Te0.01Mn0.01 | 25% | Te, Mn | 350 | 225.1 | 0.80 | −213.3 | 535.3 | 0.77 | 0.279 (FTIR) | — | — | — | [302] |
| — | — | 550 | 133.8 | 0.61 | −246.9 | 424.3 | 1.44 | — | — | — | — | ||
| — | — | 700 | 82.5 | 0.61 | −274.7 | 272.1 | 1.57 | — | — | — | |||
| Mg3.2Sb1.99Te0.01 | 0% | Te | 350 | 51.7 | 0.93 | −209.1 | 129.0 | 0.20 | 0.54 | 29.5 | 1.45 | — | [301] |
| — | — | 550 | 78.6 | 0.68 | −242.1 | 263.2 | 0.93 | — | 56.1 | 1.25 | — | ||
| — | — | 700 | 63.5 | 0.64 | −274.5 | 209.9 | 1.27 | — | 42.3 | 1.31 | — | ||
| Mg3Sb1.25Bi0.75Y0.01 | 37.50% | Y | 350 | 128.3 | 1.41 | −215.5 | 297.3 | 0.31 | — | 47.8 | 1.93 | — | [337] |
| Mg3+δ Sb1.1Bi0.89Te0.01Mn0.01 | 45% | Te, Mn | 350 | 217.7 | 0.79 | −182.7 | 740.1 | 0.70 | 0.242 (FTIR) | — | — | — | [302] |
| — | — | 550 | 115.4 | 0.69 | −241.5 | 389.6 | 1.20 | — | — | — | — | — | |
| — | — | 700 | 70.0 | 0.70 | −264.3 | 260.4 | 1.29 | — | — | — | — | — | |
| Mg3.05Sb1.0Bi0.97Te0.03 | 50% | Te | 350 | 230.9 | 0.69 | −180.9 | 801.6 | 0.79 | 0.364 (DFT) | 135.7 | 1.43 | — | [329] |
| — | — | 550 | 92.8 | 0.49 | −209.6 | 454.1 | 1.21 | — | 73.4 | 1.17 | — | — | |
| Mg3.05(Sb0.3Bi0.7)1.996Te0.004 | 70% | Te | 350 | 258.4 | 0.61 | −204.7 | 679.0 | 0.99 | 0.208 (G-S gap) | 159.3 | 1.38 | [303] | |
| 550 | 102.9 | 0.64 | −222.0 | 435.5 | 1.14 | — | 92.7 | 1.07 | |||||
| Mg3.2Sb0.5Bi1.498Te0.02 | 75% | Te | 300 | 276.6 | 0.76 | −218.9 | 488.8 | 0.70 | — | 267.9 | 1.02 | [327] | |
| 350 | 226.2 | 0.70 | −234.8 | 419.1 | 0.86 | — | 218.4 | 1.02 |
To synthesize n-type Mg3 X2 an excess of Mg (x > 0) is required, such that the typical nominal stoichiometry is Mg3+x X2, to suppress the formation of Mg vacancies which are an electron killer defect [297]. Note that the actual composition of the Mg3 X2 phase, written with nonstoichimetry as Mg3+δ X2 will have δ much smaller (possibly even δ < 0) than the nominal x needed for processing. The amount of excess Mg required varies, depending on the synthesis route and starting materials (powder, turnings, shot, granules etc). n-type conduction can be achieved as long as the sample is in the Mg-excess thermodynamic condition. This sensitivity of the thermodynamic chemical potential of Mg implies the n-type conduction can be lost during high temperature (T> 700 K) processing as the high vapor pressure and reactivity of Mg leads to a net loss of Mg at elevated temperature [306, 308, 315, 316]. The charge carrier concentration is controlled by using aliovalent substitution, i.e. group 3 elements (e.g. Sc, Y, La) substituting for Mg or group 16 elements (e.g. S, Se or Te) on anion sites, as extrinsic dopants. All good thermoelectric Mg3 X2 materials contain such an extrinsic dopant (e.g. nominal composition Mg3+x Sb1.5Bi0.49Te0.01 [295]) but for convenience they may be formulated simply as Mg3Sb1.5Bi0.5. Te substitution on the anion site has been known as an effective dopant [295–298, 317, 318], while Se and S do not have enough dopability to achieve optimum carrier concentration [319, 320]. Recently, cation site substitutions are reported with higher doping efficiency and thermal stability compared to the anion alternatives [304–310, 321–324].
As Mg3Sb2 and Mg3Bi2 make a solid solution for the entire composition range, the effect of the Sb:Bi ratio has been studied to optimize the thermoelectric performance. In addition to a more than 50% reduction in the lattice thermal conductivity because of alloy scattering [325], Mg3Bi2 alloying with Mg3Sb2 was proven to be an effective way to engineer the electronic band structure [301, 303, 326]. As the Bi content increases, the weighted mobility [245] increases with the reduced effective mass and smaller band gap. The band gap of pure Mg3Sb2 is around 0.5–0.6 eV [296, 306] and decreases with Bi content x in Mg3(Sb1−x Bix )2 [296, 302, 303]. Considering the operating temperature of thermoelectric materials, the Bi 25% composition (Mg3Sb1.5Bi0.5) is the most commonly studied and recognized as the highest zT composition for mid temperature (∼700 K) [301]. The higher Bi compositions (Bi content greater than 50%) were suggested as the optimized composition for lower temperatures including room temperature with reduced band gap [303, 326–330]. On the other hand, alloying on the cation (Mg) site causes a significant reduction in the performance due to the decreased carrier mobility [315]. The reason for this degraded performance is mainly due to disruption in the conduction band because the six degenerate U* (CB1) pockets originate from Mg orbital interaction [306, 315, 331, 332].
Grain boundaries play a significant role in the thermoelectric performance of Mg3
X2. They have been important since the initial studies where undesirable low electronic conductivity led to low performance (zT ∼ 0.1 is reported below ∼500 K [295, 317, 333]. Although often not identified as due to grain boundaries, and sometimes suggested as due to ionized impurity scattering, [298, 317, 334], the effect is significant in most polycrystalline samples. This thermally activated resistivity is particularly strong in Mg3
X2 but can be found in other high-efficiency thermoelectric materials as well [335]. The grain boundary effect can be described by a series circuit model including a bulk phase and a grain boundary phase [311, 333, 336, 337]. Highly resistive grain boundaries can be attributed to charged defects that collect at the grain boundaries that trap and scatter the mobile charge carriers. The grain boundary charge is screened by the dielectric response of the material making grain boundary resistance more observable in low dielectric constant materials such as Mg3Sb2 (DFT calculated relative isotropic dielectric constant of Mg3Sb2
 r = 32 [338]). This grain boundary effect can be mitigated by increasing the grain size through various methods. Some samples optimized for lower temperature ranges possess a performance comparable to the commercialized Bi2Te3 around room temperature [302, 303, 308, 309, 327–329]. While the thermal resistance of the grain boundaries is not very noticeable the dramatically reduced electrical conductivity of the overall sample leads one to expect the electronic contribution to the thermal conductivity within the grains to be much less than it actually is. This leads to a significant overestimation of the lattice thermal conductivity that is commonly reported in systems with grain boundary effects which includes not only Mg3
X2 but many other good thermoelectric materials as well [335].
r = 32 [338]). This grain boundary effect can be mitigated by increasing the grain size through various methods. Some samples optimized for lower temperature ranges possess a performance comparable to the commercialized Bi2Te3 around room temperature [302, 303, 308, 309, 327–329]. While the thermal resistance of the grain boundaries is not very noticeable the dramatically reduced electrical conductivity of the overall sample leads one to expect the electronic contribution to the thermal conductivity within the grains to be much less than it actually is. This leads to a significant overestimation of the lattice thermal conductivity that is commonly reported in systems with grain boundary effects which includes not only Mg3
X2 but many other good thermoelectric materials as well [335].
Some of the recent studies coupled with p-type Bi2Te3 [339] and p-type MgAgSb [340] showed a high conversion efficiency of more than 7% under a temperature difference of ∼300 K at the hot-side temperature around 573 K. Further improvement in the performance could lead to energy harvesting and Internet of things (IoT) thermoelectric devices. Mg3 X2 may have better mechanical properties [341, 342] as well as containing more abundant elements than commercially used Bi2Te3−Sb2Te3 alloys [330]. However, as with any new material, there will be some new challenges to overcome for making devices with n-type Mg3Sb2−Mg3Bi2 alloys. For example, thermal stability will be one of the most important issues in this material. With the reactive nature of Mg and its importance to the n-type behavior, the degradation of performance has been observed. Improved stability has been reported with a chemical substitution/ addition [306, 308, 315, 343] and use of coatings [316]; however, the fundamental mechanism of this degradation needs to be further studied. Beyond high zT and degradation, an assessment of mechanical robustness [302, 343] and processing cost are required to make commercial thermoelectric devices with Mg3Sb2−Mg3Bi2 alloys.
3.6. Clathrates
Clathrate thermoelectrics
Melis Ozen1,2, Kivanc Saglik1,2 and Umut Aydemir1,3
1 Koç University Boron and Advanced Materials Application and Research Center (KUBAM), Istanbul 34450, Turkey
2 Graduate School of Sciences and Engineering, Koç University, Istanbul 34450, Turkey
3 Department of Chemistry, Koç University, Istanbul 34450, Turkey
Clathrates are inclusion compounds with a three-dimensional (3D) framework of tetrahedrally-coordinated host structure, encapsulating in large polyhedral cavities guest molecules, atoms, or ions [344]. The classification of clathrate structures is based on packing of different building polyhedra of various sizes, e.g. pentagonal dodecahedron (formed by 12 pentagons: [512]), tetrakaidekahedron (formed by 12 pentagons and 2 hexagons: [51262]), pentakaidecahedron ([51262]), or hexakaidecahedron ([51264]). In polyanionic clathrates, the framework structure may bear a negative charge with cations (e.g. Na, K, Rb, Sr, Ba) residing as the guest atoms, whereas, in polycationic clathrates, the framework has a positive charge and the anions (e.g. Te, Cl, Br, I) are the guest atoms [345]. Inorganic clathrates crystallize mostly in two common structure types termed as type-I and type-II clathrates (figures 6(a) and (b)). Type-I clathrates are composed of two pentagonal dodecahedra and eight tetrakaidecahedra per unit cell leading to a general chemical formula of G'2
G''8
E46 (G' and G'' indicate guest species in pentagonal dodecahedra and tetrakaidecahedra, respectively) crystallizing in the primitive cubic space group  (no. 223). Type-II clathrates with composition G'16
G''8
E136 crystallize in the space group
(no. 223). Type-II clathrates with composition G'16
G''8
E136 crystallize in the space group  with a framework comprising 16 pentagonal dodecahedra and 8 hexakaidecahedra per unit cell.
with a framework comprising 16 pentagonal dodecahedra and 8 hexakaidecahedra per unit cell.
Figure 6. Crystal structures of (a) type-I clathrate and (b) type-II clathrate. Thermoelectric transport properties for selected clathrates in table 5: (c) Hall mobility (μH) vs Seebeck effective mass (ms *), (d) Seebeck coefficient (S) vs electrical conductivity (σ), (e) total thermal conductivity (κ) vs electrical conductivity, (f) lattice thermal conductivity (κL) vs total thermal conductivity, (g) weighted mobility (μw) vs lattice thermal conductivity, and (h) peak zT vs temperature (T).
Download figure:
Standard image High-resolution imageThe formal electronic structure of intermetallic clathrates can be in most cases adequately described by the Zintl-Klemm concept [346, 347], in which each constituent atom achieves a closed valence shell via a formal charge transfer from the more electropositive atoms to the more electronegative ones. Zintl-Klemm formalism provides a guiding relationship between stoichiometry, structure, and electronic properties. Clathrates with large and weakly bounded ions that can 'rattle' inside oversized cages of the rigid host framework have been discussed in the context of the PGEC concept [348]. These compounds display low lattice thermal conductivity as an inherent property of their complex crystal structure, which is ascribed to the interaction of the heat-carrying phonons with the local vibration modes of guest atoms in the polyhedral cages [349, 350]. The guest-host interactions do not substantially degrade the electronic properties. It is possible to finely adjust the electronic properties of clathrates from metallic to semiconducting behavior by tuning the chemical composition and forming vacancies in their crystal structures. By controlling the concentration of guest atoms in the cages and substituting the framework atoms, the charge carrier concentrations of clathrates can be adjusted effectively. Besides, vacancies in these materials' crystal structures can turn the electrical conduction from n-type to p-type even in the same material system and thus change their thermoelectric properties [351, 352].
Transport properties of different clathrate families (arranged for the majority atoms forming the framework structure) are presented in figures 6(c)–(h). Figure 6(c) shows the trend for Hall mobility (μH) vs Seebeck effective mass (ms *) at 300 K for the clathrates tabulated in table 6. Except for three clathrate compounds, K7.1Ba16.9Ga41.3Sn94.7, Cs8In27Sb19, and Ba6.4La1.6Cu16P30, μH values are almost exclusively well below 60 cm2V−1s−1, which are relatively low compared to other families of thermoelectrics. Low effective masses provide much higher Hall mobility values, mainly observed for the Sn and Ge clathrates. Clathrates can be obtained both n- and p-type as illustrated in figure 6(d) even for the same family of compounds. Clathrates with the homoatomic framework of four-bonded E14 elements do not require additional electrons based on the 8-N rule. Such compounds containing excess electrons are generally observed for silicon clathrates in which the electrons transferred from the guest atoms fill up antibonding conduction bands of the corresponding empty Si46 framework. Clathrates of the heavier homologous (Ge and Sn) may accommodate excess electrons by forming vacancies. Therefore, Si clathrates show relatively low Seebeck coefficient values due to their metallic nature. As the framework is built up of heavier (same group) elements of Ge and Sn, the variation and the absolute values of S increase. This trend can be correlated with doping behavior along with respective changes in the Fermi level of these clathrate families. Higher Seebeck values can be obtained with charge-balanced compositions, which in turn can be achieved more easily for the Ge and especially Sn clathrates thanks to easier substitution with other elements and higher possibility of vacancy formation (note that the bond strengths between the framework atoms decrease down the group for Group 14 elements).
Table 6. Clathrate thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κ (W m−1 K−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µ0 (cm2 V−1 s−1) | µH (cm2 V−1 s−1) | ms* (me) |
 r or r or  ( ( 0) 0) | n (1019 cm−3) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silicides | ||||||||||||||
| Ba8 Cu6 Si40 | 323 | 34.9 | 1.7 | 0.6 | −26 | 1250 | — | — | — | — | — | — | — | [361] |
| 723 | 15.3 | 1.3 | 0.1 | −65 | 714.3 | — | — | — | — | — | — | — | ||
| Ba8 Cu6 Si40 (4 GPa) | 300 | 49.5 | 1.6 | 0.9 | −35 | 1176 | 0.01 | — | 14.8 | 5.2 | 2.18 | — | 140 | [362] |
| 773 | 18.7 | 1.3 | 0.1 | −84.3 | 717.4 | 0.31 | — | — | — | — | — | — | ||
| Ba8 Cu6 Si40 (3 GPa) | 300 | 40 | 1.8 | 1 | −27 | 1234 | 0.01 | — | 15.7 | 4.9 | 1.84 | — | 160 | [362] |
| 773 | 17.8 | 1.4 | 0.3 | −78.3 | 744 | 0.27 | — | — | — | — | — | — | ||
| Ba8 Cu6 Si40 (2 GPa) | 300 | 37.4 | 2.1 | 1.3 | −24 | 1299 | 0.002 | — | 15.9 | 4.6 | 1.73 | — | 175 | [362] |
| 773 | 18 | 1.6 | 0.4 | −74.2 | 799 | 0.16 | — | — | — | — | — | — | ||
| Ba8 Cu6 Si40 (1 GPa) | 300 | 35 | 2.3 | 1.5 | −21 | 1389 | 0.002 | — | 15.4 | 4.2 | 1.69 | — | 205 | [362] |
| 773 | 18.2 | 1.8 | 0.4 | −69.3 | 874.9 | 0.14 | — | — | — | — | — | — | ||
| Ba8 Cu3.89 Si40.16 | 320 | 35.9 | 1.7 | 0.7 | −26.7 | 1234 | 0.02 | — | — | — | — | — | — | [361] |
| 720 | 14.5 | 1.26 | 0 | −62 | 709.7 | 0.16 | — | — | — | — | — | — | ||
| Ba7.78 Eu0.22 Cu3.83 Si36.47 | 320 | 29 | 1.9 | 1 | −21.4 | 1244 | 0.01 | — | — | — | — | — | — | [361] |
| 720 | 15.1 | 1.7 | 0 | −51.5 | 898.5 | 0.1 | — | — | — | — | — | — | ||
| Ba7.23 Eu0.73 Cu3.81 Si39.23 | 320 | 27.9 | 3.1 | 2.3 | −25 | 1026 | 0.002 | — | — | — | — | — | — | [361] |
| 720 | 6.4 | 2.2 | 1.3 | −40 | 492.4 | 0.03 | — | — | — | — | — | — | ||
| Ba8 Cu6 Si40 | 300 | 49.1 | 2.4 | — | 27 | 1515 | 0.01 | — | — | — | — | — | — | [363] |
| 673 | 27 | 2 | — | 74 | 980.4 | 0.18 | — | — | — | — | — | — | ||
| Ba8 Cu6 Ge8 Si32 | 300 | 43.5 | 1.9 | — | 45 | 800 | 0.03 | — | — | — | — | — | — | [363] |
| 673 | 22.6 | 1.5 | — | 109 | 500 | 0.28 | — | — | — | — | — | — | ||
| Ba8 Cu6 Ge16 Si24 | 300 | 46.9 | 1.5 | — | 63 | 606 | 0.05 | — | — | — | — | — | — | [363] |
| 673 | 22.7 | 1.3 | — | 125 | 409.8 | 0.37 | — | — | — | — | — | — | ||
| Ba8 Cu6 Ge24 Si16 | 300 | 41.2 | 1.1 | — | 74 | 444.4 | 0.07 | — | — | — | — | — | — | [363] |
| 673 | 20.9 | 1.2 | — | 141.2 | 308.6 | 0.42 | — | — | — | — | — | — | ||
| Ba8 Cu6 Ge15 Si25 | 323 | 66.3 | 1.7 | — | 79 | 740.7 | 0.07 | — | — | — | — | — | — | [364] |
| 773 | 20 | 1.2 | — | 121.3 | 465.1 | 0.43 | — | — | — | — | — | — | ||
| Ba8 Al6 Si40 | 298 | — | — | — | — | — | — | 0.5 | — | — | — | — | — | [365] |
| Ba8 Al10 Si36 | 320 | 11 | — | — | −9 | 1104 | — | — | — | — | — | — | — | [366] |
| 670 | 10.5 | — | — | −27 | 1079 | — | — | — | — | — | — | — | ||
| Ba8 Al16 Si30 | 320 | 11.7 | — | — | −11.5 | 925 | — | — | — | — | — | — | — | [366] |
| 670 | 9.1 | — | — | −32.8 | 769.2 | — | — | — | — | — | — | — | ||
| Ba8 Al14 Si31 | 300 | 15.7 | 1.9 | 1.2 | −13 | 1000 | 0.01 | — | 6.8 | 1.5 | — | — | — | [367] |
| 1100 | 7.7 | 1.9 | 0.9 | −80 | 534.7 | 0.18 | — | — | — | 6.94 | — | 400 | ||
| Ba8 Al15 Si30 | 300 | 45.7 | 1.9 | 1.2 | −38 | 1000 | 0.02 | 0.2 | 4.1 | 1.5 | 2.7 | — | — | [367] |
| 1000 | 9.7 | 1.7 | 0.7 | −90 | 505 | 0.24 | — | — | — | 6.62 | — | 300 | ||
| Ba8 Al13.6 Si32.4 | 317 | 53 | 3.5 | — | −24 | 2000 | 0.01 | — | — | — | — | — | — | [368] |
| 865 | 24.4 | 3.7 | — | −78.5 | 1205 | 0.17 | — | — | — | — | — | — | ||
| Ba7.7 Dy0.3 Al12.8 Si33.2 | 317 | 4.3 | 3.5 | — | −3.5 | 1053 | — | — | — | — | — | — | — | [368] |
| 865 | 9.6 | 3.6 | — | −53.8 | 719.4 | 0.05 | — | — | — | — | — | — | ||
| Ba7.5 Dy0.5 Al14.7 Si31.3 | 317 | 76 | 2.3 | — | −44.4 | 1539 | 0.04 | — | — | — | — | — | — | [368] |
| 865 | 25.4 | 2.3 | — | −105 | 865 | 0.36 | — | — | — | — | — | — | ||
| Ba7.3 Dy0.7 Al14.4 Si31.6 | 317 | 65.7 | 3.1 | — | −35.6 | 1667 | 0.02 | — | — | — | — | — | — | [368] |
| 863 | 24.3 | 3 | — | −93 | 970.8 | 0.24 | — | — | — | — | — | — | ||
| Ba7 Dy1 Al13.8 Si32.2 | 317 | 85 | 3.3 | — | −21.3 | 3610 | 0.02 | — | — | — | — | — | — | [368] |
| 863 | 38.9 | 3.3 | — | −65 | 2370 | 0.27 | — | — | — | — | — | — | ||
| Ba7 Sr1 Al16 Si30 | 300 | 60 | 2.2 | 1.1 | −30 | 1667 | 0.03 | — | — | — | — | — | — | [369] |
| 1200 | 12 | 2.6 | 0.3 | −92 | 793.6 | 0.3 | — | — | — | — | — | — | ||
| Ba7 Eu1 Al13 Si31 | 300 | — | 1.7 | 1 | −7 | 1064 | 0.01 | — | 18.6 | 3 | 0.35 | — | 100 | [370] |
| 1200 | 6.8 | 2.6 | 0.8 | −71.1 | 614.6 | 0.14 | — | — | — | — | — | — | ||
| Ba8 B0.32 Al14.2 Si30.5 | 300 | 51.5 | 2.1 | 1.1 | −30 | 1429 | 0.03 | — | — | — | — | — | — | [370] |
| 900 | 14.5 | 2.5 | 1.9 | −67 | 909 | 0.15 | — | — | — | — | — | — | ||
| Ba8 Au4.75 Si41.25 | 300 | 12.7 | 2.7 | 2.5 | −36 | 293.2 | 4 × 10−3 | — | — | — | — | — | — | [352] |
| 680 | 6.1 | 2.8 | 2.5 | −71 | 234.7 | 0.03 | — | — | — | — | — | — | ||
| Ba8 Au5.15 Si40.85 | 300 | 42.8 | 1.7 | 1.55 | −104 | 301.2 | 0.07 | 0.1 | — | — | 3.7 | — | 650 | [352] |
| 480 | 26.5 | 1.7 | 1.4 | −123.7 | 292.4 | 0.12 | — | — | — | — | — | — | ||
| Ba8 Au5.30 Si40.70 | 300 | 39.8 | 1.5 | 1.4 | 143.2 | 170.6 | 0.06 | 0.1 | — | — | 6.8 | — | 80 | [352] |
| 530 | 19.4 | 1.8 | 1.5 | 129.4 | 232 | 0.13 | — | — | — | — | — | — | ||
| Ba8 Au5.45 Si40.55 | 300 | 63.6 | 1.6 | 1.3 | 108.2 | 423.7 | 0.1 | 0.2 | — | — | 5.28 | — | 150 | [352] |
| 600 | 27.5 | 1.9 | 1.7 | 135.8 | 364.9 | 0.22 | — | — | — | — | — | — | ||
| Ba8 Au6 Si40 | 300 | 86.2 | 2.2 | 1.1 | 47 | 1515 | 0.05 | — | — | — | 8.72 | — | 720 | [352] |
| 650 | 80.3 | 2.9 | 1.2 | 127.4 | 1333 | 0.18 | — | — | — | — | — | — | ||
| Ba7.0 Eu1.0Au5.16 Si41.84 | 300 | 73.3 | — | — | 90 | 625 | — | — | — | — | — | — | — | [371] |
| Ba6.19 Eu1.68Au5.89 Si40.11 | 300 | 61.3 | — | — | −60 | 833.3 | — | — | — | — | — | — | — | [371] |
| Ba8.0 Au5.59Si39.01 | 300 | 73.1 | 3.2 | 2.9 | 107.6 | 490.2 | 0.1 | — | 5.7 | 3.4 | 7.2 | — | 150 | [372] |
| Ba7 Ce1Au5.5 Si40.5 | 300 | 36.1 | 1.8 | 1.6 | −150 | 142.8 | 0.05 | — | 1.9 | 1.3 | 6.03 | — | 92.9 | [373] |
| 500 | 29.4 | 1.8 | 1.5 | −175 | 186.2 | 0.1 | — | — | — | — | — | — | ||
| Ba7 La1Au6 Si40 | 300 | 127.5 | 1.9 | 1.8 | 275 | 117.6 | 0.1 | — | 3.3 | 2.8 | 7.91 | — | 26.9 | [373] |
| 400 | 104.5 | 1.9 | 1.8 | 300 | 111.1 | 0.11 | — | — | — | — | — | — | ||
| Ba8 Ni3.8 Si42.2 | 300 | 12.6 | — | — | −13 | 806.5 | — | — | — | — | — | — | — | [374] |
| 823 | 3.8 | 2 | 1 | −27 | 534.7 | 0.02 | — | — | — | — | — | — | ||
| Ba8 Ni3.6 Si42.4 | 300 | 78.5 | 3 | 1.6 | −32 | 2041 | 0.02 | — | — | — | — | — | — | [375] |
| 800 | 25.2 | 4 | 1 | −58.6 | 1531 | 0.1 | — | — | — | — | — | — | ||
| Ba8 Ni3.8 Si42.4 | 300 | 82.7 | — | — | −40.4 | 1698 | — | 0.1 | 7.4 | 2.8 | 5.1 | — | 400 | [375] |
| 790 | 26.8 | — | — | −70 | 1314 | — | — | — | — | — | — | — | ||
| Ba8 Ni3.8 □y Si42.2−y | 300 | 85.8 | 4.3 | 2.4 | −27.6 | 2591 | — | — | — | — | — | — | — | [376] |
| 700 | 34.5 | 3.9 | 0.7 | −54.5 | 1852 | 0.1 | — | — | — | — | — | — | ||
| Ba8 Pt1.5 Ni3.5 Si41 | 300 | 76.2 | — | — | −40 | 1580 | — | — | — | — | — | — | — | [377] |
| 1040 | 11.8 | — | — | −44 | 1429 | — | — | — | — | — | — | — | ||
| Ba8 Pt5 Si41 | 300 | 30.7 | — | — | 35 | 728.8 | — | — | — | — | — | — | — | [378] |
| 1040 | 4.8 | — | — | 20 | 1287 | — | — | — | — | — | — | — | ||
| K7 BaAl9 Si37 | 300 | — | 1.2 | 1 | — | 309.6 | — | — | — | — | — | — | 29.1 | [378] |
| 873 | 6.7 | — | — | −166 | 108.7 | 0.23 | — | — | — | — | — | — | ||
| K6.5 Ba1.5 Al9.5 Si36.5 | 300 | — | 1.1 | 0.5 | — | 826.4 | 0.35 | — | — | 1.2 | — | — | 449 | [378] |
| 873 | 8.8 | — | — | −133 | 212.7 | 0.4 | — | — | — | — | — | — | ||
| K6 Ba2 Al10 Si36 | 300 | — | 0.8 | 0.7 | — | 259.7 | — | — | — | 13.2 | — | — | 12.5 | [378] |
| 873 | 55 | — | — | −184 | 724.6 | 0.28 | — | — | — | — | — | — | ||
| K7.6 Ga3.1 Al4.6 Si38.7 | 300 | 7.2 | 1.5 | 1.4 | −300 | 5 | 0.02 | — | — | — | — | — | — | [379] |
| 550 | — | — | — | — | 5 | 0.07 | — | — | — | — | — | — | ||
| Ba8 Ga17 Si29 | 300 | 53.4 | 1.5 | 1.1 | −71.5 | 600 | 0.06 | — | 17.5 | 8.8 | 2 | — | 44 | [380] |
| 900 | 17 | — | — | −165.8 | 290 | 0.55 | — | — | — | — | — | — | ||
| Ba8 Cu0.2 Si32 Ga13.8 | 300 | 72.7 | — | — | −41 | 1471 | — | — | — | — | 0.6 | — | 16 | [381] |
| 835 | 23.9 | — | — | −101 | 813 | — | — | — | — | — | — | — | ||
| Si46 | 400 | — | 59.9 | — | — | 9 × 10−7 | — | — | — | — | — | — | — | [382] |
| Si136 | 300 | — | — | — | — | — | — | 1.7–1.9 | — | — | — | — | — | [383] |
| Si230 | 400 | — | 21.9 | — | — | — | — | — | — | — | — | — | — | [382] |
| Si644 | 400 | — | 13.4 | — | — | — | — | — | — | — | — | — | — | [382] |
| Ba8Si46 | 400 | — | 12.6 | 7.6 | — | 6 × 10−7 | — | — | — | — | — | — | — | [382] |
| BaSi46 | 300 | 125 | 2.7 | 1.7 | −60 | 1700 | 0.05 | — | — | — | 2.99 | — | 100 | [384] |
| 800 | 75.7 | — | — | −141.2 | 1450 | 0.46 | — | — | — | — | — | — | ||
| Ba8Si230 | 400 | — | 8.7 | — | — | — | — | — | — | — | — | — | — | [382] |
| Ba8Si644 | 400 | — | 8.7 | — | — | — | — | — | — | — | — | — | — | [382] |
| Na5.1Si136 | 300 | 6.7 | 7 | 6.9 | −50 | 111.1 | 1.2 × 10−3 | — | — | — | — | — | — | [385] |
| Na8.2Si136 | 300 | 16.8 | 12 | 11.7 | −35 | 400 | 1.2 × 10−3 | — | — | — | — | — | — | [385] |
| K8Al8Si38 | 300 | 1.1 | 1.8 | 1.8 | −90 | 9.1 | 1.2 × 10−3 | 1.4 | 69.8 | 39 | 0.01 | — | 0.031 | [386] |
| Cs8Ga8Si38 | 300 | 0 | 1.7 | 1.7 | 24 | 0.1 | 1 × 10−6 | 1.1 | — | — | — | — | — | [386] |
| K8Ga8Si38 | 300 | 0.2 | 0.5 | 0.5 | −33 | 4.5 | 3 × 10−4 | 1.2 | 239.6 | 82 | — | — | — | [386] |
| C8Al8Si38 | 400 | — | — | — | — | — | 1 × 10−4 | — | — | — | — | — | — | [387] |
| 300 | 0 | 0.93 | 0.9 | −300 | 1.8 × 10−3 | 2 × 10−5 | 0.8 | — | — | — | — | — | ||
| Rb8Al8Si38 | 300 | 0.3 | 1.24 | 1.2 | −252 | 0.3 | 5 × 10−4 | 0.7 | — | — | — | — | — | [387] |
| K8Al8Si38 | 300 | — | 1.65 | — | — | — | — | 0.3 | — | — | — | — | — | |
| Ca8Na16Al24Si112 | 300 | 1 | 4.5 | — | −32 | 24.8 | — | — | — | — | — | — | — | [388] |
| Si30.3P15.7Se7.93 | — | — | — | — | — | — | — | 0.7 | — | — | — | — | — | [389] |
| Ba8Al16Ga2Si26P2 | 300 | 58.8 | 1.8 | 1.3 | −60 | 800 | 0.06 | — | — | — | 1.34 | — | 30 | [390] |
| 900 | 19.5 | — | — | −150 | 400 | 0.47 | — | — | — | — | — | — | ||
| Germanides | ||||||||||||||
| Ge136 | 300 | — | — | — | — | — | — | 0.6–0.8 | — | — | — | — | — | [383] |
| Ba8Ge43□3 | 300 | — | 3.2 | 2.6 | −10 | 769.2 | 0 | — | — | — | — | — | — | [391] |
| 673 | 65.8 | 3.8 | 2 | −39.4 | 4660 | 0.04 | — | — | — | — | — | — | ||
| Ba8Zn7.66Ge36.55Sn1.79 | 300 | 70.6 | 1 | — | −74 | 762.8 | 0.13 | — | 27.5 | 14 | 1.7 | — | 30 | [392] |
| 850 | 22.3 | 0.9 | 0.4 | −187 | 272.8 | 0.82 | — | — | — | — | — | — | ||
| Ba8Au5.3Ge40.7 | 300 | 95.9 | 1.3 | 0.8 | 112.2 | 606 | 0.2 | 0.3 | — | — | 0.49 | — | 4 | [393] |
| 683 | 44 | 0.9 | 0.5 | 173.5 | 452.5 | 0.9 | — | — | — | — | — | — | ||
| Ba24Ge100 | 300 | 54.4 | 2.6 | 1.3 | −21.6 | 2100 | 0.01 | — | 8.6 | 2.4 | 3.35 | — | 550 | [394] |
| 873 | 22 | 3.8 | 0.6 | −50 | 1800 | 0.11 | — | — | — | — | — | — | ||
| Ba24Ag6Ge94 | 300 | 82 | 1.8 | 0.8 | −42.5 | 1597 | 0.04 | — | 20.2 | 7.8 | 2.25 | — | 110 | [394] |
| 873 | 24.2 | 2.3 | 0.5 | −85 | 1100 | 0.31 | — | — | — | — | — | — | ||
| Ba24Cu6Ge94 | 300 | 21.3 | 2.3 | 1.6 | −19.7 | 898 | 0.01 | — | 19.1 | 5.1 | — | — | 111 | [394] |
| 873 | 12.3 | 2.2 | 1.3 | −80 | 600 | 0.17 | — | — | — | — | — | — | ||
| Ba8Cu5.1Ge40.2Sn0.7 | 300 | 89.4 | 1.7 | 0.9 | −55 | 1333 | 0.07 | 0.2 | 27.1 | 11.9 | 1.56 | — | 43 | [395] |
| 800 | 24.3 | 1.2 | 0.6 | −153 | 403.2 | 0.62 | — | — | — | — | — | — | ||
| Ba8Cu5.7Ge40.3 | 329 | 111.1 | 1.3 | 1.1 | 210 | 250 | 0.26 | 0.3 | 12 | 9.6 | 3.9 | — | — | [396] |
| 615 | 43.2 | 1.1 | 0.9 | 229 | 199.2 | 0.62 | — | — | — | — | — | — | ||
| Ba8Cu5.13Ge40.87 | 329 | 51.4 | 1.9 | 1.1 | −44 | 1111 | 0.04 | — | — | — | — | — | — | [396] |
| 773 | 25 | 1.6 | 0.9 | −137 | 478.4 | 0.42 | — | — | — | — | — | — | ||
| Ba8Cu5.93Ge40.07 | 329 | 83 | 1.2 | 1.1 | 300 | 65.8 | 0.17 | — | 12.7 | 11 | 1.18 | — | — | [396] |
| 573 | 35.7 | 1.1 | 1.1 | 299 | 65.8 | 0.3 | — | — | — | — | — | — | ||
| Ba8Cu6Si16Ge24 | 300 | 41.2 | 1.1 | 0.9 | −74 | 444.4 | 0.07 | — | 28.3 | 14.4 | 1.26 | — | 20 | [397] |
| 673 | 20.8 | 1 | 0.6 | −141 | 307.7 | 0.43 | — | — | — | — | — | — | ||
| Eu0.5Ba7.5Cu6Si16Ge24 | 300 | 42.4 | 1 | 0.7 | −70 | 487.8 | 0.07 | — | 19.9 | 9.8 | 1.56 | — | 30 | [397] |
| 673 | 22.4 | 0.85 | 0.4 | −137 | 348.7 | 0.53 | — | — | — | — | — | — | ||
| Eu1Ba7Cu6Si16Ge24 | 300 | 44.5 | 1 | 0.65 | −67 | 537.6 | 0.07 | — | 17.3 | 8.4 | 1.81 | — | 40 | [397] |
| 673 | 24.3 | 0.7 | 0.2 | −133.6 | 393.7 | 0.66 | — | — | — | — | — | — | ||
| Eu1.5Ba6.5Cu6Si16Ge24 | 300 | 46.4 | 0.9 | 0.5 | −62 | 609.7 | 0.08 | — | 16.1 | 7.5 | 1.94 | — | 50 | [397] |
| 673 | 24.1 | 0.7 | 0.1 | −125 | 434.8 | 0.69 | — | — | — | — | — | — | ||
| Ba8Cu5Si3Ge38 | 300 | 67 | 1.6 | 1 | −55 | 1000 | — | — | — | — | — | — | — | [398] |
| 823 | 22.5 | 1.5 | 0.6 | −141 | 450.5 | 0.5 | — | — | — | — | — | — | ||
| Ba8Cu5Ge41 | 300 | 55.4 | 1.3 | 0.8 | −80 | 546.5 | 0.08 | — | 23.7 | 12.5 | 1.13 | — | — | [399] |
| 673 | 28.4 | 1.3 | 0.8 | −170 | 298.5 | 0.44 | — | — | — | — | — | — | ||
| Ba8Cu5Si6Ge45 | 300 | 60.6 | 1.4 | 0.5 | −50 | 1000 | 0.05 | — | 17.6 | 7.4 | 1.44 | — | — | [399] |
| 823 | 20.2 | 1.4 | 0.5 | −139.7 | 411.5 | 0.45 | — | — | — | — | — | — | ||
| Ba8Cu5Si10Ge31 | 300 | 61.2 | 1.7 | 0.9 | −60 | 833.3 | 0.05 | — | — | — | — | — | — | [399] |
| 773 | 23.3 | 1.7 | 0.9 | −139.7 | 431 | 0.4 | — | — | — | — | — | — | ||
| Ba8Cu5Si18Ge23 | 300 | 54.7 | 1.4 | 1.1 | −115 | 333.3 | 0.09 | — | 13.0 | 8.2 | 1.16 | — | 18 | [399] |
| 573 | 27.3 | 1.4 | 1.1 | −160 | 253.1 | 0.3 | — | — | — | — | — | — | ||
| Ba8Cu6Ge20Si20 | 300 | 32.9 | 1.3 | 0.7 | −38.2 | 715.3 | 0.03 | — | — | — | — | — | — | [400] |
| 720 | 13.2 | 1.15 | 0.6 | −112.7 | 307.7 | 0.25 | — | — | — | — | — | — | ||
| Ba8Cu6Si16Ge24 (4 GPa) | 300 | 48.3 | 1.1 | — | 64.3 | 609.7 | 0.07 | — | — | — | — | — | — | [377] |
| 673 | 23.8 | 0.8 | — | 129 | 408.1 | 0.55 | — | — | — | — | — | — | ||
| Ba8Cu6Si16Ge24 (5 GPa) | 300 | 41.8 | 1.05 | — | 50 | 689.6 | 0.05 | — | — | — | — | — | — | [377] |
| 673 | 22.4 | 0.8 | — | 116 | 452.5 | 0.52 | — | — | — | — | — | — | ||
| Ba8Cu4.5Si6Ge35.5 | 300 | 30 | 1.1 | — | −25 | 1000 | 0.06 | — | — | — | — | — | — | [401] |
| 873 | 22 | 2 | — | −115 | 666.7 | 0.45 | — | — | — | — | — | — | ||
| Ba8Cu4.8Ga1.0Ge40.2 | 300 | — | 2 | 0.8 | — | — | 0.05 | — | — | — | — | — | — | [401] |
| 900 | — | 1.4 | 0.7 | — | — | 0.9 | — | — | — | — | — | — | ||
| Ba8Cu4.63Ga1.01Ge40.35 | 300 | 91.2 | — | — | −37 | 2049 | — | — | 58.5 | — | 1.29 | — | — | [401] |
| Ba8Cu4.63Ga1.02Ge40.35 | 300 | 91.3 | 1.9 | 0.8 | −37 | 2049 | 0.04 | — | 58.5 | 21.2 | 1.29 | — | 56 | [401] |
| 900 | 29.8 | 1.4 | 0.7 | −157 | 564.8 | 0.9 | — | — | — | — | — | — | ||
| Ba8Cu4.78Ga0.89Ge40.33 | 300 | 86.5 | — | — | −40.4 | 1776 | — | — | 51 | 19.3 | 1.35 | — | — | [401] |
| 900 | 27.9 | — | — | −159 | 515.5 | — | — | — | — | — | — | — | ||
| Ba8Cu4.92Ga0.75Ge40.33 | 300 | 66.3 | — | — | −42.9 | 1280 | — | — | 38.8 | 15.1 | 1.36 | — | — | [401] |
| 900 | 24.4 | — | — | −162 | 434.8 | — | — | — | — | — | — | — | ||
| Ba8Cu4.6 In1.4Ge40.0 | 300 | 92.5 | 1 | — | −60.4 | 1250 | 0.1 | — | 97.4 | 44.8 | 1.41 | — | 40 | [402] |
| Ba8Ni3.8Ge42.2 | 300 | 1.8 | 0.9 | 0.9 | 125 | 10.1 | — | — | — | — | — | — | — | [374] |
| 823 | 0.9 | 0.9 | 0.8 | −83 | 40 | 0.02 | — | — | — | — | — | — | ||
| Ba8Ni3.8Si10Ge32.2 | 300 | 26.2 | 1.3 | 0.8 | −40.9 | 531.9 | — | — | — | — | — | — | — | [374] |
| 823 | 10.2 | 1.4 | 0.8 | −106 | 319.5 | 0.19 | — | — | — | — | — | — | ||
| Ba8Ni2Ge43−yy | 300 | 27.6 | 2.2 | — | −25.5 | 900.9 | 0.01 | — | — | — | 4.24 | — | 610 | [351] |
| 650 | 42.6 | 2.9 | 2.2 | −83.6 | 1269 | 0.2 | — | — | — | — | — | — | ||
| Ba8Ni3Ge42−yy | 300 | 23.9 | 1.9 | 1.5 | −38.5 | 515.5 | 0.01 | — | — | — | 4.5 | — | 360 | [351] |
| 650 | 15.4 | 2.5 | 2 | −104.8 | 341.3 | 0.1 | — | — | — | — | — | — | ||
| Ba8Ni3.5Ge42.5−yy | 300 | 108 | 2.3 | 1.4 | −67.2 | 1299 | 0.08 | 0.2 | — | — | 4.57 | — | 160 | [351] |
| 600 | 13 | 2.4 | — | −100 | 273.9 | 0.15 | — | — | — | — | — | — | ||
| Ba8Ni3.8Ge42.2−yy | 300 | 45.8 | 1.9 | 1.8 | −152 | 176.7 | 0.06 | 0.2 | — | — | 3.6 | — | 26 | [351] |
| 500 | 34 | 2.6 | 1.9 | −159.4 | 259 | 0.2 | — | — | — | — | — | — | ||
| Ba8Ni4Ge42−yy | 300 | 51.1 | 1.9 | 1.8 | −172 | 156 | 0.07 | 0.1 | — | — | 2.5 | — | 19 | [351] |
| 450 | 25 | 1.6 | 1.6 | −172 | 140.2 | 0.13 | — | — | — | — | — | — | ||
| Ba8Ni4.2Ge41.8−yy | 300 | 52.7 | 1.5 | 1.4 | 215 | 97.4 | 0.07 | 0.1 | — | — | 9 | — | 75 | [351] |
| Ba8Ni3.5Ge42.1□0.4 | 300 | 63.7 | 2.1 | 1.4 | −52.9 | 990 | 0.05 | — | — | — | — | — | — | [403] |
| 680 | 34.5 | 3.1 | — | −119.6 | 675.6 | 0.21 | — | — | — | — | — | — | ||
| Ba8.09Ga16.61Ge29.30 | 300 | 65.7 | 1.8 | — | 38 | 1435 | 0.03 | — | — | — | 0.53 | — | 15 | [404] |
| 773 | 56.3 | 1.7 | — | 177 | 670 | 0.93 | — | — | — | — | — | — | ||
| Ba8.16Ga16.68Ge29.16 | 300 | 75 | 1.7 | — | 46 | 1349 | 0.04 | — | — | — | 0.62 | — | 14.2 | [404] |
| 773 | 56.5 | 1.6 | — | 192 | 564 | 1.02 | — | — | — | — | — | — | ||
| Ba8.05Ga16.81Ge29.14 | 300 | 70.4 | 1.5 | — | 52 | 1115 | 0.05 | — | — | — | 0.56 | — | 10 | [404] |
| 823 | 35.1 | 1.3 | — | 200 | 351 | 0.88 | — | — | — | — | — | — | ||
| Ba7.91Ga16.90Ge29.20 | 300 | 60.9 | 1.3 | — | 62 | 800 | 0.06 | — | — | — | 0.47 | — | 6 | [404] |
| 823 | 34.7 | 1.2 | — | 206 | 324 | 0.94 | — | — | — | — | — | — | ||
| Ba8.04Ga16.98Ge28.97 | 300 | 58.2 | 1.2 | — | 77 | 600 | 0.07 | — | — | — | 0.11 | — | 0.8 | [404] |
| 823 | 13.3 | 1 | — | 165 | 200 | 0.42 | — | — | — | — | — | — | ||
| Ba8Ga15.85In0.09Ge29.03 | 321 | 25.8 | 1 | 0.9 | −126 | 151.5 | 0.08 | — | — | — | — | — | — | [405] |
| 876 | 8.9 | 1.1 | 0.9 | −212 | 84.7 | 0.31 | — | — | — | — | — | — | ||
| Ba8Ga15.59In0.15Ge27.27 | 321 | 29.2 | 1.2 | 1 | −111 | 207.5 | 0.07 | — | — | — | — | — | — | [405] |
| 876 | 9.4 | 1.2 | 0.9 | −199 | 104.7 | 0.31 | — | — | — | — | — | — | ||
| Ba8Ga15.83In0.31Ge25.94 | 325 | 50.9 | 1 | 0.5 | −82 | 549.5 | 0.13 | — | — | — | — | — | — | [405] |
| 773 | 15.7 | 0.8 | 0.6 | −203 | 137.7 | 0.52 | — | — | — | — | — | — | ||
| Ba8Ga16Ge30 (3 GPa) | 300 | 53.3 | 1.3 | 0.8 | −66.8 | 645.1 | 0.07 | — | — | — | — | — | — | [406] |
| 773 | 27.9 | 0.9 | 0.2 | −173.1 | 347.2 | 0.97 | — | — | — | — | — | — | ||
| Ba8Ga16Ge30 (4 GPa) | 300 | 59.3 | 1.1 | 0.6 | −63.6 | 757.6 | 0.08 | — | — | — | — | — | — | [406] |
| 773 | 28.2 | 0.7 | 0 | −170.2 | 363.6 | 1.14 | — | — | — | — | — | — | ||
| Ba8Ga16Ge30 (5 GPa) | 300 | 65.1 | 1.5 | 0.8 | −61 | 869.5 | 0.07 | — | — | — | — | — | — | [406] |
| 773 | 31.1 | 1 | 0.2 | −167.6 | 413.2 | 0.92 | — | — | — | — | — | — | ||
| Ba8Ga16Ge30 | 300 | 25.5 | 3 | 1.9 | −70 | 500 | 0.04 | 0.5 | — | — | 2.48 | — | 60 | [407] |
| 600 | 52.8 | 2.5 | 1.1 | −140 | 400 | 0.25 | — | — | — | 3.61 | — | 50 | ||
| Ba8(Ga1)16Ge30 | 373 | 56.2 | 1.4 | 1.2 | −230 | 200 | 0.12 | — | — | — | — | — | — | [408] |
| 823 | 27.2 | 1.1 | — | −225 | 166.6 | 0.69 | — | — | — | — | — | — | ||
| Ba8(Al0.2Ga0.8)16Ge30 | 373 | 42.7 | 1.6 | 0.9 | −58 | 833.3 | 0.07 | — | — | — | — | — | — | [408] |
| 923 | 21 | 1.4 | 0.7 | −156 | 400 | 0.67 | — | — | — | — | — | — | ||
| Ba8(Al0.23Ga0.77)16Ge30 | 373 | 51 | — | — | −53 | 1053 | 0.1 | — | — | — | — | — | — | [408] |
| 1073 | — | — | — | −178 | 518 | — | — | — | — | — | — | — | ||
| Ba8(Al0.25Ga0.75)16Ge30 | 373 | 56.2 | 1.7 | 0.6 | −49 | 1370 | 0.07 | — | — | — | — | — | — | [408] |
| 1073 | — | 2.1 | 1 | −163 | 515.5 | 0.93 | — | — | — | — | — | — | ||
| Ba8(Al0.33Ga0.67)16Ge30 | 373 | 30 | 1.9 | 1 | −32.5 | 1053 | 0.02 | — | — | — | — | — | — | [408] |
| 973 | — | 2.6 | 1.4 | −113 | 724.6 | 0.33 | — | — | — | — | — | — | ||
| Ba8(Al0.5Ga0.5)16Ge30 | 373 | 26.7 | — | — | −26 | 1163 | — | — | — | — | — | — | — | [408] |
| Ba8(Al)16Ge30 | 373 | 20 | — | — | −12.5 | 1755 | — | — | — | — | — | — | — | [408] |
| Ba8Ga16.1Zn3Ge26.9 | 300 | 35.8 | 1.4 | 1.4 | 162 | 123 | 0.07 | — | 41.4 | 30.1 | 0.27 | — | 0.7 | [409] |
| 690 | 27.3 | 1.3 | — | 230 | 148 | 0.42 | — | — | — | — | — | — | ||
| Ba8Ga16.2Zn3Ge26.8 | 300 | 43.3 | 1.4 | 1.4 | 160 | 152 | 0.08 | — | 39.6 | 28.7 | 0.71 | — | 3.3 | [409] |
| 700 | 23.8 | 1.3 | — | 208 | 170 | 0.44 | — | — | — | — | — | — | ||
| Ba8Ga16.3Zn3Ge26.7 | 300 | 39 | 1.5 | 1.5 | 140 | 174 | 0.06 | — | 36.4 | 24.9 | 0.7 | — | 4.3 | [409] |
| 650 | 23.9 | 1.3 | — | 188 | 193 | 0.34 | — | — | — | — | — | — | ||
| Ba8Ga16Zn3.0Ge27.0 | 300 | 36.1 | 1.3 | 1.3 | 230 | 56 | 0.07 | — | 59.7 | 48.9 | 0.49 | — | 0.7 | [410] |
| 790 | 24.6 | 1.3 | — | 294 | 77.9 | 0.38 | — | — | — | — | — | — | ||
| Ba8Ga16Zn3.2Ge26.8 | 300 | 27.6 | 1.4 | 1.3 | 250 | 34 | 0.06 | — | 35 | 29.3 | 0.58 | — | 0.7 | [410] |
| 700 | 18.3 | 1.2 | — | 270 | 63.7 | 0.32 | — | — | — | — | — | — | ||
| Ba8Ga16Ge28Zn2 | 300 | 50.1 | 1.3 | 0.2 | −125 | 268.8 | 0.1 | — | — | — | — | — | — | [411] |
| 773 | 24.5 | 1.2 | 1.1 | −250 | 125 | 0.51 | — | — | — | — | — | — | ||
| Ba8Ga16Ge30 | 300 | 68.5 | 0.9 | 0.5 | −79.5 | 680.2 | 0.09 | — | — | — | — | — | — | [411] |
| 873 | 22.7 | 1.4 | 0.9 | −205.7 | 232.5 | 0.65 | — | — | — | — | — | — | ||
| Ba8.01Ga15.79Al2.95Ge26.91 | 300 | 46.2 | 1.1 | 1 | 187 | 118.2 | 0.12 | — | 5.6 | 4.3 | 2.8 | — | 17.4 | [412] |
| 760 | 23.4 | 1 | 1 | 255 | 109.7 | 0.61 | — | — | — | — | — | — | ||
| Ba7.92Ga15.84Al3.38Ge26.50 | 300 | 38.8 | 1.2 | 1.2 | 168 | 123.9 | 0.09 | — | 5.9 | 4.3 | 2.43 | — | 18.3 | [412] |
| 780 | 26 | 1.1 | 1.1 | 247 | 138.8 | 0.55 | — | — | — | — | — | — | ||
| Ba7.89Ga15.92Al1.89Ge27.12 | 300 | 34.2 | 1.1 | 1.1 | 200 | 75.3 | 0.09 | — | 4.9 | 3.8 | 2.51 | — | 12.4 | [412] |
| 850 | 18.9 | 1 | 1 | 264 | 94.4 | 0.53 | — | — | — | — | — | — | ||
| Ba8Ga15Ge31 | 300 | 65.4 | 1.8 | 1.1 | −47 | 1150 | 0.05 | — | 19.6 | 8 | 2.1 | — | 5.6 | [413] |
| 723 | 30 | 1.4 | — | −121 | 632.9 | 0.45 | — | — | — | — | — | — | ||
| Ba8Ga16Ge30 | 300 | 85 | 1.8 | 0.7 | −45 | 1562 | 0.05 | 0.3 | — | — | 2.6 | — | 27.8 | [414] |
| 1043 | 22.3 | 1.7 | 0.2 | −150 | 571.4 | 0.8 | — | — | — | — | — | — | ||
| Ba8Ga16.6Ge28.7 | 300 | 58.8 | — | 1.2 | 60 | 800 | 0.06 | — | — | — | 0.29 | — | 3 | [415] |
| 823 | 44.2 | 1.2 | 0.7 | 234 | 298 | 1.1 | — | — | — | — | — | — | ||
| Yb0.3Ba7.7Ga16Ge30 | 300 | 31.8 | 1.6 | 1.2 | −56.9 | 457.1 | 0.03 | — | 23.7 | 10.6 | 1.25 | — | 29.3 | [416] |
| 950 | 24.1 | 1.3 | 0.8 | −206 | 278.2 | 0.83 | — | — | — | — | — | — | ||
| Yb0.5Ba7.5Ga16Ge30 | 300 | 34.5 | 1.5 | 1.1 | −60 | 469.6 | 0.03 | — | 17.4 | 8 | 1.78 | — | 46.2 | [416] |
| 950 | 26.6 | 1.2 | 0.6 | −198.3 | 336.8 | 1.02 | — | — | — | — | — | — | ||
| Yb0.7Ba7.3Ga16Ge30 | 300 | 62.5 | 1.7 | 0.9 | −50 | 1030 | 0.04 | — | 22.9 | 9.6 | 1.79 | — | 61.2 | [416] |
| 950 | 32.3 | 1.5 | 0.6 | −176 | 530 | 1.1 | — | — | — | — | — | — | ||
| Ba8Ni0.31Zn0.52Ga13.06Ge32.2 | 300 | 67.2 | 1.8 | 0.9 | −47.8 | 1160 | 0.06 | — | 41.8 | 17.2 | — | — | — | [417] |
| 1000 | 28 | 1.3 | — | −193 | 406.9 | 1.2 | — | — | — | — | — | — | ||
| Ba7.7Yb0.3Ni0.1Zn0.54Ga13.8Ge31.56 | 300 | 6.9 | 1.7 | 0.8 | −40 | 142.8 | 0.05 | — | — | — | — | — | — | [418] |
| 900 | 2.7 | 1.3 | 0.3 | −150 | 55.6 | 0.91 | — | — | — | — | — | — | ||
| Ba8Sb2Ga14Ge30 | 300 | 70.8 | 2 | 1.3 | −52.5 | 1110 | 0.05 | — | — | — | — | — | — | [419] |
| 900 | 39 | 1.7 | 0.4 | −154.4 | 760 | 1.05 | — | — | — | — | — | — | ||
| Ba8Ga16Si11Ge19 | 300 | 16.1 | — | 0.5 | −103 | 114.9 | 0.07 | — | 6.6 | 3.9 | 0.92 | — | 18.4 | [420] |
| Ba8Ga16Si13Ge17 | 300 | 14.4 | — | 0.4 | −88 | 126.5 | 0.06 | — | 6.7 | 3.7 | 0.86 | — | 21.6 | [420] |
| Ba8Ga16Si4Ge26 | 300 | 2 | — | 0.5 | −67 | 24.2 | 0.01 | — | 2.9 | 1.4 | 0.41 | — | 11.2 | [420] |
| Ba8Ga16Si9Ge21 | 300 | 15.5 | — | 0.6 | −112 | 98 | 0.06 | — | 6.1 | 3.8 | 0.93 | — | 16.1 | [420] |
| Sr8Ga16Ge30 | 300 | 99.3 | 1.5 | 1.2 | −100 | 738 | 0.11 | — | 13.6 | 8 | 1.28 | — | 41.4 | [420] |
| 800 | — | 1.5 | 0.9 | −160 | — | 0.72 | — | — | — | — | — | — | ||
| Sr8Ga15.5In0.5Ge30 | 300 | 104.5 | 1.3 | 0.7 | −80 | 1030 | 0.16 | — | 29.6 | 15.6 | 0.75 | — | 43 | [420] |
| 800 | 28.7 | 1.2 | 0.5 | −152 | 483 | 0.65 | — | — | — | — | — | — | ||
| Sr8Ga15In1Ge30 | 300 | 125.3 | 1.5 | 0.7 | −70 | 1440 | 0.13 | — | 37.5 | 19.8 | 0.44 | — | 40.1 | [420] |
| 800 | 32.6 | 1.5 | 0.4 | −132 | 698 | 0.51 | — | — | — | — | — | — | ||
| Na8Ga8.4Ge46−8.4 | 300 | 81.9 | 2.1 | 2 | −270 | 80 | 0.07 | — | 5.8 | 4.9 | 5.5 | — | 10.3 | [421] |
| Na8Ga8.1Ge46−8.1 | 300 | 85.4 | 2.3 | 2.1 | −160 | 300 | 0.1 | 0.5 | 10.2 | 7.4 | 4.8 | — | 26 | [421] |
| 817 | 30.3 | 1.7 | 1.5 | −242.5 | 183 | 0.48 | — | — | — | — | — | — | ||
| Na8Ga8.1Ge46−8.1 | 300 | 128.1 | 2.5 | 2.1 | −136 | 600 | 0.13 | — | 11.2 | 7.6 | 4.6 | — | 26 | [421] |
| 817 | 38.2 | 1.9 | 1.5 | −212.5 | 327 | 0.63 | — | — | — | — | — | — | ||
| Na8Ga7.8Ge46−7.8 | 300 | 126.3 | 2.7 | 2.3 | −112 | 800 | 0.1 | — | 11.1 | 6.9 | 4.8 | — | 72 | [421] |
| 817 | 36.6 | 2.1 | 1.5 | −184 | 436 | 0.56 | — | — | — | — | — | — | ||
| Na8Ga7.5Ge46−7.5 | 300 | 129.6 | 3 | 2.5 | −105 | 900 | 0.11 | — | 10.6 | 6.4 | 5.1 | — | 90 | [421] |
| 817 | 36.4 | 2.3 | 1.6 | −174 | 487 | 0.53 | — | — | — | — | — | — | ||
| Na8Ga7.4Ge46−7.4 | 300 | 175.3 | 3.3 | 2.5 | −89 | 1517 | 0.87 | — | 11.9 | 6.6 | 5 | — | 120 | [421] |
| 817 | 39.8 | 2.5 | 1.7 | −160 | 628 | 0.47 | — | — | — | — | — | — | ||
| K8Al8Ge38 | 300 | 16.8 | 1.3 | 0.9 | −35.8 | 390 | 0.01 | — | 10.5 | 3.7 | 0.28 | — | 6.3 | [422] |
| K8Ga8Ge38 | 300 | — | 1.1 | — | 4.2 | 0.9 | 2 × 10−6 | — | — | — | — | — | — | [422] |
| Rb7.88Au2.47Ge43.53 | 300 | — | 2 | 2 | −13 | 5 | 1.3 × 10−5 | — | — | — | — | — | — | [423] |
| K7Sr17Ga40Ge96 | 300 | 23.7 | 2.3 | 2.2 | −80 | 280 | 0.02 | — | 56.9 | 30 | 0.62 | — | 5.7 | [424] |
| 800 | 39.1 | 1.9 | 1.4 | −170 | 330 | 0.43 | — | — | — | — | — | — | [425] | |
| Sr7.92Ga15.04Sn0.35Ge30.69 | 298 | 117.8 | 1.7 | — | −80 | 1150 | 0.15 | — | 59.1 | 31.2 | 0.17 | — | 2.3 | |
| 750 | 61.4 | 1.6 | — | −190 | 600 | 1 | — | — | — | — | — | |||
| Stannides | ||||||||||||||
| Ba8Ga16Sn30 (SC) | 300 | 17.3 | 0.7 | — | −138.8 | 78.5 | 0.11 | — | 30.6 | 20.9 | 0.33 | — | 3.8 | [426] |
| 510 | 24.8 | 0.8 | — | −221.3 | 94.5 | 0.31 | — | — | — | — | — | — | ||
| Ba8Ga16Zn0.5Sn30 (SC) | 300 | 13.7 | 0.7 | — | −115.7 | 82.8 | 0.08 | — | 32.8 | 20.6 | 0.28 | — | 3.7 | [426] |
| 510 | 16.9 | 0.8 | — | −196 | 86.3 | 0.22 | — | — | — | — | — | — | ||
| Ba8Ga16Zn1Sn30 (SC) | 300 | 18.9 | 0.7 | — | −147.8 | 76.6 | 0.13 | — | 21 | 14.7 | 0.37 | — | 5.5 | [426] |
| 510 | 43.6 | 0.8 | — | −270 | 94.5 | 0.45 | — | — | — | — | — | — | ||
| Ba8Ga16Zn1.5Sn30 (SC) | 300 | 47.9 | 0.7 | — | −212 | 91.7 | 0.22 | — | 24 | 19.2 | 0.47 | — | 3.6 | [426] |
| 540 | 84.8 | 0.8 | — | −346 | 83 | 0.63 | — | — | — | — | — | — | ||
| Ba8Ga15.82Sn30.18 | 300 | — | — | — | −200 | 333.3 | — | — | — | — | 1.46 | — | 5.5 | [427] |
| Ba8Ga15.89Sn30.11 | 300 | 144.6 | 0.7 | 0.6 | −240 | 200 | 0.45 | 0.4 | — | — | 1.5 | — | 4.3 | [427] |
| 490 | 100.5 | 0.8 | 0.5 | −303 | 139.8 | 0.85 | — | — | — | — | — | — | ||
| Ba8Ga15.92Sn30.08 | 300 | 148.3 | — | — | 370 | 45.5 | — | — | — | — | — | — | 11 | [427] |
| Ba8Ga15.93Sn30.07 | 300 | 191.4 | — | — | 280 | 166.6 | — | — | — | — | — | — | 17 | [427] |
| Ba8Ga15.94Sn30.06 | 300 | 184.2 | — | — | −290 | 142.8 | — | — | — | — | — | — | 3.3 | [427] |
| Ba8Ga15.97Sn30.03 | 300 | 182.6 | — | — | 320 | 100 | — | 0.4 | — | — | 4.3 | — | 14 | [427] |
| Ba8Ga15.82Sn30.22 | 300 | 31.8 | 0.7 | 0.7 | 220 | 55.5 | 0.04 | — | — | — | — | — | — | [398] |
| Ba8Ga15.92Sn30.12 | 300 | 48.1 | 1.2 | 1 | −110 | 312.5 | 0.06 | — | — | — | 0.43 | — | 3.4 | [398] |
| Ba8Ga15.92Sn30.12 | 300 | 36.2 | 0.8 | 0.8 | −300 | 25 | 0.03 | — | — | — | 2.34 | — | 3.2 | [398] |
| Ba8Ga16.02Sn30.02 | 300 | 85.7 | 1 | 0.9 | −180 | 238.1 | 0.23 | — | — | — | 0.95 | — | 3.8 | [398] |
| Ba8Ga16.12Sn29.92 | 300 | 42.1 | 1 | 0.9 | 150 | 166.6 | 0.06 | — | — | — | 2.71 | — | 28 | [398] |
| Ba8Ga15.8Cu0.033Sn30.17 | 300 | 163.9 | 0.7 | 0.6 | −215 | 303 | 0.55 | — | 71.2 | 57.2 | 1.1 | — | 35 | [428] |
| 550 | 118.5 | 0.7 | — | −307 | 187.2 | 1.38 | — | — | — | — | — | — | ||
| Ba8Ga15.8Cu0.018Sn30.19 | 300 | 158.6 | 0.7 | 0.6 | −235 | 232.5 | 0.51 | — | 54.9 | 45.2 | 1.1 | — | 31 | [428] |
| 550 | 102.3 | 0.8 | — | −311 | 154.3 | 1.1 | — | — | — | — | — | — | ||
| Ba7.99Ga15.84Cu0.004Sn30.16 | 300 | 315.7 | 0.8 | — | 395.7 | 71.9 | 0.39 | — | 2.8 | 2.5 | 6.2 | — | 18 | [428] |
| 460 | 321.7 | 0.8 | — | 470 | 58.8 | 0.71 | — | — | — | — | — | — | ||
| Ba8Ga15.8In0.1Sn30.1 | 300 | 107.2 | 0.7 | 0.5 | −195 | 250 | 0.42 | — | 62.6 | 48.8 | 0.96 | — | 3.1 | [429] |
| 525 | 71 | — | 0.4 | −272 | 157 | 0.95 | — | — | — | — | — | — | ||
| Ba8Ga15.66In0.2Sn30.14 | 300 | 121.3 | 0.7 | 0.4 | −175 | 357.1 | 0.5 | — | 79.5 | 59.6 | 0.98 | — | 3.8 | [429] |
| 540 | 79.2 | 0.8 | 0.4 | −250 | 235.8 | 1.05 | — | — | — | — | — | — | ||
| Ba8Ga15.7Cu0.3Sn30 | 300 | 0 | 0.7 | 0.5 | −210 | 0 | 0.53 | — | 71.7 | 57.2 | 1.39 | — | 4.5 | [430] |
| 540 | 0 | 0.7 | 0.4 | −303 | 0 | 1.35 | — | — | — | — | — | — | ||
| Ba8Ga14Sn30 | 300 | 191.6 | — | — | −385 | 49.4 | 0.36 | — | 33.7 | 30.1 | 2.40 | — | 1.2 | [431] |
| 485 | 397.8 | — | — | −523 | 42.6 | 0.82 | — | — | — | — | — | — | ||
| Ba8Ga12Sn30 | 300 | 46.3 | — | — | −225 | 76.3 | 0.28 | — | 41.4 | 33.7 | — | — | 1.0 | [431] |
| 485 | 47.9 | — | — | −305 | 64.2 | 0.5 | — | — | — | — | — | — | ||
| Ba8Ga18Sn30 | 300 | 75.9 | — | — | 275 | 70 | 0.2 | — | 31.5 | 26.9 | 1.62 | — | 2.5 | [431] |
| 485 | 57.7 | — | — | 326 | 60.6 | 0.45 | — | — | — | — | — | — | ||
| Ba8Ga16Sn30 | 300 | — | — | — | 0 | — | 0.15 | — | — | — | — | — | — | [345] |
| Ba8.03Ga15.7Zn0.07Sn30.3 | 300 | 181.9 | — | — | −220 | 219.3 | 0.4 | — | 51.2 | 41.4 | 1.2 | — | 3.3 | [432] |
| 550 | — | — | −325 | — | 0.85 | — | — | — | — | — | — | |||
| Ba7.95Ga15.97Sn30.03 | 300 | 231.4 | — | — | 325 | 100 | 0.5 | — | — | — | 7.65 | — | 14 | [427] |
| 450 | 135.8 | — | — | 350 | 83.3 | 0.9 | — | — | — | — | — | — | ||
| Ba7.77Eu0.12Ga15.83Sn30.28 | 300 | 225.6 | — | — | 330 | 110 | — | — | 7.3 | 6.4 | 1.94 | — | 10 | [433] |
| 480 | 244.2 | — | — | 415 | 90 | 0.88 | — | — | — | — | — | — | ||
| Ba8Ga10Al6Sn30 | 300 | 196.8 | 0.7 | — | −244 | 260 | 0.63 | — | 48.4 | 40.3 | 0.67 | — | 4.6 | [434] |
| 500 | 142.7 | — | — | −300.8 | 210 | 1.2 | — | — | — | — | — | — | ||
| Ba8Ga8Al8Sn30 | 300 | 188.1 | 0.8 | — | −247 | 240 | 0.48 | — | 39.7 | 33.1 | 0.67 | — | 4.5 | [434] |
| 500 | 123.4 | — | — | −298.7 | 186 | 1.03 | — | — | — | — | — | — | ||
| Ba22Ga46Sn90 | 200 | 0 | — | — | 95 | 0.1 | — | — | — | — | — | — | — | [435] |
| 300 | 0 | 3.4 | — | −46 | 0.5 | 1 × 10−5 | — | 11.7 | 4.7 | 0.2–0.25 | — | 0.03 | ||
| 400 | 0.6 | — | — | −225 | 1.4 | 0.02 | — | — | — | — | — | — | ||
| Ba21Ga49Sn87 | 170 | 0 | — | — | 45 | 0 | — | — | — | — | — | — | — | [435] |
| 300 | 0.1 | 3.3 | — | −200 | 0.3 | — | — | 7.5 | 5.9 | 0.03 | — | 0.01 | ||
| 400 | 1.3 | — | — | −305 | 1.3 | — | — | — | — | — | — | — | ||
| Cs8Ba16Ga41Sn95 | 300 | 28.9 | 13.7 | 12.1 | −124 | 157 | 0.04 | — | 60.1 | 39 | 0.63 | — | 24 | [436] |
| 687 | 30.9 | 10.9 | 9.3 | −185 | 281 | 0.6 | — | — | — | — | — | — | ||
| Cs8Ba16Ga41Sn95 | 300 | 40 | 15 | 13 | −115 | 244 | 0.06 | — | 81.3 | 51 | 0.59 | — | 3 | [436] |
| 687 | 40.5 | 11 | 8.8 | −188 | 355 | 0.75 | — | — | — | — | — | — | ||
| Cs8Ba16Ga41Sn95 | 300 | 17.7 | 13.7 | 12.5 | −117 | 105 | 0.03 | — | 34.8 | 22 | 0.64 | — | 3.1 | [436] |
| 687 | 32.4 | 11.9 | 10.9 | −204 | 236 | 0.56 | — | — | — | — | — | — | ||
| Cs8Ba16Ga40Sn96 | 300 | 37 | 14.7 | 12.5 | −131 | 184 | 0.05 | 0.1 | 57.1 | 38 | 0.68 | — | 3.1 | [436] |
| 740 | 41.1 | 11.3 | 7.4 | −200 | 350 | 0.87 | — | — | — | — | — | — | ||
| K7Ba9Ga26Sn110 | 300 | 8.2 | 0.4 | 0.4 | −147.7 | 33.3 | 0.06 | 0.2 | — | — | 0.45 | — | 3.1 | [437] |
| 575 | 117.3 | 0.4 | 0.2 | −162.3 | 1064 | 0.58 | — | — | — | — | — | — | ||
| K3Ba13Ga23Sn113 | 300 | 185.1 | 0.3 | 0.2 | −114.4 | 1136 | 0.24 | 0.2 | — | — | 0.51 | — | 6.2 | [437] |
| 575 | 88.5 | 0.4 | 0.1 | −133.3 | 1136 | 0.6 | — | — | — | — | — | — | ||
| K7.1Ba16.9Ga41.3Sn94.7 | 300 | 133.4 | 1.3 | 0.7 | −100 | 991 | 0.23 | — | 240.1 | 141 | 0.6 | — | — | [438] |
| 640 | 56.3 | 1.2 | 0.5 | −167 | 567 | 0.93 | — | — | — | — | — | — | ||
| K9Ba15Al31Ga8Sn97 | 300 | 77.3 | 0.5 | 0.45 | −170 | 150 | 0.25 | — | 54 | 40 | 0.9 | — | 2.3 | [439] |
| 640 | 35.4 | 0.6 | 0.45 | −250 | 100 | 0.82 | — | — | — | — | — | — | ||
| Ba13.2K10.8Ga36.7Sn89.4 | 300 | 202.2 | — | — | −50 | 3333 | — | — | — | — | — | — | — | [440] |
| Rb8Sn44 | 300 | 3.7 | 2 | 1 | −7.3 | 6291 | — | — | 3 | 0.5 | 1.32 | — | — | [441] |
| Cs8Sn44 | 300 | 5.8 | 1.8 | 1.8 | −160 | 12.5 | 4.7 × 10−3 | — | 13.8 | 10 | 0.38 | — | — | [442] |
| Cs8Zn4Sn42 | 300 | 16.1 | 2.7 | 2.7 | −212.5 | 20.8 | 0.02 | — | — | 8.3 | 0.45 | — | — | [442] |
| Rb8Ga8Sn38 | 300 | 5.6 | — | — | −143.8 | 14.3 | — | — | — | 0.6 | 0.14 | — | — | [442] |
| Rb8Ga8Sn38 | 300 | — | 1.2 | 1.2 | −145 | — | 8 × 10−4 | — | — | — | — | — | — | [345] |
| K8Ga7Sn39 | 300 | 105.9 | 1.3 | 1.2 | −175 | 195.7 | 0.12 | — | — | — | — | — | — | [443] |
| K8Ga8Sn38 | 300 | 185.6 | 1.2 | 1.1 | −235 | 194.2 | 0.17 | — | — | — | — | — | — | [443] |
| K8Ga8Sn38 | 300 | — | 1.2 | 1.1 | −230 | — | 0.17 | — | 30.5 | 25 | 1.7 | — | — | [443] |
| K8Ga7.9Sn37.9 | 300 | 754 | 2 | 2 | −220 | 909.1 | 0.07 | — | — | — | — | — | — | [444] |
| K8Ga8Sn38 | 300 | 167.3 | — | 0.12 | −270 | 125 | 0.16 | — | 36.4 | 31 | 1.8 | — | 3.2 | [445] |
| K8Al8Sn38 | 300 | 70.1 | — | 0.14 | −265 | 55 | 0.09 | — | 4.8 | 4.1 | 4.3 | — | — | [445] |
| K8In8Sn38 | 300 | 110.3 | — | 0.13 | −250 | 100 | 0.13 | — | 11.7 | 9.8 | 3.2 | — | — | [445] |
| K8Zn4Sn42 | 300 | 45.8 | 1.6 | 1.6 | −200 | 66.7 | 0.08 | — | — | — | — | — | — | [446] |
| Na2ZnSn5 | 295 | 258.2 | 1.9 | 1.1 | −111 | 1070 | 0.21 | — | — | — | — | — | — | [447] |
| 400 | 212.8 | 2.2 | 1.3 | −130 | 1000 | 0.3 | — | — | — | — | — | — | ||
| Phosphides | ||||||||||||||
| Ba8Cu16P30 | 300 | 13 | 1.3 | 0.8 | 12.7 | 847.5 | 0.02 | 0.5 | 19.6 | 4.3 | 17.1 | — | 33 | [448] |
| 900 | 6 | 1.4 | 0.4 | 50.7 | 510.2 | 0.09 | — | — | — | — | — | — | ||
| Ba7.3La0.7Cu16P30 | 300 | 22.4 | 1 | 0.7 | 30 | 621 | 0.03 | — | 90.3 | 29.5 | 0.17 | — | 3.8 | [448] |
| 900 | 10.5 | 1.2 | 0.6 | 111 | 348.4 | 0.32 | — | — | — | — | — | — | ||
| Ba7.1La0.9Cu16P30 | 300 | 25.8 | 1.1 | 0.8 | 39 | 549.5 | 0.03 | — | 83.4 | 31 | 0.09 | — | 1 | [448] |
| 900 | 12 | 1.1 | 0.5 | 128 | 321.5 | 0.43 | — | — | — | — | — | — | ||
| Ba6.4La1.6Cu16P30 | 300 | 44.5 | 1 | 0.9 | 73 | 487.8 | 0.08 | 0.4 | 449.8 | 227 | 16.1 | — | 0.5 | [448] |
| 900 | 13.9 | 0.9 | 0.6 | 170 | 225.2 | 0.63 | — | — | — | — | — | — | ||
| Ba8Cu16P30 | 300 | 0 | 1.2 | — | 12.8 | 0 | 4 × 10−3 | — | — | — | — | — | — | [449] |
| 812 | 1.3 | 1.4 | — | 46 | 102 | 0.07 | — | — | — | — | — | — | ||
| Ba8Cu14Ge6P26 | 300 | 9.2 | 0.8 | — | 101.5 | 66.7 | 0.03 | — | — | — | — | — | — | [449] |
| 812 | 0 | 0.7 | — | 234 | 0 | 0.63 | — | — | — | — | — | — | ||
| Ba8Cu14Zn2P30 | 298 | — | — | — | 80 | — | — | — | — | — | — | — | — | [450] |
| Ba8Cu14Zn2P30 | 800 | 17.4 | 0.9 | 0.6 | 202 | 162.6 | 0.62 | — | — | — | — | — | — | [451] |
| Ba8Cu14Ge6P26 (SC) | 300 | 9.2 | 0.8 | 0.7 | 102 | 66.6 | 0.02 | — | — | — | — | — | — | [449] |
| 812 | 15.4 | 0.7 | 0.6 | 234 | 102 | 0.63 | — | — | — | — | — | — | ||
| Ba8Cu16P30 | 275 | 15.1 | 1.1 | — | 12.5 | 877.1 | 3.5 × 10−3 | — | — | — | — | — | — | [452] |
| 300 | — | — | — | 12.8 | — | — | — | — | — | — | — | — | ||
| Ba8Cu4Au12P30 | 275 | 9 | 0.7 | 0.4 | 14.3 | 458.7 | — | — | — | — | — | — | — | [452] |
| 300 | — | — | — | 15.3 | — | 3.8 × 10−3 | — | — | — | — | — | — | ||
| Ba8Cu8Au8P30 | 275 | 9.8 | 1 | 0.6 | 14.8 | 483 | — | — | — | — | — | — | — | [452] |
| 300 | — | — | — | 16.1 | — | 3.1 × 10−3 | — | — | — | — | — | — | ||
| Ba8Cu12Au4P30 | 275 | 8.3 | 1 | 0.6 | 11 | 543.5 | 1.9 × 10−3 | — | — | — | — | — | — | [452] |
| Ba8Au16P30 | 275 | 12.9 | 0.6 | — | 15.3 | 613.5 | 0.01 | — | — | — | — | — | — | [452] |
| 300 | — | — | — | 15.3 | — | — | — | — | — | — | — | — | ||
| Ba8Au16P30 | 300 | 11.1 | 0.6 | 0.2 | 15.8 | 588.2 | — | — | — | — | — | — | — | [453] |
| 390 | 7.5 | 0.7 | 0.2 | 16.2 | 571.4 | 0.01 | — | — | — | — | — | — | ||
| EuNi2P4 | 300 | 3.9 | 5.2 | 3.1 | 0.9 | 2857 | — | — | — | — | — | — | — | [454] |
| BaNi2P4 | 300 | — | 4.5 | 0.3 | — | 11 933 | — | — | — | — | 0.37 | — | — | [455] |
| BaNi2P4 | 300 | 27.3 | 4.2 | — | 1.6 | 12 500 | 2.3 × 10−3 | — | — | — | — | — | — | [456] |
| BaCu2P4 | 300 | 15 | 1.7 | 1.5 | 30 | 416.6 | 6.6 × 10−3 | — | — | — | — | — | — | [456] |
| SrNi2P4 | 300 | 7.2 | 3 | — | 0.6 | 7143 | 3 × 10−5 | — | — | — | — | — | — | [456] |
| Ba8Cu16P30 | 300 | 0 | 1 | — | 15 | 1000 | 0.05 | — | — | — | — | — | — | [457] |
| Antimonides | ||||||||||||||
| K58Zn122Sb207 | 300 | 1.4 | 0.4 | 0.4 | 389 | 0.3 | 4 × 10−4 | 0.2 | — | — | — | — | — | [458] |
| Cs8In27Sb19 | 300 | 0.2 | 0.9 | 0.9 | 250 | 0.2 | 4 × 10−4 | 0.3 | 1051.5 | 880 | 0.002 | — | 0.0001 | [459] |
| Cs8Ga27Sb19 | 300 | — | 0.8 | 0.8 | 150 | — | — | 0.7 | — | — | — | — | — | [459] |
| Rb8Ga27Sb19 | 300 | — | 0.9 | 0.9 | 150 | — | — | 0.6 | — | — | — | — | — | [459] |
| Cs8Zn18Sb28 | 300 | 0.4 | 0.8 | — | 80 | 4 | 1 × 10−3 | 0.9 | — | — | — | — | — | [460] |
| Cs8Zn13.5Cd4.5Sb28 | 300 | 0.3 | 0.6 | — | 76 | 3.2 | 1 × 10−3 | 0.1 | — | — | — | — | — | [460] |
| Cs8Zn9Cd9Sb28 | 300 | 0.2 | 0.5 | — | 63 | 2.6 | 6 × 10−4 | 0.1 | — | — | — | — | — | [460] |
| Cs8Zn4.5Cd13.5Sb28 | 300 | 0.2 | 0.5 | — | 26 | 5.3 | 2 × 10−4 | 0.1 | — | — | — | — | — | [460] |
| Cs8Cd18Sb28 | 300 | 17 | 0.7 | — | 17 | 833.3 | 0.01 | 2.5 × 10−2 | — | — | — | — | — | [460] |
| Inverse clathrates | ||||||||||||||
| Si32.1P13.9Te6.6Br | 300 | 382.3 | 5 | — | 270 | 285.7 | 0.03 | — | — | — | — | — | — | [461] |
| 750 | 205.4 | 3.5 | — | 330 | 333.3 | 0.1 | — | — | — | — | — | — | ||
| Si30.3P15.6Te6.6Se1.46 | 300 | 52.3 | 4.2 | — | 310 | 26.3 | 0 | — | — | — | — | — | — | [461] |
| 750 | 19.9 | 3 | — | 350 | 26.3 | 0.01 | — | — | — | — | — | — | ||
| Ge30P16Te8 | 300 | — | — | — | 800 | 0.1 | — | 0.3 | — | — | — | — | — | [462] |
| Ge38P8I8 | 300 | — | — | — | — | 0 | — | 0.2 | — | — | — | — | — | [462] |
| Si131.8P40.4Te21.5 | 300 | 19.1 | 1.5 | — | 170 | 37 | 0.02 | — | — | — | — | — | — | [463] |
| 1100 | 3.3 | 1.4 | — | 210 | 30.3 | 0.36 | — | — | — | — | — | — | ||
| Si31.9P13.9Te7.00 | 300 | 85.9 | 3.2 | 3.1 | 170 | 166.7 | 0.05 | — | — | — | — | — | — | [463] |
| 870 | 38.7 | 2.9 | 2.6 | 270 | 142.8 | 0.3 | — | — | — | — | — | — | ||
| Sn38Sb8I8 | 300 | — | 0.7 | — | −600 | — | — | 0.8 | — | — | — | — | — | [464] |
| Ge38Sb8I8 | 300 | — | 1.2 | — | −800 | — | — | 1.2 | — | — | — | — | — | [464] |
GPa: applied pressure for the high pressure synthesis.□: vacancy.SC: single crystal.
All known P clathrates show p-type conduction. Sn clathrates generally show much lower σ values in comparison to those of Si and Ge clathrates due to their tendency to have charge-balanced compositions. Figure 5(e) reveals the phonon-glass nature of the clathrates' thermal conductivity with a majority of phases possessing κ below 3 W K–1m–1 irrespective of the framework atoms. Heavier Sn clathrates with large cage volumes display the lowest κ values compared to Si and Ge homologous. Based on a linear fit analysis, κL contributes on average ∼70% to κ (figure 6(f)). High μw (calculated by equation (5)) and low κL are desirable to achieve better thermoelectrics efficiencies. Sn clathrates satisfy this condition best among all families of clathrates (figure 6(g)), which manifest themselves with the highest peak zT values attained at low-to-mid temperatures (figure 6(h)). Ge clathrates show moderate μw and little higher κL values compared to the Sn counterparts and display high peak zT values at mid-to-high temperatures. Si clathrates have relatively low μw and peak zT values but are the most stable phases (TmSi > TmGe > TmSn; Tm: melting T) make them attractive for high-temperature thermoelectrics applications. P and Sb cationic clathrates possess the lowest μw values and generally show low thermoelectric efficiencies.
Widespread use of thermoelectric generators necessitates stable n- and p-type materials of almost equal thermoelectric potential and compatibility in thermal expansion to minimize stress effects. For such module applications, inorganic clathrates displaying both n- and p-type conduction in the same material systems with high thermoelectric efficiencies are very suitable materials. However, in targeting large-scale industrial applications, cheap, earth-abundant, and non-toxic raw materials should be preferred during their synthesis. As mentioned above, Sn, Ge, and Si clathrates show the best thermoelectric performances at low, mid, and high temperatures, respectively. Combinatorial use of these framework elements in the same clathrate framework could lead to superior thermoelectric properties along with better thermal management. Besides, band structure engineering through proper substitution of the framework atoms or tuning the vacancy concentration on the framework sites and the guest atom concentrations inside the polyhedral cages with novel synthetic techniques, e.g. low-temperature redox reactions [353, 354] melt-centrifugation [44, 314] or liquid phase sintering [355, 356] may potentially lead to enhanced thermoelectric properties. Leveraging high-throughput calculations and data mining [357–360], the selection process of inorganic clathrates can be accelerated, and unexplored clathrate phases may be uncovered with high thermoelectric performance. Exploring novel clathrate types with unique cage structures should be another motivation for discovering high-efficiency materials for this family of compounds.
Acknowledgment
This work is supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK) with Grant Number 118M371.
3.7. FeGa3-type
Intermetallic compounds with crystal structure of the FeGa3 type
Raúl Cardoso-Gil
Max-Planck-Institut für Chemische Physik fester Stoffe, Nöthnitzer Straße 40, 01187 Dresden, Germany
Intermetallic compounds with composition T(8)Tr3, where T(8) is a TM of the group 8 and Tr being gallium or indium, are rather unexpectedly semiconductors with narrow band gap, originated by the hybridization of d and p orbitals of the participant atoms [465]. They crystallize in the tetragonal FeGa3 structure type (space group P42/mnm, nr.139). In the crystal structure, the Ga2 (8j) atoms form double trigonal prisms capped by four Ga1 (4c) atoms and filled by two TM atoms (4f). The FeGa3 type considered as a simple crystal structure, shows its complexity in its electronic structure. In FeGa3 itself, the valence electron count of 17 ve/fu can be rationalized with atomic interactions isolobal to one Fe−Fe and eight Fe−Ga two-center-two electron bonds. The detailed bonding analysis shows the presence of three-center Fe–Ga–Ga' bonding having simultaneously a direct influence on the Fe–Fe bonding in the dumbbell [466]. The knowledge on the atomic interactions and bonding scenario is the key for the regulation of charge carrier and transport properties considering chemical insights. Additionally, the presence of different bonding types (bonding inhomogeneity) allows a reduction of the lattice thermal conductivity [467]. Thus, this particular electronic condition offers a suitable setup to tune the thermoelectric properties via chemical substitution. The consequent improvement of the thermoelectric properties has been accomplished tailoring the band gap with the synthesis of partially substituted derivatives. This concerning, the data compilation (table 7) shows a direct comparative view on the resulting thermoelectric properties of intermetallic compounds of the FeGa3-type.
Table 7. FeGa3 -type thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT {T} | Eg (eV) | µH (cm2 V−1 s−1) | ms* (me) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| FeGa3 | — | — | — | — | — | — | 0.31t | — | — | [465] |
| FeGa3 | — | — | — | — | — | — | 0.50t | — | — | [477] |
| FeGa3 | — | — | — | — | — | — | 0.45–0.29e | — | — | [478] |
| FeGa3 | — | — | — | — | — | — | 0.8e | — | — | [478] |
| FeGa3 | — | — | — | — | — | — | 0.4e | — | — | [479] |
| FeGa3 | — | — | — | — | — | — | 0.5t | — | — | [476] |
| FeGa3 | 313 | 123.1 | 3.7 | −563 | 4.3 | — | 0.3t 0.26e | 12 (295) | 5 | [471] |
| FeGa3 | 298 | 0.4 | 5.3 | 1.47 | 183 | — | — | — | — | [480] |
| FeGa3 | 300 | 1.3 | ∼6.4 | −350 | 0.5 | 0.000 14 | 0.47e | — | 0.2 | [481] |
| FeGa3 | 290 | 10.8 | 2.14 | −455 | 1.18 | 0.0023 {390 K} | — | — | — | [473] |
| FeGa2.95 | 290 | 9.5 | — | −518 | 0.5 | — | — | — | — | [473] |
| FeGa3.05 | 290 | 4.0 | — | −400 | 0.83 | — | — | — | — | [473] |
| FeGa2.991Ge0.009 | 290 | 2.8 | — | −153 | 10 | — | — | — | — | [473] |
| FeGa2.952Ge0.048 | 290 | 5.9 | — | −98 | 43 | — | — | — | — | [473] |
| FeGa2.901Ge0.099 | 290 | 3.4 | — | −73 | 35 | — | — | — | — | [473] |
| Fe0.99 Co0.01Ga3 | 290 | 8.0 | — | −196 | 17.5 | — | — | — | — | [473] |
| Fe0.95 Co0.05Ga3 | 290 | 4.2 | — | −93 | 33 | — | — | — | — | [473] |
| Fe0.90 Co0.10Ga3 | 290 | 5.6 | — | −95 | 42 | — | — | — | — | [473] |
| Fe0.99Co0.01Ga2.991Ge0.009 | 290 | 6.8 | 1.37 | −130 | 32.6 | 0.013 {390 K} | — | — | — | [473] |
| FeGa3 | 300 | 11.6 | — | −5 | 1876 | — | — | — | — | [482] |
| CoGa3 | 300 | 42.0 | — | −6.3 | 5434 | — | — | — | — | [482] |
| Fe0.75 Co0.25Ga3 | 300 | 22.1 | — | −8.1 | 2242 | — | — | — | — | [482] |
| FeGa3 | 290 | 11.8 | 4.4 | −360 | 3.85 | — | — | — | — | [483] |
| FeGa3 [001] | 290 | 201.1 | 4.4 | −605 | 3.85 | — | — | — | — | [483] |
| FeGa3 [100] | 290 | 162.4 | 5.7 | −590 | 3.7 | — | — | — | — | [483] |
| FeGa2.95Al0.05 | 315 | 19.5 | 4.0 | −550 | 0.8 | 0.020 {750 K} | — | — | — | [484] |
| FeGa2.90Al0.10 | 315 | 0.8 | 4.3 | −140 | 4 | 0.015 {750 K} | — | — | — | [484] |
| FeGa2.97In0.03 | 315 | 0.4 | 4.6 | −118 | 2.6 | 0.010 {750 K} | — | — | — | [484] |
| FeGa2.94In0.06 | 315 | 0.2 | 4.4 | −48 | 2.9 | 0.006 {750 K} | — | — | — | [484] |
| FeGa2.97Zn0.03 | 315 | 2.6 | 3.6 | 310 | 1.7 | 0.016 {750 K} | — | — | — | [484] |
| FeGa2.94Zn0.06 | 315 | 31.9 | 3.2 | 420 | 5.9 | 0.016 {750 K} | — | — | — | [484] |
| FeGa2.95Ge0.05 | 315 | 53.7 | 3.1 | −120 | 330 | 0.13 {667 K} | — | — | — | [484] |
| FeGa2.90Ge0.10 | 315 | 31.1 | 2.3 | −70 | 385 | 0.10 {716 K} | — | — | — | [484] |
| FeGa2.80Ge0.20 | 315 | 51.1 | 1.9 | −82 | 526 | 0.21 {765 K} | — | — | — | [484] |
| Fe0.995 Co0.005Ga3 | 300 | 21.1 | 4.0 | −213 | 40 | 0.014 {400 K} | — | — | — | [485] |
| Fe0.985 Co0.015Ga3 | 300 | 18.2 | 3.6 | −109 | 120 | 0.012 {400 K} | — | — | — | [485] |
| Fe0.975 Co0.025Ga3 | 300 | 25.3 | 2.8 | −109 | 167 | 0.029 {400 K} | — | — | — | [485] |
| Fe0.950 Co0.050Ga3 | 300 | 28.9 | 3.4 | −94 | 233 | 0.052 {400 K} | — | — | — | [485] |
| Fe0.875 Co0.125Ga3 | 300 | 30.3 | 2.9 | −79 | 303 | 0.037 {400 K} | — | — | — | [485] |
| Fe0.500 Co0.500Ga3 | 300 | 34.9 | 2.6 | −49 | 588 | 0.027 {400 K} | — | — | — | [485] |
| Fe25Ga75 | 373 | 11.6 | 4.0 | −334 | 7.5 | ∼0.002 {773 K} | 0.25e | 9 (300) | [486] | |
| Fe25Ga74Sn1 | 373 | 50.8 | 3.1 | −256 | 81 | 0.09 {473 K} | — | — | — | [486] |
| Fe25Ga73Sn2 | 373 | 49.3 | 2.8 | −202 | 147 | 0.11 {573 K} | — | — | — | [486] |
| Fe25Ga72Sn3 | 373 | 40.1 | 2.3 | −178 | 158 | 0.14 {524 K} | — | — | — | [486] |
| Ru25Ga75 | 373 | 19.2 | 4.7 | −522 | 1.4 | 0.09 {773 K} | 0.33e | 10 (300) | [486] | |
| Fe25Ga74Zn1 | 373 | 117.3 | 4.5 | 322 | 87 | 0.14 {737 K} | — | — | — | [486] |
| Fe25Ga73Zn2 | 373 | 100.9 | 5.0 | 307 | 89 | 0.21 {737 K} | — | — | — | [486] |
| Fe25Ga72Zn3 | 373 | 81.9 | 5.1 | 294 | 84 | 0.14 {737 K} | — | — | — | [486] |
| FeGa3 | 300 | — | — | — | 2.5 | — | 0.35t 4.0e | — | — | [487] |
| FeGa2.98Zn0.02 | 300 | — | — | — | 5.9 | — | 0.2e | — | — | [487] |
| FeGa2.96Zn0.04 | 300 | — | — | — | 22 | — | 0.2e | — | — | [487] |
| FeGa2.94Zn0.06 | 300 | — | — | — | 43 | — | 0.18e | — | — | [487] |
| Fe0.986Mn0.014Ga3 | 300 | — | — | — | 0.14 | — | 0.33e | — | — | [487] |
| Fe0.966Mn0.034Ga3 | 300 | — | — | — | 0.37 | — | 0.23e | — | — | [487] |
| Fe0.916Mn0.084Ga3 | 300 | — | — | — | 20 | — | 0.14e | — | — | [487] |
| Fe0.913Mn0.087Ga3 | 300 | — | — | — | 2.5 | — | 0.13e | — | — | [487] |
| FeGa2.99Ge0.01 | 293 | — | — | — | — | — | 24 (300) | — | [488] | |
| FeGa2.93Ge0.07 | 293 | — | — | — | — | — | 11.5 (300) | — | [488] | |
| FeGa2.85Ge0.15 | 293 | 58.8 | 4.9 | −118 | 333 | 0.14 {623 K} | — | 9.5 (300) | — | [488] |
| FeGa2.75Ge0.25 | 293 | 66.5 | 4.5 | −85 | 588 | 0.17 {673 K} | — | 2.9 (300) | — | [488] |
| FeGa2.65Ge0.35 | 293 | 59.1 | 4.1 | −77 | 588 | 0.20 {673 K} | — | — | — | [488] |
| Fe0.90Co0.10Ga2.65Ge0.35 | 293 | 54.3 | 2.7 | −67 | 633 | — | — | — | — | [488] |
| Fe0.85Co0.15Ga2.65Ge0.35 | 325 | 53.2 | 2.7 | −73 | 658 | 0.25 {823 K} | — | — | — | [488] |
| Fe0.75Co0.25Ga2.65Ge0.35 | 293 | 65.5 | 2.5 | −47 | 1111 | 0.24 {873 K} | — | — | — | [488] |
| Fe0.50 Co0.50Ga2.65Ge0.35 | 293 | 68.7 | 2.0 | −45 | 1219 | 0.23 {873 K} | — | — | — | [488] |
| Fe0.95 Co0.05Ga3 | 322 | 48.9 | 3.75 | −143 | 234 | 0.14 {620 K} | — | — | — | [489] |
| Fe0.975Ni0.025Ga3 | 322 | 37.7 | 4.1 | −222 | 71.4 | 0.09 {620 K} | — | — | — | [489] |
| FeGa3 | 373 | — | — | — | 0.36 | — | 0.46e | — | — | [490] |
| FeAl0.276Ga2.724 | 373 | — | — | — | 0.71 | — | 0.42e | — | — | [490] |
| FeAl0.366Ga2.634 | 373 | — | — | — | 1.25 | — | 0.4e | — | — | [490] |
| FeAl0.534Ga2.466 | 373 | — | — | — | 4.35 | — | 0.35e | — | — | [490] |
| Fe0.96Re0.04Ga3 | 327 | 9.0 | 3.7 | 217 | 18.5 | 0.06 {975 K} | 0.46 | — | — | [491] |
| Fe0.92Re0.08Ga3 | 327 | 0.3 | 3.6 | 38.4 | 8.3 | 0.05 {825 K} | 0.4 | — | — | [491] |
| RuGa3 | — | — | — | — | — | — | 0.3t | — | — | [465] |
| RuGa3 | — | — | — | — | — | — | 0.26t | — | — | [477] |
| RuGa3 | 300 | — | — | — | 3.3 | — | ∼0.5t | — | — | [487] |
| RuGa3 | 313 | 1.6 | 3.3 | −274 | 1.6 | 0.18 {940 K} | 0.3t 0.32e | 7.8 (295) | 0.5 | [471] |
| RuGa3 | 373 | 66.9 | 4.3 | −546 | 3.7 | 0.13 {973 K} | 0.4t 0.33e | 10 (300) | — | [469] |
| RuGa3 | 300 | 20.3 | ∼7 | −477 | 1.8 | — | 0.33t | — | — | [492] |
| RuGa3 | 300 | 31.6 | ∼7 | −463 | 3.3 | 0.08 {845 K} | 0.33t | — | — | [492] |
| OsGa3 | — | — | — | — | — | — | 0.68t | — | — | [477] |
| OsGa3 | 313 | 84.2 | 3.5 | −577 | 2.5 | — | 0.42e | 12 (300) | 1.8 | [471] |
| ReGa2Ge i | 300 | 2.1 | 1.11 | −43.5 | 40 | — | 0.23–0.4t | — | — | [474] |
| RuIn3 | — | — | — | — | — | — | 0.31t | — | — | [465] |
| RuIn3 | — | — | — | — | — | — | 0.3t | — | — | [477] |
| RuIn3 | — | — | — | — | — | — | 0.41t 0.46–0.51e | — | — | [493] |
| RuIn3 | — | — | — | — | — | — | 0.3t | — | — | [470] |
| RuIn3 | 300 | 14.8 | 5.7 | −400 | 3.2 | 0.073 {753 K} | 0.45e | — | — | [468] |
| RuIn2.99Sn0.01 | 300 | 66.0 | 3.2 | −170 | 206 | 0.10 {464 K} | — | — | — | [468] |
| RuIn2.975Sn0.025 | 300 | 41.0 | 2.1 | −115 | 250 | 0.14 {565 K} | — | — | — | [468] |
| RuIn2.950Sn0.050 | 300 | 26.9 | 2.3 | −100 | 200 | 0.084 {628 K} | — | — | — | [468] |
| RuIn2.90Sn0.10 | 300 | 47.0 | 2.3 | −82 | 450 | 0.17 {676 K} | — | — | — | [468] |
| RuIn2.99Zn0.01 | 300 | 0.1 | 3.1 | 54 | 2 | 0.13 {789 K} | — | — | — | [468] |
| RuIn2.975Zn0.025 | 300 | 84.0 | 3.6 | 253 | 100 | 0.24 {235 K} | — | — | — | [468] |
| RuIn2.950Zn0.050 | 300 | 212.9 | 2.6 | 140 | 950 | 0.45 {629 K} | — | — | — | [468] |
| RuIn2.90Zn0.10 | 300 | 44.1 | 2.6 | 175 | 130 | 0.18 {637 K} | — | — | — | [468] |
| RuIn2.99Sn0.01 | 300 | — | — | −99t | — | — | — | — | — | [470] |
| RuIn2.975Sn0.025 | 300 | — | — | −133t | — | — | — | [470] | ||
| RuIn2.950Sn0.050 | 300 | — | — | −174t | — | — | — | [470] | ||
| RuIn2.90Sn0.10 | 300 | — | — | −218t | — | — | — | [470] | ||
| RuIn2.875Sn0.125 | 300 | — | — | −274t | — | — | — | [470] | ||
| RuIn2.99Zn0.01 | — | — | 222t | — | — | — | [470] | |||
| RuIn2.975Zn0.025 | 300 | 316.0 | 4.0 | 164t 150e | 1250 | 0.76 {620 K} | — | — | — | [470] |
| RuIn2.950Zn0.050 | 300 | 219.0 | 3.9 | 124t 146e | 909 | 0.41 {620 K} | — | — | — | [470] |
| RuIn2.90Zn0.10 | 300 | 477.0 | 4.4 | 98t 140e | 2128 | 0.60 {620 K} | — | — | — | [470] |
| RuIn2.875Zn0.125 | 300 | — | — | 85t | — | — | — | — | — | [470] |
| Ru0.95Rh0.05In3 | 300 | 36.1 | 3.3 | −150 | 143 | 0.05 {472 K} | — | — | — | [494] |
| Ru0.95Ir0.05In3 | 300 | 29.2 | 2.8 | −120 | 167 | 0.04 {444 K} | — | — | — | [494] |
| Ru0.95Re0.05In3 | 300 | 1.5 | 3.7 | −120 | 8.3 | 0.15 {786 K} | — | — | — | [494] |
| Ru0.95Ir0.05In2.95Zn0.05 | 300 | 32.8 | 2.9 | −115 | 200 | 0.04 {428 K} | — | — | — | [494] |
| RuIn3 | 300 | 1.6 | 3.0 | 207 | 3.2 | 0.17 {773 K} | 0.2t 0.19e | 11 (300) | — | [469] |
| Ru24In76 | 373 | 1.4 | 3.5 | 138 | 9.1 | 0.07 {773 K} | — | — | — | [469] |
| Ru25In75 | 373 | 15.2 | 3.5 | 324 | 11 | 0.11 {873 K} | — | — | — | [469] |
| Ru25.4In74.6 | 373 | 7.5 | 4.2 | 278 | 9.3 | 0.096 {773 K} | — | — | — | [469] |
| Ru26In74 | 373 | 7.7 | 3.6 | 295 | 7.8 | 0.10 {673 K} | — | — | — | [469] |
| Co1Ru25In74 | 373 | 44.4 | 2.9 | −193 | 147 | 0.077 {473 K} | — | — | — | [469] |
| Co2Ru24In74 | 373 | 27.2 | 2.5 | −175 | 111 | 0.10 {473 K} | — | — | — | [469] |
| RuIn3 | 290 | 16.5 | 2 | −420 | 2.7 | 0.0077 {347 K} | — | — | — | [472] |
| Ru0.995Ir0.005In3 | 290 | 10.7 | — | −172 | 31 | — | — | — | — | [472] |
| Ru0.99Ir0.01In3 | 290 | 21.0 | 1.6 | −172 | 61 | 0.053 {380 K} | — | — | — | [472] |
| Ru0.95Ir0.05In3 | 290 | 11.2 | — | −83 | 100 | — | — | — | — | [472] |
| Ru0.80Ir0.20In3 | 290 | 13.4 | — | −37 | 286 | — | — | — | — | [472] |
| Ru0.60Ir0.40In3 | 290 | 21.6 | — | −40.5 | 420 | — | — | — | — | [472] |
| Ru0.20Ir0.80In3 | 290 | 12.1 | — | −24 | 400 | — | — | — | — | [472] |
| IrIn3 i | 290 | 31.2 | — | −17 | 1449 | — | — | — | — | [472] |
Temperature for highest zT in bracket (e.g. 0.14 {524 K}).t: theoretical.e: experimental.i: isostructural compound with 17 ve and transition metal ≠ T(8).
The narrow band gap of the binary semiconductors ranges between 0.2 and 0.5 eV. Their electrical conductivity increases significantly by substitution, e.g. from σ(300K) = 3.2 Ω−1cm−1 in RuIn3 [468, 469] to σ(300K) = 2128 Ω−1cm−1 in RuIn2.90Zn0.10 [470].
RuIn3 and RuGa3 are n-type semiconductors at T < 360 K and T < 468 K and p-type above these temperatures, respectively. The n- to p-type transition is selectively suppressed in RuIn3 upon electron or hole doping, reaching |S| values around 200 μV K−1 [468, 470]. FeGa3 and OsGa3 do not present this behavior. Noteworthy, the original binary compounds show high values of Seebeck coefficients at room temperature, e.g. S = −563 μV K−1 and S = −477 μV K−1 in FeGa3 and OsGa3, respectively [471].
The thermal conductivity of binary FeGa3-type compounds is reduced to ∼30% by substitutions, where Ru0.99Ir0.01In3 with κ= 1.55 W m−1K−1 [472], Fe0.99 Co0.01Ga2.991Ge0.09 with κ = 1.37 W m−1K−1 [473] and ReGa2Ge with κ = 1.1 W m−1K−1 [474] are those representatives with the lowest thermal conductivity at room temperature, attributed to the singular chemical substitutions. The ternary derivative ReGa2Ge is a rather unusual chemical variant of the FeGa3-type with a TM atom from the group 7 (Re) and Ge substituting gallium. Its stoichiometric composition, yields likewise a defined valence electron count of 17 ve/fu. The presence of Re−Ga and Re−Re interactions is also consistent with the reported band gap (0.23−0.4 eV) and the semiconductor behavior [474].
An essential condition for good thermoelectric materials is a reasonable electrical conductivity associated to a low electronic contribution to the thermal conductivity, this is fulfilled by certain intermetallic compounds with small band gap [475]. For further isostructural TTr3 intermetallic compounds a theoretical study on their thermoelectric properties has been performed [476], based on these results, the corresponding experimental study is main part of an ongoing project. The acquired knowledge should strengthen the search and the development of further intermetallic compounds with high potential as thermoelectric materials, as well as the better understanding and control of their physical properties.
3.8. Actinides and lanthanides
Actinide- and lanthanide-based thermoelectrics
E Svanidze
Max-Planck-Institut für Chemische Physik fester Stoffe, Nöthnitzer Straße 40, 01187 Dresden, Germany
The idea of using lanthanide- or actinide-based materials for thermoelectric applications is rather unconventional and, perhaps, even far-fetched. On the one hand, the chemical and radiological attributes of these materials require non-trivial experimental conditions which render scaling-up efforts questionable, while, on the other hand, the complexity of 4f- and 5f-orbitals often makes their theoretical assessment quantitatively challenging. However, much like other branches of fundamental research on f-electron-based alloys and compounds, understanding their complex properties can provide a convenient avenue towards a targeted discovery of 'simpler' materials containing only s-, p-, and d-electrons.
Previous work on lanthanide- [65, 495–550], thorium- [551–568], and uranium-based [521, 551, 557, 560, 564, 569–594] systems is summarized in table 8 and figures 7–9. Some variations in the reported data can probably be attributed to the fragile ground states of f-electron materials and, consequently, sample quality issues [467]. Moreover, for the majority of these systems, values of the thermal conductivity κ, are missing. In order to estimate the value of zT for those compounds, a value of κ = 10 W m−1 K−1 was used. Based on the existing data, this estimate of κ is comparable to what has been observed in these materials, with average values κave(R) = κave(Th) = 6.7 W m−1 K−1, κave(U) = 10.6 W m−1 K−1, but, of course, experimentally determined values of κ are highly desired in order to properly assess the thermoelectric potential of these systems. As for the values of the effective mass, a distinction must be made between those obtained from the low-temperature specific heat, ARPES, or de Haas-van Alphen measurements and those extracted from room-temperature Hall or specific heat data. In the case of heavy-fermion lanthanide- and uranium-based systems, it was previously noted [595] that the assumption of a spherical Fermi surface, that is needed for this analysis, is quantitatively not accurate. Moreover, experimentally obtained values of the lattice thermal conductivity, weighed mobility, bandgap, mobility parameter, as well as static dielectric constant are currently lacking. Filling such gaps will enable deeper insight into thermoelectric properties of lanthanide- and actinide-based compounds.
Figure 7. Thermoelectric figure of merit zT of lanthanide-based compounds for various temperatures [65, 495–550].
Download figure:
Standard image High-resolution imageFigure 8. Thermoelectric figure of merit zT for Th-based compounds for various temperatures. Given the toxicity and mild radioactivity of Th compounds and alloys, a possible application domain is the aerospace industry, with the yellow region marking the suitable temperatures range [551–568].
Download figure:
Standard image High-resolution imageFigure 9. Thermoelectric figure of merit zT for U-based compounds for various temperatures. Given the toxicity and mild radioactivity of U compounds and alloys, a possible application domain is the aerospace industry, with the yellow region marking the suitable temperatures range [521, 551, 557, 560, 564, 569–594].
Download figure:
Standard image High-resolution imageTable 8. Actinide and lanthanide thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κ (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µ0 (cm2 V−1 s−1) | ms* (me) |
 r or r or  ( ( 0) 0) | zT max | T (K) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc2Te3 | 300 | 46.9 | — | 90 | 400 | 0.02 | — | — | — | — | 0.3 | 500 | [495] |
| YB6 | 300 | 22.2 | — | −0.5 | 25 000 | 0.000 02 | — | — | — | — | — | — | [496, 507, 517] |
| YB66 | 300 | 0.3 | 2 | 450 | 0.03 | 0.0001 | — | — | — | — | 0.1 | 1000 | [528] |
| YC2 | 300 | 482.5 | — | 12 | 33 300 | 0.01 | — | — | — | — | — | — | [539, 547] |
| Y2O3 | 300 | 1.8 | — | 30 | 50 | 0.0001 | — | — | — | — | — | — | [548] |
| Y2S3 | 300 | 360.2 | — | 30 | 10 000 | 0.03 | — | — | — | — | — | — | [548] |
| Y5Ge3 | 300 | 75.9 | — | −8.1 | 7690 | 0.002 | — | — | — | — | — | — | [549] |
| YGe | 300 | 14.3 | — | 0.7 | 12 700 | 0.000 02 | — | — | — | — | — | — | [549] |
| YGe2 | 300 | 12.7 | — | 0.8 | 10 200 | 0.000 02 | — | — | — | — | — | — | [549] |
| YNiSb | 300 | 86.8 | 6 | 40 | 1800 | 0.01 | — | — | — | — | — | — | [550] |
| Y3Ru4Ge13 | 300 | 49.4 | 3.8 | 37 | 1110 | 0.01 | — | — | — | — | — | — | [497] |
| i-Zn-Mg-Y QC | 300 | 55.6 | 6–7 | 8 | 5700 | 2.5 × 10−7 | — | — | — | — | — | — | [498, 499] |
| i-Zn-Mg-Y QC | 300 | 35.1 | 2.6 | 8 | 3600 | 0.003 | — | — | — | — | — | — | [500] |
| LaB6 | 300 | 27.9 | — | 0.1 | 66 700 | 2.0 × 10−6 | — | — | — | — | — | — | [496, 507, 517] |
| LaC2 | 300 | 242.7 | — | 9 | 22 200 | 0.005 | — | — | — | — | — | — | [539, 547] |
| LaN | 300 | 323.7 | — | 35 | 7690 | 0.03 | — | — | — | — | — | — | [501] |
| La2O3 | 300 | 2.0 | — | 33 | 50 | 0.0002 | — | — | — | — | — | — | [548] |
| LaSi2 | 300 | 2228.4 | — | −4.2 | 424 000 | 0.02 | — | — | — | — | — | — | [502] |
| LaS | 300 | 63.2 | 22.6 | 4 | 12 600 | 0.0003 | — | — | — | — | — | — | [503] |
| LaS2 | 300 | — | — | — | — | — | — | — | — | — | 0.7 | 1200 | [504] |
| La2S3 | 300 | 1924.5 | 9 | 100 | 14 300 | 0.5 | — | — | — | — | — | — | [548, 505] |
| La3S4 | 300 | 32.2 | 1.3 | −40 | 667 | 0.03 | — | — | — | — | 0.3 | 1000 | [506] |
| La5Ge3 | 300 | 13.6 | — | −1.7 | 5920 | 0.000 05 | — | — | — | — | — | — | [549] |
| LaGe | 300 | 73.0 | — | −3.9 | 14 900 | 0.0007 | — | — | — | — | — | — | [549] |
| LaGe2 | 300 | 139.4 | — | −10 | 11 500 | 0.004 | — | — | — | — | — | — | [549] |
| LaSe | 300 | 202.3 | 24.3 | 9 | 18 500 | 0.002 | — | — | — | — | — | — | [503] |
| La2Se3 | 300 | 1.6 | 340 | 0.7 | 0.0002 | — | — | — | — | — | — | [548, 508] | |
| La2Te3 | 300 | 8.1 | 1.1 | 40 | 169 | 0.007 | — | — | — | — | — | — | [548, 509, 510] |
| La3Te4 | 300 | 30.0 | 17 | −25 | 1000 | 0.001 | — | — | — | — | 1.2 | 1250 | [65, 511] |
| LaTe1.5 | 300 | — | — | — | — | — | — | — | — | — | 1 | 1000 | [504] |
| LaCoO3 | 300 | 0.0 | — | 300 | 0.001 | 2.7 × 10−7 | — | — | — | — | — | — | [512] |
| La6Pd12In5 | 300 | 16.1 | 7.2 | −1 | 10 900 | 0.000 05 | — | — | — | — | — | — | [513] |
| La3Cu3Sb4 | 300 | — | 2.8 | 60 | — | 0.02 | — | — | — | — | 0.05 | 400 | [514] |
| LaCrSe3 | 300 | 40.6 | 12.5 | 250 | 50 | 0.008 | — | — | — | — | — | — | [515] |
| CeC2 | 300 | 52.3 | — | −2.4 | 16 700 | 0.0003 | — | — | — | — | — | — | [539, 547] |
| CeB6 | 300 | 122.5 | — | 2.8 | 34 000 | 0.0008 | — | — | — | — | — | — | [496, 507, 517] |
| CeN | 300 | 960.5 | — | 20 | 40 000 | 0.05 | — | — | — | — | — | — | [501] |
| CeSi2 | 300 | 56.8 | — | 19.3 | 2450 | 0.003 | — | — | — | — | — | — | [502] |
| CeS | 300 | 169.5 | 16.3 | 12 | 11 700 | 0.003 | — | — | — | — | — | — | [503] |
| CeTe | 300 | 50.8 | 11.3 | 5 | 8200 | 0.0006 | — | — | — | — | — | — | [503] |
| Ce5Ge3 | 300 | 9.8 | — | 1.7 | 4270 | 0.000 04 | — | — | — | — | — | — | [549] |
| CeGe | 300 | 60.5 | — | −6.1 | 8060 | 0.0009 | — | — | — | — | — | — | [549] |
| CeGe2 | 300 | 6.8 | — | 0.7 | 6060 | 9.0 × 10−6 | — | — | — | — | — | — | [549] |
| CeO2 | 300 | 0.6 | — | 47 | 11.1 | 0.000 07 | — | — | — | — | — | — | [548] |
| CeSe2 | 300 | 1.2 | 1 | 480 | 0.10 | 0.0007 | — | — | — | — | — | — | [516, 518] |
| Ce0.9Cu0.1Se2 | 300 | 26.1 | 1.3 | 320 | 14.3 | 0.03 | — | — | — | — | 0.2 | 800 | [516] |
| CeS1.42 | 300 | 1.7 | 0.01 | −34 | 40.8 | 0.1 | — | — | — | — | 0.6 | 1400 | [519] |
| Ce3Se4 | 300 | 31.6 | 2.4 | −150 | 125 | 0.04 | — | — | — | — | — | — | [518] |
| Ce2Se3 | 300 | 21.1 | — | −57 | 303 | 0.003 | — | — | — | — | — | — | [510, 518] |
| Ce2Te3 | 300 | 31.2 | 1.1 | −176 | 90.9 | 0.08 | — | — | — | — | — | — | [509] |
| Ce3Te4 | 300 | 26.8 | 1.9 | −40 | 556 | 0.01 | — | — | — | — | — | — | [509] |
| CeRhSn | 300 | 453.1 | — | 45 | 8330 | 0.05 | — | — | — | — | — | — | [520] |
| CeIrSn | 300 | 87.7 | — | 40 | 1820 | 0.009 | — | — | — | — | — | — | [520] |
| Ce6Pd12In5 | 300 | 0 | 4.2 | 2 | 8330 | 2.4 × 10−10 | — | — | — | — | — | — | [513] |
| CeCu4Al8 | 300 | 278.2 | — | 12 | 19 200 | 0.008 | — | — | — | — | — | — | [521] |
| Ce3Cu3Sb4 | 300 | — | 2 | 55 | — | 0.03 | — | — | — | — | 0.06 | 400 | [514] |
| CeFe4−x Cox Sb12 | 300 | — | — | — | — | — | — | — | — | — | 1.2 | 1000 | [522] |
| Ce0.65Fe2 Co2Sb11Sn | 300 | 63.4 | 2 | 80 | 625 | 0.06 | — | — | — | — | — | — | [550] |
| Ce0.65Fe2 Co2Sb10Ge2 | 300 | 31.9 | 3.2 | 20 | 1330 | 0.005 | — | — | — | — | — | — | [550] |
| Ce0.65Fe2 Co2Sb12 | 300 | 84.1 | 3.5 | 100 | 625 | 0.05 | — | — | — | — | — | — | [550] |
| PrB6 | 300 | 51.7 | — | −0.6 | 51 300 | 0.000 06 | — | — | — | — | — | — | [496, 507, 517] |
| PrSe | 300 | 10.6 | 0.9 | 1 | 7200 | 0.0002 | — | — | — | — | — | — | [503] |
| PrS | 300 | 1923.8 | — | −19.3 | 83 000 | 0.09 | — | — | — | — | — | — | [503, 523] |
| PrTe | 300 | 19.7 | 8.0 | 2 | 7400 | 0.0001 | — | — | — | — | — | — | [503] |
| PrC2 | 300 | 282.9 | — | −9.1 | 25 600 | 0.006 | — | — | — | — | — | — | [539, 547] |
| PrN | 300 | 389.1 | — | −34 | 9520 | 0.03 | — | — | — | — | — | — | [501] |
| Pr3Te4 | 300 | 30.0 | 31 | −25 | 1000 | 0.0006 | — | — | — | — | 1.7 | 1200 | [65] |
| Pr2Te3 | 300 | 28.1 | 1.8 | −35 | 667 | 0.01 | — | — | — | — | — | — | [509, 510] |
| PrSi2 | 300 | 20.2 | −3.2 | 4950 | 0.0002 | — | — | — | — | — | — | [502] | |
| PrCoO3 | 300 | 0 | 1 | 210 | 0.05 | 0.000 07 | — | — | — | — | 0.04 | 700 | [524] |
| NdB6 | 300 | 38.6 | — | 0.4 | 50 000 | 0.000 02 | — | — | — | — | — | — | [496, 507, 517] |
| NdS | 300 | 34.6 | 19.3 | 2 | 13 000 | 0.000 08 | — | — | — | — | — | — | [503] |
| NdC2 | 300 | 275.1 | — | −10 | 22 700 | 0.007 | — | — | — | — | — | — | [539, 547] |
| NdN | 300 | 576.0 | — | −36 | 13 300 | 0.05 | — | — | — | — | — | — | [501] |
| Nd5Ge3 | 300 | 47.3 | — | −7 | 5520 | 0.0008 | — | — | — | — | — | — | [549] |
| NdGe2 | 300 | 40.8 | — | −3.9 | 8330 | 0.0004 | — | — | — | — | — | — | [549] |
| Nd3Te4 | 300 | 60.6 | 14 | −50 | 1000 | 0.005 | — | — | — | — | 1.2 | 1273 | [525] |
| Nd2Te3 | 300 | 10.6 | 1.3 | −15 | 588 | 0.003 | — | — | — | — | — | — | [509, 510] |
| NdCoO3 | 300 | 0.0 | 1.3 | 300 | 0.01 | 0.000 02 | — | — | — | 0.04 | 800 | [524] | |
| Nd2CuO4 | 300 | 62.7 | 24 | −720 | 0.3 | 0.0002 | — | — | — | — | — | — | [526] |
| Nd1.85Th0.15CuO4 | 300 | 1.6 | — | −10 | 133 | 0.000 04 | — | — | — | — | — | — | [527] |
| SmB6 | 300 | 44.8 | — | 7.6 | 4830 | 0.0008 | — | — | — | — | — | — | [496, 507, 517] |
| SmB66 | 300 | 0.6 | 2.1 | 590 | 0.01 | 0.000 07 | — | — | — | — | 0.1 | 1000 | [528] |
| Sm2Te3 | 300 | 0 | — | 130 | 0.004 | 2.0 × 10−7 | — | — | — | — | — | — | [510] |
| Sm2O3 | 300 | 4791.3 | — | 30 | 133 000 | 0.4 | — | — | — | — | — | — | [548] |
| Sm3Au3Sb4 | 300 | 31.1 | — | 125 | 167 | 0.008 | — | — | — | — | — | — | [550] |
| Sm2CuO4 | 300 | 75.6 | 20 | −840 | 0.1 | 0.0001 | — | — | — | — | — | — | [526] |
| EuB6 | 300 | 251.0 | — | −17.7 | 11 800 | 0.01 | — | — | — | — | — | — | [496, 507, 517] |
| Eu8Ga16Ge30 | 300 | 101.2 | — | −152 | 391 | 0.1 | — | — | — | — | 0.3 | 600 | [529] |
| Eu11−x Ybx Cd6Sb12 | 300 | 21.3 | 0.8 | 120 | 122 | 0.07 | — | — | — | — | 0.1 | 320 | [530] |
| Eu2Pb2Bi6Se13 | 300 | 12.6 | — | −35 | 300 | 0.001 | — | — | — | — | — | — | [550] |
| Eu2Pb2Bi4Se10 | 300 | 112.1 | — | −140 | 500 | 0.03 | — | — | — | — | — | — | [550] |
| GdC2 | 300 | 299.0 | 3 | −7.4 | 33 300 | 0.02 | — | — | — | [531] | |||
| GdB6 | 300 | 9.4 | — | 0.1 | 22 400 | 7.0 × 10−7 | — | — | — | — | — | — | [496, 507, 517] |
| Gd3Se4 | 300 | 15.3 | — | −14 | 909 | 0.0005 | — | — | — | — | — | — | [518] |
| Gd2Te3 | 300 | 19.0 | — | 160 | 66.7 | 0.005 | — | — | — | — | — | — | [518] |
| Gd2O3 | 300 | 7.2 | — | 42 | 143 | 0.0008 | — | — | — | — | — | — | [548] |
| Gd1−x Srx CoO3 | 300 | 6.7 | 0.3 | 100 | 50.0 | 0.06 | — | — | — | — | — | — | [532] |
| Gd3Cu3Sb4 | 300 | — | 3.7 | 89 | 0.04 | — | — | — | — | 0.09 | 400 | [514] | |
| Gd3Au3Sb4 | 300 | 35.7 | — | 50 | 588 | 0.004 | — | — | — | — | — | — | [550] |
| Gd14.34Au67.16Ge18.5 | 300 | 18.7 | 5.2 | 4.5 | 3330 | 0.0004 | — | — | — | 0.03 | 400 | [533] | |
| Gd14.19Au69.87Si15.94 | 300 | 5.2 | 3.1 | 1.5 | 2500 | 0.000 05 | — | — | — | — | 0.01 | 400 | [533] |
| Gd2CuO4 | 300 | 7.5 | 30 | −700 | 0.05 | 0.000 03 | — | — | — | — | — | — | [526] |
| 1/1-Au-Ge-Gd AC | 300 | 14.6 | 5.5 | 3.5 | 3300 | 0.0002 | — | — | — | — | — | — | [533] |
| 1/1-Au-Si-Gd AC | 300 | 5.2 | 3 | 1.5 | 2500 | 0.000 06 | — | — | — | — | — | — | [533] |
| 1/1-Au-Al-Gd AC | 300 | 37.1 | 3.7 | 11 | 2790 | 0.003 | — | — | — | — | 0.006 | 800 | [499] |
| TbC2 | 300 | 270.4 | 3.0 | −7.8 | 28 600 | 0.02 | — | — | — | — | — | — | [531] |
| TbB6 | 300 | 42.6 | — | −1.1 | 26 700 | 0.0001 | — | — | — | — | — | — | [496, 507, 517] |
| TbCoO3 | 300 | 0 | 1.8 | 600 | 50 000 | 3.0 × 10−6 | — | — | — | — | 0.05 | 800 | [524] |
| i-Zn-Mg-Tb QC | 300 | 36.2 | 5–6 | 6 | 4900 | 1.2 × 10−7 | — | — | — | — | — | — | [498] |
| DyC2 | 300 | 266.3 | 3.1 | −6.8 | 32 200 | 0.01 | — | — | — | — | — | — | [531] |
| DyN | 300 | 360.8 | — | −36 | 8330 | 0.03 | — | — | — | — | — | — | [501] |
| Dy3Te4 | 300 | 19.2 | 2.2 | −49 | 323 | 0.01 | — | — | — | — | — | — | [509] |
| Dy3Ru4Ge13 | 300 | 52.6 | 3 | 35 | 1250 | 0.02 | — | — | — | — | — | — | [497] |
| DyCoO3 | 300 | 0.2 | 2 | 800 | 0.0005 | 4.8 × 10−6 | — | — | — | — | 0.05 | 850 | [524] |
| HoN | 300 | 445.3 | — | −37 | 10 000 | 0.04 | — | — | — | — | — | — | [501] |
| HoB66 | 300 | 0.3 | 1.9 | 460 | 0.03 | 0.0001 | — | — | — | — | 0.09 | 1000 | [528] |
| HoPdSb | 300 | 35.9 | 6 | 170 | 112 | 0.02 | — | — | — | — | — | — | [550, 534] |
| HoNiSb | 300 | 72.3 | 5.5 | 40 | 1500 | 0.01 | — | — | — | — | — | — | [550] |
| Ho3Au3Sb4 | 300 | 40.8 | — | 140 | 182 | 0.01 | — | — | — | — | — | — | [550] |
| Ho3Ru4Ge13 | 300 | 55.7 | 3.2 | 37 | 1250 | 0.02 | — | — | — | — | — | — | [497] |
| i-Zn-Mg-Ho QC | 300 | 52.6 | 7–8 | 8 | 5400 | 2.3 × 10−7 | — | — | — | — | — | — | [498] |
| EuB6 | 300 | 251.0 | — | −17.7 | 11 800 | 0.01 | — | — | — | — | — | — | [496, 507, 517] |
| ErC2 | 300 | 156.7 | 2.1 | −6.0 | 21 400 | 0.01 | — | — | — | — | — | — | [531] |
| ErN | 300 | 412.3 | — | −36 | 9520 | 0.04 | — | — | — | — | — | — | [501] |
| Er2O3 | 300 | 2.0 | — | 33 | 50.0 | 0.0002 | — | — | — | — | — | — | [548] |
| Er3Cu3Sb4 | 300 | — | 3.5 | 38 | — | 0.02 | — | — | — | — | 0.04 | 400 | [514] |
| ErNiSb | 300 | 113.9 | 4 | 160 | 400 | 0.07 | — | — | — | — | — | — | [550] |
| ErNi1−x Pdx Sb | 300 | — | — | — | — | — | — | — | — | — | 0.3 | 600 | [535] |
| ErNiSb | 300 | 101.1 | 5 | 150 | 400 | 0.05 | — | — | — | — | — | — | [550] |
| i-Zn-Mg-Er QC | 300 | 53.1 | 5–6 | 7 | 6200 | 0.002 | — | — | — | — | — | — | [498] |
| TmC2 | 300 | 17.1 | 0.3 | −7.2 | 1940 | 0.01 | — | — | — | — | — | — | [531] |
| TmB66 | 300 | 0.3 | 2.1 | 460 | 0.03 | 0.0001 | — | — | — | — | 0.09 | 1000 | [528] |
| TmNiSb | 300 | 28.4 | 3 | 58 | 400 | 0.01 | — | — | — | — | — | — | [550] |
| i-Au-Al-Tm QC | 300 | 33.4 | 4.7 | 8.7 | 3160 | 0.002 | — | — | — | — | — | — | [499] |
| 1/1-Au-Al-Tm AC | 300 | 49.5 | 4.7 | 13.6 | 3020 | 0.004 | — | — | — | 0.01 | 800 | [499] | |
| TmAgTe2 trigonal | 300 | 11.5 | 0.5 | 500 | 0.8 | 0.01 | — | — | — | — | 0.4 | 650 | [536] |
| TmAgTe2 tetragonal | 300 | 29.4 | 0.8 | 570 | 0.9 | 0.01 | — | — | — | — | 0.2 | 650 | [536] |
| YbB6 | 300 | 658.0 | −25.5 | 21 500 | 0.04 | — | — | — | — | — | — | [496, 507, 517] | |
| YbB66 | 300 | 0.5 | 2.3 | 570 | 0.01 | 0.000 06 | — | — | — | — | 0.08 | 1000 | [528] |
| Yb2Te3 | 300 | 6018.8 | 35 | 143 000 | 0.5 | — | — | — | — | — | — | [548] | |
| Yb2O3 | 300 | 1081.3 | 18 | 50 000 | 0.05 | — | — | — | — | — | — | [548] | |
| YbAl3 | 300 | 1424.0 | 14 | −88 | 12 500 | 0.2 | — | — | — | — | — | — | [537] |
| YbMnxAl3 | 300 | — | — | — | — | — | — | — | — | — | 0.5 | 300 | [537] |
| i-Cd-Yb QC | 300 | 134.7 | 5.1 | 16 | 7000 | 0.01 | — | — | — | — | — | — | [500] |
| i-Cd-Yb QC | 300 | 87.8 | 9 | 13 | 5600 | 0.003 | — | — | — | — | — | — | [538] |
| 1/1Cd-Yb AC | 300 | 102.9 | 9 | 12 | 7100 | 0.003 | — | — | — | — | — | — | [538] |
| i-Cd-Yb QC | 300 | 107.8 | 5–7.5 | 6–16 | 2900–5600 | 0.007 | — | — | — | — | — | — | [540] |
| 1/1Cd-Yb AC | 300 | 48.9 | 7.5 | 14 | 2900 | 0.002 | — | — | — | — | — | — | [540] |
| YbNiSb | 300 | 60.0 | 4.5 | 20 | 2500 | 0.007 | — | — | — | — | — | — | [550] |
| Yb15.78Au65.22Ge19.00 | 300 | 0.6 | 2.5 | −1.2 | 370 | 6.0 × 10−6 | — | — | — | — | 6.0 × 10−6 | 400 | [533] |
| Yb14ZnSb11 | 300 | 6.2 | 12 | 5 | 1000 | 0.000 06 | — | — | — | — | 0.1 | 900 | [541, 542] |
| Yb14MnSb11 | 300 | 30.3 | 1.4 | 50 | 500 | 0.01 | — | — | — | — | — | — | [542] |
| Yb13.5La0.5ZnSb11 | 300 | 24.0 | 10.5 | 20 | 1000 | 0.001 | — | — | — | 0.7 | 1275 | [541] | |
| Yb13.5Y0.5ZnSb11 | 300 | 36.0 | 10.5 | 30 | 1000 | 0.003 | — | — | — | — | 0.7 | 1300 | [541] |
| Yb4−x Smx Sb3 | 300 | 48.5 | 35 | −10 | 4000 | 0.0003 | — | — | — | — | 0.7 | 1300 | [543] |
| Yb4−x Lax Sb3 | 300 | 60.0 | 25 | 20 | 2500 | 0.001 | — | — | — | — | 0.8 | 1000 | [543, 544] |
| i-Au-Al-Yb QC | 300 | 45.9 | — | 9 | 4200 | 0.001 | — | — | — | — | — | — | [545] |
| 1/1-Au-Ge-Yb AC | 300 | 0.5 | 3 | −1 | 370 | 4.0 × 10−6 | — | — | — | — | — | — | [533] |
| i-Au-Al-Yb QC | 300 | 72.7 | — | 11.4 | 5280 | 0.002 | — | — | — | — | — | — | [499] |
| 1/1-Au-Al-Yb AC | 300 | 80.9 | 3.7–3.9 | 9.7–12.9 | 3010–5200 | 0.006 | — | — | — | — | 0.01 | 900 | [499] |
| Yb14Mn0.6Zn0.4Sb11 | 300 | — | — | — | — | — | — | — | — | — | 1.1 | 1275 | [542] |
| Lu3Ru4Ge13 | 300 | 70.3 | 3.7 | 35 | 1670 | 0.02 | — | — | — | — | — | — | [497] |
| Th | 300 | 251.0 | — | −4 | 50 000 | 0.002 | — | — | — | — | — | — | [551, 552] |
| ThO2 | 300 | — | 11 | — | — | 0 | — | — | — | — | — | — | [561] |
| ThC | 300 | 1279.7 | — | 50 | 21 100 | 0.2 | — | — | — | — | 0.09 | 1200 | [562, 563] |
| ThN | 300 | 191.9 | — | −3 | 50 000 | 0.001 | — | — | — | — | 0 | 400 | [564] |
| ThS | 300 | 73.2 | — | −5 | 11 800 | 0.0009 | — | — | — | — | 0.01 | 1273 | [565] |
| ThBe13 | 300 | 29.5 | — | −2.5 | 9090 | 0.0002 | — | — | — | — | — | — | [566] |
| ThB4 | 300 | 91.2 | — | −5 | 14 700 | 0.001 | — | — | — | — | — | — | [567] |
| ThB6 | 300 | 344.8 | — | −5 | 55 600 | 0.004 | — | — | — | — | — | — | [567] |
| Th3P4 | 300 | 108.0 | 2.8 | −223 | 182 | 0.1 | — | — | — | — | 0.3 | 1273 | [568, 553] |
| Th3As4 | 300 | 228.8 | 5.4 | −380 | 62.5 | 0.05 | — | — | — | — | 0.1 | 1273 | [554, 555] |
| Th3Sb4 | 300 | 215.1 | 7.5 | −52.5 | 3370 | 0.04 | — | — | — | — | 0 | 1273 | [554] |
| ThAsSe | 300 | 31.0 | — | −5 | 5000 | 0.0004 | — | — | — | — | — | — | [556, 557] |
| ThAsS | 300 | 136.8 | — | −3.8 | 28 600 | 0.001 | — | — | — | — | — | — | [558] |
| ThPS | 300 | 159.2 | — | −8.5 | 15 400 | 0.003 | — | — | — | — | — | — | [559] |
| Th3 Co3Sb4 | 300 | 23.6 | — | 5 | 3800 | 0.0003 | — | — | — | — | — | — | [560] |
| U metal | 300 | 316.9 | — | 7 | 37 000 | 0.005 | — | — | — | — | — | — | [551, 590, 591] |
| U2N3 | 300 | 37.0 | — | −107 | 250 | 0.009 | — | — | — | — | 0.1 | 383 | [592, 593] |
| U2S3 | 300 | — | — | — | 0.005 | — | — | — | — | — | — | [593] | |
| U2Se3 | 300 | 1.2 | — | −2 | 435 | 0.02 | — | — | — | — | — | — | [594] |
| USb2 | 300 | 31.2 | 3.1 | 20 | 1300 | 0.005 | — | — | — | — | 0.007 | 700 | [569, 593] |
| USe2 | 300 | 1.0 | — | 21 | 40.0 | 0.02 | — | — | — | — | — | — | [570, 594] |
| US2 | 300 | — | — | — | 7.5 | 0.005 | — | — | — | — | — | — | [569, 570] |
| UO2 | 300 | 4.0 | 6.5 | 500 | 0.3 | 0.0003 | — | — | — | — | 0.1 | 1000 | [569, 571, 593] |
| UB2 | 300 | — | — | — | — | 0.04 | — | — | — | — | [572] | ||
| US | 300 | 284.8 | 9.6 | 55 | 4250 | 0.04 | — | — | — | — | 0.1 | 700 | [573] |
| UP | 300 | 297.0 | 10.5 | 59.2 | 4100 | 0.04 | — | — | — | — | — | — | [557, 573] |
| UC | 300 | 1903.6 | 23.0 | 45 | 35 000 | 0.09 | — | — | — | — | — | — | [573] |
| UN | 300 | 517.4 | 13.4 | 75 | 5500 | 0.07 | — | — | — | — | 0.2 | 700 | [573, 574] |
| UAs | 300 | 304.5 | 8.4 | 62 | 4000 | 0.06 | — | — | — | — | — | — | [564, 573] |
| USb | 300 | 204.9 | 4.2 | 40 | 4250 | 0.05 | — | — | — | — | — | — | [573, 575] |
| USe | 300 | 197.6 | 6.7 | 40 | 4100 | 0.03 | — | — | — | — | — | — | [573] |
| UB4 | 300 | 620.1 | — | 5 | 100 000 | 0.03 | — | — | — | — | — | — | [576, 577] |
| UAl3 | 300 | — | — | — | — | 0.06 | — | — | — | — | 0.06 | 773 | [593] |
| USi3 | 300 | 648.4 | 21 | 20 | 27 000 | 0.02 | — | — | — | — | 0.4 | 1273 | [578] |
| UGe3 | 300 | 244.9 | 18 | 12 | 16 900 | 0.004 | — | — | — | — | 0.0005 | 1200 | [578] |
| U3P4 | 300 | 28.3 | 1.9 | 30 | 785 | 0.01 | — | — | — | — | — | — | [560] |
| Ni-doped U3As4 | 300 | 3.3 | 2.5 | −0.6 | 3300 | 0.000 01 | — | — | — | — | 0.4 | 1100 | [560, 593] |
| U3Sb4 | 300 | 11.1 | 3.4 | −3.5 | 2500 | 0.0003 | — | — | — | — | — | — | [560] |
| U3Se4 | 300 | 15.6 | 25.5 | −8.4 | 1530 | 0.0001 | — | — | — | — | — | — | [560] |
| U3Te4 | 300 | 21.4 | 30.6 | −7.4 | 2370 | 0.0001 | — | — | — | — | — | — | [560] |
| UBe13 | 300 | 153.3 | — | 14 | 9090 | 0.005 | — | — | — | — | — | — | [521] |
| URu2Si2 | 300 | 48.0 | — | −20 | 2000 | 0.02 | — | — | — | — | 0.1 | 1100 | [578, 579] |
| UFe2Si2 | 300 | 21.6 | 0.5 | −30 | 600 | 0.03 | — | — | — | — | — | — | [578] |
| UNi2Si2 | 300 | 0.2 | — | −4 | 37.0 | 1.8 × 10−6 | — | — | — | — | — | — | [580] |
| URhGa5 | 300 | 612.2 | 0.3 | 60 | 8330 | 0.07 | — | — | — | — | — | — | [581] |
| U3Pt3Sb4 | 300 | 9.8 | 2.0 | 193.7 | 23.3 | 0.02 | — | — | — | — | — | — | [582] |
| U3Ni3Sb4 | 300 | 8.2 | 2.5 | 10.2 | 667 | 0.0006 | — | — | — | — | — | — | [582] |
| U3Pd3Sb4 | 300 | 4.1 | 2.9 | 80.8 | 40 | 0.003 | — | — | — | — | — | — | [582] |
| U3Pt3Sn4 | 300 | 8.4 | 69 | −6.9 | 1000 | 0.000 01 | — | — | — | — | — | — | [582] |
| U2Ru3Si5 | 300 | 12.4 | — | −5 | 2000 | 0.001 | — | — | — | — | 0.04 | 1100 | [579] |
| U2Fe3Si5 | 300 | 34.7 | — | −10 | 2860 | 0.0009 | — | — | — | — | — | — | [583] |
| U2FeSi3 | 300 | 20.7 | — | 12 | 1430 | 0.0006 | — | — | — | — | — | — | [583] |
| U3Fe2Si7 | 300 | 88.7 | — | 11 | 6670 | 0.002 | — | — | — | — | — | — | [583] |
| U1.2Fe4Si9.7 | 300 | 5.3 | — | 4 | 1050 | 0.000 05 | — | — | — | — | — | — | [583] |
| UOS | 300 | 0.3 | 3.8 | 150 | 1.3 | 0.0002 | — | — | — | — | 0.7 | 1200 | [569, 593] |
| UOSe | 300 | 14.7 | 0.8 | 166 | 48.0 | 0.05 | — | — | — | — | 0.2 | 1200 | [569, 593] |
| USiGe2 | 300 | — | — | — | — | — | — | — | — | — | 0.5 | 1473 | [584, 593] |
| UBaO3 | 300 | — | 0.8 | 170 | — | — | — | — | — | — | 0.0002 | 900 | [585] |
| Fe-doped UCo3Sb12 | 300 | 60.6 | 3.5 | 50 | 1000.0 | 0.02 | — | — | — | — | 0.6 | 800 | [586] |
| U2Ru2Sn | 300 | — | — | — | 37.0 | — | — | — | — | — | 0.004 | 140 | [587, 588] |
| U2Rh2In | 300 | 0.6 | — | 12.5 | 37.0 | 0.000 02 | — | — | — | — | — | — | [589] |
Among lanthanide-containing compounds and alloys, the majority of previous reports have focused on the introduction of large lanthanide atoms into existing materials which show good thermoelectric properties; see, for example, section 3.2 covering skutterudite materials and section 3.9 covering oxide systems. In the current section, we focus on materials for which the starting compound is based on a lanthanide element. As evident from figure 7, room-temperature values of the thermoelectric figure of merit are rather modest, with zTmax ∼ 0.5 for the HH compound TmNiSb. Interestingly, the high-temperature region shows that more promising materials are likely to be compounds with the Th3P4 structure type [65, 506, 511, 525], Zintl phase Yb14(Mn,Y,La)x Zny Sb11 [541, 542], skutterudite compound CeFe4−x Cox Sb12 [522], as well as lanthanide-based dichalcogenides [504, 519].
When it comes to actinide-based materials, some work has been done for compounds and alloys containing uranium and thorium. Among Th-based materials, very little has been published regarding investigations of their thermoelectric properties. In particular, for the majority of materials provided in table 8, even their thermal conductivity values are missing. Overall, the values of thermoelectric figure of merit, summarized in figure 8, appear to be rather modest (notice a three-fold vertical axis decrease in figure 8 compared to figure 7). For both room temperature and high-temperature regions, two groups of Th-based materials appear to be promising—ThC [562, 563] and compounds with the Th3P4 structure type [553–555, 568]. However, more Th-based materials need to be examined in order to evaluate the viability of using these materials for thermoelectric applications.
While the possibility of using uranium-based materials for thermoelectric applications has been proposed over half a century ago [569], very little work has been done in this field in the meantime [582, 593]. The stagnation of this topic is probably due to several factors: the limited number of facilities that carry out uranium work, possible health concerns, as well as the inability to predict new thermoelectric materials using computational means. Nonetheless, given the low cost and abundance of depleted uranium, development of functional uranium-based materials can perhaps contribute to the solution of the nuclear waste problem. Moreover, toxicity and radiological danger of these materials can be avoided if they are used, for example, for aerospace applications [582].
As evident from figure 9, more experimental studies are needed in order to adequately enhance the current maximum value of the thermoelectric figure of merit in uranium-based materials. Given the currently available data, the most promising systems appear to be compounds with the Th3P4 structure type [560, 593], AuCu3 structure type [578, 584, 593] as well as the skutterudite compound U0.2FeCo3Sb12 [586].
Acknowledgment
Eteri Svanidze would like to acknowledge the support of the Christiane Nüsslein-Volhard-Stiftung.
3.9. Oxides
Oxide thermoelectrics
Dursun Ekren1, Robert Freer2 and Ryoji Funahashi3
1 Department of Metallurgy and Materials Engineering, Iskenderun Technical University, Iskenderun 31200, Hatay, Turkey
2 Department of Materials, University of Manchester, Oxford Road, M13 9PL, United Kingdom
3 National Institute of Advanced Industrial Science and Technology, 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan
Oxides, developed from earth abundant materials, having structural/chemical flexibility and high temperature stability, are well suited to medium and high temperature thermoelectric applications. Today there is a wide variety of oxide thermoelectrics (table 9) [596–668], but work on oxide thermoelectrics only began in the 1990s after the discovery of a high PF (5000 μW m−1 K−2) in single crystal Nax CoO2 (NCO) combined with both high μ (∼13 cm2 V−1 s−1 at 300 K) and a large S (∼100 μV K−1 at 300 K). Whilst polycrystalline NCO presents additional processing challenges and the presence of microstructural features can seriously degrade the transport properties, resulting in very modest zT in the pure material [653], the performance of these p-type materials can, under ideal conditions, reach zTmax of 0.92 at 960 K, particularly when prepared as a composite containing 10%Ag [655]. NCO tends to physically degrade at elevated temperatures, but the related p-type, layered compounds Ca3Co4O9 (CCO) and Bi2Sr2Co2Oy do not suffer in the same way and have been exploited in prototype modules [669]. Once again the highest thermoelectric performance has been reported for single crystals, but polycrystalline CCO has achieved zTmax of ∼0.43 at 1073 K for Bi or Ba doped ceramics [640, 641]. For the closely related misfit layered cobaltite Bi2Sr2 Co2Oy , the orientation-dependent properties mean that texturing of ceramics is essential to maximize performance. By use of partial melting and/or doping of Bi2Sr2 Co2Oy, zTmax of 0.27 has been achieved at 973 K [649, 650].
Table 9. Oxides—thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κ (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | µH (cm2 V−1 s−1) a | ms* (me) | n (1020 cm−3) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| n-Type oxides | ||||||||||
| La-SrTiO3 | 300 | 219.7 | 9.5 | −150.0 | 1000.0 | 0.08 | 9.2 | — | 6.8 | [596] |
| 1073 | 15.6 | 3.0 | −291.7 | 80.0 | 0.27 | — | — | — | ||
| Sr0.92La0.08TiO3 | 300 | 456.8 | 5.9 | −107.3 | 3070.6 | 0.19 | — | — | — | [597] |
| 1045 | 20.6 | 3.0 | −233.1 | 200.0 | 0.37 | — | — | — | ||
| La0.1Sr0.83Dy0.07TiO3 | 300 | 107.9 | 6.1 | −70.7 | 921.1 | 0.04 | — | — | — | [598] |
| 1045 | 16.5 | 2.6 | −200.9 | 234.9 | 0.36 | — | — | — | ||
| Sr0.85Pr0.15TiO3 | 323 | 309.9 | 5.5 | −72.2 | 3838.2 | 0.12 | 11.8 | — | 23.0 | [599] |
| 723 | 45.4 | 3.8 | −161.4 | 647.4 | 0.35 | — | — | — | ||
| La0.067Sr0.9TiO3−δ + 0.6G | 323 | 173.0 | 1.8 | −122.6 | 1066.7 | 0.42 | — | — | — | [600] |
| 1023 | 9.7 | 2.1 | −221.2 | 105.0 | 0.36 | — | — | — | ||
| Sr0.8La0.067Ti0.8Nb0.2O3 | 310 | 50.6 | 4.6 | −69.2 | 622.8 | 0.02 | 1.6 | 4.0 | 28.3 | [601] |
| 1023 | 15.1 | 3.1 | −167.8 | 303.4 | 0.27 | — | — | — | ||
| Sr0.9La0.1Ti0.9Nb0.1O3 | 323 | 48.1 | 4.2 | −98.5 | 411.8 | 0.01 | 0.9 | 9.8 | 15.0 | [602] |
| 1100 | 31.9 | 2.8 | −287.8 | 201.8 | 0.66 | — | — | — | ||
| Sr0.8La0.06Ti0.8Nb0.2O3 + 2.5 wt%Fe | 315 | 71.0 | — | −69.6 | 966.2 | 0.02 | 0.9 | 6.7 | 56.0 | [603] |
| 1000 | 50.8 | 2.9 | −169.6 | 882.4 | 0.38 | — | — | — | ||
| Sr0.9Nd0.1TiO3 + 0.5%wt B2O3 + 0.3 wt% ZrO2 | 310 | 168.9 | 6.9 | −94.3 | 1418.9 | 0.06 | 2.1 | 42.0 | [604] | |
| 1015 | 18.9 | 2.9 | −215.1 | 217.6 | 0.37 | — | — | — | ||
| Sr0.8La0.067Ti0.8Nb0.2 O3−d + 1 wt% G | 310 | 2.3 | 4.2 | −327.2 | 1.2 | 0.002 | — | — | — | [605] |
| 965 | 0.9 | 2.8 | −360.3 | 1.8 | 0.03 | — | — | — | ||
| SrTiO3 + 0.7 wt% RGO | 300 | 231.7 | 7.7 | −356.1 | 85.3 | 0.04 | 25.4 | 0.9 | [606] | |
| 750 | 11.1 | 3.6 | −343.6 | 18.2 | 0.05 | — | — | — | ||
| Sr0.98Ti0.90Nb0.10O3±δ + 0.6 wt% rGO | 315 | 240.2 | 6.7 | −97.1 | 1993.6 | 0.09 | 23.7 | — | 5.2 | [607] |
| 1160 | 11.9 | 2.9 | −221.1 | 155.6 | 0.29 | — | — | — | ||
| Sr0.9La0.1TiO3 + 20 wt % Ti | 330 | 119.3 | 2.4 | −183.3 | 370.4 | 0.08 | 161.4 | — | 0.1 | [608] |
| 1073 | 16.2 | 1.3 | −321.4 | 59.0 | 0.50 | — | — | — | ||
| SrTi0.85Nb0.15 O3 + 1.5 wt% GO | 323 | 49.6 | 7.1 | −66.2 | 676.9 | 0.007 | 9.0 | 1.7 | 4.7 | [609] |
| 1200 | 6.3 | 3.4 | −187.7 | 126.9 | 0.50 | — | — | — | ||
| Sr0.9La0.1Ti0.9Nb0.1O3 | 315 | 102.2 | 5.6 | −89.2 | 947.5 | 0.15 | 7.2 | 7.0 | [610] | |
| 793 | 38.0 | 3.9 | −162.7 | 554.1 | 0.39 | — | — | — | ||
| Sr0.95(Ti0.8Nb0.2)0.95Ni0.05O3 | 305 | 88.4 | — | −63.9 | 1150.0 | — | — | — | — | [611] |
| 1073 | 13.0 | 1.4 | −206.9 | 177.3 | 0.60 | — | — | — | ||
| La0.08Sr0.9TiO3−δ | 330 | 182.9 | 8.0 | −114.7 | 1287.7 | 0.07 | 10.9 | 4.2 | 7.4 | [612] |
| 867 | 18.8 | 4.3 | −209.2 | 182.0 | 0.16 | — | — | — | ||
| CaMn0.98Nb0.02O3 | 340 | 15.4 | 0.9 | −192.6 | 44.4 | 0.09 | — | — | — | [613] |
| 1060 | 3.7 | 0.8 | −247.0 | 31.4 | 0.32 | — | — | — | ||
| Ca0.92Pr0.04Yb0.04MnO3 | 330 | 32.0 | 1.7 | −145.7 | 153.3 | 0.07 | — | — | — | [614] |
| 973 | 6.3 | 1.3 | −196.3 | 84.4 | 0.24 | — | — | — | ||
| Ca0.97Bi0.03MnCu0.04O3−δ | 377 | 200.3 | 1.8 | −196.4 | 645.2 | 0.51 | — | — | — | [615] |
| 1073 | 9.5 | 1.5 | −213.9 | 120.7 | 0.44 | — | — | — | ||
| Ca0.89Pr0.08Sr0.03MnO3 | 373 | 25.4 | 1.8 | −179.5 | 98.2 | 0.07 | 24.4 | — | 1.3 | [616] |
| 973 | 7.1 | 1.8 | −143.8 | 176.6 | 0.20 | — | — | — | ||
| Ba0.1Eu0.9TiO3−δ | 323 | — | 7.9 | −853.2 | — | — | — | — | — | [617] |
| 1123 | 12.6 | 2.7 | −300.0 | 62.9 | 0.24 | 0.4 | — | 9.6 | ||
| Zn0.98Al0.02O | 300 | 107.7 | 40.2 | −97.1 | 831.2 | 0.007 | 73.5 | — | 0.7 | [618] |
| 1273 | 16.3 | 5.3 | −179.4 | 396.8 | 0.30 | — | — | — | ||
| Ca-doped (ZnO)3.5In2O3 | 545 | 9.7 | 2.9 | −86.0 | 215.1 | 0.03 | — | — | — | [619] |
| 1053 | 8.0 | 2.2 | −168.4 | 166.7 | 0.23 | — | — | — | ||
| Zn0.96Al0.02Ga0.02O | 305 | 139.3 | 13.1 | −135.3 | 672.7 | 0.03 | — | — | — | [620] |
| 1073 | 38.0 | 4.8 | −236.0 | 371.1 | 0.52 | — | — | — | ||
| Zn0.96Ga0.04O1.02 | 350 | 9.6 | 8.1 | −88.8 | 105.3 | 0.003 | 6.5 | — | 1.0 | [621] |
| 1000 | 5.7 | 3.6 | −174.5 | 102.6 | 0.09 | — | — | — | ||
| Ni-coated In2O3(ZnO)5 | 373 | 1.8 | −89.0 | 24.3 | — | 0.1 | [622] | |||
| 973 | 7.7 | 1.3 | −172.0 | 136.0 | 0.39 | — | — | — | ||
| In2O3(ZnO)3 | 330 | 34.5 | 3.2 | −64.1 | 503.8 | 0.02 | — | — | — | [623] |
| 973 | 12.9 | 2.6 | −133.8 | 361.5 | 0.24 | — | — | — | ||
| Sr0.61Ba0.39Nb2O5.95F0.05 | 323 | 3.1 | — | −152.9 | 13.1 | 0.006 | — | — | — | [624] |
| 1073 | 6.7 | 2.0 | −204.3 | 94.4 | 0.21 | — | — | — | ||
| Sr0.50La0.20Ba0.30Nb2O6−δ | 323 | 4.7 | 1.4 | −140.2 | 23.5 | 0.01 | — | — | — | [625] |
| 1073 | 7.1 | 2.1 | −180.9 | 131.6 | 0.22 | — | — | — | ||
| Ba5.19Nd8.54Ti18O54 | 350 | 14.2 | — | −110.0 | 115.8 | — | — | — | [626] | |
| 1000 | 5.0 | 1.5 | −210.0 | 60.0 | 0.16 | — | — | — | ||
| Ba6Ti2Nb8O30 | 350 | 5.9 | 1.8 | −102.1 | 54.0 | 0.01 | 0.4 | — | 14.5 | [627] |
| 873 | 6.4 | 1.7 | −176.6 | 90.9 | 0.14 | — | — | — | ||
| Ti8(O,N)15 | 300 | 13.0 | 1.6 | −125.5 | 69.2 | 0.02 | — | — | — | [628] |
| 550 | 13.8 | 1.8 | −131.9 | 167.7 | 0.09 | — | — | — | ||
| Ti0.83Nb0.17(O,N)2±δ | 323 | 46.3 | 2.0 | −230.0 | 80.0 | 0.08 | — | — | — | [629] |
| 973 | 8.8 | 2.6 | −198.4 | 115.0 | 0.35 | — | — | — | ||
| TiO1.76 | 323 | 19.2 | 2.3 | −85.3 | 195.4 | 0.02 | — | — | — | [630] |
| 973 | 14.9 | 2.1 | −148.3 | 350.0 | 0.35 | — | — | — | ||
| Ti9O17 | 305 | 22.1 | — | −178.1 | 64.2 | 0.2 | 12.6 | 18.3 | [631] | |
| 764 | 8.6 | 2.6 | −168.8 | 110.0 | 0.16 | — | — | — | ||
| TiAl0.02O1.78 | 320 | 37.4 | 2.7 | −33.9 | 1007.5 | 0.01 | — | — | — | [632] |
| 973 | 14.0 | 2.6 | −87.6 | 721.6 | 0.20 | — | — | — | ||
| p-Type oxides | ||||||||||
| Ca2.7Y0.3 Co4O9+δ | 373 | 16.6 | 1.8 | 137.1 | 106.2 | 0.03 | — | — | — | [633] |
| 973 | 6.6 | 1.5 | 171.7 | 117.2 | 0.22 | — | — | — | ||
| Ba0.1Ag0.1Ca2.8 Co4O9 | 373 | 14.2 | 1.9 | 143.3 | 84.3 | 0.04 | — | — | — | [634] |
| 973 | 7.8 | 1.4 | 172.0 | 138.9 | 0.29 | — | — | — | ||
| Ca2.8Lu0.2 Co4O9+δ | 300 | 19.4 | 1.5 | 155.1 | 72.0 | 0.04 | — | — | — | [635] |
| 1073 | 6.6 | 1.2 | 194.0 | 105.0 | 0.36 | — | — | — | ||
| Ca2.97Sr0.03 Co4O9 | 1000 | 5.9 | 1.8 | 172.0 | 110.0 | 0.23 | — | — | — | [636] |
| Ca3 Co3.9Cr0.1O9+δ | 323 | 16.2 | 3.4 | 155.4 | 67.0 | 0.02 | — | — | — | [637] |
| 1073 | 6.0 | 2.0 | 194.1 | 94.6 | 0.19 | — | — | — | ||
| Ca2.9Cd0.1 Co4O9 | 323 | 19.4 | 2.1 | 141.2 | 94.9 | 0.03 | — | — | — | [638] |
| 1000 | 10.0 | 1.5 | 207.9 | 121.9 | 0.36 | — | — | — | ||
| Ca2.7Gd0.3 Co4O9+δ | 373 | 10.9 | 1.7 | 145.1 | 63.2 | 0.03 | — | — | — | [639] |
| 973 | 7.6 | 1.4 | 179.2 | 124.5 | 0.24 | — | — | — | ||
| Ca2.8Ba0.2 Co4O9 | 300 | 30.3 | 1.7 | 139.6 | 135.3 | 0.05 | — | — | — | [640] |
| 973 | 14.7 | 1.9 | 181.5 | 233.6 | 0.43 | — | — | — | ||
| Ca2.8Ba0.2 Co4O9 | 370 | 91.2 | 2.1 | 142.9 | 537.0 | 0.15 | — | — | — | [641] |
| 1073 | 12.4 | 1.9 | 192.1 | 201.4 | 0.43 | — | — | — | ||
| Bi0.15Ca2.85 Co4O9 | 323 | 28.1 | 2.1 | 152.8 | 119.9 | 0.04 | — | — | — | [642] |
| 973 | 9.1 | 1.8 | 181.3 | 144.6 | 0.25 | — | — | — | ||
| Ca2.5Tb0.5 Co4O9 | 300 | 8.0 | 1.3 | 132.6 | 39.2 | 0.01 | — | — | — | [643] |
| 800 | 48.2 | 1.2 | 321.7 | 112.0 | 0.74 | — | — | — | ||
| Ca2.95Sr0.05 Co4O9 | 367 | 19.1 | 2.2 | 156.8 | 94.0 | 0.04 | — | — | — | [644] |
| 957 | 9.2 | 1.2 | 180.5 | 143.6 | 0.38 | — | — | — | ||
| Ca3 Co4O9 | 367 | 12.6 | 1.9 | 144.9 | 71.6 | 0.03 | — | — | — | [645] |
| 773 | 6.5 | 1.3 | 162.0 | 92.0 | 0.14 | — | — | — | ||
| Bi2Sr1.8 Co2Ox | 800 | 2.9 | 0.9 | 105.0 | 87.0 | 0.09 | — | — | — | [646] |
| Bi2Sr2 Co2Oy (Ag2O powder 3 wt%) | 380 | 9.8 | 0.8 | 109.0 | 91.6 | 0.07 | — | — | — | [647] |
| 973 | 3.7 | 0.9 | 167.1 | 68.7 | 0.26 | — | — | — | ||
| Bi2Sr1.96La0.04 Co2O9 | 300 | 9.3 | 1.0 | 110.6 | 59.7 | 0.03 | — | — | — | [648] |
| 737 | 4.2 | 1.3 | 177.9 | 46.2 | 0.15 | — | — | — | ||
| Bi2Sr2 Co2Ox | 300 | 28.5 | 0.9 | 111.7 | 181.1 | 0.07 | — | — | — | [649] |
| 1000 | 5.7 | 1.2 | 162.4 | 118.1 | 0.27 | — | — | — | ||
| Bi2Sr2 Co2Ox | 300 | 27.9 | 0.9 | 112.1 | 176.2 | 0.07 | — | — | — | [650] |
| 1000 | 5.8 | 1.2 | 163.5 | 119.0 | 0.27 | — | — | — | ||
| La0.95Sr0.05Ni0.2 Co0.8O3 | 300 | 37.3 | 1.6 | 31.0 | 1000.0 | 0.006 | — | — | — | [651] |
| NaCo1.9Pd0.1O4 | 323 | 13.9 | 3.2 | 74.2 | 166.7 | 0.01 | — | — | — | [652] |
| 723 | 4.6 | 2.9 | 108.7 | 113.6 | 0.05 | — | — | — | ||
| Nax Co2O4 | 300 | 0.8 | 3.7 | 67.5 | 9.6 | 0.001 | — | — | — | [653] |
| 973 | 8.0 | 2.2 | 210.7 | 90.9 | 0.11 | — | — | — | ||
| NaCo0.9Ni0.1O2 | 373 | 17.7 | 2.3 | 116.6 | 145.9 | 0.01 | — | — | — | [654] |
| 773 | 9.6 | 2.2 | 154.3 | 149.2 | 0.13 | — | — | — | ||
| Nax Co2O4 + 10 wt% Ag | 500 | 53.0 | 2.0 | 155.0 | 424.3 | 0.29 | — | — | — | [655] |
| 900 | 39.2 | 1.9 | 223.1 | 373.7 | 0.92 | — | — | — | ||
| BiCuSeO | 300 | 2.9 | 0.6 | 349.0 | 1.1 | 0.01 | 22.0 | 0.01 | [656] | |
| 923 | 16.0 | 0.4 | 425.0 | 13.9 | 0.50 | — | — | — | ||
| Bi0.875Ba0.125CuSeO | 300 | 49.4 | 0.7 | 84.9 | 452.8 | 0.12 | 3.0 | 11.0 | [657] | |
| 923 | 13.1 | 0.5 | 184.0 | 186.8 | 1.11 | — | — | — | ||
| Bi0.94Pb0.06CuSeO | 323 | 84.2 | 0.7 | 159.0 | 333.3 | 0.47 | 7.5 | 4.8 | 3.4 | [658] |
| 823 | 17.3 | 0.5 | 221.0 | 135.0 | 1.14 | — | — | — | ||
| Bi0.985Na0.015CuSeO | 300 | 108.2 | 0.8 | 255.2 | 125.0 | 0.31 | 9.9 | — | 0.6 | [659] |
| 923 | 15.7 | 0.5 | 317.7 | 47.5 | 0.91 | — | — | — | ||
| Bi0.94Pb0.06CuSeO | 300 | 106.6 | 1.0 | 135.7 | 500.0 | 0.30 | — | — | 7.8 | [660] |
| 923 | 17.3 | 0.6 | 223.6 | 155.6 | 1.18 | — | — | — | ||
| Bi0.96Pb0.04CuSe0.95Te0.05O | 323 | 73.5 | 0.8 | 171.9 | 250.0 | 0.28 | 8.6 | 2.8 | 1.8 | [661] |
| 873 | 15.4 | 0.4 | 245.0 | 99.5 | 1.20 | — | — | — | ||
| Bi0.875Ba0.125Cu0.85Ni0.15SeO | 315 | 61.5 | 1.1 | 358.0 | 23.2 | 0.08 | 4.0 | 0.3 | [662] | |
| 923 | 30.4 | 0.5 | 402.0 | 34.5 | 0.97 | — | — | — | ||
| Bi0.88Mg0.06Pb0.06CuSeO | 300 | 181.6 | 1.1 | 100.0 | 1346.9 | 0.24 | — | — | 2.5 | [663] |
| 750 | 51.6 | 0.7 | 183.1 | 544.7 | 1.19 | — | — | — | ||
| Bi0.94Pb0.06Cu0.99Fe0.01SeO | 310 | 124.1 | 0.8 | 169.8 | 406.7 | 0.47 | 11.5 | — | 2.2 | [664] |
| 873 | 23.1 | 0.5 | 237.7 | 162.1 | 1.46 | — | — | — | ||
| Bi0.90Ba0.10CuSe0.90Te0.10O | 300 | 69.7 | 1.2 | 110.2 | 451.1 | 0.47 | 4.9 | — | 6.9 | [665] |
| 873 | 71.4 | 0.8 | 295.2 | 257.5 | 1.17 | — | — | — | ||
| Bi0.94Y0.06CuSeO | 300 | 14.5 | 0.7 | 91.2 | 121.7 | 0.04 | — | — | — | [666] |
| 873 | 12.0 | 0.5 | 161.8 | 204.5 | 0.93 | — | — | — | ||
| Bi0.92Pb0.08CuSeO + 8% SiC | 300 | 116.4 | 0.8 | 120.1 | 662.8 | 0.36 | 1.2 | 3.1 | 3.8 | [667] |
| 873 | 19.2 | 0.6 | 187.2 | 243.2 | 1.32 | — | — | — | ||
| Bi0.88Li0.06Ca0.06CuSeO | 300 | 103.6 | 1.4 | 192.3 | 248.7 | 0.17 | 4.0 | — | 10.0 | [668] |
| 873 | 20.5 | 0.7 | 289.0 | 79.5 | 0.87 | — | — | — | ||
a All data in the µH column are for Hall mobility, except for: SPB = intrinsic mobility—single parabolic band model.
Whilst p-type oxides have been predominantly limited to cobalt-based, layered structured compounds, the n-type materials include a number of different structural families, amongst which, the perovskites have attracted considerable attention. CaMnO3 (CMO) was one of the first n-type perovskites explored and like many oxides, the undoped material suffers from inherently high thermal conductivity. There is also a metal-insulator transition at high temperature due to a change in the spin state of Mn ions. Substituting Ca with heavy rare earths has been particularly successful in reducing thermal transport [613]; dual doping on both Ca and Mn sites can also enhance charge transport [614, 616]. Theoretical work suggests that zTmax of greater than 1.0 can be achieved in CMO [670], but to date the use of soft chemistry processing to develop sub-micron grains with nanosized twinned domains, has produced the best performing CMO with a zTmax of 0.32 at 1060 K [613].
The transport properties of perovskite SrTiO3 (STO) depend critically on processing conditions and composition. Undoped STO processed in air is an insulator, exhibiting a high Seebeck coefficient (S −380 μV K−1 at 300 K), but also exceptionally high thermal conductivity (9–12 W m−1 K−1) at room temperature [596]. By processing under reducing conditions a high PF, comparable with that of Bi2Te3 can be achieved, but reducing thermal conductivity is much more of a challenge as nanostructuring is less effective than in many other materials. Doping on the cation A site, with La in place of ∼10% of the Sr has been popular and effective, which under reducing conditions leads to the formation of oxygen vacancies, which enhance electrical conductivity and reduce thermal conductivity [596–612]. On the cation B site, doping with higher valent Nb leads to metallic conduction and simultaneously increases S because the effective mass m* is increased; consequently, the PF σS2 is enhanced, with values of ∼1500 μW m−1 K−2 at 1000 K recorded for SrTi0.8Nb0.2O3 epitaxial films and a zTmax of 0.37 [596].
By optimized doping of A and or B sites of STO, zTmax values at high temperatures have remained stubbornly around 0.38 [597–599, 603, 604, 610]. There have been isolated reports of zTmax values above 0.5 for STO-based materials [602, 608, 611], but an interesting development in recent years has been the enhancement of transport properties at lower temperatures through additions of carbon-based species. Lin et al [600] showed that incorporation of small amounts of graphene (<1 wt%) into STO enabled single crystal-like electronic transport behavior, with high electrical conductivity at temperatures of 373 K or less. The presence of the graphene at the grain boundaries promoted oxygen deficiency, increasing charge transport through an increase in the weighted mobility; at the same time the graphene helped reduce thermal transport. Increased zT values, around 0.4 were achieved over a wide temperature range up to 873 K, thereby greatly enhancing the operational thermal window. Other studies with graphene/graphene oxide support the enhancement behavior [605–607, 609]. It is predicted that zT values above 0.5 could be achieved for STO-graphene composites by optimizing the grain boundary structure [671].
Semiconducting zinc oxide, ZnO (ZO) is a further oxide having considerable potential because of its high mobility and high PF, but again there is the disadvantage of high thermal conductivity (∼5 W m−1 K−1). Doping with trivalent elements is usually employed to increase thermoelectric performance; a zTmax of 0.30 at 1273 K was achieved with 2% Al doping [618], whilst dual doping with Al and Ga (the latter preventing second phase formation, which limits electrical conductivity) enabled one of the highest zT values to be achieved for an oxide—zTmax 0.65 for Zn0.96Al0.02Ga0.02O at 1273 K [620].
The success with improving the properties of ZnO led to work on the related homologous compounds In2O3(ZnO)m (IZO) and Ga2O3(ZnO)m (GZO), having structures comprising layers of ZnO separated by integer numbers of layers of gallium or indium oxide. The attraction of these materials is that by changing the number of layers the PF can be adjusted whilst maintaining low thermal conductivity. For many of the simple binary compounds, the zTmax is still quite modest, but for In2O3(ZnO)3 and Ni coated In2O3(ZnO)5 very useful zTmax values of 0.24 and 0.39 at 973 K respectively have been reported [623, 622]. Increasing the value of m can be beneficial for developing superlattice and twin structures for reducing thermal conductivity, but unfortunately there is often a concomitant reduction in electrical conductivity as well.
Non-stoichiometric titania, specifically the Magnéli phases Tin O2n−1 (n =2, 3,...) have significantly better thermoelectric properties than TiO2, as a result of the presence of planar shear defects and oxygen vacancies acting as effective phonon scatterers. With increasing non-stoichiometry electrical conductivity increases and S reduces, but there is often a beneficial reduction in thermal conductivity as well; in TiO1.76 the highest zTmax of 0.35 was achieved at 973 K [630]. Simultaneous co-doping by Nb and N has the double benefit of increasing the PF and reducing κ for Magnéli phases, leading to zTmax of 0.35 at 973 K for Ti0.83Nb0.17(O,N)2±δ [629].
There have also been investigations of materials with the complex tungsten bronze structure based on (Sr,Ba)Nb2O6. With the more complex crystal structure they are expected to have inherently low thermal conductivity; structural anisotropy means that they need to be textured to optimize performance. Both cation and anion doping has been explored; replacing oxygen by fluorine increases the PF through increases in carrier concentration, and helps to reduce κ, leading to a zTmax of 0.21 at 1073 K for Sr0.61Ba0.39Nb2O5.95F0.05 [624]. Somewhat surprisingly, ferroelectric Ba6−x Nd8+2x Ti18O54 has both a high Seebeck coefficient (−210 μV K−1) and an exceptionally low thermal conductivity (∼1.45 W m−1 K−1); indeed this κ value is one of the lowest for an oxide. With modest electrical conductivity the zTmax was limited to 0.16 at 1000 K for Ba5.19Nd8.54Ti18O54 [626]. Nevertheless, it is clear that oxides offer considerable opportunities for medium and high temperature thermoelectrics if the natural advantages of oxides can be exploited to the full. Indeed, the recent work on composites including carbon species [600] suggests that oxides could potentially be used over very much wider temperature ranges, in principle from room temperature.
Finally, new materials from the oxyselenide family, mainly BiCuSeO, have attracted a lot of attention as p-type material candidates due to their intrinsically low thermal conductivity and moderate PF values [657]. These materials have a complex crystal structure consisting of alternating insulating (Bi2O2)2+ layers and conductive (Cu2Se2)2− layers along the c-axis [672]. The occurrence of low thermal conductivity is linked to the layered crystal structure with low Young's modulus and speed of sound [673]. BiCuSeO exhibits a large Seebeck coefficient varying from 353 μV K−1 at 300 K to 420 μV K−1 at 923 K [657]. The high S values is linked to the two dimensional confinement of charge carriers due to the layered crystal structure and alternated stacking of insulating and conductive layers [658]. BiCuSeO has typically low electrical conductivity and exhibits semiconductor-like conduction behavior with temperature [657–659]. PF values generally improved with doping at the Bi site as a result of the significant increase in electrical conductivity; reasonable values were obtained for compositions containing Bi and/or O vacancies. Moreover, co-doping strategies generally resulted in further improvement in PF values due to the increased contribution of charge carriers; thermal conductivity generally increased with doping. However, the increase in the PF with doping compensates for relatively small increase in thermal conductivity, which leads to higher zT values. For example, a zTmax value of 1.46 at 873 K is reported for Bi0.94Pb0.06Cu0.99Fe0.01SeO [664].
Transport properties for the various oxides (based on data collected for 300 K) are summarized in figure 10. Currently, there are considerably more n-type oxides than p-type materials available and this is reflected in figure 10(a), showing the relationship between Seebeck coefficient and electrical conductivity. Materials such STO and BiCuSeO which inherently have exceptionally low electrical conductivities exhibit the highest Seebeck coefficients (above 300 μV K−1). However, by doping, electrical conductivity can be increased for all oxides, and indeed by careful processing, very high σ values (towards 4000 S cm−1) and single-crystal like electrical conductivity have been achieved in STO.
Figure 10. Thermoelectric transport properties for selected oxides in table 9: (a) Seebeck coefficient (S) vs electrical conductivity (σ), (b) total thermal conductivity (κ) vs electrical conductivity, (c) weighted mobility (μw) vs lattice thermal conductivity and (d) peak zT vs temperature.
Download figure:
Standard image High-resolution imageWhilst there is a desire for phonon-glass behavior, and high thermoelectric performance in oxides, it is clear from figure 10(b) that whilst high electrical conductivity can be achieved (e.g. for STO), the problem continues to be high thermal conductivity, exceptionally high in some cases with the exception of BiCuSeO. However, by selective doping and nanostructuring there has been progress in recent years, and many of the layered structured compounds and the complex tungsten bronze structured materials do exhibit low thermal conductivities (down to 1.45 W m−1 K−1). Alternatively, intrinsically low thermal conductivity (as low as 0.6 W m−1 K−1) and moderate electrical conductivity of oxyselenides leads to relatively high zT values. Figure 10(c) shows the weighted mobility (which was calculated via experimental S and σ values using equation (4)) as a function of lattice thermal conductivity for eight oxide families. As noted earlier (in sections 1.4, 3.4 and 3.6) the ratio of weighted mobility to lattice thermal conductivity (denoted by m) is directly proportional to the thermoelectric quality factor, B (equation (3)), which in turn is proportional to the thermoelectric figure of merit, zT. The highest value of m (i.e. high μw and low κL) is observed for BiCuSeO materials (the highest m≈ 330) whilst it is approximately 160–180 for STO and CMO materials. These values are broadly comparable with many of the metallic counterparts, although the best Zintls reach m values in excess of 350 (figure 5) similar to that for BiCuSeO. Finally figure 10(d) summarizes peak zT values as a function of temperature. Several oxide materials have peak zT values above 0.6 at temperatures above 800 K whereas zTmax values are typically >0.8 and even reaching to ∼1.5 for oxyselenides around 900 K.
With the advances in computational material science [674, 675], many materials can first be theoretically screened and then the promising compositions can be evaluated experimentally. Such studies suggest that oxides with zT values above 1 are possible and the utilization of new material design concepts, such as introducing/controlling interfaces at both atomic [676] and micro [677] scales, will allow control of both phonon and charge carrier transport in oxides. This will enable oxides to be competitive with more established materials, since they can offer much more once they can be utilized to full capacity, including their stability over a wide temperature range, lower density, lower toxicity, and cheaper material production.
The mobility data in table 9 are predominantly obtained from Hall measurements; only one entry has been obtained by SPB method; both types of entry are clearly identified.
3.10. Sulfides and selenides
Sulfide and selenide thermoelectrics
Anthony V Powell, Shriparna Mukherjee, Sahil Tippireddy and Paz Vaqueiro
Department of Chemistry, University of Reading, RG6 6DX Reading, United Kingdom
The terrestrial abundance of both sulfur (3.5 × 105 ppb) and selenium (50 ppb) exceeds that of tellurium (1 ppb), making metal sulfides and selenides attractive candidates for large-scale thermoelectric applications. While the higher vibrational frequencies associated with the lighter chalcogens are expected to result in a higher thermal conductivity than in the tellurides, a number of materials-design strategies [678] have been applied to generate high-performance materials. The decrease in electronegativity on progressing down the chalcogen group raises the energy of the anion valence orbitals, thus reducing the bandgap (Eg) between the predominantly anion-based orbitals of the valence band and the cation-derived conduction band. The reduced separation between anion- and cation-derived orbitals leads to more effective orbital overlap and broadening of the bands. This is exploited in partial substitution of sulfur by selenium to fine-tune the electronic structure through band broadening, thereby reducing the carrier effective mass (m*) and increasing the mobility (μ). Sulfide and selenide thermoelectrics are not without challenges. In particular, volatilization of the chalcogen during preparation or processing may occur, producing compositional changes and the formation of inclusions of secondary phases. In favorable cases, the resulting interfaces may increase phonon scattering and reduce thermal conductivity. The majority of metal sulfide and selenide thermoelectrics are p-type semiconductors; n-type conduction generally occurring in low-dimensional structures and amongst chalcopyrite-related phases.
In seeking to translate the high-performance of metal tellurides into the more abundant sulfides and selenides, attention has focused on the lighter congeners of tellurides of proven thermoelectric performance. This includes the rocksalt-structured phases PbQ (Q = S, Se) for which both n- and p-type derivatives can be created through appropriate doping, and figures of merit, zT ⩾ 1. Increasing concerns over the toxicological and environmental impact of lead has prompted investigation of the analogous tin and germanium sulfides and selenides, the layered structures of which arise from distortion of the rocksalt structure. The figure of merit, zT > 2.4, achieved in single crystalline SnSe [679], has motivated a wider investigation of tin chalcogenides. Although the corresponding sulfide, SnS, adopts a similar structure, the maximum figure of merit is somewhat lower at zT = 0.8 at 873 K [680]. While conventional doping of GeSe leads to modest improvements in performance, alloying with AgSbSe2 results in a p-type material with a figure of merit, zT = 0.9 at 710 K [681] while alloying with AgBiSe2 produces an n-type variant which exhibits a maximum figure of merit, zT = 0.44 at 677 K [682].
The more structured DOS associated with a low-dimensional structure has stimulated efforts to increase the Seebeck coefficient by tuning the Fermi level to sharp discontinuities in the DOS [683], although reductions in thermal conductivity due to interface scattering of phonons appears to play a more dominant role. One-dimensional chain structures [684] and two-dimensional structures, including intercalates of dichalcogenides, Ax TiS2 (A = Co, Cu, Ag) [685–687] and a variety of pavonite-related materials [688, 689], have been investigated, together with materials possessing low-dimensional structural motifs within a 3D structure, as exemplified by the derivatives of shandite, Co3Sn2S2.
The high polarizability of the sulfide and selenide anions favors cation diffusion, which at high temperatures can induce the cation sub-lattice to enter a liquid-like state. The term phonon-liquid electron-crystal (PLEC) has been applied to such phases. The PLEC-type phases Cu1.97S and Cu2−x Se exhibit an exceptional thermoelectric performance, with figures of merit reaching zT = 1.7 [690] and zT = 1.5 [691] respectively (figure 11). The cation mobility that promotes PLEC behavior introduces an instability into the materials, due to copper migration and deposition, resulting in compositional changes that cause cracking and mechanical degradation. Efforts to overcome stability problems have motivated investigation of alternative structure types, in which a proportion of the tetrahedral sites are occupied by other cations. A wide range of cation-ordered derivatives of zinc blende, including chalcopyrite, kesterite and stannite, has been investigated, along with structurally more complex phases such as bornite, in which antifluorite- and zinc-blende-type sub-cells alternate. A combination of hole doping and the creation of cation vacancies in bornite, leads to a figure of merit, zT ≈ 0.8 [692], at 550 K, with no significant degradation in performance on thermal cycling.
Figure 11. The maximum thermoelectric figure of merit, zT of various sulfides and selenides: Cu0.05TiS1.5Se0.5 (n-type) [699], Cu4.972Fe0.968S4 (p-type) [692], [Cu26Cr2Ge6]1.024S32 (p-type) [700], Ag0.9InZn0.1Se2 (n-type) [701], Cu13.5Sb4S12Se (p-type) [702], Cu1.85Ag0.15Sn0.9In0.1Se3 (p-type) [703], SnS0.91Se0.09 (p-type) [704], Pb0.93Sb0.05S0.5Se0.5 (n-type) [695], Cu1.97S (p-type) [690].
Download figure:
Standard image High-resolution imageThe beneficial effect of increasing structural complexity is exemplified by tetrahedrite, the structure of which may be considered as a defective derivative of zinc-blende containing transition-metal cations in both tetrahedral and trigonal-planar sites. The parent sulfide Cu12Sb4S13 is a p-type metal, with a low thermal conductivity, associated with anharmonic localized vibrational modes. Reduction of the hole carrier concentration through chemical substitution of copper, increases the figure of merit to zT ≈ 1.0 at relatively modest temperatures (575 ⩽ T/K ⩽ 725). Tuning the electronic structure through the partial replacement of sulfide with selenium decreases the resistivity, without a significant impact on the Seebeck coefficient. This appears to have the greatest impact on performance at temperatures close to ambient.
The structure of colusite (Cu26V2Ge6S32) may also be considered to be derived from an ordered variant of zinc blende. The complex structure and large unit cell contribute to a low thermal conductivity, κ ≈ 0.5 W m−1 K−1. Substitution of copper in colusite and its congeners, with dipositive transition-metal cations, decreases the hole carrier concentration and improves the Seebeck coefficient, leading to figures of merit at elevated temperatures in the range 0.6 ⩽ zT ⩽ 0.9. Colusite provides a striking example of the impact of consolidation conditions on thermoelectric properties [693]. Hot pressing (HP 1023 K) Cu26V2Sn6S32 results in sulfur loss and the formation of intergrowths that help reduce the thermal conductivity to κ ≈ 0.66 W m−1 K−1, increasing the figure of merit by a factor of three (zT = 0.93 at 675 K [693]) over that of the same material subjected to spark plasma sintering (SPS 873 K) at a lower temperature.
As illustrated in table 10, there has been considerable progress in the discovery of sulfides and selenides for thermoelectric applications. There are now several families of p-type sulfides containing Earth-abundant elements, with figures of merit approaching or exceeding unity at moderate temperatures, including tetrahedrites and colusites. Among p-type selenides, Cu2Se and SnSe, with maximum figures of merit of zT = 1.54 and zT = 2.6 at 1000 and 923 K respectively, stand out [679, 691]. Furthermore, it has been demonstrated very recently that exceptional thermoelectric performance (zT = 3.1 at 783 K [694]) can be achieved in polycrystalline SnSe when feedstock reagents are purified to remove all traces of oxides. By contrast, it is evident from table 10 that there are few examples of n-type sulfides and selenides with comparable performances. The few exceptions including Pb0.93Sb0.05S0.5Se0.5, with a maximum figure of merit of zT = 1.65 at 900 K [695] and Ag2Se, with zT = 1.2 near room temperature [696]. The discovery of environmentally friendly n-type sulfides, containing abundant elements and with good thermoelectric performance, remains a challenge to be addressed.
Table 10. Sulfide and selenide thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κL (W m−1 K−1) | κ (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µH (cm2 V−1 s−1) | ms* (me) |
 r or r or  ( ( 0) 0) | nH (cm−3) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binary phases | |||||||||||||
| Cu2Se (p) | 420 | 40.6 | 0.6 | 1 | 100 | 500 | 0.2 | 1.23 a | — | 4.2 | — | 3.2 × 1020 | [691, 705] |
| Cu2Se (p) | 1000 | 29.8 | 0.5 | 0.75 | 300 | 125 | 1.54 | 1.23 a | — | — | — | — | [691] |
| Cu1.94Se0.5S0.5 (p-type) | 300 | 37.7 | 0.45 | 0.7 | 100 | 280 | 0.1 | — | — | 4.2 | 1.0 × 1021 | [706] | |
| Cu1.94Se0.5S0.5 (p-type) | 1000 | 27.3 | 0.2 | 0.6 | 220 | 290 | 2.3 | — | — | — | — | — | [706] |
| Cu1.97S (p-type) | 300 | 10.8 | 0.55 | 0.6 | 100 | 80 | 0.04 | — | — | — | — | 7.27 × 1020 | [690] |
| Cu1.97S (p-type) | 1000 | 22.6 | 0.35 | 0.5 | 300 | 95 | 1.7 | — | — | 5.7 | — | — | [690] |
| Cu2S (p-type) | 300 | 14.5 | 0.35 | 0.39 | 300 | 10 | 0.07 | — | — | — | — | 0.48 × 1019 | [690] |
| Cu2S (p-type) | 1000 | 13.5 | 0.32 | 0.35 | 450 | 10 | 0.65 | — | — | — | — | — | [690] |
| Cu2Se0.92S0.08 (p-type) | 300 | 134.6 | 0.28 | 0.75 | 100 | 1000 | 0.4 | — | — | 2.3 | 2.8 × 1020 | [707] | |
| Cu2Se0.92S0.08 (p-type) | 1000 | 23.7 | 0.37 | 0.5 | 275 | 133 | 2 | — | — | — | — | — | [707] |
| Cu1.98Ag0.2Se (p-type) | 300 | 24.1 | 0.6 | 0.8 | 40 | 500 | 0.03 | — | 300 e | — | — | — | [111] |
| Cu1.98Ag0.2Se (p-type) | 900 | 4.4 | 0.25 | 0.4 | 260 | 25 | 0.4 | 0.45 | 3.16e | — | — | — | [111] |
| PbSe(p-type) | 300 | 414.6 | — | 1.8 | 320 | 227 | 0.38 | 0.26 | — | 0.11 | 210 | 9.0 × 1017 | [708–710] |
| PbSe(n-type) | 900 | 5.4 | — | 1.6 | −150 | 110 | 0.13 | — | — | — | — | — | [710] |
| Pb0.975Cu0.005Na0.02Te0.1Se0.9 (p-type) | 300 | 121.3 | 0.95 | 2.5 | 50 | 2000 | 0.06 | — | — | — | — | 1.5 × 1020 | [710] |
| Pb0.975Cu0.005Na0.02Te0.1Se0.9 (p-type) | 860 | 50.1 | 0.7 | 0.89 | 294 | 180 | 1.5 | — | — | — | — | — | [710] |
| Pb0.993Na0.007Se (p-type) | 300 | 102.2 | 2 | 3.8 | 34 | 2500 | 0.02 | — | — | — | — | 0.93 × 1020 | [711] |
| Pb0.993Na0.007Se (p-type) | 850 | 37.2 | 0.6 | 1.1 | 200 | 390 | 1.2 | — | — | — | — | — | [711] |
| Pb0.98Sr0.02Se 1% Na (p-type) | 300 | 83.6 | 1.59 | 3.68 | 24 | 2901 | 0.01 | — | — | — | — | 1.6 × 1020 | [712] |
| Pb0.98Sr0.02Se 1% Na (p-type) | 923 | 38.5 | 0.73 | 1.14 | 246 | 268 | 1.3 | — | — | — | — | — | [712] |
| PbS (p-type) | 300 | 33.3 | — | 2.5 | 300 | 23 | 0.02 | 0.37 | — | 0.16 | 169 | — | [708, 709, 695] |
| PbS (n-type) | 900 | 2.0 | — | 1.25 | −200 | 23 | 0.066 | — | — | — | — | — | [695] |
| Pb0.93Sb0.05S0.5Se0.5 (n-type) | 300 | 78.3 | 1.1 | 1.6 | −70 | 900 | 0.08 | — | — | — | — | — | [695] |
| Pb0.93Sb0.05S0.5Se0.5 (n-type) | 900 | 32.0 | 0.3 | 0.8 | −188 | 421 | 1.65 | — | — | — | — | — | [695] |
| Pb0.9865Ga0.0125In0.001S (n-type) | 300 | 242.2 | — | 3 | −100 | 1800 | 0.18 | — | — | — | — | 1.5 × 1019 | [713] |
| Pb0.9865Ga0.0125In0.001S (n-type) | 920 | 34.6 | — | 1 | −283 | 156 | 1.1 | — | — | — | — | — | [713] |
| Sn0.98Na0.02S (p-type) | 300 | 5.7 | — | 1 | 175 | 16.67 | 0.02 | 0.4 a | 630 e | 0.35 | 1.67 × 1019 | [714] | |
| Sn0.98Na0.02S (p-type) | 850 | 13.2 | — | 0.45 | 325 | 32.5 | 0.65 | — | — | 1.2 | — | — | [714] |
| SnS (p-type) | 300 | 0.0 | — | 1 | 250 | 0.005 | 9.3 × 10−6 | 1.12 a | — | — | — | 2.0 × 1019 | [715] |
| SnS (p-type) | 848 | 8.7 | — | 0.3 | 400 | 9 | 0.41 | — | — | — | — | — | [715] |
| SnS0.98Br0.02 (n-type) | 300 | 113.4 | — | 1 | −875 | 0.1 | 2.30 × 10−3 | — | — | — | — | 1.0 × 1017 | [716] |
| SnS0.98Br0.02 (n-type) | 823 | 44.4 | — | 0.6 | −627 | 3.15 | 0.17 | — | — | — | — | — | [716] |
| SnS0.91Se0.09 (p-type) | 300 | 568.0 | 1.7 | 2.4 | 200 | 1250 | 0.6 | — | — | — | — | — | [704] |
| SnS0.91Se0.09 (p-type) | 873 | 53.9 | 0.5 | 0.61 | 370 | 82 | 1.6 | — | — | — | — | — | [704] |
| SnS (p-type) | 300 | 0.0 | — | 1.3 | 400 | 0.008 | 2.90 × 10−5 | 1.21 a | 5 e | — | — | 1.37 × 1014 | [717] |
| SnS (p-type) | 820 | 14.8 | — | 0.5 | 516 | 3.78 | 0.16 | — | — | — | — | — | [717] |
| SnS-0.5%Ag (p-type) | 300 | 10.3 | — | 0.88 | 350 | 4 | 0.02 | 1.18 a | 3.5 e | 1.42 | 2.72 × 1018 | [717] | |
| SnS-0.5%Ag (p-type) | 870 | 15.1 | — | 0.42 | 377 | 21.1 | 0.6 | — | — | — | — | — | [717] |
| SnSe (p-type) | 300 | 147.0 | 0.68 | 0.7 | 500 | 10 | 0.1 | 0.86 | — | — | — | 3 × 1017 | [679] |
| SnSe (p-type) | 923 | 38.3 | 0.25 | 0.35 | 350 | 80 | 2.6 | — | — | — | — | 1.0 × 1019 | [679] |
| Sn0.85Se (p-type) | 325 | 4.9 | 0.95 | 0.95 | 217 | 10 | 0.02 | — | — | — | — | 7 × 1019 | [680] |
| Sn0.85Se (p-type) | 873 | 20.9 | 0.44 | 0.46 | 375 | 30 | 0.8 | — | — | — | — | — | [680] |
| Sn0.98Na0.016Ag0.004Se (p-type) | 300 | 55.6 | — | 1.3 | 150 | 220 | 0.1 | ∼0.86 | — | — | — | 6.23 × 1019 | [718] |
| Sn0.98Na0.016Ag0.004Se (p-type) | 785 | 21.1 | — | 0.45 | 250 | 110 | 1.2 | — | — | — | — | — | [718] |
| Ag1.96S (n-type) | 300 | 424.4 | — | 0.47 | −850 | 0.5 | 0.02 | 0.9 a | — | — | — | — | [719] |
| Ag1.96S (n-type) | 560 | 21.9 | — | 0.45 | −143 | 240 | 0.62 | — | — | — | — | 1.2 × 1019 | [719] |
| Ag2Se1.06 (n-type) | 300 | 338.0 | — | 1.08 | −153 | 1290 | 0.84 | 0.21 | — | — | — | 5.59 × 1018 | [720] |
| Ag2Se (n-type) | 300 | 409.1 | — | 1.1 | −133 | 1988 | 0.96 | 0.16 | — | — | — | 1.07 × 1018 | [721] |
| Ag2Se (n-type) | 300 | 256.2 | 0.6 | −170 | 800 | 1.2 | — | — | — | — | 6.63 × 1018 | [696] | |
| Bi2S3 (n-type) | 300 | 5.2 | 1.31 | 1.31 | −351 | 2 | 0.01 | — | — | — | — | 3.7 × 1016 | [684] |
| Bi2S3 (n-type) | 625 | 2.5 | 0.86 | 0.86 | −323 | 4 | 0.03 | — | — | — | — | — | [684] |
| Bi2S3+0.5 mol% BiCl3 (n-type) | 300 | 86.7 | 1.02 | 1.37 | −103 | 619 | 0.15 | — | — | — | — | 2.6 × 1019 | [684] |
| Bi2S3+0.5 mol% BiCl3 (n-type) | 772 | 17.3 | 0.63 | 0.76 | −234 | 106 | 0.57 | — | — | — | — | — | [684] |
| Bi2S2.7Se0.3+0.5 mol% BiCl3 (n-type) | 300 | 58.7 | 1.18 | 1.38 | −130 | 296 | 0.11 | — | — | — | — | — | [684] |
| Bi2S2.7Se0.3+0.5 mol% BiCl3 (n-type) | 710 | 15.2 | 0.68 | 0.77 | −257 | 63 | 0.39 | — | — | — | — | — | [684] |
| Bi2Se3 (n-type) | 300 | 97.6 | — | 2.42 | −35 | 2320 | 0.04 | — | — | — | — | — | [74] |
| Bi2Se3 (n-type) | 523 | 33.6 | — | 2.08 | −48 | 1329 | 0.08 | — | — | — | — | — | [74] |
| Bi2Se2S1 (n-type) | 300 | 9.2 | — | 0.7 | −193 | 22 | 0.03 | — | — | — | — | — | [74] |
| Bi2Se2S1 (n-type) | 523 | 17.2 | — | 0.5 | −295 | 29 | 0.23 | — | — | — | — | — | [74] |
| Bi2Se1S2 (n-type) | 300 | 53.6 | — | 0.71 | −195 | 125 | 0.2 | — | — | — | — | — | [74] |
| Bi2Se1S2 (n-type) | 523 | 23.7 | — | 0.65 | −224 | 91 | 0.37 | — | — | — | — | — | [74] |
| Cu0.01Bi2Se3 (n-type) | 300 | 89.7 | 0.64 | 0.74 | −171 | 277 | 0.33 | — | — | — | — | — | [722] |
| Cu0.01Bi2Se3 (n-type) | 590 | 34.3 | 0.31 | 0.78 | −104 | 666 | 0.54 | — | — | — | — | — | [722] |
| Bi2Se1.5Te1.5 (n-type) | 300 | 126.3 | 0.6 | 0.83 | −159 | 449 | 0.41 | — | — | — | — | — | [723] |
| Bi2Se1.5Te1.5 (n-type) | 600 | 32.5 | 0.44 | 0.85 | −171 | 284 | 0.59 | — | — | — | — | — | [723] |
| (PbSe)5Bi2Se3 (n-type) | 300 | 5.2 | 0.59 | 0.61 | −90 | 44 | 0.02 | — | — | — | — | — | [724] |
| (PbSe)5Bi2Se3 (n-type) | 700 | 8.7 | 0.34 | 0.39 | −229 | 49 | 0.48 | — | — | — | — | — | [724] |
| (PbSe)5(Bi2Se3)2 (n-type) | 300 | 5.0 | 0.63 | 0.72 | −30 | 139 | 0.01 | — | — | — | — | — | [724] |
| (PbSe)5(Bi2Se3)2 (n-type) | 700 | 6.8 | 0.41 | 0.57 | −128 | 125 | 0.25 | — | — | — | — | — | [724] |
| GeSe (p-type) | 300 | 29.2 | 2.9 | 2.90 | 628 | 0.45 | 0.001 | — | — | — | — | — | [725] |
| GeSe (p-type) | 700 | 25.8 | 0.87 | 0.87 | 590 | 2.2 | 0.05 | — | — | — | — | — | [725] |
| GeSe(AgSbTe2)0.2 (p-type) | 300 | 58.0 | 0.63 | 0.71 | 177 | 167 | 0.22 | — | — | — | — | — | [725] |
| GeSe(AgSbTe2)0.2 (p-type) | 750 | 39.4 | 0.74 | 0.90 | 230 | 242 | 0.96 | — | — | — | — | — | [725] |
| Ge0.97Ag0.03Se (p-type) | 300 | 1.1 | 1.16 | 1.16 | 350 | 0.44 | 0.002 | — | — | — | — | — | [726] |
| Ge0.97Ag0.03Se (p-type) | 650 | 20.4 | 0.43 | 0.45 | 515 | 3.71 | 0.18 | — | — | — | — | — | [726] |
| Ge0.79Ag0.01Sn0.2Se (p-type) | 300 | 5.3 | 0.74 | 0.74 | 309 | 3.3 | 0.01 | — | — | — | — | — | [726] |
| Ge0.79Ag0.01Sn0.2Se (p-type) | 650 | 15.5 | 0.4 | 0.40 | 475 | 4.5 | 0.2 | — | — | — | — | — | [726] |
| (GeSe)0.50(AgBiSe2)0.50 (n-type) | 300 | 16.0 | 0.43 | 0.47 | −208 | 32 | 0.09 | 0.4 | — | — | — | 3.29 × 1018 | [682] |
| (GeSe)0.50(AgBiSe2)0.50 (n-type) | 677 | 11.9 | 0.48 | 0.61 | −175 | 119 | 0.44 | — | — | — | — | — | [682] |
| GeSe(AgSbSe2)0.2 (p-type) | 300 | 23.1 | 0.85 | 0.88 | 153 | 88 | 0.07 | — | 5 e | — | — | 1.1 × 1020 | [681] |
| GeSe(AgSbSe2)0.2 (p-type) | 710 | 40.9 | 0.74 | 0.9 | 263 | 158 | 0.86 | — | — | — | — | — | [681] |
| Chalcopyrites, tetrahedrites and bornites | |||||||||||||
| CuFeS2 (n-type) | 300 | 157.5 | 8.42 | 8.46 | −362 | 53 | 0.02 | 0.7 b | — | 1.8 | — | 3.2 × 1019 | [727, 728] |
| CuFeS2 (n-type) | 630 | 41.6 | 2.35 | 2.35 | −384 | 33 | 0.13 | — | — | — | — | — | [727] |
| Cu0.92Zn0.08FeS2 (n-type) | 300 | 94.5 | 5.3 | 5.4 | −187 | 242 | 0.04 | — | — | 4.9 | — | 39.6 × 1019 | [727] |
| Cu0.92Zn0.08FeS2 (n-type) | 630 | 37.8 | 1.92 | 2 | −263 | 122 | 0.26 | — | — | — | — | — | [727] |
| Cu0.92Cd0.08FeS2 (n-type) | 300 | 111.4 | 3.91 | 3.98 | −230 | 173.1 | 0.077 | — | 4.08 e | 9 | — | 3.18 × 1020 | [729] |
| Cu0.92Cd0.08FeS2 (n-type) | 723 | 26.1 | 1.2 | 1.32 | −265 | 101 | 0.39 | — | — | — | — | — | [729] |
| AgInSe2 (n-type) | 370 | 164.9 | 0.71 | 0.72 | −692 | 1.66 | 0.04 | 0.9 a | — | — | — | 2.04 × 1017 c | [701] |
| AgInSe2 (n-type) | 800 | 10.3 | 0.33 | 0.35 | −417 | 8 | 0.35 | — | — | — | — | — | [701] |
| Ag0.95In0.95Zn0.1Se2 (n-type) | 300 | 98.1 | 0.68 | 0.7 | −436 | 14 | 0.1 | 0.9 a | — | — | — | 6.68 × 1017 | [701] |
| Ag0.95In0.95Zn0.1Se2 (n-type) | 815 | 16.6 | 0.28 | 0.37 | −306 | 48 | 0.95 | — | — | — | — | — | [701] |
| Ag0.9InZn0.1Se2 (n-type) | 300 | 16.7 | 0.38 | 0.42 | −170 | 52 | 0.12 | 0.97 a | — | — | — | 4.96 × 1018 | [701] |
| Ag0.9InZn0.1Se2 (n-type) | 815 | 10.7 | 0.11 | 0.34 | −195 | 112 | 1.05 | — | — | — | — | — | [701] |
| CuIn3Se5 (n-type) | 300 | 14.1 | 1.1 | 1.12 | −600 | 0.3 | 0.003 | 0.75 d | — | 0.007 | — | 3 × 1016 | [730] |
| CuIn3Se5 (n-type) | 930 | 2.9 | 0.64 | 0.68 | −270 | 15.3 | 0.15 | — | — | — | — | — | [730] |
| CuIn3Se4.9Te0.1 (n-type) | 300 | 1447.9 | 0.58 | 0.59 | −1000 | 0.3 | 0.01 | 1.05 d | 0.34 | 6 × 1017 | [730] | ||
| CuIn3Se4.9Te0.1 (n-type) | 930 | 5.0 | 0.4 | 0.42 | −292 | 20.5 | 0.4 | — | — | — | — | — | [730] |
| CuInSe2 (n-type) | 323 | 0.4 | 3.98 | 4 | −100 | 3.5 | 0.01 | 0.894 a | 32.6 c , e | — | — | 9 × 1017 c | [731, 732] |
| CuInSe2 (n-type) | 773 | 0.2 | 0.9 | 0.92 | −152 | 2.8 | 0.08 | — | — | — | — | — | [731] |
| CuIn0.98Zn0.02Se2 (p-type) | 323 | 47.1 | 3.7 | 3.72 | 400 | 11.4 | 0.02 | — | 10.7 c , e | 0.7 c | 6.6 × 1018 c | [731] | |
| CuIn0.98Zn0.02Se2 (p-type) | 773 | 15.8 | 0.9 | 0.92 | 448 | 8.1 | 0.18 | — | — | — | — | — | [731] |
| Cu0.99InSe2.05 (p-type) | 323 | 1.8 | — | 1.52 | 300 | 1.4 | 0.002 | 0.892 a | — | — | — | — | [732] |
| Cu0.99InSe2.05 (p-type) | 620 | 33.3 | — | 0.38 | 510 | 6 | 0.31 | — | — | — | — | — | [732] |
| Cu5FeS4 (p-type) | 300 | 12.7 | 0.45 | 0.48 | 156 | 46.7 | 0.04 | 1.25 a | 0.16 e | — | — | 1.9 × 1021 | [733, 734] |
| Cu5FeS4 (p-type) | 540 | 17.4 | 0.25 | 0.4 | 174 | 125 | 0.47 | — | — | — | — | — | [733] |
| Cu5FeS3.8Se0.2 (p-type) | 300 | 24.0 | 0.38 | 0.47 | 120 | 137 | 0.1 | — | 1.15 e | 2.42 | 6.24 × 1020 | [733] | |
| Cu5FeS3.8Se0.2 (p-type) | 540 | 18.6 | 0.24 | 0.46 | 150 | 178 | 0.5 | — | — | — | — | — | [733] |
| Cu4.972Fe0.968S4 (p-type) | 320 | 5.8 | 0.44 | 0.47 | 138 | 29.4 | 0.04 | — | — | — | — | — | [692] |
| Cu4.972Fe0.968S4 (p-type) | 550 | 25.0 | 0.18 | 0.3 | 236 | 90 | 0.79 | — | — | — | — | — | [692] |
| Cu0.96 Co0.04Fe0.96Zn0.04S4 (p-type) | 323 | 5.9 | 0.33 | 0.36 | 106 | 45.5 | 0.05 | — | — | — | — | — | [735] |
| Cu0.96 Co0.04Fe0.96Zn0.04S4 (p-type) | 590 | 14.7 | 0.23 | 0.36 | 171 | 125 | 0.6 | — | — | — | — | — | [735] |
| CuCrSe2 (p-type) | 300 | 54.5 | — | 1.12 | 95 | 434 | 0.1 | — | — | — | — | 5.8 × 1019 | [736] |
| CuCrSe2 (p-type) | 673 | 16.6 | — | 0.82 | 172 | 170 | 0.44 | — | — | — | — | — | [736] |
| CuCr0.99In0.01Se2 (p-type) | 300 | 53.7 | — | 1.11 | 97 | 416 | 0.1 | — | — | — | — | 5.18 × 1019 | [736] |
| CuCr0.99In0.01Se2 (p-type) | 673 | 24.3 | — | 0.73 | 210 | 160 | 0.65 | — | — | — | — | — | [736] |
| Cu12Sb4S13 (p-type) | 300 | 115.7 | 0.49 | 1.25 | 90 | 987 | 0.2 | 1.7 a | — | — | — | — | [737, 738] |
| Cu12Sb4S13 (p-type) | 723 | 41.2 | 0.5 | 1.41 | 131 | 767 | 0.65 | — | — | — | — | — | [737] |
| Cu10.5Ni1Zn0.5Sb4S13 (p-type) | 300 | 38.8 | 0.42 | 0.5 | 156 | 143 | 0.2 | — | — | — | — | — | [739] |
| Cu10.5Ni1Zn0.5Sb4S13 (p-type) | 723 | 28.9 | 0.4 | 0.58 | 215 | 200 | 1.03 | — | — | — | — | — | [739] |
| Cu13.5Sb4S12Se (p-type) | 300 | 17.7 | 0.42 | 0.46 | 150 | 70 | 0.1 | — | — | — | — | — | [702] |
| Cu13.5Sb4S12Se (p-type) | 723 | 35.0 | 0.25 | 0.78 | 174 | 390 | 1.1 | — | — | — | — | — | [702] |
| Tetrahedral based structures | |||||||||||||
| Cu2Sn0.9Zn0.1S3 (p-type) | 323 | 42.0 | 1.50 | 1.90 | 70 | 540 | 0.05 | — | — | — | — | — | [740] |
| Cu2Sn0.9Zn0.1S3 (p-type) | 723 | 19.6 | 0.43 | 0.80 | 150 | 290 | 0.58 | — | — | — | — | — | [740] |
| Cu2Sn0.85Mn0.15S3 (p-type) | 323 | 70.3 | 1.50 | 2.20 | 65 | 980 | 0.06 | — | 1.5 e | 8 | — | 6 × 1021 | [741] |
| Cu2Sn0.85Mn0.15S3 (p-type) | 723 | 28.2 | 0.32 | 1.00 | 135 | 500 | 0.68 | — | — | — | — | — | [741] |
| Cu2Sn0.8 Co0.2S3 (p-type) | 323 | 51.9 | 0.95 | 1.40 | 67 | 700 | 0.07 | — | 1.01 e | 7 | — | 4.35 × 1021 | [742] |
| Cu2Sn0.8 Co0.2S3 (p-type) | 723 | 29.4 | 0.33 | 0.80 | 155 | 410 | 0.85 | — | — | — | — | — | [742] |
| Cu2SnSe3 single crystal (p-type) | 300 | — | — | — | — | — | — | 0.843 a | 4 e | 1.2 | 13 | 1.5 × 1019 | [743] |
| Cu2Sn0.9In0.1Se3 (p-type) | 300 | 64.7 | 2.66 | 3.20 | 57 | 930 | 0.03 | — | — | — | — | 8.3 × 1020 | [744] |
| Cu2Sn0.9In0.1Se3 (p-type) | 850 | 28.9 | 0.48 | 0.93 | 210 | 270 | 1.14 | — | — | — | — | — | [744] |
| Cu1.85Ag0.15Sn0.9In0.1Se3 (p-type) | 323 | 48.2 | — | 1.60 | 73 | 590 | 0.07 | — | — | — | — | 3.43 ×1020 | [703] |
| Cu1.85Ag0.15Sn0.9In0.1Se3 (p-type) | 823 | 27.0 | — | 0.56 | 240 | 170 | 1.42 | — | — | — | — | — | [703] |
| Cu1.875SnSe3 (p-type) | 300 | 11.0 | 1.43 | 1.46 | 145 | 46 | 0.02 | — | 0.2 f | — | — | 7 × 1020 | [745] |
| Cu1.875SnSe3 (p-type) | 800 | 10.4 | 0.18 | 0.26 | 290 | 35 | 0.95 | — | — | — | — | [745] | |
| Cu2Ga0.07Ge0.93Se3 (p-type) | 300 | 49.8 | — | 2.20 | 54 | 758 | 0.03 | — | 4.37 e | — | — | 10.8 × 1020 | [746] |
| Cu2Ga0.07Ge0.93Se3 (p-type) | 745 | 24.1 | — | 1.33 | 140 | 420 | 0.5 | — | — | — | — | — | [746] |
| Cu3Sb0.85Sn0.15S4 (p-type) | 300 | 32.7 | 2.80 | 3.00 | 71 | 370 | 0.02 | 0.9 a | 2.9 e | 3 | 7.8 × 1020 | [747] | |
| Cu3Sb0.85Sn0.15S4 (p-type) | 573 | 21.2 | 2.10 | 2.30 | 125 | 300 | 0.12 | — | — | — | — | — | [747] |
| Cu3Sb0.9Ge0.1S4 (p-type) | 300 | 71.6 | 1.90 | 2.10 | 151 | 280 | 0.09 | — | 5.22 e | — | — | 3.07 × 1020 | [748] |
| Cu3Sb0.9Ge0.1S4 (p-type) | 623 | 38.8 | 0.76 | 0.92 | 243 | 155 | 0.63 | — | — | — | [748] | ||
| Cu3Sb0.97Ge0.03Se2.8S1.2 (p-type) | 300 | 91.7 | 1.54 | 1.88 | 127 | 480 | 0.11 | — | 14.8 e | 2.33 | 2.03 × 1020 | [749] | |
| Cu3Sb0.97Ge0.03Se2.8S1.2 (p-type) | 650 | 47.0 | 0.52 | 0.89 | 235 | 220 | 0.89 | — | — | — | — | — | [749] |
| Cu2.1Cd0.9GeSe4 (p-type) | 336 | 4.2 | 1.57 | 1.59 | 121 | 28 | 0.01 | — | — | — | — | — | [750] |
| Cu2.1Cd0.9GeSe4 (p-type) | 723 | 11.8 | 0.56 | 0.64 | 226 | 72 | 0.42 | — | — | — | — | — | [750] |
| Cu2.1Cd0.9SnSe4 (p-type) | 300 | 39.8 | 1.92 | 2.04 | 129 | 203 | 0.05 | 0.95 a | — | — | — | — | [751] |
| Cu2.1Cd0.9SnSe4 (p-type) | 700 | 14.5 | 0.23 | 0.49 | 156 | 190 | 0.65 | — | — | — | — | — | [751] |
| Cu2.075 Co0.925GeS4 (p-type) | 300 | 6.7 | 2.00 | 2.01 | 123 | 37 | 0.01 | — | — | — | — | — | [752] |
| Cu2.075 Co0.925GeS4 (p-type) | 723 | 17.2 | 0.90 | 1.01 | 239 | 90 | 0.37 | — | — | — | — | — | [752] |
| Cu2 CoSnS4 (p-type) nanocrystals | 300 | 8.2 | — | 0.51 | 243 | 11 | 0.04 | — | — | — | — | — | [753] |
| Cu2 CoSnS4 (p-type) nanocrystals | 700 | 11.3 | — | 0.37 | 283 | 34 | 0.51 | — | — | — | — | — | [753] |
| Cu2.15 Co0.8Mn0.05SnS4 (p-type) | 300 | 35.7 | 1.98 | 2.25 | 69 | 417 | 0.03 | — | — | — | — | — | [754] |
| Cu2.15 Co0.8Mn0.05SnS4 (p-type) | 800 | 24.4 | 0.64 | 0.98 | 185 | 278 | 0.77 | — | — | — | — | — | [754] |
| Cu2 CoSnSe4 (p-type) | 300 | 29.8 | — | 1.74 | 104 | 210 | 0.03 | — | — | 1.7 | 1.9 × 1020 | [755] | |
| Cu2 CoSnSe4 (p-type) | 850 | 16.9 | 0.60 | 0.80 | 225 | 133 | 0.74 | — | — | — | — | — | [755] |
| Cu2FeSnS4 (p-type) nanocrystals | 300 | 6.6 | — | 0.60 | 232 | 10 | 0.03 | — | — | — | — | — | [753] |
| Cu2FeSnS4 (p-type) nanocrystals | 700 | 7.5 | — | 0.44 | 270 | 26 | 0.31 | — | — | — | — | — | [753] |
| Cu2FeSnSe4 (p-type) | 300 | 24.9 | — | 2.90 | 227 | 40 | 0.01 | — | — | 3.5 | 0.9 × 1020 | [755] | |
| Cu2FeSnSe4 (p-type) | 850 | 8.6 | 0.65 | 0.73 | 234 | 61 | 0.4 | — | — | — | — | — | [755] |
| Cu2.1Fe0.9SnSe4 (p-type) | 300 | 48.6 | 2.62 | 2.72 | 151 | 190 | 0.05 | — | — | 3.2 | 2.3 × 1020 | [756] | |
| Cu2.1Fe0.9SnSe4 (p-type) | 800 | 14.9 | — | 0.91 | 180 | 180 | 0.52 | — | — | — | — | — | [756] |
| Cu2MnSnS4 (p-type) nanocrystals | 300 | 2.2 | — | 0.70 | 234 | 3.3 | 0.01 | — | — | — | — | — | [753] |
| Cu2MnSnS4 (p-type) nanocrystals | 700 | 4.1 | — | 0.53 | 265 | 15 | 0.14 | — | — | — | — | — | [753] |
| Cu2MnSnSe4 (p-type) | 300 | 12.0 | — | 2.60 | 189 | 30 | 0.01 | — | — | 1.2 | — | 0.3 × 1020 | [755] |
| Cu2MnSnSe4 (p-type) | 850 | 10.3 | 0.49 | 0.57 | 268 | 49 | 0.53 | — | — | — | — | — | [755] |
| Cu2.1(Fe0.5Mn0.5)0.9SnSe4 (p-type) | 300 | 23.5 | — | 2.40 | 150 | 93 | 0.01 | — | — | 1.5 | — | 0.6 × 1020 | [757] |
| Cu2.1(Fe0.5Mn0.5)0.9SnSe4 (p-type) | 800 | 16.4 | — | 0.84 | 204 | 150 | 0.6 | — | — | — | — | — | [757] |
| Cu2.1Mn0.9SnSe4 (p-type) | 300 | 20.6 | 2.55 | 2.71 | 67 | 249 | 0.01 | — | — | 1.44 | — | 2.89 × 1020 | [758] |
| Cu2.1Mn0.9SnSe4 (p-type) | 800 | 18.2 | 0.67 | 0.91 | 186 | 205 | 0.6 | — | — | — | — | — | [758] |
| Cu2ZnGeS4 (p-type) | 300 | — | 3.80 | 3.80 | 187 | — | — | 2.0 a | — | — | — | — | [759] |
| Cu2ZnGeS4 (p-type) | 670 | 2.6 | 1.00 | 409 | 1.7 | — | — | — | — | — | — | [759] | |
| Cu2ZnGeSe4 (p-type) | 300 | 2.0 | 3.20 | 3.20 | 320 | 1.1 | — | 1.4 a | — | — | — | — | [759] |
| Cu2ZnGeSe4 (p-type) | 670 | 7.0 | 0.83 | 325 | 12 | — | — | — | — | — | — | [759] | |
| Cu2.075Zn0.925GeSe4 (p-type) | 300 | 5.5 | 2.45 | 2.50 | 83 | 52 | 0.005 | — | — | — | — | 1.0 × 1020 | [760] |
| Cu2.075Zn0.925GeSe4 (p-type) | 670 | 19.3 | 0.66 | 0.86 | 195 | 150 | 0.45 | — | — | — | — | — | [760] |
| Cu1.8Zn1.05Sn0.95S4 (p-type) single crystal | 300 | 43.6 | — | 2.90 | 420 | 7.5 | 0.04 | — | 80.8 e | 5.8 × 1017 | [761] | ||
| Cu1.8Zn1.05Sn0.95S4 (p-type) single crystal | 400 | 563.1 | — | 2.40 | 506 | 55 | 0.2 | — | — | — | — | — | [761] |
| Cu2.125Zn0.875SnS4 (p-type) Hot forged | 300 | 5.5 | 1.16 | 1.20 | 40 | 115 | 0.005 | — | — | — | — | — | [762] |
| Cu2.125Zn0.875SnS4 (p-type) Hot forged | 725 | 14.3 | 0.07 | 0.33 | 155 | 200 | 1.1 | — | — | — | — | — | [762] |
| Cu2ZnSnSe4 (p-type) single crystal | 300 | 117.5 | — | 3.90 | 420 | 20.2 | 0.06 | — | 141.2 e | — | — | 8.8 × 1017 | [763] |
| Cu2ZnSnSe4 (p-type) single crystal | 400 | 738.8 | — | 2.80 | 505 | 73 | 0.32 | — | — | — | — | — | [763] |
| Cu2.1Zn0.9SnSe4 (p-type) coated | 300 | 57.6 | — | 3.66 | 55 | 860 | 0.02 | 1.4 a | — | — | — | — | [764] |
| Cu2.1Zn0.9SnSe4 (p-type) coated | 860 | 31.9 | — | 1.28 | 206 | 318 | 0.91 | — | — | — | — | — | [764] |
| Cu2ZnSn0.90In0.10Se4 (p-type) coated | 300 | 34.5 | 3.2 | 3.3 | 93 | 282 | 0.015 | — | — | — | — | — | [765] |
| Cu2ZnSn0.90In0.10Se4 (p-type) coated | 850 | 30.4 | — | 0.82 | 300 | 100 | 0.95 | — | — | — | — | — | [765] |
| Colusites | |||||||||||||
| Cu26V2Ge6S32 (p-type) | 350 | 41.0 | 0.41 | 0.63 | 125 | 277 | 0.26 | — | — | — | — | — | [766] |
| Cu26V2Ge6S32 (p-type) | 663 | 23.0 | 0.5 | 0.57 | 215 | 140 | 0.73 | — | — | — | — | — | [766] |
| Cu24Ni2V2Ge6S32 (p-type) | 300 | 38.0 | 0.88 | 1.2 | 79 | 380 | 0.06 | — | — | — | — | — | [767] |
| Cu24Ni2V2Ge6S32 (p-type) | 690 | 19.2 | 0.36 | 0.8 | 145 | 281 | 0.5 | — | — | — | — | — | [767] |
| Cu24 Co2V2Ge6S32 (p-type) | 300 | 19.2 | 0.68 | 0.7 | 179 | 54 | 0.07 | — | — | — | — | — | [767] |
| Cu24 Co2V2Ge6S32 (p-type) | 690 | 13.3 | 0.45 | 0.5 | 265 | 48 | 0.4 | — | — | — | — | — | [767] |
| Cu26Nb2Ge6S32 (p-type) | 310 | 47.0 | 0.53 | 0.79 | 125 | 265 | 0.16 | — | — | — | — | 1.2 × 1021 | [768] |
| Cu26Nb2Ge6S32 (p-type) | 673 | 18.1 | 0.34 | 0.58 | 202 | 131 | 0.65 | — | — | — | — | — | [768] |
| Cu26Nb2Ge5.5S32 (p-type) | 300 | 48.4 | 0.4 | 0.53 | 132 | 238 | 0.24 | — | — | — | — | — | [769] |
| Cu26Nb2Ge5.5S32 (p-type) | 670 | 20.1 | 0.41 | 0.53 | 220 | 117 | 0.73 | — | — | — | — | — | [769] |
| Cu26.5Nb2Ge5.5S32 (p-type) | 330 | 70.1 | 0.51 | 0.72 | 125 | 434 | 0.29 | — | — | 9.6 | 2.1 × 1021 | [770] | |
| Cu26.5Nb2Ge5.5S32 (p-type) | 670 | 28.4 | 0.45 | 0.67 | 199 | 211 | 0.84 | — | — | — | — | — | [770] |
| Cu26Nb2Ge6 Co0.5S32 (p-type) | 310 | 51.0 | 0.56 | 0.82 | 135 | 254 | 0.22 | — | — | — | — | 1.8 × 1021 | [768] |
| Cu26Nb2Ge6 Co0.5S32 (p-type) | 673 | 20.7 | 0.41 | 0.55 | 210 | 136 | 0.71 | — | — | — | — | — | [768] |
| Cu26Nb2Ge6Ni0.5S32 (p-type) | 310 | 47.8 | 0.68 | 0.83 | 120 | 287 | 0.15 | — | — | — | — | 1.2 × 1021 | [768] |
| Cu26Nb2Ge6Ni0.5S32 (p-type) | 673 | 20.1 | 0.45 | 0.60 | 194 | 159 | 0.66 | — | — | — | — | — | [768] |
| Cu26Nb2Ge6Fe0.5S32 (p-type) | 310 | 33.2 | 0.77 | 0.82 | 166 | 114 | 0.12 | — | — | — | — | 9.0 × 1020 | [768] |
| Cu26Nb2Ge6Fe0.5S32 (p-type) | 673 | 16.5 | 0.44 | 0.55 | 212 | 106 | 0.57 | — | — | — | — | — | [768] |
| Cu26Ta2Ge6S32 (p-type) | 300 | 38.4 | 0.56 | 0.65 | 143 | 165 | 0.15 | — | — | — | — | — | [769] |
| Cu26Ta2Ge6S32 (p-type) | 670 | 18.1 | 0.4 | 0.49 | 229 | 95 | 0.66 | — | — | — | — | — | [769] |
| Cu26Ta2Ge5.5S32 (p-type) | 300 | 45.3 | 0.46 | 0.57 | 136 | 212 | 0.22 | — | — | — | — | — | [769] |
| Cu26Ta2Ge5.5S32 (p-type) | 670 | 20.0 | 0.36 | 0.48 | 222 | 114 | 0.79 | — | — | — | — | — | [769] |
| Cu26V2Sn6S32 (p-type) | 300 | 49.9 | 0.6 | 0.76 | 116 | 300 | 0.16 | — | — | 3.2 | — | 4.2 × 1020 | [771] |
| Cu26V2Sn6S32 (p-type) | 670 | 20.2 | 0.47 | 0.64 | 194 | 159 | 0.64 | — | — | — | — | — | [771] |
| Cu26V2Sn6S32 (SPS 873) (p-type) | 300 | 55.5 | 1.4 | 2.08 | 45 | 1020 | 0.03 | — | — | — | — | — | [693] |
| Cu26V2Sn6S32 (SPS 873) (p-type) | 675 | 27.3 | 0.58 | 1.45 | 102 | 667 | 0.33 | — | — | — | — | — | [693] |
| Cu26V2Sn6S32 (HP 1023) (p-type) | 300 | 63.1 | 0.35 | 0.66 | 91 | 531 | 0.19 | — | — | — | — | — | [693] |
| Cu26V2Sn6S32 (HP 1023) (p-type) | 675 | 25.2 | 0.21 | 0.57 | 162 | 292 | 0.93 | — | — | — | — | — | [693] |
| Cu26V2Sn5.5S32 (p-type) | 300 | 30.1 | — | 0.66 | 130 | 152 | 0.18 | — | — | — | — | — | [772] |
| Cu26V2Sn5.5S32 (p-type) | 673 | 20.9 | — | 0.60 | 205 | 146 | 0.63 | — | — | — | — | — | [772] |
| Cu24Zn2V2Sn6S32 (p-type) | 300 | 58.2 | 1.53 | 1.96 | 66 | 714 | 0.05 | — | — | — | — | — | [773] |
| Cu24Zn2V2Sn6S32 (p-type) | 700 | 20.6 | 0.71 | 1.1 | 143 | 316 | 0.44 | — | — | — | — | — | [773] |
| Cu26Nb2Sn6S32 (p-type) | 300 | 44.4 | 0.51 | 0.65 | 116 | 267 | 0.18 | — | — | 3.6 | — | 4.7 × 1020 | [771] |
| Cu26Nb2Sn6S32 (p-type) | 670 | 19.3 | 0.43 | 0.57 | 203 | 137 | 0.66 | — | — | — | — | — | [771] |
| Cu26Nb2Sn5.5S32 (p-type) | 300 | 59.1 | 0.55 | 0.76 | 117 | 351 | 0.2 | — | — | — | — | — | [769] |
| Cu26Nb2Sn5.5S32 (p-type) | 670 | 25.0 | 0.44 | 0.62 | 194 | 197 | 0.76 | — | — | — | — | — | [769] |
| Cu26Ta2Sn6S32 (p-type) | 300 | 63.5 | 0.47 | 0.69 | 116 | 382 | 0.23 | — | — | 4.3 | — | 6.6 × 1020 | [771] |
| Cu26Ta2Sn6S32 (p-type) | 670 | 23.3 | 0.4 | 0.59 | 198 | 175 | 0.78 | — | — | — | — | — | [771] |
| Cu26Ta2Sn5.5S32 (p-type) | 300 | 68.4 | 0.41 | 0.64 | 115 | 417 | 0.27 | — | — | — | — | — | [769] |
| Cu26Ta2Sn5.5S32 (p-type) | 670 | 27.3 | 0.35 | 0.55 | 206 | 187 | 0.96 | — | — | — | — | — | [769] |
| Cu26Cr2Ge6S32 (p-type) | 300 | 231.9 | 1.59 | 2.93 | 83 | 2188 | 0.15 | — | — | — | — | — | [774] |
| Cu26Cr2Ge6S32 (p-type) | 700 | 62.1 | 0.49 | 1.56 | 150 | 875 | 0.86 | — | — | — | — | — | [774] |
| Cu26Cr2Ge6S32 (p-type) | 300 | 232.8 | 1.52 | 2.84 | 84 | 2164 | 0.16 | — | 2.93 f | — | — | — | [775] |
| Cu26Cr2Ge6S32 (p-type) | 700 | 60.3 | 0.46 | 1.52 | 149 | 860 | 0.89 | — | — | — | — | — | [775] |
| [Cu26Cr2Ge6]1.024S32 (p-type) | 300 | 66.6 | 1.35 | 1.58 | 114 | 411 | 0.1 | 0.66 f | — | — | [700] | ||
| [Cu26Cr2Ge6]1.024S32 (p-type) | 700 | 50.3 | 0.52 | 1.14 | 178 | 510 | 1 | — | — | — | — | — | [700] |
| Cu25ZnCr2Ge6S32 (p-type) | 300 | 128.9 | 1.79 | 2.26 | 110 | 838 | 0.12 | — | — | — | — | — | [774] |
| Cu25ZnCr2Ge6S32 (p-type) | 700 | 35.8 | 0.75 | 1.20 | 176 | 371 | 0.67 | — | — | — | — | — | [774] |
| Cu26CrMoGe6S32 (p-type) | 300 | 93.0 | 1.44 | 1.94 | 87 | 828 | 0.1 | 1.39 f | — | — | — | [775] | |
| Cu26CrMoGe6S32 (p-type) | 700 | 46.0 | 0.49 | 1.24 | 154 | 619 | 0.83 | — | — | — | — | — | [775] |
| Cu26CrWGe6S32 (p-type) | 300 | 112.3 | 1.09 | 1.74 | 81 | 1091 | 0.13 | — | 1.56 f | — | — | — | [775] |
| Cu26CrWGe6S32 (p-type) | 700 | 47.9 | 0.37 | 1.20 | 151 | 668 | 0.9 | — | — | — | — | — | [775] |
| Low Dimensional Materials | |||||||||||||
| TiS2 (n-type) | 300 | 167.6 | 4.22 | 4.5 | −277 | 151 | 0.08 | — | — | — | — | 0.11 × 1021 | [776] |
| TiS2 (n-type) | 700 | 42.7 | 2.3 | 2.43 | −390 | 37 | 0.16 | — | — | — | — | — | [776] |
| Ti1.025S2 (n-type) | 300 | 144.8 | 1.54 | 2.3 | −103 | 1033 | 0.14 | — | — | — | — | 1.11 × 1021 | [776] |
| Ti1.025S2 (n-type) | 700 | 33.8 | 1.08 | 1.58 | −192 | 291 | 0.48 | — | — | — | — | — | [776] |
| Cu0.10TiS2 (n-type) | 300 | 227.1 | 1.68 | 3.89 | −50 | 3745 | 0.05 | — | — | — | — | 3.67 × 1021 | [685] |
| Cu0.10TiS2 (n-type) | 800 | 27.6 | 0.77 | 1.8 | −142 | 524 | 0.47 | — | — | — | — | — | [685] |
| Ag0.1TiS2 (n-type) | 300 | 111.8 | 1.63 | 3.5 | −62 | 1469 | 0.05 | — | — | — | — | — | [686] |
| Ag0.1TiS2 (n-type) | 700 | 35.1 | 0.85 | 1.72 | −153 | 478 | 0.46 | — | — | — | — | — | [686] |
| Co0.04TiS2 (n-type) | 321 | 81.4 | — | 2.76 | −112 | 571 | 0.08 | — | — | — | — | — | [687] |
| Co0.04TiS2 (n-type) | 573 | 47.7 | — | 1.98 | −200 | 277 | 0.3 | — | — | — | — | — | [687] |
| TiS1.5Se0.5 (n-type) | 300 | 150.2 | 2.2 | 2.6 | −141 | 662 | 0.15 | — | — | — | — | 3.5 × 1021 | [699] |
| TiS1.5Se0.5 (n-type) | 700 | 29.8 | 1.58 | 1.48 | −232 | 161 | 0.33 | — | — | — | — | — | [699] |
| Cu0.05TiS1.5Se0.5 (n-type) | 300 | 179.5 | 1.54 | 2.92 | −77 | 1851 | 0.1 | — | — | — | — | 7 × 1021 | [699] |
| Cu0.05TiS1.5Se0.5 (n-type) | 700 | 39.8 | 0.92 | 1.61 | −167 | 459 | 0.55 | — | — | — | — | — | [699] |
| Co3Sn2S2 (n-type) | 310 | 215.6 | 1.9 | 4.56 | −52 | 3584 | 0.07 | — | — | — | — | — | [777] |
| Co3Sn2S2 (n-type) | 677 | 80.7 | 0.59 | 4.74 | −85 | 2506 | 0.26 | — | — | — | — | — | [777] |
| Co3Sn1.6In0.4S2 (n-type) | 310 | 201.2 | 1.7 | 3.15 | −79 | 2115 | 0.13 | — | — | — | — | — | [777] |
| Co3Sn1.6In0.4S2 (n-type) | 677 | 65.9 | 1.06 | 3.62 | −104 | 1574 | 0.32 | — | — | — | — | — | [777] |
| Co2.6Fe0.4Sn2S2 (n-type) | 300 | 178.0 | 2.38 | 3.97 | −70 | 2046 | 0.08 | — | — | — | — | — | [778] |
| Co2.6Fe0.4Sn2S2 (n-type) | 575 | 81.6 | 1.39 | 4.08 | −88 | 1901 | 0.2 | — | — | — | — | — | [778] |
| Co2.667Fe0.333Sn1.6In0.4S2 (n-type) | 300 | 219.8 | 2.12 | 3.47 | −102 | 1590 | 0.14 | — | — | — | — | — | [779] |
| Co2.667Fe0.333Sn1.6In0.4S2 (n-type) | 575 | 88.1 | 1.12 | 3.45 | −103 | 1669 | 0.29 | — | — | — | — | — | [779] |
| MnBi4S7 (n-type) | 300 | 21.0 | 0.89 | 1 | −87 | 187 | 0.04 | — | — | — | — | — | [688] |
| MnBi4S7 (n-type) | 700 | 6.6 | 0.61 | 0.67 | −200 | 52 | 0.21 | — | — | — | — | — | [688] |
| FeBi4S7 (n-type) | 300 | 17.0 | 0.74 | 0.82 | −93 | 139 | 0.05 | — | — | — | — | — | [688] |
| FeBi4S7 (n-type) | 700 | 6.1 | 0.75 | 0.81 | −191 | 53 | 0.17 | — | — | — | — | — | [688] |
| CdPb2Bi4S9 (n-type) | 300 | 5.7 | 0.73 | 0.78 | −59 | 79 | 0.02 | — | — | — | — | 1.6 × 1018 | [689] |
| CdPb2Bi4S9 (n-type) | 775 | 7.5 | 0.49 | 0.52 | −253 | 37 | 0.53 | — | — | — | — | — | [689] |
| CdAg2Bi6Se11 (n-type) | 300 | 18.7 | 0.36 | 0.54 | −57 | 269 | 0.05 | — | — | — | — | 1.8 × 1019 | [689] |
| CdAg2Bi6Se11 (n-type) | 775 | 14.0 | 0.13 | 0.43 | −149 | 232 | 0.95 | — | — | — | — | — | [689] |
| Pb5Bi6Se14 (n-type) | 300 | 17.9 | 0.48 | 0.58 | −88 | 157 | 0.06 | — | — | — | — | 4.8 × 1019 | [780] |
| Pb5Bi6Se14 (n-type) | 705 | 9.7 | 0.34 | 0.46 | −211 | 68 | 0.46 | — | — | — | — | — | [780] |
| Pb3Bi2S6 (n-type) | 300 | 13.7 | 0.79 | 0.94 | −58 | 193 | 0.02 | — | — | — | — | 1.2 × 1020 | [780] |
| Pb3Bi2S6 (n-type) | 715 | 7.5 | 0.56 | 0.68 | −197 | 63 | 0.26 | — | — | — | — | — | [780] |
| PbBi2S4 (n-type) | 300 | 19.2 | 0.63 | 0.64 | −136 | 90 | 0.08 | — | — | — | — | 4.6 × 1019 | [780] |
| PbBi2S4 (n-type) | 710 | 10.3 | 0.53 | 0.57 | −269 | 37 | 0.33 | — | — | — | — | [780] | |
| CdPbBi4Se8 (n-type) | 325 | 7.3 | 0.43 | 0.45 | −125 | 44 | 0.05 | — | 15.2 e | — | — | 3.21 × 1019 | [781] |
| CdPbBi4Se8 (n-type) | 850 | 6.1 | 0.27 | 0.32 | −254 | 34 | 0.63 | — | — | — | — | — | [781] |
| CdSnBi4Se8 (n-type) | 325 | 19.9 | 0.61 | 0.72 | −109 | 148 | 0.08 | — | 32.9 e | 2.85 × 1019 | [781] | ||
| CdSnBi4Se8 (n-type) | 850 | 7.1 | 0.44 | 0.64 | −151 | 132 | 0.4 | — | — | — | — | — | [781] |
| Cu1.62Bi4.61S8 (n-type) | 300 | 10.6 | 0.57 | 0.58 | −222 | 18 | 0.05 | — | — | — | — | — | [782] |
| Cu1.62Bi4.61S8 (n-type) | 660 | 7.4 | 0.49 | 0.55 | −231 | 37 | 0.21 | — | — | — | — | — | [782] |
| Cu1.595Zn0.025Bi4.61S8 (n-type) | 300 | 13.9 | 0.46 | 0.58 | −66 | 170 | 0.04 | — | — | — | — | — | [782] |
| Cu1.595Zn0.025Bi4.61S8 (n-type) | 660 | 7.7 | 0.35 | 0.54 | −137 | 116 | 0.26 | — | — | — | — | — | [782] |
a Band gap determined by optical spectroscopy. b Band gap determined by x-ray photoelectron spectroscopy. c Measured at room temperature. d Bandgap measured from Seebeck coefficient (S) using Eg = 2SmaxeT. e Mobility data based on Hall measurements. f Technique of measuring mobility not defined explicitly.
As noted above, stability problems, due to cation migration, are a concern for PLEC-type phases such as Cu2Se and Ag2Se. In a thermoelectric device, electromigration of the mobile cations occurs when an electrical current flows through the PLEC material. This results in a compositional gradient along the thermoelectric leg, together with cracking and loss of performance [697]. Possible approaches to minimize degradation due to ionic diffusion, which need to be fully investigated, include the introduction of additional cations to block the migration path of the mobile cations, or tuning the geometry of the thermoelectric legs to ensure that the voltage applied to the PLEC material remains below a critical threshold [698].
In the case of sulfides or selenides, volatilization of the chalcogen at the elevated temperatures at which a device operates, may result in materials degradation and hence deterioration of the thermoelectric performance. Development of protective coatings may be required to address this issue. Device construction will also require the identification of suitable diffusion-barrier materials and solders, for these new families of materials. Matching the coefficients of thermal expansion of the different device components will be also essential, to avoid the possible fracture of the thermoelectric device during operation. To achieve progress in the implementation of thermoelectric technology, the research efforts that have led to the discovery of new sulfides and selenides should be followed by work that addresses the device level challenges.
Acknowledgments
We thank the UK Engineering and Physical Sciences Research Council (EP/T020040) and the Leverhulme Trust (RPG-2019-288) for financial support.
3.11. Silicides
Silicide thermoelectrics
Franck Gascoin1 and Theodora Kyratsi2
1 Laboratoire CRISMAT UMR 6508 CNRS ENSICAEN, 14050 Caen Cedex 04, France
2 Department of Mechanical and Manufacturing Engineering, University of Cyprus, Nicosia 2109, Cyprus
Since the 1960s, a multitude of silicides based thermoelectric materials have been studied for their potential or promising thermoelectric properties. Indeed, their combination with virtually any other metal provides an ideal playground for thermoelectricians [783]. The alloys Si–Ge certainly stand out from the crowd as they have been used in the fabrication of radio-isotope thermoelectric generators and thus utilized by NASA for powering a large number of space missions. Their stability over time is undeniable and that makes up for their high cost and rather poor efficiency. If Si-Ge could be seen as a model compound for high temperature applications, other silicides are now scrutinized against them.
Amongst the different class of silicides, present and probable future investigations focus on the cheap and non-toxic Mg2Si and MnSix based materials. Evidently, cost and environmentally friendly materials are today crucial parameters as they largely compete with efficiency. Moreover, silicides are often low-density materials, another key aspect of their potential for industrial applications. Therefore, combining the n-type Mg2Si and p-type MnSix into a thermoelectric module is indeed very appealing and would represent a major achievement. All these positive arguments have resulted in a myriad of investigations that focused first on the improvement of the thermoelectric figure of merit of these two materials.
Magnesium silicide Mg2Si and the related solid solutions Mg2(Six Ge1−x ) and Mg2(Six Sn1−x ) were first identified as promising by Nicolau during the first international congress on thermoelectric energy conversion in 1976 [784] and these predictions were quickly confirmed by the experimental results of different groups in the early 1990s [785–787]. Since then, efforts have been devoted to optimize materials in terms of synthesis, performance, and stability over time. The best results are obtained using multiple substitutions on both the magnesium and the silicon sites. These have led to materials with zT often exceeding 1.3 at 700 K with a record high zT of 1.7 for Mg1.98Cr0.02(Si0.3Sn0.7)0.98Bi0.02 at 680 K [788], although the stability, at the operating temperature, of the silico-stannides still being under investigation.
Higher manganese silicides (HMS) have been considered as promising p-type thermoelectric materials because they are ecologically benign but also because they possess high mechanical strength and they are stable in air up to 1023 K. HMS exist as several incommensurate phases with chemical formulae of Mn4Si7, Mn11Si19, Mn15Si26, and Mn27Si47, all crystallizing in the Nowotny chimney-ladder structures. These structures are constructed by the two Mn and Si sublattices, where the Mn atoms form the chimneys in which the Si atoms spiral as ladders. The tetragonal unit cells of different HMS compounds have similar a parameter, and different c parameter, depending on the cSi/cMn ratio of the two sublattices.
Despite all the efforts undertaken to improve the thermoelectric efficiency, most of the HMS have relatively mediocre zT, typically between 0.4 and 0.5 at the most, which is detrimental to a module hypothetically made of an n-type Mg2Si counter leg. Only recently, via addition of rhenium or introduction of high density dislocation, zTs reaching the unity value at 825 K have been found [789, 790].
Only a few investigation and projects have tried to tackle the design and construction of an all silicide thermoelectric module. Not surprisingly, the major issue is the difference between the coefficients of thermal expansion of HMS and Mg2Si, which often leads to violent breakage of the module upon cycling over the operating temperature range. This inescapable problem might necessitate the use of buffer layers or other engineering tactics that remain to be proven efficient but also triggered efforts toward discovering an efficient p-type Mg2Si that could therefore advantageously replace HMS [791, 792]
Table 11. Silicides thermoelectric properties.
| Material | T (K) | µw (cm2 V−1 s−1) | κL (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | k (W m−1 K−1) | References |
|---|---|---|---|---|---|---|---|---|
| Magnesium silicides | ||||||||
| n-type | ||||||||
| Mg1.98Cr0.02(Si0.3Sn0.7)0.98Bi0.02 | 300 | 421.0 | 1.55 | −129 | 2149 | 0.4 | 2.7 | [788] |
| 680 | 213.1 | 1.08 | −230 | 1130 | 1.7 | 2.3 | ||
| Mg2.08Si0.4−x Sn0.6Bix, x = 0.030 | 300 | 452.2 | — | −130 | 2280 | 0.44 | 2.7 | [822] |
| 773 | 121.7 | — | −205 | 1045 | 1.55 | 2.2 | ||
| Mg2.08Si0.364Sn0.6Sb0.036 | 300 | 455.4 | 1.38 | −121 | 2570 | 0.45 | 2.7 | [789] |
| 716 | 149.0 | 1.03 | −207 | 1115 | 1.5 | 2.04 | ||
| Mg2Si0.3Sn0.67Bi0.03/3.0 wt % SiC | 323 | 370.3 | 1.97 | −133 | 2010 | — | 3.14 | [823] |
| 773 | 130.9 | 1.3 | −230 | 841 | 1.45 | 2.4 | ||
| Mg2(Si0.3GeySn0.7−y )0.98Sb0.02, y = 0.05 | 300 | 383.9 | 2 | −125 | 2060 | 0.3 | 3.16 | [824] |
| 800 | 118.5 | 1.25 | −220 | 900 | 1.45 | 2.4 | ||
| Mg2Si0.55−x Sn0.4Ge0.05Sbx, x = 0.0125 | 300 | 221.7 | 2.5 | −107 | 1500 | 0.15 | 3.4 | [825] |
| 800 | 87.5 | 1.3 | −220 | 665 | 1.2 | 2.15 | ||
| Mg2Si0.55−x Sn0.4Ge0.05Bix, x = 0.02 | 300 | 212.4 | 1.6 | −94.5 | 1702 | 0.15 | 2.96 | [821] |
| 800 | 93.1 | 1 | −216 | 741 | 1.4 | 2 | ||
| Mg2.16(Si0.4Sn0.6)0.97Bi0.03 | 300 | 361.5 | 1.64 | −121 | 2040 | 0.3 | 2.78 | [826] |
| 800 | 111.8 | 1 | −215 | 900 | 1.4 | 2.3 | ||
| Mg2(Si0.4Sn0.6)Sbx, x = 0.18 | 300 | — | — | — | — | — | — | [820] |
| 673 | 135.3 | 0.66 | −232 | 690 | 1.4 | 1.85 | ||
| Mg2Si0.3Sn0.665Bi0.035 | 300 | — | — | — | — | — | — | [827] |
| 323 | 349.6 | 1.75 | −115 | 2380 | 0.35 | 2.9 | ||
| 773 | 118.8 | 1 | −205 | 1020 | 1.4 | 2.4 | ||
| Mg2Si0.5875Sn0.4Sb0.0125 | 300 | 328.2 | — | −115 | 2000 | 0.3 | 2.8 | [828] |
| 883 | 91.0 | — | −210 | 900 | 1.4 | 2.2 | ||
| Mg2Si0.35Sn0.65−x Bix, x = 0.03 | 300 | 426.9 | 1.95 | −120 | 2440 | 0.3 | 3.3 | [819] |
| 780 | 116.9 | 1.3 | −210 | 960 | 1.3 | 2.5 | ||
| Mg2.16(Si0.4Sn0.6)0.985Sb0.015 | 300 | 317.3 | 1.7 | −130 | 1600 | 0.3 | 2.54 | [829] |
| 740 | 107.3 | 1.1 | −220 | 725 | 1.3 | 2 | ||
| Mg2.16(Si0.3Sn0.7)0.98Sb0.02 | 300 | 482.5 | 2.2 | −115 | 2940 | 0.3 | 3.85 | [830] |
| — | — | 1.4 | −205 | 1180 | 1.3 | 2.8 | ||
| Mg2.15(Si0.3Sn0.7)0.99Sb0.01 | 300 | 483.7 | 1.45 | −158 | 1740 | 0.45 | 2.75 | [831] |
| 700 | 156.9 | 0.95 | −235 | 820 | 1.3 | 2.3 | ||
| Mg2(Si0.3Sn0.7)1−y Biy, y = 0.15 | 300 | 412.5 | 1.75 | −130 | 2080 | 0.35 | 2.9 | [832] |
| 700 | 136.0 | 1.3 | −210 | 950 | 1.3 | 2.45 | ||
| (Mg2.06Si0.3Sn0.68Bi0.02 | 300 | — | — | — | — | 0.22 | 3.6 | [818] |
| 773 | — | — | — | — | 1.3 | 2.5 | ||
| Mg2Si0.6−x Sn0.4Bix, x = 0.03 | 300 | 218.5 | — | −93.3 | 1780 | 0.16 | 3.21 | [816] |
| 850 | 88.1 | 1.5 | −213 | 795 | 1.2 | 2.51 | ||
| Mg2Si0.55−x Sn0.4Ge0.05Sbx, x = 0.0125 | 300 | 221.7 | 2.5 | −107 | 1500 | 0.15 | 3.4 | [833] |
| 800 | 87.5 | 1.3 | −220 | 665 | 1.2 | 2.15 | ||
| Mg2.16(Si0.3Sn0.7)0.98Sb0.02 | 300 | 437.9 | — | −125 | 2350 | 0.31 | — | [834] |
| 800 | 114.3 | — | −215 | 920 | 1.3 | — | ||
| Mg2(Si0.4Sn0.6)Bix, x = 0.03 | 373 | 618.3 | 0.85 | −205 | 1780 | 0.6 | 1.28 | [815] |
| 573 | 0.95 | −275 | 1.2 | 1.33 | ||||
| Mg2.16(Si0.30Sn0.70)0.98Sb0.02 | 300 | 370.9 | 2.2 | −130 | 1870 | 0.3 | 3.4 | [835] |
| 750 | 118.9 | 1.5 | −220 | 820 | 1.2 | 2.5 | ||
| Mg2.2Si0.49Sn0.5Sb0.01 | 300 | 253.7 | 1.9 | −120 | 1450 | 0.25 | 2.7 | [817] |
| 873 | 73.9 | 1.2 | −230 | 570 | 1.25 | 2.1 | ||
| Mg2.08Si0.8Sn0.2/3%Bi | 300 | 133.3 | 2.45 | −145 | 560 | 0.06 | 2.8 | [836] |
| 850 | 91.7 | 0.75 | −260 | 480 | 1.17 | 1.8 | ||
| Mg2Si0.4−x Sn0.6Sbx, x = 0.0075 | 300 | — | — | — | — | 0.3 | 2.85 | [814] |
| 773 | — | — | — | — | 1.1 | 2.35 | ||
| Mg2Si0.4Sn0.6−x Sbx, x = 0.1, 2 at% Zn | 300 | — | — | — | — | 0.2 | — | [837] |
| 750 | — | — | — | — | 1.1 | — | ||
| Mg2Si0.4Sn0.6−y Gey, y = 0.1 | 300 | 249.0 | 2 | −100 | 1850 | 0.17 | 3.15 | [838] |
| 800 | 96.1 | 1.2 | −210 | 820 | 1.1 | 2.6 | ||
| Mg2Si0.6−y Sn0.4Sby, y = 0.0125 | 300 | 217.3 | 1.65 | −95 | 1729 | 0.15 | 2.9 | [839] |
| 860 | 80.1 | 1 | −225 | 640 | 1.11 | 2.4 | ||
| Mg2.2Si0.392As0.008Sn0.5925Sb0.0075/2% TiO2 nanoparticles | 300 | 303.8 | — | −125 | 1630 | — | 3.2 | [839] |
| 823 | 76.6 | — | −235 | 510 | 1.1 | 3.1 | ||
| Mg2.2Si0.392As0.008Sn0.5925Sb0.0075/5% TiO2 nanoparticles | 300 | 293.5 | — | −130 | 1480 | — | 3 | [840] |
| 823 | 74.8 | −240 | 470 | 1.1 | 3 | |||
| Mg2Si0.4−x Sn0.6Sbx, x = 0.015 | 300 | 275.5 | 1.5 | −150 | 1090 | 0.3 | 2.8 | [813] |
| 773 | 102.0 | 1.4 | −250 | 520 | 1.1 | 2.35 | ||
| Mg2(1+x)Si0.45Sn0.537Sb0.013, x = 0.08 | 300 | 297.5 | 2 | −130 | 1500 | 0.3 | 2.9 | [841] |
| 725 | 96.8 | 1.55 | −200 | 800 | 1 | 2.5 | ||
| Mg2Si0.6Ge0.4Bi0.02 | 300 | 109.1 | — | −85 | 1000 | 0.05 | 3 | [812] |
| 800 | 41.8 | — | −190 | 450 | 1 | 1.8 | ||
| Mg2(Si0.3Sn0.7)0.975Sb0.025 | 300 | 380.3 | 2.1 | −175 | 1120 | 0.35 | 2.9 | [811] |
| 640 | 156.5 | 1.4 | −260 | 535 | 1 | 2.25 | ||
| Mg1.96Al0.04Si0.97Bi0.03 | 300 | 243.0 | 3.1 | −89.91 | 2076 | 0.15 | 4.75 | [842] |
| 873 | — | 1 | — | 900 | 1 | 3 | ||
| Mg2.2Si0.5925Sn0.4Sb0.0075 | 300 | 179.7 | — | −95 | 1430 | 0.12 | 3 | [843] |
| 673 | 93.3 | — | −200 | 690 | 1 | 1.95 | ||
| Mg2Si0.785Sn0.2Sb0.015 | 340 | 123.9 | 2 | −85 | 1370 | 0.2 | 3.25 | [844] |
| 740 | 74.1 | 1.2 | −190 | 710 | 0.95 | 2 | ||
| Mg2Si0.5−x Sn0.5Sbx, x = 0.013 | 300 | 188.6 | 2 | −105.6 | 1300 | 0.17 | 2.8 | [810] |
| 740 | 86.8 | 1.35 | −200 | 740 | 0.9 | 2.3 | ||
| Mg2Si:Bi = 1−x, x = 2 at % | 300 | 156.8 | 5.5 | −100 | 1165 | 0.05 | 6.3 | [845] |
| 850 | 89.9 | 2.4 | −245 | 560 | 0.86 | 3.5 | ||
| Mg2.10Si0.38Sn0.6Sb0.02 | 300 | 218.4 | 2.2 | −103.9 | 1540 | 0.2 | 3.12 | [809] |
| 700 | 87.3 | — | −215 | 575 | 0.85 | 2.3 | ||
| Mg2−x Lax Si0.58Sn0.42, x = 0.005 | 300 | 82.0 | — | −105 | 570 | 0.05 | 3.1 | [808] |
| 810 | 48.8 | — | −205 | 450 | 0.8 | 2.3 | ||
| Mg2Si + 2 at% Bi | 300 | 229.2 | — | −85 | 2100 | 0.05 | 6.2 | [846] |
| 840 | 74.7 | −200 | 770 | 0.74 | 3.8 | |||
| Mg2Si + 2 at% Bi | 300 | 15.8 | 4.8 | −105 | 110 | 0.05 | 4.9 | [847] |
| 823 | 57.0 | 1.8 | −200 | 570 | 0.7 | 2.9 | ||
| Mg2Si + 0.15 at% Bi | 300 | 168.2 | 6 | −100 | 1250 | 0.05 | 6.8 | [848] |
| 775 | 81.4 | 2.4 | −230 | 525 | 0.7 | 3 | ||
| Mg2Si0.5875Sn0.4Sb0.0125 | 300 | 84.4 | — | −135 | 400 | 0.1 | 2.5 | [807] |
| 775 | 55.0 | — | −235 | 335 | 0.7 | 2 | ||
| Mg2Si0.970Bi0.030 | 300 | 222.9 | 5.1 | −89 | 1929 | 0.07 | 6.61 | [806] |
| 810 | 73.1 | 1.8 | −187 | 830 | 0.68 | 3.5 | ||
| Mg2Si0.6–y Sby Sn0.4, y = 0.005 | 300 | 70.7 | 2.35 | −110 | 460 | 0.07 | 2.7 | [849] |
| 724 | 49.7 | 1.3 | −220 | 325 | 0.68 | 1.8 | ||
| Mg2Si + 2 at% Al | 300 | 122.4 | — | −180 | 340 | 0.05 | 9.2 | [850] |
| 970 | — | — | — | −225 | 460 | 0.66 | ||
| p-type | — | — | — | — | — | — | ||
| Mg1.98Li0.02Si0.4Sn0.6 | 300 | 101.2 | 1.6 | 135 | 480 | 0.15 | 2 | [791] |
| 700 | 41.9 | 1.25 | 205 | 310 | 0.55 | 1.6 | ||
| Mg2Li0.025Si0.4Sn0.6 | 300 | 99.7 | — | 125 | 535 | 0.13 | 1.9 | [792] |
| 675 | 52.2 | — | 210 | 345 | 0.7 | 1.3 | ||
| Manganese silicides | ||||||||
| MnSi1.73 | 823 | 29.7 | 2.5 | 234 | 200 | 0.3 | — | [793] |
| Mn0.99Re0.01Si1.75Ge0.025 | 825 | 70.6 | 1.5 | 230 | 500 | 1 | — | [789] |
| Mn0.95Cr0.05Si1.74 + 2%mol V17Ge31 | 900 | 46.1 | 2.2 | 180 | 666 | 0.7 | — | [851] |
| Mn(Si0.992Ge0.008)1.73 | 723 | 73.1 | — | 225 | 450 | 0.58 | 2.8 | [800] |
| MnSi1.73 with 5% Al doping | 800 | 42.9 | — | 252 | 225 | 0.82 | — | [805] |
| MnSi1.73Ge0.02 | 823 | 31.5 | — | 220 | 250 | 0.44 | 2.8 | [795] |
| (Mn15Si26)99(MnS) | 800 | 40.8 | — | 220 | 310 | 0.59 | — | [852] |
| Re0.04Mn0.96Si1.8 | 800 | 38.4 | 1.8 | 230 | 260 | 0.57 | — | [799] |
| Mn(Si0.992Ge0.008)1.73 | 833 | 35.9 | 2 | 220 | 290 | 0.6 | — | [853] |
| MnSi1.75 | 800 | 46.1 | 1.4 | 220 | 350 | 0.62 | — | [802] |
| Mn0.95Cr0.05Si1.74 | 850 | 53.5 | 2.3 | 210 | 500 | 0.6 | — | [801] |
| Mn0.99Ag0.01Si1.8 | 823 | 42.1 | 2.0 | 210 | 375 | 0.55 | — | [798] |
| MnSi1.8 + 1.5% Ag/Pt QDs | 823 | 49.0 | 1.92 | 234 | 330 | 0.64 | — | [798] |
| Mn0.96V0.04Si1.73 | 873 | 40.3 | — | 170 | 625 | 0.52 | 3.1 | [797] |
| MnSi1.75 | 777 | 35.5 | — | 230 | 230 | 0.47 | 2.7 | [796] |
| MnSi1.787Al0.01 + 0.6% Fe NPs | 773 | 45.7 | 1.9 | 220 | 330 | 0.53 | — | [854] |
| MnSi1.74 | 773 | 38.9 | 2 | 230 | 250 | 0.4 | — | [794] |
| V0.04Mn+A120Si1.74 | 773 | 36.4 | 2 | 210 | 295 | 0.4 | — | [794] |
| Mn30.36Re6Si63.64 | 873 | 57.2 | 1.27 | 235 | 416 | 1.15 | — | [790] |
| MnSi1.746Te0.03 | 823 | 43.9 | 1.5 | 230 | 310 | 0.7 | — | [804] |
| MnSi1.73 + 5 at% Al | 873 | 30.8 | 1.1 | 210 | 300 | 0.65 | — | [803] |
| MnSi1.733 | 850 | 0.2 | 2 | 240 | 330 | 0.55 | — | [855] |
A summary of the best zT values as a function of temperature for magnesium silicides and manganese silicides is presented in figure 12 (p-type) and figure 13 (n-type).
Figure 12. The maximum thermoelectric figure of merit, zT, of selected p-type magnesium silicides and manganese silicides: MnSi1.73 [793], V0.04Mn0.96Si1.74 [794], MnSi1.74 [794], MnSi1.73Ge0.02 [795], MnSi1.75 [796], Mn0.96V0.04Si1.73 [797], Mg1.98Li0.02Si0.4Sn0.6 [791], Mn0.99Ag0.01Si1.8 [798], Re0.04Mn0.96Si1.8 [799], Mn(Si0.992Ge0.008)1.73 [800], Mn0.95Cr0.5Si1.74 [801], MnSi1.75 [802], MnSi1.73 + 5 at% Al [803], Mg2Li0.025Si0.4Sn0.6 [792], MnSi1.746Te0.03 [804], 5% Al doped MnSi1.73 [805], Mn0.99Re0.01Si1.75Ge0.025 [789], Mn30.36Re6Si63.64 [790].
Download figure:
Standard image High-resolution imageFigure 13. The maximum thermoelectric figure of merit, zT, of selected n-type magnesium silicides: Mg2Si0.97Bi0.03 [806], Mg2Si0.5875Sn0.4Sb0.0125 [807], Mg1.995La0.005Si0.58Sn0.42 [808], Mg2.10Si0.38Sn0.6Sb0.02 [809], Mg2Si0.487Sn0.5Sb0.013 [810], Mg2(Si0.3Sn0.7)0.975Sb0.025 [811], Mg2Si0.6Ge0.4Bi0.02 [812], Mg2Si0.385Sn0.6Sb0.015 [813], Mg2Si0.3925Sn0.6Sb0.0075 [814], Mg2(Si0.4Sn0.6)0.97Bi0.03 [815], Mg2Si0.57Sn0.4Bi0.03 [816], Mg2.2Si0.49Sn0.5Sb0.01 [817], (Mg2.06Si0.3Sn0.68Bi0.02 [818], Mg2Si0.35Sn0.62Bi0.03 [819], Mg2(Si0.4Sn0.6)0.82Sb0.18 [820], Mg2Si0.53Sn0.4Ge0.05Bi0.02 [821], Mg2.08Si0.364Sn0.6Sb0.036 [789], Mg2.08Si0.37Sn0.6Bi0.03 [822], Mg1.98Cr0.02(Si0.3Sn0.7)0.98Bi0.02 [788].
Download figure:
Standard image High-resolution imageAcknowledgments
Theodora Kyratsi acknowledges support from the THERMOSS Project funded by EU network M-ERA.NET (KOINA/M-ERA.NET/0316/03).
3.12. Borides and carbides
Boride and carbide thermoelectrics
Philipp Sauerschnig1,2 and Takao Mori1,3
1 International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), Tsukuba, Ibaraki 305-0044, Japan
2 Global Zero Emission Research Center, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Ibaraki 305-8569, Japan
3 Graduate School of Pure and Applied Science, University of Tsukuba, Tsukuba, Ibaraki 305-8671, Japan
A couple of notable application directions for thermoelectric materials are: thermal energy harvesting, to power innumerable sensors and mobile devices expected to be utilized in future society [856–859], and high temperature waste heat thermoelectric conversion for energy saving and carbon reduction [860, 861]. For the latter to be serviceable, the thermoelectric materials themselves need to be high temperature stable refractory materials such as oxides [601, 862–864], silicides [865–868], nitrides [869, 870], borides and carbides. The latter two classes of materials are gathered in this section. Thermoelectric properties of boride and carbide compounds collected from the literature are shown in table 12.
Table 12. Borides and carbides thermoelectric properties.
| Material | µw (cm2 V−1 s−1) | κ (W m−1 K−1) | S (µV K−1) | σ (Ω−1 cm−1) | zT | Eg (eV) | µH (cm2 V−1 s−1) | Power factor (mW m−1 K−2) | T (K) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| β-rhombohedral B (C doping) (p-type) | 3.6 | 9.7 | 401 | 4.7 | 0.0078 | — | — | 0.076 | 1000 | [880] |
| β-rhombohedral B (Fe doping) (n-type) AM | 0.02 | — | −17.5 | 4.6 | — | — | — | 0.000 14 | 750 | [881] |
| β-rhombohedral B (p-type) SC | 3.1 | 240 (262 K) | 396 | 4.5 | — | — | — | 0.071 | 1030 | [882] a |
| β-rhombohedral B | — | — | — | — | — | 1.32(1)/1.50(1) for E ∥ c, 1.29(1)/1.46(1) eV for E ┴ c | — | — | 300 | [883] |
| β-rhombohedral B (0.5 at.%V doping) (n-type) SC | — | — | −399 | — | — | — | — | — | 300 | [884] |
| β-rhombohedral B (1.23 at.%V doping) SC | — | — | — | 42.4 | — | — | — | — | 300 | [884] |
| β-rhombohedral B (2 at.%Hf doping) SC | — | 2.9 | — | — | — | — | — | — | 963 | [884] |
| β-rhombohedral B (2.5 at.%Fe doping) (p-type) SC | 1.7 | — | 89 | 146 | — | — | — | 0.12 | 1400 | [884] |
| β-rhombohedral B (1 at.%Zr doping) (p-type) SC | 6.6 | — | 271 | 71.6 | — | — | — | 0.53 | 1500 | [884] |
| β-rhombohedral B (1 at.%Zr doping) (p-type) SC | 0.2 | 4.7 | 589 | 0.00799 | 2.9 × 10−5 | 0.000 28 | 500 | [884] | ||
| β-rhombohedral B (5 at.%Si doping) (p-type) SC | 8.9 | — | 262 | 118 | — | — | — | 0.81 | 1600 | [884] |
| Β-rhombohedral B (4.8 at.%Si doping) SC | — | 18.5 | — | — | — | — | — | — | 500 | [884] |
| β-rhombohedral B105 (p-type) HP | 0.5 | 4.1 | 342 | 1.4 | 0.0042 | — | — | 0.016 | 1040 | [885] |
| β-rhombohedral B105V1.5 (n-type) HP | 0.4 | 2.1 | −104 | 15.7 | 0.0078 | — | — | 0.017 | 963 | [885] |
| β-rhombohedral B105 Co1.5 (p-type) HP | 0.3 | 4.1 | 188 | 4.3 | 0.0039 | — | — | 0.015 | 1040 | [885] |
| β-rhombohedral B105Cu5 (p-type) SPS | 0.8 | 2.3 | 208 | 9.8 | 0.018 | — | — | 0.042 | 973 | [886] |
| β-rhombohedral B98.12Zr1.88 (p-type) SPS | 0.1 | — | 332 | 0.13 | — | — | — | 0.0014 | 850 | [887] |
| β-rhombohedral B97.26Zr2.74 (n-type) SPS | 0.02 | — | −516 | 0.0053 | — | — | — | 0.000 14 | 850 | [887] |
| B4C (p-type) HP | 2.8 | — | 223 | 40.8 | — | — | 1.1 | 0.20 | 1273 | [888] |
| B4.7C (p-type) HP | 7.7 | — | 219 | 119 | — | — | — | 0.57 | 1273 | [888] |
| B6.7C (p-type) HP | 16.5 | — | 221 | 249 | — | — | — | 1.2 | 1273 | [888] |
| B4C HP | — | 6.3 | — | — | — | — | — | — | 1700 | [889] |
| B6.5C HP | — | 5.5 | — | — | — | — | — | — | 1700 | [889] |
| B7.5C HP | — | 5.4 | — | — | — | — | — | — | 1700 | [889] |
| B4C (p-type) HP | 3.4 | 15.7 | 235 | 32.4 | 0.012 | — | — | 0.18 | 1053 | [890] |
| B7.13C b (p-type) HP | 26.6 | 3.2 | 230 | 272 | 0.47 | — | — | 1.4 | 1053 | [890] |
| B7.70C (p-type) HP | 8.6 | 3.9 | 247 | 72.2 | 0.12 | — | — | 0.44 | 1053 | [890] |
| B4C + 2 mol.% TiB2 (p-type) AM | 59.0 | 8.7 | 304 | 273 | 0.32 | — | — | 2.5 | 1100 | [891] |
| B4C + 6 mol.% TiB2 (p-type) AM | 161.9 | 10.6 | 355 | 415 | 0.54 | — | — | 5.2 | 1100 | [891] |
| B4C-SiB14-Si (B80C18Si2) (p-type) AM | 54.5 | 11.0 | 341 | 156.5 | 0.18 | — | — | 1.8 | 1065 | [892] |
| B4C-SiB14-Si (B80C5Si15) (p-type) AM | 73.8 | 5.6 | 412 | 93.1 | 0.30 | — | — | 1.6 | 1065 | [892] |
| B4C (62.4% rel.density) (p-type) HP | 0.6 | 3.8 | 283 | 3.9 | 0.0096 | — | — | 0.031 | 1173 | [893] |
| B4C (0.2 at.% Si) (78.3% rel. density) (p-type) HP | 2.6 | 6.5 | 230 | 30.8 | 0.029 | — | — | 0.16 | 1173 | [893] |
| B4C (2 wt.% W2B5) (p-type) HP | 1.5 | 7.5 | 216 | 21.9 | 0.016 | — | — | 0.10 | 1173 | [893] |
| B4C—2 mol.%TiB2 (parallel) (p-type) FZ | 7.5 | — | 222 | 80.6 | — | — | 2.3 | 0.40 | 1023 | [894] |
| B4C—2 mol.%TiB2 (perpendicular) (p-type) FZ | 9.5 | — | 219 | 105.8 | — | — | 2.3 | 0.51 | 1023 | [894] |
| B4C (p-type) FZ SC | 11.2 | 16.3 | 248 | 85.7 | 0.032 | — | 1 | 0.53 | 1000 | [895] |
| B4.3C SC (parallel C) (p-type) FZ SC | 0.03 | — | 160 | 0.11 | — | — | — | 0.000 28 | 300 | [896] |
| B4.3C SC (perpendicular C) (p-type) FZ SC | 0.01 | — | 160 | 0.05 | — | — | — | 0.000 13 | 300 | [896] |
| B6.5C (p-type) IBE thin film | 12.0 | — | 238 | 111 | — | — | — | 0.63 | 1050 | [897] |
| B4C | — | — | — | — | — | 2.09 | — | — | 300 | [898] |
| B4C (p-type) nanowires | 23.9 | — | 295 | 30 | — | — | — | 0.26 | 430 | [899] |
| B13C2 (10 wt.% HfB2) c (p-type) SPS | 18.6 | 5.1 | 157 | 414 | 0.20 | — | — | 1.0 | 1003 | [900] |
| B4C (p-type) SPS | 9.0 | 13 | 271 | 50.8 | 0.028 | — | — | 0.37 | 973 | [901] |
| B6P | — | — | — | — | — | 3.35 | — | — | 300 | [902] |
| B6As | — | — | — | — | — | 3.48 | — | — | 300 | [902] |
| B12P2CVD (Si doped) (p-type) CVD | 0.1 | 3.6 | 738 | 0.0015 | 2.2 × 10−5 | — | 31.7 (Si(100))/10.8 (Si(111)) at 300 K | 8.2 × 10−5 | 963 | [903] |
| B6O (p-type) HP | 4.5 | 5.5 | 545 | 1.1 | 0.0059 | — | 1.2 | 0.033 | 993 | [904] |
| B12As2 | — | — | — | — | — | — | 80 | — | 300 | [905] |
| B12As2 CVD (p-type) CVD thin film | 0.2 | — | 107 | 1.4 | — | — | 18.8 | 0.0016 | 300 | [906] |
| B12As2 CVD thin film | — | 15.3/27.0 | — | — | — | — | — | — | 300 | [906] |
| B12As2 (p-type) SC (solution growth) | 0.7 | — | 471 | 0.23 | — | 3.47 | 20 (300 K) | 0.0051 | 663 | [907] |
| B6S (p-type) (sintering) | 0.02 | — | 211 | 0.15 | — | — | — | 0.000 67 | 800 | [908] |
| α-rhombohedral B (p-type) polycrystalline | 0.6 | — | 501 | 0.38 | — | — | 20 (680 K) | 0.0095 | 1400 | [882] a |
| amorphous B (p-type) | 0.04 | — | 332 | 0.092 | — | — | — | 0.0010 | 853 | [880] |
| SmB66 (p-type) (zone melting) | 0.001 | — | 100 | 0.01 | — | 0.8 | 15 | 0.000 01 | 300 | [909] |
| GdB66 (p-type) (zone melting) | 0.008 | — | 390 | 0.002 | — | 0.87 | 15 | 3.0 × 10−5 | 300 | [909] |
| YbB66 (p-type) (zone melting) | 0.006 | — | 270 | 0.006 | — | 1.27 | 5 | 4.4 × 10−5 | 300 | [909] |
| YB66 (p-type) (zone melting) | 0.007 | — | 340 | 0.003 | — | 1 | — | 3.5 × 10−5 | 300 | [882] a |
| DyB66 (p-type) (zone melting) | 0.002 | — | 140 | 0.007 | — | 0.72 | — | 1.4 × 10−5 | 300 | [882] a |
| ErB66 (p-type) FZ SC | 0.1 | — | 225 | 1.2 | — | — | — | 0.0061 | 1035 | [910] |
| YB66 (p-type) FZ SC | 0.2 | — | 206 | 3 | — | — | — | 0.013 | 1035 | [911] |
| YB48 (p-type) FZ SC | 4.6 | 2.5 | 202 | 59.7 | 0.096 | — | — | 0.24 | 990 | [912] |
| SmB66 (p-type) FZ SC | 5.4 | 2.7 | 210 | 69.1 | 0.12 | — | — | 0.30 | 1050 | [913] |
| YbB66 (p-type) FZ SC | 5.2 | 2.6 | 241 | 41.7 | 0.091 | — | — | 0.24 | 973 | [914] |
| HoB66 (p-type) SPS | 5.2 | 2.6 | 235 | 44.4 | 0.092 | — | — | 0.25 | 973 | [528] |
| TmB66 (p-type) SPS | 5.0 | 2.6 | 235 | 42.9 | 0.089 | — | — | 0.24 | 973 | [528] |
| YB41Si1.2 (p-type) FZ SC | 0.6 | — | 184 | 1.4 | — | — | <0.1 | 0.0047 | 290 | [915] |
| YbB44Si2 (p-type) FZ SC | 0.8 | — | 209 | 10 | — | — | — | 0.044 | 1023 | [910] |
| ErB44Si2 (p-type) FZ SC | 1.1 | 2.7 (300 K) | 222 | 12 | — | — | — | 0.059 | 1023 | [910] |
| TbB44Si2 (p-type) FZ SC | 0.8 | 185 | 14 | — | — | — | 0.048 | 1023 | [910] | |
| ErB44Si2 FZ SC | — | 1.6 | — | — | — | — | — | — | 873 | [916] |
| YbB44Si2 FZ SC | — | 3.3 | — | — | — | — | — | — | 300 | [917] |
| TbB44Si2 (p-type) sintering | 0.04 | — | 242 | 0.35 | — | — | — | 0.0020 | 1023 | [918] |
| Tb0.9Lu0.1B44Si2 (p-type) sintering | 0.05 | — | 258 | 0.32 | — | — | — | 0.0021 | 1023 | [918] |
| Tb0.8Lu0.2B44Si2 (p-type) sintering | 0.05 | — | 299 | 0.21 | — | — | — | 0.0019 | 1023 | [918] |
| YB44Si2 (Zn doping) (p-type) AM | 2.0 | — | 190 | 32.4 | — | — | — | 0.12 | 1040 | [919] |
| YB41Si1.3 ([510] direction) (p-type) FZ SC | 2.7 | 4.2 | 225 | 27 | 0.033 | — | — | 0.14 | 1000 | [920] |
| YB41Si1.3 ([052] direction) (p-type) FZ SC | 1.0 | — | 301 | 4.1 | — | — | — | 0.037 | 1000 | [920] |
| α-AlB12 (p-type) | 19.8 | — | 398 | 59 | — | — | — | 0.93 | 1693 | [882] a |
| MgAlB14 (Ni doping) (p-type) | 0.3 | — | 144 | 17.4 | — | — | — | 0.036 | 1553 | [882] a |
| AlMgB14 (p-type) SPS | 1.6 | — | 338 | 4.5 | — | — | — | 0.051 | 1030 | [921] |
| Al0.55Y0.58B14 (Al added) (n-type) SPS | 9.5 | 4.5 | −123 | 304 | 0.099 | — | — | 0.46 | 973 | [922] |
| Y0.55B14 (p-type) SPS | 1.0 | 2.1 | 387 | 1.5 | 0.010 | — | — | 0.022 | 973 | [922] |
| Y1−x B28.5C4 (n-type) sintering | 0.1 | 1.4 | −18 | 10.6 | 0.000 17 | — | — | 0.000 34 | 673 | [925] |
| LuB22C2N (n-type) HP | 0.005 | — | −58 | 0.47 | — | — | — | 0.000 16 | 1040 | [926] |
| YB22C2N (n-type) HP | 0.0001 | — | −35 | 0.015 | — | — | — | 1.8 × 10−6 | 973 | [927] |
| YB22C2N (n-type) SPS | 0.004 | 4.5 | −33 | 0.57 | 1.3 × 10−5 | — | — | 6.2 × 10−5 | 973 | [927] |
| HoB17CN (n-type) SPS | 0.000 06 | −36 | 0.0081 | — | — | 1.0 × 10−6 | 973 | [927] | ||
| ErB22C2N (n-type) HP | 0.000 04 | 1.8 | −21 | 0.0092 | 2.2 × 10−7 | — | — | 4.1 × 10−7 | 973 | [927] |
| YB22C2N (12% VB2) (n-type) sintering | 0.1 | — | −57 | 5.4 | — | — | — | 0.0018 | 1040 | [928] |
| YB22C2N (16% VB2) (n-type) sintering | 0.03 | 0.9 | −29 | 5.1 | 0.0005 | — | — | 0.000 43 | 1040 | [928] |
| B14Si (p-type) | 23.2 | — | 443 | 27.6 | — | — | — | 0.54 | 1300 | [882] a |
| SiB6 d (p-n transition) sintering | 0.3 | 2.5 | 140 | 11 | 0.011 | — | — | 0.022 | 1273 | [930] |
| SiBn (n = 15–49, 90 at.% B) (p-type) SPS | 1958.9 | 5.2 | 897 | 9.4 | 0.16 | — | — | 0.76 | 1100 | [931] |
| YCrB4 (n-type) AM | 36.5 | — | −110 | 510.4 | — | 0.17 | — | 0.62 | 500 | [933] |
| YMoB4 (n-type) AM | 2.6 | — | −85 | 130.8 | — | 0.28 | — | 0.095 | 923 | [933] |
| YWB4 (n-type) AM | 2.2 | — | −70 | 123.9 | — | — | — | 0.061 | 873 | [933] |
| YMo0.8Fe0.2B4 (n-type) AM | 6.9 | — | −72 | 447.3 | — | — | — | 0.23 | 973 | [933] |
| GdCrB4 (n-type) AM | 31.2 | — | −116 | 403.5 | — | — | — | 0.54 | 500 | [933] |
| HoCrB4 (n-type) AM | 25.9 | — | −79 | 556.8 | — | — | — | 0.35 | 500 | [933] |
| TiB2(n-type) SPS | 60.0 | — | −14 | 17 675 | — | — | — | 0.35 | 873 | [894] |
| ZrB2-SiC (20 vol.%) (n-type) HP | 201.1 | 67.0 | −23.8 | 29 126 | 0.019 | — | — | 1.6 | 773 | [932] |
| ZrB2-SiC (20 vol.%)-WC (5 vol.%) (n-type) HP | 91.1 | 47.0 | −20.3 | 15 457 | 0.010 | — | — | 0.64 | 773 | [932] |
| CaB6 (n-type) SPS | 25.9 | — | −273 | 160 | — | — | — | 1.2 | 1050 | [921] |
| SrB6 (n-type) SPS | 48.5 | — | −145 | 1335 | — | — | — | 2.8 | 1050 | [921] |
| YbB6 (n-type) SPS | 24.6 | — | −111 | 1033 | — | — | — | 1.3 | 1050 | [921] |
| CeB6 (p-type) SPS | 5.1 | — | 2 | 12 617 | — | — | — | 0.0050 | 1050 | [921] |
| SmB6 (p-type) SPS | 3.1 | — | 3 | 5211 | — | — | — | 0.0047 | 1050 | [921] |
| CaB6 (n-type) SPS | 44.3 | 15.2 | −165 | 992 | 0.19 | — | — | 2.7 | 1073 | [934] |
| SrB6 (n-type) SPS | 47.9 | 12.0 | −195 | 756 | 0.026 | — | — | 2.9 | 1073 | [934] |
| Ca0.5Sr0.5B6 (n-type) SPS | 45.7 | 8.6 | −169 | 976 | 0.035 | — | — | 2.8 | 1073 | [934] |
| CaB6 (n-type) SPS | 53.7 | 11.7 | −162 | 1181 | 0.027 | — | — | 3.1 | 1035 | [935] |
| SrB6 (n-type) SPS | 16.9 | 9.1 | −246 | 140 | 0.096 | — | — | 0.85 | 1035 | [935] |
| BaB6 (n-type) SPS | 31.7 | 10.7 | −209 | 403 | 0.17 | — | — | 1.8 | 1035 | [935] |
| SrB6 (n-type) CVD thin film | 4.0 | — | −74 | 140 | — | — | — | 0.077 | 660 | [936] |
| YbB6 (n-type) HPCVD thin film | 16.1 | 2.54 (at 296 K) | −60 | 563 | — | 0.44 | 0.20 | 563 | [937] | |
| UB4 (p-type) AM | 23.2 | 40 | 2300 | — | — | — | 0.37 | 850 | [577] | |
| α-SiC (n-type) sintering (N2 atmosphere) | 0.001 | 1.4 | −76 | 0.073 | 3.2 × 10−5 | — | — | 0.000 042 | 1065 | [938] |
| α-SiC (p-type) sintering (Ar atmosphere) | 0.2 | 0.5 | 301 | 0.7 | 0.014 | — | — | 0.0063 | 1065 | [938] |
| α-SiC n-type (monocrystalline) | — | — | −29 to −108 | — | — | — | 20–100 | — | 300 | [943] |
| α-SiC (0.5 wt.% B4C, 2.5 wt.%C) (p-type) sintering | 70.0 | — | 615 | 8.5 | — | — | — | 0.32 | 1073 | [947] |
| α-SiC (0.5 wt.% B4C, 2.5 wt.%C, 3 wt.% Al2O3) (p-type) sintering | 81.7 | — | 500 | 37.6 | — | — | — | 0.94 | 1073 | [947] |
| β-SiC (n-type) sintering (N2 atmosphere) | 0.6 | 1.2 | −65 | 49.8 | 0.019 | — | — | 0.021 | 1065 | [938] |
| β-SiC (n-type) sintering (Ar atmosphere) | 0.7 | 3.5 | −121 | 25.6 | 0.011 | — | — | 0.037 | 1065 | [938] |
| β-SiC (n-type) (polycrystalline film) | — | — | −9 to −28 | — | — | — | 0.1–3 | — | 300 | [943] |
| β-SiC + 7 wt.% Si3N4 (n-type) SPS | 4.3 | — | −76 | 265 | — | — | — | 0.15 | 973 | [944] |
| β-SiC/C composite (p-type) SPS | 0.5 | 4.1 | 15.8 | 56.6 | 0.000 16 | — | — | 0.0014 | 473 | [945] |
| β-SiC (B,N-doped) (p-type) vapor phase growth | 12.5 | 32 | 89 | 730 | 0.019 | — | — | 0.58 | 1072 | [946] |
| β-SiC/Si/Au polysilastyrene composite (p-type) sintering | 36.1 | 52.4 | 92.4 | 298 | 0.0015 | — | 5.74 | 0.25 | 300 | [948] |
| β-SiC (n-type) CVD (He atmosphere) | 0.02 | 4.6 | −67 | 1.1 | 0.0001 | — | — | 0.000 49 | 950 | [949] |
| β-SiC (n-type) nanowire | 6.5 | 6 | −67 | 107 | 0.0030 | — | — | 0.048 | 370 | [950] |
| Mo2CTx (n-type, annealed) solution synthesis | 9.7 | — | −31 | 1132 | — | — | 0.016 (pristine) d , 1.86 (annealed) d | 0.11 | 800 | [939] |
| Mo2TiC2Tx (n-type) solution synthesis | 18.2 | — | −47 | 1392 | — | — | 0.323 (pristine) d , 2.85 (annealed) d | 0.31 | 800 | [939] |
| Mo2Ti2C3Tx (n-type) solution synthesis | 5.8 | — | −28 | 754 | — | — | 0.451 (pristine) d , 2.05 (annealed) d | 0.059 | 800 | [939] |
| TiC0.7N0.3 (n-type) sintering | 24.3 | 18.0 | −18 | 5587 | 0.0088 | — | — | 0.18 | 873 | [940] |
| WC/Polylactic Acid composite (n-type) additive manufacturing | 0.6 | 28.0 | −12 | 42 | 6.5 × 10−6 | — | — | 0.000 60 | 300 | [941] |
| Fe-2.3C-Si-5Mn-7 V-8Cr (VC/Cr23−x Fex C6) (n-type) induction melting | 70.0 | 21.6 | −25 | 10 155 | 0.024 | — | — | 0.63 | 800 | [942] |
| Zr2 [Al3.56Si0.44]C5 (n-type) SPS | 12.3 | — | −13 | 4427 | — | — | — | 0.075 | 953 | [952] |
a And references therein. b κ of sample composition B6.81C. c κ of sample calculated from zT, resistivity and Seebeck coefficient. d Data from [953].AM = arc melting; CVD = chemical vapor deposition; FZ = floating Zone; HP = hot press; HPCVD = hybrid physical chemical vapor deposition; IBE = intense pulsed-ion beam evaporation; SC = single crystal; SPS = spark plasma sintering.
In general, borides have excellent mechanical properties including high temperature stability, chemical stability, and low compressibility due to the strong covalent bonding of boron. It is a particularly refractory class of materials with borides like RB66 typically having melting points above 2400 K. In addition, boron cluster compounds, formed with the boron icosahedron as a structural unit, have been found to exhibit intrinsic low thermal conductivity, which is advantageous for thermoelectrics, despite the compounds possessing strong bonding and generally high speed of sound. Many of the compounds exhibit large Seebeck coefficients, and electrical conductivities that increase with an increase in temperature due to hopping conduction. Therefore, despite their relatively low zT at lower temperatures, they are considered a promising system as ultra-high temperature thermoelectric materials [871–879].
We first briefly cover the very boron-rich compounds, namely, those which possess the B12 icosahedron as main building block of the boron cluster atomic network. The thermoelectric properties of the most common form of elemental boron, beta-boron, have been extensively studied through modification of properties via TM doping [880–887]. As the excellent properties of p-type boron carbide became clear [888–890], effort was focussed on the search for a viable n-type counterpart. We mention here that because of the electron deficient nature of the boron atomic network as reviewed previously [871, 873], the boron icosahedral borides are predominantly p-type. The n-type characteristics, in even a limited range of temperature, in boron cluster compounds were first found by Werheit with Fe doping [881] and Slack with V doping [884]. Later both p-type and n-type characteristics with very large absolute values of Seebeck coefficients were found for Zr doped beta-boron with variation of the Zr content [887]. Boron carbide has been reported with the highest performance of zT∼ 1 for boron carbide and TiB2 composites [891, 892]. In addition to the wide range of compositions of boron carbide [888–890], there has also been extensive work investigating the thermoelectric properties of boron carbide in different forms like single crystals, thin films, nanowires, and various composites [893–901]. Boron carbide belongs to the alpha-boron rhombohedral structure type, and various compounds of this family such as boron phosphide, boron arsenide, boron oxide, boron sulfide, in addition to alpha-boron itself, have been investigated [882, 902–908]. Amongst the boron cluster compounds, Si doped B12P2 has been reported to have high mobility because of the hole carriers [903], although mobility has not been measured for many of these borides, presumably due to low mobility. Amorphous boron also has been measured with low electrical conductivity as would be expected [880]. The RB66 compounds are found to have amorphous-like behavior of thermal conductivity; relatively high thermoelectric performance has been observed for some rare earths like SmB66 and compounds with relatively high metal content [882, 909–915]. The RB44Si2 compounds have also been investigated and shown to have moderate thermoelectric performance [528, 910, 916–920], behavior of general interest, the possibility to control morphology through addition of volatile element [919], anisotropic properties related to interesting crystal structure channels [920], and insight into the importance of disorder as the origin of amorphous-like behavior of thermal conductivity in such crystalline compounds [917]. It should be mentioned that for most of the boron icosahedral compounds, the measurements with conventional facilities were typically limited to a maximum of 1100 K, but thermoelectric performance of compounds like RB66 and RB44Si2 were showing steep increases in thermoelectric properties toward higher temperatures, with melting points of these compounds above 2400 K and 2300 K, respectively,
In the aluminoborides [882, 921, 922], a striking discovery was made for Alx YB14, in that, unusually, it was found to be possible to vary the Al content in a relatively wide range and as a result, both p-type and n-type characteristics could be obtained with relatively high performance for a boride [923]. Liquid phase sintering was also recently discovered for this important compound, simplifying processing and reducing synthesis time [924]. The RB22C2N and homologous series of compounds has also attracted interest with its structural similarities and as the n-type counterpart compounds to boron carbide [925–928]. The synthesis process for this compound has also been radically improved by using a gaseous reaction which may also be useful for the synthesis of nitride compounds [929].
Silicon boride compounds have also been investigated as potential high temperature thermoelectric materials [882, 930, 931]. For a SiBn (n = 15–49)/SiB6 composite prepared by SPS a remarkably high Seebeck coefficient close to 900 µV K−1 and a zT approaching 0.2 at 1100 K with an upwards trend with increasing temperature have been reported [931]. Unlike the boron-rich compounds discussed so far, diboride, tetraboride and hexaboride compounds are not built from B12 icosahedra and generally show n-type behavior [577, 894, 921, 932–937]. TM diborides, e.g. TiB2 [894] and ZrB2 [932] show metallic behavior and while PFs >1 mW m−1 K−2 have been observed in ZrB2–SiC composite materials [932], very high thermal conductivity values limit the overall thermoelectric performance. Layered REMB4 compounds (RE = rare earth element, M = transition metal) have been studied by applying the mno electron counting rule to find semiconducting compounds. REMB4 (RE = Y, Gd, Ho; M = Cr, Mo, W) compounds of the YCrB4-type were found to be n-type semiconducting materials [577]. TM hexaborides MB6 (M = Ca, Sr, Ba) are among the best performing boride thermoelectric materials with zT values of 0.3 around 1000 K [934, 935]. To reduce thermal conductivity of these compounds thin films have been deposited for YbB6 and SrB6 [936, 937].
Besides borides, another group of materials which has been investigated for the use as high temperature thermoelectric applications due to their good thermal stability are carbides [938–951]. Efforts have been focused on the compound SiC which exists in several different modifications, the most prominent being the hexagonal 6-H α-SiC [938, 939, 947] and the cubic 3-C β-SiC [938, 939, 944–946, 948–950]. Depending on the synthesis conditions both p-type and n-type behavior have been reported. Strategies for improving the thermoelectric properties have included the addition of secondary phases for the fabrication of composite materials, e.g. B4C [947], C [945, 947], Al2O3 [947], Si3N4 [944], Si [948] and Si/Au/polysilastyrene [948]. Thermoelectric properties of carbide materials in addition to SiC have been reported for layered Mo-based MXene carbides [951], TiC0.7C0.3 [940], flexible WC/polylactic acid composites [941], VC/Cr23−x Fex C6 containing Fe-2.3C-Si-5Mn-7V-8Cr alloy [942] and Zr2[Al3.56Si0.44]C5 [943].
Boride and carbide thermoelectric materials have great structural and chemical variety and have been studied thoroughly from a fundamental point of view. Their thermoelectric performance compared to the established thermoelectrics is however relatively low. Many of the borides have very large Seebeck coefficients and relatively low electrical conductivities. Compositing partial metallic networks has been shown to largely enhance the performance for several cases, like YB22C2N [952] and boron carbide [891, 892], and this should also be attempted in future with other borides with relatively high performance. Besides necessary improvements in the thermoelectric performance, in order to exploit the advantages of this class of thermoelectrics, i.e. high thermal and chemical stability and generally increasing performance with increasing temperature, appropriate evaluation and application systems need to be established; these are currently limited to fairly moderate temperature ranges. For example, most commercial thermoelectric facilities used to measure Seebeck coefficients and electrical conductivity typically have a maximum measuring temperature of 800 °C or 1000 °C at most. Whereas, for example, most of the borides presented in this work have zT values showing a large increasing trend at these temperatures and possess melting points above 2000 °C. Thereby, for ultra-high temperature applications in the range 1000 °C–2000 °C, such as occurring in jet engine exhausts, topping cycle for fusion power generation, etc, borides are one of the few thermoelectric materials which are actually stable at these temperatures and may potentially possess higher zT values than has been given so far in the literature. As two prominent refractory thermoelectric material systems, consideration and investigations of these two materials should continue.
Acknowledgments
Support from JSPS JP16H06441 and JST Mirai Program JPMJMI19A1 are acknowledged.
4. Challenges and future perspectives
In this compilation we have sought to provide an overview of the thermoelectric properties of a wide range of inorganic materials. These include current state-of-the-art thermoelectric materials but also a number of more 'exotic' materials which could become important in the coming years or provide insights into routes to improve the more established materials. Whilst many publications in the field perhaps give undue attention to the maximum figure of merit, zTmax, as the critical parameter, we have tried to stress the importance of the average zT, particularly over the intended temperature range for the application, and indeed the other important thermoelectric parameters (documented here) which indicate the strengths and potential limitations of the material.
With the advances in modeling and experimental instrumentation over the past decade there are considerable opportunities in the development and discovery of new materials and understanding the structures and mechanisms controlling properties from the atomic to the macroscopic level. Materials discovery through data mining, machine learning or high throughput calculations can point the way in the selection of potential new materials with high thermoelectric performance, enabling candidates to be screened theoretically, and the most promising evaluated experimentally. A further constraint here is that the work should target Earth-abundant, non-toxic starting materials to minimize cost and environmental impact. Atomistic and Density of States calculations along with band structure engineering can be used to define the most effective additives for enhancing electrical conductivity or reducing thermal conductivity and ways to induce band convergence, thus improving the PF.
Whatever new materials appear in the coming years there are many important challenges that will need to be addressed if the thermoelectrics are to reach their full potential at the material and device level and be fully exploited in a growing range of applications. For maximum output from a thermoelectric module the n-type and p-type materials should exhibit comparable performance. Many of the existing materials have significant imbalance between their best n-type and p-type candidates; for example, for Tellurides and Zintls the p-type performance is much better than the n-type, whilst for Skutterudites it is the reverse. Work is necessary to develop more, better matched thermoelectric materials in the different systems, but also to extend the temperature range of operation through enhancing performance away from the peak temperature. In many cases this is particularly desirable at lower temperatures, even down to room temperature; for example with HHs, skutterudites and oxides, a reduction in thermal conductivity is highly desirable to help improve the average zT.
Looking beyond zT and PF performance, another very important challenge to be addressed is the mechanical properties and stability of the materials. To avoid serious mechanical stresses in the modules, the p-type and n-type materials should have similar coefficients of thermal expansion, but greater attention also needs to be paid to a variety of mechanical properties including brittleness and mechanical strength at elevated temperatures. This is especially true of the metal-based thermoelectrics. A closely related, more general challenge is that of controlling degradation at elevated temperature, or as a result of thermal cycling. Materials that contain volatile species (e.g. Na, Sb, S, Pb) tend to degrade via the physical loss of material or via electromigration when a current flows. The former can be addressed by some form of coating or encapsulation, and the latter by cation doping to block migration pathways, but greater understanding of corrosion and degradation mechanisms are essential if viable, long-lasting solutions are to be achieved.
As the construction of thermoelectric modules involves multiple interfaces, between dissimilar materials in many cases, greater understanding of these interfaces and transport processes across them is essential to avoid physical degradation and unnecessary power losses in device operation. Identification of most suitable diffusion-barrier materials and solders, for the various families of materials will be important.
In order to ensure that thermoelectrics become more competitive in the market place, a detailed analysis of processing costs at each step in module production, and the development of cheaper material-processing and manufacturing routes are critical. The availability of cost-effective thermoelectrics, with increased conversion efficiencies, covering wider temperature ranges would open up new markets from the room temperature IoT to ultra-high temperature engineering systems.
Acknowledgments
Robert Freer gratefully acknowledges support of the UK Engineering and Physical Sciences Research Council for the provision of funding through EP/L014068/1, EP/L017695/1 and EP/T020040/1.
Disclaimer and declaration
The information presented in this review was selected in good faith by all the authors. We accept that it is impossible to include all the available data. However, errors and omissions will be corrected in the subsequent editions of the tables. Corrections and more complete information from the scientific community are most welcome in order to improve the value and scope of the data presented in the tables.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.