Abstract
In the crucial area of sustainable energy storage, solid-state batteries (SSBs) with nonflammable solid electrolytes stand out due to their potential benefits of enhanced safety, energy density, and cycle life. However, the complexity within the composite cathode determines that fabricating an ideal electrode needs to link chemistry (atomic scale), materials (microscopic/mesoscopic scale), and electrode system (macroscopic scale). Therefore, understanding solid-state composite cathodes covering multiple scales is of vital importance for the development of practical SSBs. In this review, the challenges and basic knowledge of composite cathodes from the atomic scale to the macroscopic scale in SSBs are outlined with a special focus on the interfacial structure, charge transport, and mechanical degradation. Based on these dilemmas, emerging strategies to design a high-performance composite cathode and advanced characterization techniques are summarized. Moreover, future perspectives toward composite cathodes are discussed, aiming to facilitate the develop energy-dense SSBs.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Future perspectives
A multiscale understanding of composite cathodes in solid-state batteries is of particular importance to achieve composite cathodes with stable structure, high mass loading, and fast charge transport kinetics. Based on these comprehensive understandings and related analysis, reconstructing interface structure at the atomic scale, manipulating the internal stress/strain of cathode, and tailoring composite cathode architecture from materials level (microscale, mesoscale) to electrode level (macroscale) have been proven to be successful strategies for designing high-performance composite cathodes and show great potential for advanced solid-state batteries.
1. Introduction
Advanced battery technologies are enabling the clean energy storage and transport electrification. In recent years, liquid-based lithium-ion batteries (LLIBs) have gained success in large-scale promotion [1–3]. However, the increasing combustion accidents and the current bottleneck of one charge distance range for electric vehicles are arousing tremendous attention to developing next-generation batteries with enhanced safety and energy density. Thereinto, solid-state batteries where the combustible liquid electrolytes are replaced with nonflammable inorganic solid electrolytes (SEs) is one of the most promising candidates [4–11].
SEs, which provide ionic conductivity, high electrochemical stability, and mechanical strength, enable solid-state lithium batteries (SSBs) to radically solve the safety problems [11–15]. In particular, using SEs is deemed to be the most promising way to prevent dendrite growth, thus allowing for matching with metal lithium (Li) anode that possesses high theoretical capacity (3860 mAh g−1) [3, 5, 16–23]. Theoretically, great advantages in SEs and Li anode could enable SSBs to achieve high energy density when coupled with the high-energy cathode [24]. Therefore, it is imperative to employ cathode materials that possess a high specific capacity and/or high average operating potentials in SSBs. For example, LiNix Coy Mnz O2 (x + y + z =1, NCM) with moderate capacity and high operating potential, Li-rich layered oxide (LRLO) with high capacity and wide operating potential, and Li-free cathode based on conversion reaction such as FeF3 with superior capacity are desirable for high-energy-density SSBs [25–29].
However, when paired with a high-energy cathode, the SSBs are usually found to behave differently from theoretical expectations and are still far from practical applications [30, 31]. The crux of this dilemma originates from the high complexity of composite cathodes, involving chemistry (atomic scale), materials (microscopic/mesoscopic scale), and electrode system (macroscopic scale) [32, 33]. For example, ionic exchange, interfacial side reaction, nonuniform mixing, mechanical degradation, etc often occur within composite cathodes during the electrochemical and manufacturing processing, resulting in poor electrochemical performance [33, 34]. To bridge the gap from macroscopic electrochemical performances to microscopic properties, it is necessary to consider multiscale issues and design corresponding strategies. In this case, a more comprehensive and in-depth understanding of surface/interface structure evolution at the atomic scale, multiscale charge transport, and multiscale mechanical evolutions in working SSBs are indispensable for constructing an ideal composite cathode for high-energy-density SSBs, as illustrated in figure 1. Compared with LLIBs (figure 2), the composite cathodes in SSBs faced more challenges, such as the continuous ionic/electronic conductivity networks, the influence of morphology/architecture and crystallographic orientations, etc. In addition, although several insightful reviews have been conducted on the cathode, [35–38] little attention has been paid to decoupling the intertwined multiscale issues within composite cathodes and summarizing multiscale effective strategies for high-energy-density SSBs.
Figure 1. Understanding the composite cathodes in solid–state batteries from the atomic scale to macroscopic scale. The properties of interfacial atoms and ions, such as ionic interdiffusion and vacancy, determine the interfacial chemical/electrochemical stability. Situated at a larger scale, the crystal structure of cathode materials involving surface structure and crystallographic orientations affect the interfacial charge transport kinetics and stability. A further step towards the macroscopic scale including cathode materials and electrode design requires extensive engineering aimed at establishing continuous electronic and ionic networks, tuning materials' morphology, designing advanced electrode architecture, and avoiding mechanical issues like crack and delamination. Note that to design a high-performance composite cathode, considering these issues comprehensively is of particular importance.
Download figure:
Standard image High-resolution imageFigure 2. Understanding the composite cathodes in liquid–state batteries from the atomic scale to macroscopic scale.
Download figure:
Standard image High-resolution imageHerein, we present an overview of recent progress on high-energy cathodes in SSBs with a special focus on how to design a high-performance composite cathode based on a comprehensive understanding of multiscale issues within the composite cathode. This review will firstly introduce typical high-energy cathode materials and provide a comparison in the respects of volume change, specific capacity, rate capability, working voltage, cost and cycle performance. The second part of this review summarizes multiscale challenges facing composite cathodes ranging from atomic/ionic scale to macroscopic scale. On the basis of these comprehensive understandings and related analysis, recently reported strategies for designing an ideal composite cathode, including reconstructing interface structure at the atomic scale, manipulating the internal stress/strain of cathode, and tailoring composite cathode architecture, are described. Furthermore, a combination of advanced characterization techniques from atomic probes to macroscopic probes is also summarized to investigate the cathodic behaviors in SSBs. Finally, a general conclusion and future research directions of SSBs are discussed according to our understandings.
2. Overview of high-energy cathode materials in SSBs
Advanced cathode active materials (CAMs; e.g. LiCoO2, Ni-rich layered oxides, LRLO, and Li-free cathodes) are enabling the construction of high-energy-dense SSBs [33]. Figure 3 schematically presents their properties in terms of volume change, specific capacity, rate capability, working voltage, cost, and cycle performance (in ascending order of specific capacity) [27, 28, 39–44]. The upper cutoff voltage of commercial cathode material LiCoO2 (LCO) was only 4.2 V vs. Li+/Li, which possesses only a specific capacity of 137 mAh g−1 [45]. However, LCO holds advantages in its high theoretical capacity (∼274 mAh g−1) and high safety [46, 47]. Nevertheless, a layered LCO could provide only half of its theoretical capacity owing to the structural instability at high voltage [39, 40]. Furthermore, owing to the irreversible movement of O2− sheets from LCO to CoO2, the theoretical capacity of LCO is unreachable. Nevertheless, the increasing demand for energy means that the LCO must break through the limit capacity and cycle steadily. In addition, the fierce competition from the new darling and the added cost of the Co source also compels researchers to increase the energy density of LCO. Considering the low volume change, good cycle performance, and high-rate capability, LCO is still a promising candidate for SSBs.
Figure 3. Performances of four typical cathode materials in solid-state batteries. Radar plots of different high-energy cathode materials: LiCoO2 (LCO), LiNixCoyMnzO2 (NMC), Li[LixTM1-x]O2 (0 ⩽ x ⩽ 0.33) (LRLO), and Li-free cathode materials FeF3 (in ascending order of specific capacity).
Download figure:
Standard image High-resolution imageNi-rich layered NCM has gained increasing interest to achieve high-energy SSBs, especially for NCM with low Co content (like LiNi0.8Co0.1Mn0.1O2, NCM811) possessing a relatively high capacity and high potential [41]. For Ni-rich layered NCM cathodes, Li-ion is extracted from the structural lattice accompanied by the oxidation process of TMs during the charging process. The capacity generates from the reversible cations redox like Ni2+/3+/4+. Generally, only cations redox takes place during electrochemical processes [48]. LRLO with a general formula of Li[Lix TM1−x ]O2 (0 ⩽ x ⩽ 0.33) offers anomalous high specific capacity (>250 mAh g−1) and wide potential window (2–4.8 V vs. Li/Li+), which are regarded as the most promising choices for high-energy-density SSBs [43, 49]. When the voltage is below 4.5 V vs. Li/Li+, Li+ ions are extracted from the component of LRLO, and cations are oxidized to higher valence, similar to Ni-rich layered NCM. When the voltage surpasses 4.5 V vs. Li/Li+, oxygen ions are involved in the redox process. The unique anionic redox in LRLOs is made up of the reversible and irreversible processes. The irreversible oxygen species escaping from the structure will result in severe irreversible phase transformation, contributing to fast capacity and voltage decay. However, LRLO cathodes decrease the use of expensive Cobalt and thus have the advantages of environmental friendliness, low cost, and high thermal stability [50]. However, employing LRLO to SSBs has been rarely reported, probably stemming from their unstable crystal structure and interfacial side reactions that lead to large charge-transfer resistance [44, 45]. Li-free cathodes based on conversion reactions demonstrate their superior specific capacity and moderate redox potential, which can realize the high energy densities of SSBs [46, 47]. Among various types of conversion cathodes, metal fluorides are a promising Li-free cathode owing to their high capacity (450 mAh g−1 for FeF2), suitable theoretical potential (2.8 V vs. Li/Li+), and extremely low cost [51–53]. However, the inherently poor ionic conductivity and substantial volume change (up to 30%) result in fast capacity degradation.
3. Challenges facing composite cathodes in SSBs
Liquid electrolytes possess outstanding wetting ability to electrodes, while SEs have poor solid–solid contact owing to their intrinsic characteristics. Therefore, the composite cathodes in SSBs need to be fabricated by intermixing ionic/electronic conductors (SEs and carbon materials) and CAMs to ensure efficient Li-ion and electron conductivity pathways, so as to break limitation of kinetics behaviors over multiple lengths [30, 54–56]. Nevertheless, nonuniform mixing, unexpected agglomeration, ion interdiffusion, microstructure instability, mechanical failure, etc. would commonly occur during the manufacturing and electrochemical processing of composite cathodes, resulting in poor electrochemical performance [57–60]. Therefore, understanding multiscale mechanisms of degradation is of prime importance in order to overcome the challenges facing composite cathodes for high-performance SSBs. This chapter mainly describes three cathode-related challenges, including surface/interface structure evolution at the atomic scale, multiscale charge transport, and multiscale mechanical degradation.
3.1. Surface/interface structure evolution at the atomic scale
Different cathode materials have distinct properties due to their different electronic and crystal structure, which leads to different electrochemical processes. However, there is one thing in common for all cathode materials that no electrode can escape the effect of surface/interface structure evolution at the atomic scale during battery cycling. In SSBs, it has been reported that many transition metals (TMs) at a high oxidation state have been found to be unstable against SEs [61–63]. The instability of surface phase and side reactions at CAM–SE interface could cause severe structure evolution, resulting in fast capacity fading. An example can be found in a high-voltage CAM LiNi0.5Mn1.5O4 (LNMO), which undergoes the evolution of both electronic and atomic structures during the delithiated process owing to the migration of oxygen and TM ions [64]. Specifically, at the beginning of delithiation, Li ions were extracted by the electrochemical force. However, the varing contact condition between CAM and SEs results in different Li ions migration rates in composite cathodes, which causes different delithiation states (oxidation state) of LNMO lattices along the electric field [65, 66]. In particular, the area with high-level delithiation (under high voltage) has more TM ions migration than an area with low-level delithiation owing to the instable structure after the formation of lithium vacancy. In this case, oxygen easily flows from the unstable structure. According to density functional theory (DFT) calculations, the formation of oxygen vacancies will further contribute to the reduction of defect formation energy, indicating that oxygen vacancies and Li vacancy concentration (VLi) are two synergetic factors for forming defects (figure 4(a)), thus accelerating the structural degradation [59, 67].
Figure 4. Surface/interface structure evolutions in SSBs. (a) Theoretical calculations of the energy difference between bulk and antiphase boundary with/without considering oxygen vacancies at a high delithiation state. Reproduced with permission [64]. Copyright 2018, Springer Nature, CC BY 4.0. (b) The voltage profile of LGPS during the electrochemical process according to the first-principles calculation. Reproduced with permission [71]. John Wiley & Sons. [© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim]. (c) Ionic interdiffusion at the heterogeneous AM–SE interface in an SSBs. Reproduced with permission [72]. Copyright (2019) American Chemical Society. (d) Interfacial structure evolution of NMC532 cathode at a high voltage in SSBs based on Li10GeP2S12 SEs. Reproduced with permission [42]. John Wiley & Sons. [© 2021 Wiley-VCH GmbH].
Download figure:
Standard image High-resolution imageAdditionally, when a SE is not thermodynamically stable at a high voltage, it tends to react with CAMs and induces phase change at the SE–CAM interfaces (figure 4(b)) [54, 68–71]. DFT calculations based on the atomic structure of the β–Li3PS4 (LPS)(010)/LCO(110) interface has demonstrated that Li+ in the LPS side with high Li chemical potentials begins to transfer toward the anode side during the initial charging process, resulting in the growth of a Li+-depleted layer [72, 73]. Moreover, the energetically preferable formation of P–O bond indicates that the interfacial reaction tends to occur along with the interdiffusion of cations (Co and P) and anions (O and S), which further enhances this Li-ion depletion (figure 4(c)). As a result, the interfacial resistance is increasing and results in the fast capacity fading. Wang et al [74] found that the layered structure of LiNi0.5Mn0.3Co0.2O2 (NCM532) would be restructured to rock-salt phase after repetitive cycling and became fatigued, which is attributed to the CAM–SE reactions and surface oxygen loss at the high voltage (figure 4(d)). The wide electrochemical window (2.5–4.4 V vs. Li/Li+) beyond that of Li10GeP2S12-based SEs (1.7–2.1 V vs. Li/Li+) would induce the generation of non-oxygen species such as elemental sulfur and polysulfides at the CAM–SE interface. The highly oxidized species, together with interfacial structural evolution (like layered-to-rock salt) substantially impede the interfacial charge transport kinetics, leading to the large interfacial resistance in composite cathodes.
3.2. Multiscale ionic transport at CAM–SE interface
In conventional batteries, the excellent wettability of liquid electrolytes enables Li ions to transport through the grains in active materials. Cathodes with different properties like crystallographic presentation, pore size, contact area, and morphology can still achieve high performance in LLIBs [74, 75]. However, in SSBs, different cathode properties may imply tortuous and/or blocked pathways for ionic/electronic transport [48, 76, 77]. With the good tunability on nanoscale geometries and dimensions, cathode properties could be regulated at atomic levels and unique phenomena could be observed. Significant achievements have been made like the manipulation of interfacial crystallographic orientation, which can improve ionic transport kinetics due to increased charge accessibilities and shortened diffusion lengths [78–80]. While desired transport kinetics can be achieved at the microscale, the shifting of this kinetics from microscale to macroscale is usually impeded originating from intrinsic complexity within composite cathodes, including the distribution of CAMs and SEs, the existance of pores, etc. Therefore, multiscale investigations should be carried out in order to augment comprehension of ionic transport in composite cathode. It is also imperative to break the space or time resolution limitation of multi-technique approaches for probing ionic and electronic transport at varying scales.
3.2.1. Microscopic scales.
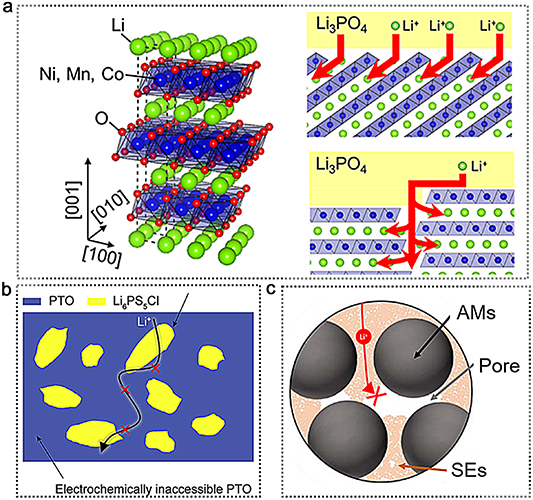
Structural inhomogeneities inside the composite cathode ranging from nanometer to micrometer scales can often dominate the ionic conductivity [37]. The prime example is the influence of interface crystallographic orientation between SEs and CAMs [78, 80, 81]. In composite cathodes, CAM and SE often possess different crystal structures, which usually causes the mismatch of interfaces, further seriously blocking the transportation of Li+ at the interface, especially for CAMs with high specific surface area. To demonstrate the effect of CAM surface crystallography on SSBs performance, epitaxial growth of the cathode materials is used to control the crystallographic orientation. Such interfacial structure design can serve as a model electrode to demonstrate the properties of ionic transport at the CAMs–SEs interfaces [82, 83]. Manipulating the microstructure enables us to quantitatively study the impact of the crystallographic orientations on charge transport kinetics. For example, Nishio et al fabricated two types of NMC films with (001)- and (104)-oriented crystals (figure 5(a)) [84]. The batteries with (104)-oriented NCM thin film exhibited higher interface resistances than those of (001)-exhibited NCM thin film, suggesting the importance of the crystallographic presentation of SEs and CAMs on interfacial diffusion kinetics. To further prove the effect in larger format SSBs concepts, Zahiri et al prepared a series of highly crystallographically oriented, crystalline, thick, and dense alkali ion TM oxide cathodes contacted with various SEs to comprehensively study the role that crystallography and interface morphology plays in the performance of SSBs [81]. As expected, a direct effect of interfacial crystallography on SSBs performance is shown by the linear correlation between interfacial ion transport and capacity fade. This linear relationship also demonstrated that interfacial resistance is the dominant factor of the cycling performance and even a predictor for the future performances of SSBs.
Figure 5. Multiscale charge transport in composite cathodes. (a) NMC (104)–Li3PO4 and NMC (001)–Li3PO4 interface with an antiphase inversion grain boundary. Red arrows represent Li-ion transfer pathways. Reproduced with permission [84]. Copyright 2020, American Chemical Society. (b) Schematic illustration of disconnected Li+ percolating network in unfavorable microstructure that leads to an electrochemically inaccessible PTO. Reproduced with permission [131]. Copyright 2021, Elsevier. (c) Schematic illustration of a pore hindering the ionic transport. Reproduced with permission [87]. © The Author(s) 2019. Published by ECS. CC BY 4.0.
Download figure:
Standard image High-resolution imageIn addition, an interphase will form between CAM and SE in the most case due to their chemical incompatibility, which usually impeded the charge transfer at the interface. For example, oxide-based SEs are usually rigid and thus a co-sintering process at high temperature between CAMs and SEs is required to have intimate contact. Nevertheless, co-sintering requires a high temperature to obtain sufficient contact areas. High temperatures are generally favorable for severe side reactions, including, interfacial decomposition, interdiffusion of elements, and structural reorganization, thus resulting in high interfacial resistance in most cases. Furthermore, the electronic network is also important to boost the interfacial charge transport. However, Zhang et al recently found that conductive carbon could accelerate the electrochemical decomposition of LGPS by providing sufficient electronic pathways. Therefore, instabilities caused by electrolyte decomposition will eventually lead to increased interfacial resistance and degraded performance in SSBs.
3.2.2. Mesoscopic and macroscopic scale.
Different from liquid electrolytes in lithium-ion batteries that can easily penetrate into the porous electrodes and diffuse across the interface, it is hard for SEs to access the voids, which further impedes Li-ion transport across the CAM–SE interfaces in composite cathodes [60, 85]. Therefore, the source of resistance to ion transport beyond the atomic/microscopic scale in cathode composites is inadequate physical contact between CAM and SE solid particles. It worth noting that adequate physical contact between CAMs and SEs are necessary to support the interface ionic transport.
Using the large solid-electrolyte fraction in cathode composites is a simple way to provide sufficient ionic diffusion, but, to achieve high energy density, the fraction of SEs in composite cathode should be minimized [86, 87]. However, if the distribution of SEs and CAMs is unfavorable for Li-ion transport, as indicated schematically in figure 5(b), the conductive network will result in low utilization of CAMs. Froboese et al detailedly studied the impact of the volume fraction and particle size of CAMs on ionic conductivity inside the composite cathodes [87]. To make sure that the ionic conductivity was mainly affected by the electrode structure, they employed the electrochemically inert glass particles as CAMs instead of typical cathode materials like NCM. Without CAMs in electrode, ionic conductivity of composite cathode can reach its maximum (6.19 × 10−4 S cm−1), which is comparable to reported values in composite cathodes with CAMs (2 × 10−4 S cm−1–9 × 10−4 S cm−1) [88–90]. However, the ionic conductivity decreases exponentially with increasing the volume fraction of CAMs.
In addition, when the fraction of CAMs is less than 35%, the ionic conductivity has negligible relationship with particle sizes. Once surpassing a volume fraction of 40%, the particle size of CAMs should be considered to improve ionic conductivity. The CAMs with coarser particles can achieve relatively higher ionic conductivity than the small particles. For instance, at a volume fraction of 60% of CAMs, the lowest ionic conductivity (∼2.49 × 10−6 S cm−1) can be observed for small particle sizes whereas the high ionic conductivity (∼2 × 10−5 S cm−1) is capable of being achieved for coarser particles. This kind of situation arises from the fact that different particle sizes of CAMs induce different physical contact between CAMs and SEs, which further affects porosity within composite cathodes. The electrode porosity should be as low as possible due to its electrochemical hindrance for transported ions (figure 5(c)) [87, 91]. In terms of coarser particle size of CAMs (>40 μm), the porosity keeps constant even at a volume fraction of 30%, following by an exponential increase up to 14.15% for 40–70 μm and 16.45% for 70–110 μm respectively. For small particle size, the porosity keeps on increasing even at small volume fractions and reaching the highest porosity of 19.72% at the volume fraction of 60%. When a moving Li+ encounters a pore, the transport path is interrupted. For the high porosity (>13%), the ionic conductivity of the electrode decreases to the low values of approximately 10−6 S cm−1. As discussed above, the existence of porosity and a high fraction of CAMs implies the occurrence of tortuous pathways for ionic transport and inhomogeneous current densities, resulting in a huge increment of resistance to ion transport in composite cathodes [92, 93].
3.3. Multiscale mechanical degradation
Apart from surface/interface structure evolution at the atomic scale and multiscale ion/electron transport encountered in the composite cathode, another critical challenge is mechanical degradation. In the case of materials level (microscale and mesoscale), most of CAM will experience volume shrinkage/expansion during (charge) delithiation and (discharge) lithiation processes. Considering the fact that SEs possess low plasticity and thus cannot infiltrate or flow crack caused by the volume evolution of CAMs during the electrochemical process, rigid contact of solid–solid materials in composite cathode will be prone to generating macroscale strains/stresses, which further affects the evolution of microstructure and performance of the battery [94–96]. Both fracture and delamination are catastrophic and dependent on the response of the CAMs matrix and CAM–SE interface to the developed stresses [97]. For example, the NCM underwent volume shrinkage during the delithiation process [98]. This volume contraction becomes more severe along with the increment of Ni content in NCM [99]. Owing to the rigid feature of solid materials in composite cathodes, even slight volumetric change of CAMs during electrochemical process causes serious stress problems. Notably, it will be worse for the polycrystalline cathode particles composed of densely packed primary grains with random orientations due to the anisotropic volumetric strains of these primary grains [100, 101]. The microstructural evolution of CAMs characterized by cross-sectional SEM–BSE measurements (as displayed in figure 6(a)) demonstrates that polycrystalline LiNi0.88Co0.11Al0.01O2 (NCA) particles show obvious internal cracks, stemming from severe internal stress [102].
Figure 6. Mechanical properties of composite cathodes. (a) Cross-sectional SEM images of NCA electrodes after first charge/discharge process. Reproduced with permission [102]. John Wiley & Sons. [© 2021 Wiley-VCH GmbH]. (b) The equivalent stress inside the NCM particles after delithiation of cathode. (c) Illustration of solid-solid interface models and kinetics. Reproduced with permission [48]. Copyright 2020, Springer Nature, CC BY 4.0. (d) Reconstructed 3D structures of composite cathode before cycling and after 50 cycles. Reproduced with permission [107]. Copyright 2020, Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageTo facilitate the understanding of the mechanical degradation at materials level, Lou et al employed finite element modeling (FEM) to explore the stress evolution induced by chemomechanical impact within NCM polycrystalline particles (poly–NCM), as detailedly shown in figure 6(b) [48]. In consideration of the practical operation condition, theoretical models of poly–NCM in the SSBs were established by using aggregated primary grains with random orientations. Heterogeneous stress distribution can be clearly observed in the partially charged primary particles (50% SOC). Along the radial direction of the particles, stress was shown as a function of NCM particle radius. The anisotropic crystallographic orientation and Li+ transport are mainly attributed to the local stress along the grain boundaries. During delithiated process, core region with a higher Li content than outer shell induces an apparent gradient of Li concentration, resulting in compressive stress at the center and tensile stress near the surface for poly–NCM [103, 104]. Thus, the nonequilibrium in repeated delithiation/lithiation processes will destroy particle structure and accelerate the capacity decay of SSBs. Furthermore, the severe stress within CAMs will cause CAM–SE interface to form crack and delamination, and prevent Li+ from synchronously transporting across the solid–solid interface (figure 6(c)). In this case, mechanical stability is of particular importance and must be considered for designing and fabricating a high-performance composite cathode.
Meanwhile, for the electrode level, contact loss will not only form during the first charge process but also in the following cycles, which is also responsible for the continuing capacity fade in addition to side reactions and interphase formation at the interface [96, 105, 106]. Shi and Zhang have reported that many small voids are generated during the cold-pressing process [107]. However, after 50 cycles, the void morphology has changed substantially and the total void volume increased to 9.5% of the total volume, which is more than three times that in the pristine sample (figure 6(d)). These voids are also more connected and form large fake-like cracks near the cathode particles, resulting in a significant increase in interfacial resistance [108, 109]. Herein, surface adhesion as an important parameter, which means the binding state between CAM and SE in cathode, can be employed to evaluate the interface. It is a typical example of chemical–mechanical coupling that is necessary to understand the contributions from [110]: (a) mechanical strain deriving from the lattice mismatch between the contacted phase of CAMs and SEs, (b) the chemical interfacial energy originating from the difference in coordination and bonding at the interface in comparison to the bulk and (c) electrical attraction owing to interfacial charge reorganization. All togther, the unequilibrated charge distribution leads to the non-uniform stress field inside the CAMs, which further exacerbates CAM–SE interface, facilitating thegeneration of initiation, propagation of microcracks, contact loss, and high porosity within compsoite cathodes.
4. Manipulating the structure of composite cathode from nanoscale to macroscale
In light of the challenges described above, it is imperative to employ effective methods to achieve stable, robust, ionically/electronically conductive, and electrochemically/chemically favorable solid–solid interfaces at the cathode side. Recentky, substantial progress has been made at the cathode interfaces in SSBs. For instance, reconstructing CAM–SE interface structure at atomic scale within composite cathodes like the introduction of the protective coatings has been shown to improve the electrochemical stability of both CAMs and SEs. In addition, regulating the internal stress/strain of CAM is able to alleviate the mechanical failure concerns. More importantly, tailoring the architecture of composite cathodes from materials level to electrode level has been demonstrated as an effective way to build sufficient ionic/electronic transport pathways, so as to greatly boost the performance of SSBs.
4.1. Reconstructing CAM–SE interface structure at atomic scale
Coating as an important method is able to reconstruct the AM–SE interface structure while forming two new interfaces: (a) the coating–CAM interface and (b) the SE–coating interface. Since CAMs usually undergo substantial volume change during the delithiation/lithiation processes, an ideal coating layer can effectively confine strain by elastic deformation. However, conventionally physical coating enables the interface to have weak interaction with CAMs, which easily delaminates from the CAM upon the formation of crack or the contraction of CAM during the electrochemical process. Accordingly, the advanced coating technology based on interatomic interaction is a requirement toward mechanically 'plastic' and deformable interface structure. Wang et al established the chemical interaction between LCO and TiO2, in-situ forming a continuous Li2CoTi3O8 (LCTO) layer with relatively high lithium diffusion coefficient (8.22 × 10−7 cm2 s−1), low electronic conductivity (2.5 × 10−8 S cm−1), and stable 3D network of spinel structure, as shown in figure 7(a)[111]. As a consequence, the pristine LCO–Li10GP2S12 (LGPS) interface is replaced by two new interfaces LCTO–LGPS and LCO–LCTO. Particularly, LCTO coating has interatomic interaction with LCO and thus exhibits large work of adhesion for LCTO–LCO interface, suggesting a high chemical affinity between the two surfaces. In addition, LCTO–LGPS interface is not only electrochemically and thermodynamically more compatible but also has higher interfacial affinity than LCO/LGPS interface. Therefore, the ASSLB with a modified cathode shows significantly reduced interfacial impedance. It thus delivers a high capacity of 140 mAh g−1 and shows excellent cycling stability which retains 83% capacity after 200 cycles at 0.1 C. In comparison, the ASSB with a pristine cathode exhibits a capacity of 98 mAh g−1 and only delivers poor stability (22.4% capacity after 100 cycles at 0.1 C). Moreover, interposing coating layer is also an effective strategy to restrain the formation of space charge layer (SCL) to some extent, which is generally regarded as one of the reasons for the sluggish interfacial charge transport kinetics in SSBs [112, 113]. Particularly, chemical potential coupling strategy via coating dielectric materials, such as BaTiO3 nanoparticles, can establish the built-in electric field, greatly suppressing the SCL effect [114, 115].
Figure 7. Reconstructing AM–SE interface structures. (a) Schematic illustrating the in situ formation of the LCTO coating layer at the surface of LCO core at high temperatures. Reproduced with permission [111]. Copyright 2021, Royal Society of Chemistry. (b) Embedding AM particles within the grains of SE to establish seamless solid-solid electrode-electrolyte interface. Reproduced with permission [116]. Copyright 2019, Elsevier.
Download figure:
Standard image High-resolution imageApart from introducing a coating layer with chemical interactions with CAMs, it is of particular importance to construct a seamless interface between CAMs and SEs. Although the fabrication of CAM–SE interface with atomic interaction may not be feasible in commercialization, understanding the fundamental mechanisms at atomic scale is indispensable to offer crucial insights into enabling fabrication of a high-performance composite cathode. For example, Li et al prepared an epitaxial interface between 0.54Li2TiO3–0.46LiTiO2 (LLO) CAMs and perovskite SEs [116]. Such a seemingly impossible intimate contact between CAMs and SEs nearly surpasses the one-based solid-liquid contact (figure 7(b)). Therefore, with LLO embedded within the SEs matrix in such a way, the epitaxial composite electrode showed an outstanding rate capability comparable to the slurry-cast electrode composite in conventional LLIBs.
4.2. Regulating the internal stress/strain of cathode
As described in section 3.3, polycrystalline CAMs with randomly oriented grains undergo conspicuous internal stress during repeated delithiation and lithiation processes, as illustrated in figure 8(a), which not only causes the disintegration of the particles within secondary particles but also induces the contact loss between CAMs and SEs. In contrast, by microstructural manipulation of polycrystalline LiNi0.75Co0.10Mn0.15O2 (FCG75), the FCG75 with radially oriented rod-shaped grains is able to withstand the internal stress and keep mechanical integrity [33]. In addition, by making the concentration gradient of Ni, the Ni content at the surface of FCG75 is lower than internal, remarkably prevents the occurrence of interfacial side reactions. As a consequence, the SSB with FCG75 cathode delivers a high initial Coulombic efficiency (84.9%) at 0.1 C and excellent cycling stability (79.1% retention at 0.5 C after 200 cycles).
Figure 8. Regulating the internal stress/strain of composite cathodes. (a) Schematic representation of the different microstructural and interfacial evolutions after structural manipulation in all-solid-state batteries. Reproduced with permission [33]. John Wiley & Sons. [© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim]. (b) Relative volume change in unit cell versus the molar ratio of Co/(Ni + Co) for different layered cathode active materials. (c) Relative volume changes in unit cell during the electrochemical process for different layered cathode active materials. Reproduced with permission [39]. Copyright 2019, American Chemical Society.
Download figure:
Standard image High-resolution imageAlthough rationally designing microstructure of CAM is capable of keeping mechanical integrity, the certain stress/strain inside CAMs can have a cooperative effect and may lead to severe contact loss between CAMs and SEs. Thus, the most promising way is to design zero-strain CAMs to achieve excellent mechanical stability. For instance, Strauss et al prepared a quasi-zero-strain Co-rich NCMs, NCM271 (70% Co) and NCM361 (60% Co) [39]. As opposed to NCM811 and LCO, both NCM271 and NCM361 exhibit negligible volume change (<1%) during charging up to 4.5 V vs Li/Li+ (figure 8(b)). Pressure changes in operating SSBs with NCM271 and NCM361 are shown in figure 8(c), indicating no substantial changes in linear elastic stress during cycling processes, which is consistent with the volume changes. Consequently, the use of these quasi-zero-ztrain (active) CAMs can effectively prevent gap formation between the SE and CAM during SSBs operation.
4.3. Tailoring composite cathode architecture
As mentioned above, in LLIBs, cathode possessing a porous architecture is beneficial as Li ions can diffuse with liquid electrolytes to reach active materials. However, pores in SSBs are ionic blocking and thus a dense cathodic architecture is necessary to ensure continuous electronic and ionic pathways. Meanwhile, robust architectures of composite cathodes are able to accommodate the mechanical evolution associated with lithiation/delithiation of the CAMs [96, 105]. Therefore, optimizing composite cathode architecture from material level (microscale/mesoscale) and electrode level (macroscale) is important to achieve the goals of high energy density and power density for next-generation solid-state batteries. Especially, advanced manufacturing technology at electrode level is indispensible to optimize the architectures of the composite cathode. Therefore, a multi-objective optimization routine should be employed to manipulate the architecture of composite cathodes for maximizing CAMs loading, energy density, mechanical stability, and three-phase contact area, as well as minimizing void phases and tortuosity.
4.3.1. Material level.
To break through the dilemma of electrode architecture, 3D material configurations were proposed [20]. Such 3D structure effectively enhances the areal contact between the CAMs and SEs, enabling a robust electrode with interconnected ion transportation pathways. Thus, when the thickness of the electrode is increased, the mechanical integrity of 3D structure is not sacrificed [117]. For example, Yi et al prepared a composite cathode by infiltrating NCM622 into 3D porous Li7La3Zr2O12 (LLZO) scaffolds [118]. The unidirectional pores within this 3D scaffold promote the infiltration of components cathode and significantly shorten the Li-ion transport lengths, while the utilization of the soft ionically conductive materials within the scaffold guarantees a good mechanical stability among these components. With this unique design, the SSB achieves a reversible capacity of 125–135 mAh g−1 at 0.1 C and can surpass the energy density of state-of-the-art LLIBs by 1.8–2.6 times if the thick composite cathode is used. Similarly, Zhang et al designed a new electrode with 3D interpenetrating structure containing a NCM811 cathode, a tri-layer SE, and the Li anode, as illustrated in figure 9(a) [110]. Therein, NCM811 CAMs were filled in porous Li1.5Al0.5Ge1.5(PO4)3 (LAGP) layer, enabling high-loading CAMs (∼13 mg cm−2) due to the stable mechanical support porous layer.
Figure 9. Tailoring composite cathode architectures from materials level. (a) Schematic illustration of the 3D interpenetrating structure of electrode with high mass loading of NCM811 cathode. Reproduced with permission [159]. Copyright 2020, Elsevier. (b) Cross sectional SEM images of single- (b1–b2) and poly-crystalline (b3–b4) NCM composite cathode before and after electrochemical cycling. Reproduced with permission [60]. John Wiley & Sons. [© 2021 Wiley-VCH GmbH]. (c) Comparative simulation results of the Li+ ion density in cathode structure with NCM 60% (top) and NCM 80 wt% (bottom). Reproduced with permission [124]. John Wiley & Sons. [© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim]. (d) Cathode utilization according to both particle size and CAM volume loading. First-cycle voltage curves of SSBs using different-sized SE particles in the composite cathode with fixed MCM size (5 µm) and the ratio of CAMs (60 wt%). Reproduced with permission [91]. Copyright 2020, Wiley-VCH, CC BY 4.0.
Download figure:
Standard image High-resolution imageAdditionally, morphological control is also required in the CAMs themself. Commercial-grade NCM/NCA was susceptible to serious disintegration of the particles even at the first delithiation/lithiation process owing to the anisotropic volumetric strains from grains with random orientations. Consequently, emerging researchers are developing cracking-free single-crystalline NCM/NCA for practical SSBs [119, 120]. As shown in figure 9(b), no obvious change was observed for the composite cathode using single-NCA after the first delithiation. In contrast, severe internal cracks were clearly observed within poly-NCA particles, which originates from lattice shrinkage, associated with the detrimental H2–H3 phase transition at ⩾4.1 V vs. Li/Li+ [60, 102, 121–123]. Moreover, high energy and power density need appropriate electronic and ionic transport pathways within composite cathodes. Tuning the ratio of CAMs and SEs is an effective way to ensure the electronic and ionic networks inside the composite cathode. Park et al found that 80 wt% NCM within the composite cathode shows higher electron density than that of the 60 wt% NCM. However, in the composite cathode with 80 wt% NCM, the ionic pathways become more limited and localized than that of the 60 wt% NCM (figure 9(c)), significantly reducing the utilization of CAMs [124]. Thus, carefully designing the blending ratios of CAMs to SEs is of particular importance to ensure the high performance of SSBs.
In the case of particle size of CAMs, it also has a significant effect on the architecture of composite cathodes. For small particle sizes of CAMs, they possess better electronic contacts and short ionic pathways [125]. However, some studies have presented that the size of both the CAM and SE significantly influences the morphology of the pressed composite cathodes and full-cell performance [86, 125, 126]. An ideal morphology is able to guarantee good CAM–SE contact and minimal void space. Shi et al have found that the key to achieving high energy density is to increase the ratio of CAM to SE particle size (λ). While the λ is less than 1, ionic percolation always substantially worsens [82]. In any case, the CAM particle size should be kept larger than the SE particle size. By increasing the CAM particle size to 2–3 times larger than SE, the CAM utilization can be enhanced from 20% to 100% even at a high loading. As shown in figure 9(d), both decreasing the fraction of CAMs and increasing λ are able to consistently improve the ionic percolation in composite cathode. Consequently, a large value of λ is necessary to achieve high capacity with high mass loading. Nevertheless, the benefits of increasing λ to improve the CAM's utilization are strongly dependent on the fraction of CAMs: for 80% CAMs fraction in the composite cathode, enhancing λ from 1 to 3 can increase the utilization of CAMs from 30% to 60%; however, at 85% CAMs fraction, only slight increment of the utilization of CAMs from 16% to 20% is achieved even by controlling the λ from 1 to 8.
4.3.2. Electrode level.
Manufacturing technology determines the ionic/electronic, chemical, and mechanical properties of electrodes, and also decides their potential for scale-up. Advanced manufacturing technology can not only fabricate composite cathode at low cost but also establish advanced ionic/electronic networks and achieve stable mechanical stability in composite cathodes. Therefore, scalable manufacturing of composite cathode with advanced manufacturing technology is an essential part to further improve battery performances.
4.3.2.1. Solution infiltration for scalable manufacturing of composite cathodes.
For composite cathode in SSBs, large fractions of the SEs are typically necessary to ensure that all CAMs are homogeneously surrounded by SEs. However, the low fraction of CAMs severely limits the electrode-level energy density. In order to break the limitation of the conventional composite cathode, solution infiltration of SE into electrode was proposed. The SE solution with good wetting ability enables intimate contact with CAMs like in LLIBs and thus greatly enhances the electrochemical performance of SSBs [127–130]. Yao et al employed a solvent-mixing process to perform core–shell pyrene-4,5,9,10-tetraone (PTO)–Li6PS5Cl particles, which was demonstrated to be an effective way to manipulate the structure of electrode (figure 10(a)) [131]. Compared with the solvent-mixing method, dry mixing will form undesired architecture, substantially limiting the ionic diffusion kinetics. As a result, this solvent-assisted process increases the CAM fraction from 20% to 40 wt% in combination with the high utilization (97.6%). In addition, Jung et al prepared iodine-based Li-argyrodites in a new solution-processable way (figure 10(b)) [132]. Simultaneously, they applied solution-processed Li6.5P0.5Ge0.5S5I (LPGeSI) for the infiltration of LCO electrodes. It can be confirmed that LPGeSI makes intimate contact with LCO and generates negligible void spaces. Notably, the mass fraction of LPGeSI in the composite cathode was only 12 wt% and initial Coulombic efficiency is up to 88.0%. At the same time, the SE-infiltrated LCO electrodes show excellent cycling stability of 94.9% capacity retention at 0.2 °C after 100 cycles.
Figure 10. Tailoring composite cathode architectures from electrode level. (a) The cathodes prepared by two steps for dry- and solvent-mixed. (i) Mixing PTO AMs and Li6PS5Cl SEs in a dry or solvent-assisted methods. (ii) Powder compaction via uniaxial pressing. Reproduced with permission [131]. Copyright 2021, Elsevier. (b) Schematic of the infiltration of slurry-cast LCO cathode materials with LPGeSI–EtOH solutions and corresponding cross-sectional FESEM image and EDXS elemental maps. Reproduced with permission [132]. Copyright (2020) American Chemical Society. (c) The concept of 'all-electrochem-active' (AEA) electrodes: conventional SSBs (80 wt% AMs, anode: Li metal) (left); the proposed SSBs based AEA cathode (100 wt% AEA cathode, anode: Li metal) (right). (d) Li-ion diffusion coefficients of AEA electrode detected by the potentiostatic intermittent titration technique method compared with the typical SEs and available traditional cathodes. Reproduced with permission [135]. John Wiley & Sons. [© 2021 Wiley-VCH GmbH].
Download figure:
Standard image High-resolution image4.3.2.2. Melt infiltration for scalable manufacturing of composite cathodes.
Infiltrating low-melting-point SEs into the composite cathode is another promising way to control the electrode structure. Moderately elevating temperatures will make low-melting-point SEs in a liquid state and infiltrate the electrode. Then, this composite cathode is solidified after cooling. This method imitates the low-cost manufacturing of commercial LLIBs where dense electrodes could be produced in the ambient environment before electrode drying and electrolyte filling. As such, almost all the commercial equipment could be used for the fabrication of electrodes and SSBs, which significantly decreases the obstacle for industry adoption and enables the potential for scalable fabrication of SSBs. Moreover, rapid filling of molten SEs into dense cathode is easy to generate a uniform and conformal CAM–SE interface without porosity remaining. Therefore, such an approach is beneficial to attaining electrodes with high densification and CAM–SE interfaces with low resistances, without stress concentration, resulting in high volumetric energy density, high power density, and outstanding cycling performance. Xiao et al proposed the melt-infiltration technology for the disruptive and scalable fabrication of inorganic SSBs [130]. They firstly prepared NCM111 as a cathode material and Li4Ti5O12 as an anode, using commercial manufacturing of electrodes [130]. Subsequently, the low-melting-point Li1.9OHCl0.9 SE (∼300 °C) is quickly molten at 300 °C and rapidly infiltrates into the NCM111 and Li4Ti5O12 via the capillary effect. As a result, this ASSLB delivered a high reversible capacity of 150 mAh g−1 and over 80% capacity retention after 100 cycles at the current rate of 70 mA g−1. Additionally, this method was also employed in the production of Li2S–C cathodes where molten 3LiBH4–1LiCl was infiltrated and achieved outstanding cycling stability (∼80% capacity retention after 1000 cycles) with conversion-type cathodes.
4.3.2.3. All-electrochem-active cathode design for scalable manufacturing of composite cathode.
The equivalent capacity (
 and C represents the capacity of cathode) is defined to evaluate the electrode capacity. In conventional SSBs, they perform a low
and C represents the capacity of cathode) is defined to evaluate the electrode capacity. In conventional SSBs, they perform a low  owing to involving many non-electrochemical active components within the composite cathode, containing SEs and conductive agents, which are regarded as irreducible and indispensable parts of the construction of electronic/ionic transport network (figure 10(c)). As a function of reported data, the weight fraction of CAMs in the composite cathode for SSBs is less than 80 wt% [133, 134]. In addition, the introduction of SEs would induce various interfacial issues between CAMs and SEs. Therefore, designing an all-electrochem-active (AEA) electrode with high enough ionic and electronic conductivity can increase the volume and weight percentages of CAMs to 100%. To realize this concept, Li et al designed the crystal TM sulfides, i.e. chevrel-phase Mo6S8 and layer-structured TiS2, which not only possess high conductivity but also a very stable structure [135]. Specifically, both TiS2 and Mo6S8 have high electronic conductivity comparable to the conductivity additive (super P) and such high electronic conductivity allows TiS2 and Mo6S8 to abandon the use of conductive carbon in the cathode. Meanwhile, TiS2 and Mo6S8 also possess a high Li-ion diffusion coefficient (8 × 10−9–9 × 10−10 and 1.8–9.8 × 10−8 cm2 s−1), which is several orders of magnitude higher than that of the conventional cathode materials (LiCoO2: 10−11–10−12 cm2 s−1, NCM 2.8–8 × 10−11 cm2 s−1, LiFePO4: 6.8 × 10−16–1.8 × 10−14 cm2 s−11), and comparable to the SEs (Li6.25Al0.25La3Zr2O12, 1–1.1 × 10−8 cm2 s−11, Li10GeP2S12, 8.8–9 × 10−8 cm2 s−11) [136–139]. As a consequence, they can act as a SE rather than introducing excess SE in the cathode. Based on these physicochemical properties, the TiS2-based AEA-SSBs can exhibit an initial discharge capacity of 213 mAh g−1 and Mo6S8-based AEA–SSBs deliver a reversible capacity of 130 mAh g−1. To further demonstrate the advantage of AEA concept, a hybrid S8–Mo6S8 cathode was fabricated owing to the high theoretical capacity of S cathode. By matching S8–Mo6S8–AEA cathode with the Li metal, the SSBs can deliver the capacity of 483 mAh g−1, with volumetric and gravimetric energy densities of 2778 W h l−1 and 905.5 W h kg−1, respectively. Nagao et al also designed a novel Li-rich cathode materials Li2Ru0.8S0.2O3.2 [80Li2RuO3 · 20Li2SO4] through the amorphization of Li2RuO3 with Li2SO4, which enables the CAMs to have high ductility and conductivity for obtaining favorable interfaces, resulting in the stable operation of SSBs [27].
owing to involving many non-electrochemical active components within the composite cathode, containing SEs and conductive agents, which are regarded as irreducible and indispensable parts of the construction of electronic/ionic transport network (figure 10(c)). As a function of reported data, the weight fraction of CAMs in the composite cathode for SSBs is less than 80 wt% [133, 134]. In addition, the introduction of SEs would induce various interfacial issues between CAMs and SEs. Therefore, designing an all-electrochem-active (AEA) electrode with high enough ionic and electronic conductivity can increase the volume and weight percentages of CAMs to 100%. To realize this concept, Li et al designed the crystal TM sulfides, i.e. chevrel-phase Mo6S8 and layer-structured TiS2, which not only possess high conductivity but also a very stable structure [135]. Specifically, both TiS2 and Mo6S8 have high electronic conductivity comparable to the conductivity additive (super P) and such high electronic conductivity allows TiS2 and Mo6S8 to abandon the use of conductive carbon in the cathode. Meanwhile, TiS2 and Mo6S8 also possess a high Li-ion diffusion coefficient (8 × 10−9–9 × 10−10 and 1.8–9.8 × 10−8 cm2 s−1), which is several orders of magnitude higher than that of the conventional cathode materials (LiCoO2: 10−11–10−12 cm2 s−1, NCM 2.8–8 × 10−11 cm2 s−1, LiFePO4: 6.8 × 10−16–1.8 × 10−14 cm2 s−11), and comparable to the SEs (Li6.25Al0.25La3Zr2O12, 1–1.1 × 10−8 cm2 s−11, Li10GeP2S12, 8.8–9 × 10−8 cm2 s−11) [136–139]. As a consequence, they can act as a SE rather than introducing excess SE in the cathode. Based on these physicochemical properties, the TiS2-based AEA-SSBs can exhibit an initial discharge capacity of 213 mAh g−1 and Mo6S8-based AEA–SSBs deliver a reversible capacity of 130 mAh g−1. To further demonstrate the advantage of AEA concept, a hybrid S8–Mo6S8 cathode was fabricated owing to the high theoretical capacity of S cathode. By matching S8–Mo6S8–AEA cathode with the Li metal, the SSBs can deliver the capacity of 483 mAh g−1, with volumetric and gravimetric energy densities of 2778 W h l−1 and 905.5 W h kg−1, respectively. Nagao et al also designed a novel Li-rich cathode materials Li2Ru0.8S0.2O3.2 [80Li2RuO3 · 20Li2SO4] through the amorphization of Li2RuO3 with Li2SO4, which enables the CAMs to have high ductility and conductivity for obtaining favorable interfaces, resulting in the stable operation of SSBs [27].
5. Multi-scale characterization techniques for cathodes in SSBs
An in-depth understanding of microstructure evolution, multiscale ionic transport, interfacial reactions and mechanical properties in the composite cathode is the key to improving the electrochemical performances of SSBs, which needs advanced characterization techniques [140–142]. Nevertheless, the limited permeability of rigid electrodes poses insurmountable obstacles to characterize interface reactions and bulk structure by most conventional characterization methods. Despite these challenges, tremendous progresses in advanced characterization techniques have been achieved to detect cathodes from different scales [143]. By elucidating the complex relationships between physicochemical property and electrochemical performance, new mechanisms insights into composite cathode can offer guidelines for electrode and SSBs optimization. Since structure evolution, Li-ion transportand mechanical behavior in composite cathodes involve a multi-scale process, a combination of different tools with different spatial resolutions is of necessity to comprehensively probe the true behavior in an electrode.
5.1. Atomic and microscopic scale
It is a long-term dream for researchers to observe the physicochemical evolution of composite cathode at an atomic scale. High-resolution electron microscopy techniques enable us to detect the structure evolution at the atomic or microscopic scale [5, 144, 145]. Meanwhile, in combination with EELS analysis, it is able to detect the structural, chemicaland morphological information of electrodes. Gong et al performed in situ aberration-corrected scanning transmission electron microscope to uncover the evolution of the electronic and atomic structure of the spinel LiNi0.5Mn1.5O4 (LNMO) during the charging process in SSBs (figure 11(a)) [64]. It is found that the uneven extraction of Li+ induces localized migrations of TM ions and forms antiphase boundaries. Dislocations accelerate TM ions migration as well. By coupling EELS, Wang et al observed the charge transfer in SSBs (figure 11(b)) [146]. This microscale spectroscopic characterization demonstrated the severe ion diffusion and the formation of disordered phase between LiCoO2 and LiPON, which results in performance decay and high ion-transport resistance. Additionally, it is also important to understand the spontaneous ion transport at microscale over the CAM–SE interface owing to the abundant interface within composite cathodes. The nuclear magnetic resonance (NMR) technique has the prominent ability to probe the ionic exchange between different phases [147–149]. Recently, 2D Li-ion exchange NMR was employed to quantitatively evaluate the transport rate over the interface between the Li2S CAMs and Li6PS5Br SEs, offering new insight into the ion transport at the interface (figure 11(c)) [150]. These results demonstrate that preparing the composite cathode by ball milling and nanosizing CAMs can significantly speed up the spontaneous exchange of Li+ at the interface.
Figure 11. Atomic and microscopic scale characterizatin techniques for cathodes in SSBs. (a) Configuration of the all-solid-state battery fabricated by FIB and the atomic structure of LNMO at four different zone axes. Reproduced with permission [64]. Copyright 2018, Springer Nature, CC BY 4.0. (b) The in situ TEM for the characterization of STEM and EELS. Reproduced with permission [146]. Copyright 2016, American Chemical Society. (c) Solid state NMR techniques in the research of interfacial morphology and charge transport evolution and corresponding 2D-Exchange spectroscopy. Reproduced with permission [150]. Copyright 2018, Springer Nature, CC BY 4.0.
Download figure:
Standard image High-resolution image5.2. Meso- and macroscopic scales
Time-of-flight secondary-ion (SI) mass spectrometry (ToF-SIMS) is a semiquantitative technique to characterize the local enrichments of some fragments at interfaces [151, 152]. Moreover, it is feasible to reconstruct the depth profiles in 3D for the exhibition of the spatial fragment distribution (figure 12(a)). Walther et al demonstrated that NCM622–LPSCl composite cathode undergoes an interfacial phase change and elemental interdiffusion, which induces high interfacial resistance and deteriorates the cycle stability [153]. By 3D reconstruction of the cycled composite cathode, it was found that phosphates and sulfates play a significant role in the formation of an SEI within the composite cathode. Apart from interfacial chemical reactions, the distribution of CAMs and SEs, and mechanical behavior in the composite cathode are also crucial to the electrochemical performance of SSBs. SEM is a simple technique to observe the generation of contact loss and crack during electrochemical cycling [154]. However, it is a non-invasive characterization technique. To further insight the internal structural evolution at the 3D view, x-ray tomography was employed to show the 3D structure of the sample through nondestructive visualization. It allows the quantification and visualization of 3D morphological information, including spatial distribution, particle crackingand volume/contact area [155]. By visualizing various components of CAMs, SEs, pore space, and carbon black in the composite cathode, physical parameters of the real electrodes at different cycles are able to be quantified. Subsequently, the corresponding mathematical models are established and explain the observed degradation mechanism at meso- and macroscopic scales. In addition, with directly showing 3D morphological changes and geometrical properties by x-ray tomography, it can quantitatively map the distribution of Li-ion concentration and inhomogeneous composition in SSBs (figure 12(b)) [156, 157]. One disadvantage of x-ray tomography is the low contrast between the carbon and pores in electrode [108, 158]. Nonetheless, more accurate 3D information inside cathode could be obtained with the assistance of other techniques, such as the aforementioned ToF-SIMS.
Figure 12. Meso- and macroscopic scale characterizatin techniques for cathode in SSBs. (a) Three-dimensional reconstruction of the depth profile via time-of-flight secondary-ion mass spectrometry for the composite cathode. Reproduced with permission [153]. Copyright 2019, American Chemical Society. (b) 3D characterisation based on x-ray CT for the NMC cathode data: reconstructed volume of the cathode with different components represented by greyscale values (black: pore; dark grey: CBD; white: NMC AMs;); Simulated lithiation of the reconstructed composite cathode at 1.25 and 5 °C. Reproduced with permission [156]. Copyright 2020, Springer Nature, CC BY 4.0.
Download figure:
Standard image High-resolution image6. Conclusion and outlook
High-energy cathodes coupling with SEs and Li-metal anode are strongly considered as one of the most promising energy-storage devices with high energy density, adequate safety assurance, and long lifespan. However, the design and fabrication of an ideal composite cathode for high-energy-density SSBs still remains multiscale challenges mainly originating from surface/interface structure evolution at the atomic scale, multiscale charge transfer, and multiscale mechanical degradations. It is clear that various components and complex interface inside the composite cathode excludes single materials design. A promising research trajectory for cathode science in SSBs should link chemistry (atomic scale), materials (microscopic/mesoscopic scale), and electrode systems (macroscopic scale). Therefore, a multi-scale understanding of composite cathodes in SSBs is of particular importance to achieve composite cathodes with stable structure, high mass loading, and fast charge transport kinetics. On the basis of these comprehensive understandings and related analysis, reconstructing interface structure at the atomic scale, manipulating the internal stress/strain of cathode, and tailoring composite cathode architecture from materials level (microscale, mesoscale) to electrode level (macroscale) have been proven to be successful strategies for designing high-performance composite cathodes.
Although deeper insights have been made in improving the performances of composite cathodes in recent years, unremitting efforts are still needed to design and develop more efficient strategies to promote SSBs into practical applications. In addition to the main issues covered in this review, several aspects can be considered for future research:
- (1)Multifunctional interface design: Interface manipulation in composite cathodes is still the main research direction. The coating has been investigated to effectively suppress interfacial reactions and improve interfacial contact. In consideration of the possible surface structure transformation of CAMs such as lattice oxygen escape of Ni-rich CAMs at high voltage, coating CAM to simultaneously realize surface structure doping and uniform surface coating is beneficial, which subsequently improve surface structure stability and induce stable interface buffer layers to solve the interfacial side reactions and mechanical instability.
- (2)Energy-dense electrode fabrication: Owing to the intrinsic superiority of Li-rich Mn-based oxide cathode including high theoretical capacity (>350 mAh g−1), wide voltage windows (2–4.8 V vs. Li/Li+), low toxicity, and low cost, insertion-based Li-rich Mn-based oxide cathodes are promising candidates to achieve high energy density in SSBs (>500 Wh kg‒1). In the future, it is promising to explore this cathode material and corresponding modified strategies for highly compatible mechanics and chemistry/electrochemistry at the solid–solid interface inside the composite cathode. In addition, composite cathodes with high mass loading provide opportunities to achieve high energy, however, a trade-off exists between the electrochemical performances and thickness, which mainly lies in our incomprehensive understanding of ionic/electronic transport kinetics at multiscale. Solution-processable and melt-processable SEs with high ionic conductivity are very a promising research direction in the future, enabling these ionic conductors to percolate around CAMs for constructing an advanced ionic network with a low ratio of SEs. In this case, escalating the thickness of electrode with high energy and high power can be achieved.
- (3)In-operando characterizations of the composite cathode: The evolution of multiscale structure and charge transport inside the composite cathode has been comprehensively investigated by using various advanced characterization techniques like in-situ TEM and x-ray tomography. However, it is indispensable for SSBs to properly operate under certain pressure. Developing in-operando characterization techniques that couples mechanics and electric is a precise way to reveal the internal evolution in real-time, especially for the ionic transport at the interface. Therefore, the combination of mechanics and in-situ characterization techniques such as mechanics–TEM and mechanics–XRD is next-generation in-operando characterization techniques.
- (4)Advanced characterizations in temporal scale: Different diffusion length with specific relaxation features is able to be clearly identified in time scales. The characterizations of distribution of relaxation time can distinguish the time constant of the major electrochemical process, which can simplify the impedance analysis and substantially improve the accuracy for kinetics interpretation in time scale. Thus, time scale characterization is a promising power tool for complex electrode systems like composite cathodes.
- (5)Theoretical prediction: Future efforts could focus on the understanding of the charge transport rules at different scales. With the help of multiscale theoretical calculations like DFT (atomic scale), FEM (microscale), and phase field simulation (macroscale), it is very likely to shed light on accurately tracking the surface/interface evolutions at the atomic scale, multiscale charge transfer, and multiscale mechanical evolution, which provides guidelines for design an ideal composites cathode. In addition, the combination of experiment and simulation from nano to mesoscale level is also necessary to reveal the influence of crystal structure, particle size, the ratio of CAMs and SEs within electrode, porosity, and strain/stress on the electrochemical properties.
- (6)Safety prediction: Safety is a prerequisite for battery applications. Besides numerous methods to avoid risks, establishing a model and sensing device to predict the thermal runaway constitutes the last defense for the users. Furthermore, air and moisture stability related to electrolyte storage and calendar aging must also be considered.
The development of SSBs is reaching a critical time where many techniques are transiting from laboratory to manufacturing level. This transition faces enormous challenges. Consequently, an interdisciplinary effort to understand chemistry, material science, and energy storage systems from atomic scale to macroscopic scale are required to boost the progresses, shedding a fresh light in the practical applications of SSBs.
Funding
This work was supported by National Key Research and Development Program (2021YFB2500300),National Natural Science Foundation of China (22108151, 22075029, 21805161, 21808124, 21825501, 22109084, and U1801257), China Postdoctoral Science Foundation (BX2021135, 2021TQ0164, 2021M701827), Beijing Municipal Natural Science Foundation (Z20J00043), and the 'Shuimu Tsinghua Scholar Program of Tsinghua University'.
Conflicts of interests
The authors declare no conflict of interest.