-
PDF
- Split View
-
Views
-
Cite
Cite
Phyllis G Weintraub, Eitan Recht, Lilach Lily Mondaca, Ally R Harari, Beatriz Maria Diaz, Jude Bennison, Arthropod Pest Management in Organic Vegetable Greenhouses, Journal of Integrated Pest Management, Volume 8, Issue 1, January 2017, 29, https://doi.org/10.1093/jipm/pmx021
Close - Share Icon Share
Abstract
We present a comprehensive discussion of pest management in organic greenhouse vegetable production. Greenhouse structures and production practices vary greatly in different regions of the world. In northern Europe and North America, they are closed heated structures because of the long periods of cold weather and biological control is highly developed. In Israel, commercial greenhouses are made of netting or plastic, are not heated because the winters are generally mild in comparison with northern climes and hot in the summers, and biological control is used almost exclusively on some crops. In South America, greenhouses are simple structures covered with plastic material without nets or heating/cooling systems. We limit our discussion to properly closed and ventilated greenhouses, exclusive of structures that are opened for any period during the day or season. Our discussion covers greenhouse structure; the first line of defense, regulatory, and phytosanitary measures; various management methods; and finally specific management of primary pest groups, mites, thrips, hemipterans (aphids, mealybugs, and whiteflies), and small Lepidoptera.
Pest management in organic greenhouses is conceptually the same as pest management elsewhere; however, due to the limitations and restrictions in organic agriculture, the techniques and strategies used tend to focus on augmenting natural processes and hence focus on tactics that produce longer term effects. For example, the land for organic agriculture either must have never been used for crop production or there must have been a minimum of 3 (in some places 5) years without the use of any synthetic soil amendments, fertilizers, pesticides, genetically modified crops, or sewage sludge. The selection of available materials from the preceding list is far fewer than those for nonorganic Integrated Pest Management (IPM) programs, so decisions must be made more carefully. Finally, meticulous records and periodic on-site inspections ensure that regulations are adhered to and organic certification maintained.
In preparation for cultivation, cultural control methods such as soil solarization can be used in warm climates. Crop-free periods, while not an exclusive organic control method, can be used in extremely hot or cold climates to reduce pest levels. In addition to organic pesticides, semiochemicals (behavior-modifying compounds) and biopesticides (microbial control agents including entomopathogenic fungi, bacteria and viruses, and botanical biopesticides) are increasing in importance in organic production strategies. There are a limited number of organic pesticides available and most have short persistence on plants and in the environment. A primary management tactic is the manipulation of natural enemies: releases of commercially reared predators and parasitoids and occasional augmentation by providing alternative host plants and push-pull methods of directing pests away from economic crops and into areas where they are more vulnerable to management.
Greenhouse structures and production practices vary in different regions of the world. In northern Europe and North America, closed ventilated, heated structures are used because of the long periods of cold weather; crops are either planted directly in the ground or in growing media or in soil-free culture systems; and biological control is highly developed. In Israel, commercial greenhouses are covered with insect netting or plastic sheeting; are not heated because the winters are generally mild by comparison to northern climes and hot in the summers; crops are grown either in the ground or in soil-free culture systems on the ground or suspended, and biological control is used almost exclusively on some crops. In South America, commercial greenhouses are simple structures covered with plastic materials without nets or heating/cooling systems and biological control is generally underdeveloped but is growing in many countries and currently implemented in countries such as Brazil and Colombia (Bueno 2005). Vegetable crops are grown mainly in ground, but soil-free production methods are increasing for conventional crops with the use of hydroponic systems or Nutrient Film Technique in arid or semiarid regions due to the limited amount of water available or to poor local soils. In South America, hydroponics are mainly used in Brazil (Rodriguez-Delfin 2012).
The following review presents a comprehensive view of pest management in organic vegetable production. The discussion will be limited to closed structures that may have covered opening vents but whose walls are not opened during any part of the day. The authors represent perspectives from researchers, regulators, and practicing commercial biological control industry advisors.
Greenhouse Structure
Greenhouses are simply covered structures in which plants are grown. Initially and for hundreds of years, greenhouses were created to produce flowers and vegetables out of season. In cooler temperate climes, greenhouses are still primarily used for that purpose but also to produce higher quality produce. In southern temperate, subtropical and tropical climes, the primary purpose of greenhouses is for plant protection and to prevent immigration of pests. These structures range from having very simple passive heating to computer-controlled wall and roof openings, illumination, irrigation, and heating. Commercial greenhouses are constructed from a variety of building and cladding materials and in sizes from walk-in tunnels enclosing relatively few square meters to large structures of a hectare or more.
Solid walls, glass or plastic, are effective for retaining heat and are best used in cooler temperate climates. An unfortunate development in northern climes with short day lengths was the development and utilization of high-pressure sodium lamps. While these lights extended the growing season, they also allowed greenhouses to become warm incubators for a variety of arthropod pests all year round. At an International Organization for Biological Control meeting in Finland in 2005, there was a special submeeting for the purpose of discussing how to manage Bemisia tabaci (Gennadius), a warm-weather, Mediterranean pest. Johansen et al. (2011) have extensively reviewed the development of sodium lamps, light-emitting diodes, and other irradiance sources on plant-mediated effects on arthropods and potential alternative control methods.
Structures covered with netting or screening allow better ventilation and cooling than those covered with glass or plastic. Since these screens are used in warm temperate through tropical climates, mesh hole size and shape are important factors to consider to maintain ventilation. There is extensive literature (c.f., Fatnassi et al. 2006, Rigakis et al. 2015 and references therein) on the dynamics of air movement through insect screening. Table 1 provides a range of the screen hole sizes and the insects excluded from an academic and commercial perspective.
Insect exclusion screening hole-size recommendations
| Insect pest . | mm . |
|---|---|
| Aphids | 0.34–0.3411 |
| 0.266 × 0.8182 | |
| 0.266 × 0.8183 | |
| Whiteflies | 0.46–0.4621 |
| 0.266 × 0.8182 | |
| 0.230 × 0.9003 | |
| Dipteran leafminers | 0.61–0.641 |
| 0.266 × 0.8182 | |
| 0.530 × 0.5303 | |
| Thrips | 0.19–0.1921 |
| 0.150 × 0.1502 | |
| 0.135 × 0.1353 |
| Insect pest . | mm . |
|---|---|
| Aphids | 0.34–0.3411 |
| 0.266 × 0.8182 | |
| 0.266 × 0.8183 | |
| Whiteflies | 0.46–0.4621 |
| 0.266 × 0.8182 | |
| 0.230 × 0.9003 | |
| Dipteran leafminers | 0.61–0.641 |
| 0.266 × 0.8182 | |
| 0.530 × 0.5303 | |
| Thrips | 0.19–0.1921 |
| 0.150 × 0.1502 | |
| 0.135 × 0.1353 |
1Bethke and Paine (1991), 2Green-Tek (2015), and 3Stansly and Naranjo (2010).
Insect exclusion screening hole-size recommendations
| Insect pest . | mm . |
|---|---|
| Aphids | 0.34–0.3411 |
| 0.266 × 0.8182 | |
| 0.266 × 0.8183 | |
| Whiteflies | 0.46–0.4621 |
| 0.266 × 0.8182 | |
| 0.230 × 0.9003 | |
| Dipteran leafminers | 0.61–0.641 |
| 0.266 × 0.8182 | |
| 0.530 × 0.5303 | |
| Thrips | 0.19–0.1921 |
| 0.150 × 0.1502 | |
| 0.135 × 0.1353 |
| Insect pest . | mm . |
|---|---|
| Aphids | 0.34–0.3411 |
| 0.266 × 0.8182 | |
| 0.266 × 0.8183 | |
| Whiteflies | 0.46–0.4621 |
| 0.266 × 0.8182 | |
| 0.230 × 0.9003 | |
| Dipteran leafminers | 0.61–0.641 |
| 0.266 × 0.8182 | |
| 0.530 × 0.5303 | |
| Thrips | 0.19–0.1921 |
| 0.150 × 0.1502 | |
| 0.135 × 0.1353 |
1Bethke and Paine (1991), 2Green-Tek (2015), and 3Stansly and Naranjo (2010).
Insect exclusion screens are not all interchangeable. It has been demonstrated that screens with higher ultraviolet-absorbing properties are more effective barriers for a variety of pest insects (aphids, leafhoppers, thrips, whiteflies) and can reduce virus spread within a greenhouse (Antignus 2000, Weintraub et al. 2008, Kigathi and Poehling 2012, Legarrea et al. 2014). Since a significant proportion of insect vision is in the UV range (Briscoe and Chittka 2001), research has proceeded apace on the effects of UV deprivation on the ability of predators and parasitoids to function (Chiel et al. 2006, Weintraub 2009).
Phytosanitation
For many plant and animal pest species, greenhouses provide a plethora of food coupled with environmental conditions that favor their population development over time. In organic production, the easiest, least expensive, and most effective practice is to prevent pests from entering the greenhouse is phytosanitation. Internationally, regulations provide most national plant protection organizations with guidance and the measures available to restrict pest movement across borders and in trade (Ebbels 2003, Burgman et al. 2014). At the greenhouse level, phytosanitation practices include measures aimed at reducing the chance of pest establishment. These guidelines include: 1) double access doors (interlocking) to limit the entrance of arthropods (and improve thermal insulation). 2) Footbaths filled with disinfectants at points of access to the greenhouse, to reduce pathogen movement. 3) Washing stations with soap and water for sanitization of hands and agricultural implements before movement from one area to another and thus reducing secondary spread of pathogens. 4) Removal of weeds within and immediately surrounding the greenhouse that can be a source of both arthropod pests and plant pathogens. 5) Removal and disposal of infested and infected plant material that may harbor pests and pathogens. Disposal methods include burial, burning (although this is not permitted in some countries), or compositing at sufficient temperatures to kill pests and pathogens, and 6) avoiding conditions that will cause water condensation on the crop (e.g., overhead irrigation); since the environmental conditions in the greenhouse (high temperatures and relative humidity) favor the development of many diseases. These factors should be taken into account when choosing the irrigation systems and systems to control the greenhouse environment to prevent the dew point being reached (Hanan et al. 1978, Berlinger et al. 1999).
Cultural Control Methods
Soil or Growing Medium
The advantage of soil-free growing medium is that the incidence of soil-borne pests and pathogens is virtually zero since the growing medium is discarded after the growing season. There are several types of growing media, and they may be inert such as sand, perlite, and rock wool or natural products such as pine bark, coconut coir, or peat, all of which would require organic certification. Soil-free agriculture, as opposed to traditional cultivation, requires a constant supply of water and nutrients (Schnitzler 2003) and hence a more sophisticated irrigation and feeding system. It should be noted that in the United Kingdom, all organic agriculture must be in soil systems.
Amendments for sanitizing soils in organic greenhouses are few in number and often focus on nematode management (Oka 2010) or plant pathogens (Bailey and Lazarovits 2003). Vermicompost, produced through the breakdown of organic matter by earthworms and microorganisms can be used as an amendment to strengthen the plant and reduce pest levels. Vermicompost is high in microbial diversity, contains considerable amount of humic substances, and increases host plant resistance to pests, including aphids, mealybugs, and spider mites (Arancon et al. 2005, Arancon et al. 2007, Edwards et al. 2010). Organic crops that are grown in fertile soils with high organic matter and active soil biology generally exhibit a lower abundance of several pests due to a lower nitrogen content. (Altieri and Nicholls 2003).
Solarization
Solarization, is accomplished by covering soils with clear plastic to allow solar radiation to heat the soil, and can be an efficient and effective nonchemical treatment for pests and pathogen control in warm climates (Katan 1983). Moist heat is more effective than with dry soils, and the length of effective solarization varies according to location, soil type, and organic material (Lombardo et al. 2012). Hagimori et al. (2012) demonstrated that soil solarization killed eggs, larvae, and pupae of a variety of pests of organic cruciferous vegetables. Athalia rosae ruficornis Jakovlev (Hymenoptera: Tenthredinidae), the striped flea beetle (Phyllotreta striolata (Fabricius)) (Coleoptera: Chrysomelidae) and various Lepidoptera all have soil-borne stages, and these were eradicated by covering wet soil with transparent plastic film for 1 mo during the summer months. They recorded temperatures of 70, 50, and 45°C at soil depths of 0, 10 and 20 cm, respectively.
Lombardo et al. (2012) investigated the effect of solarization on plant parasitic nematodes, Meloidogyne spp. in tomato, Solanum lycopersicum L. greenhouses. Solarization of moist soil with incorporated organic supplement (e.g., compost or mulch) covered with clear plastic for 52 or 50 d reduced the number of infested plants to 6 and 5%, respectively, compared with 25 and 20% in untreated control. These researchers noted that the solarized greenhouses produced fruit with higher yield and quality compared with untreated and conventional soil treatments, when all other factors remained constant.
Mulching
Mulching is usually used for open fields where insects are not physically restricted and plants are subjected to unfiltered sunlight, but it also has utility in organic greenhouse production. Hagimori et al. (2012) demonstrated that 1.5-m wide plastic sheets circumscribing a greenhouse was effective in preventing weed infestation, and therefore, flea beetles associated with those weeds were prevented from entering the greenhouse.
Biological Control Methods
One characteristic shared by all major pests of greenhouse production is increasing pesticide resistance due to repeated application of the few pesticide groups available for use in organic greenhouses. This problem is exacerbated for organic growers because there are even fewer registered chemicals. The result of the declining availability of effective pesticides and the expanding market for organic produce (Willer et al. 2013) is driving the search for and development of effective biological control agents (BCAs). Today, commercial BCAs are commercially available around the world for the control for the major pest species of these groups (van Lenteren et al. 2017). In the simplest definition, biological control uses one or more organism against another. For the purposes of this article, the host organism is an arthropod and the BCA another arthropod or pathogen (bacteria, fungus, nematode, or protozoan).
Parasitoids, predators, and entomopathogens are widely used in biological control programs around the world. On occasion there may be negative interactions between BCAs in the form of intraguild predation (IGP), thus reducing the efficacy of pest control (Rosenheim et al. 1995). When IGP occurs, one or more predator species may feed on another predator in addition to the host, or a hyperparasitoid (secondary parasitoid) may reduce primary parasitoid populations, e.g., with aphid parasitoids. In addition to IGP, side effects of insecticides and fungicides on BCAs and natural enemies should be taken in account when planning and managing IPM programs, and these interactions must be carefully monitored.
Secondary Plants
Noncrop plants can serve functions in a greenhouse in the form of insectary plants and banker plants (see reviews: Parolin et al. 2012, Messelink et al. 2014). Insectary plants serve as a source of food, mating sites, and shelter for BCAs. These plants are frequently rich in extra floral nectaries and/or pollen and can serve to sustain natural enemies in periods when host populations are low (Messelink et al. 2014). Banker plants are open rearing units for producing large numbers of parasitoids and were first developed in the United Kingdom for control of the melon/cotton aphid, Aphis gossypii Glover in cucumber (Bennison and Corless 1993). Banker plants consisted of barley (Hordeum vulgare L.) infested with bird cherry-oat aphid, Rhopalosiphum padi (L.) to which Aphidius colemani Viereck were released (Fig. 1). Large numbers of parasitoids were produced on the banker plants, which then migrated to the crop and led to improved biological control of the pest aphid. Banker plants using barley infested with R. padi or wheat (Triticum sp.) infested with the English grain aphid, Sitobion avenae (F.) have also been used in sweet pepper, Capsicum annuum L., for control of green peach aphid, Myzus persicae (Sulzer) and the foxglove aphid, Aulacorthum solani (Kaltenbach), by A. colemani and Aphidius ervi Haliday or Aphelinus abdominalis (Dalman), respectively.
Aphdius colemani. (A) adult, photo credit: Alex Protasov. (B) Mummy in Myzus persicae, photo by Dovik Oppenheim.
In recent years, biological control of aphids on sweet pepper has been threatened by the increasing incidence of hyperparasitoids, including Asaphes spp. (Jacobson 2011, Messelink et al. 2014). High levels of hyperparasitism of the primary parasitoid ‘mummies’ has occurred in crops both with and without banker plants, but because the banker plants can be a source of large numbers of hyperparasitoids early in the season, the banker plant system is not widely used in the United Kingdom. Hyperparasitism of primary aphid parasitoids has also been recorded in other protected crops where banker plants are not used, including strawberry (Fragaria x ananassa Duchesne) and ornamentals and on outdoor organic lettuce (Bennison and Hough 2013, Bennison and Hough 2014), and research is needed to overcome this problem for sustainable biological control of aphids within IPM programs.
A number of different plants have been used to establish generalist predators and parasitoids. Sweet alyssum (Lobularia maritime L.) Desvaux and coriander (Coriandrum sativum L.) have been used in greenhouse pepper for the generalist predatory hoverfly, Sphaerophoria rueppellii (Wiedemann), (Hogg et al. 2011, Amoros-Jimenez et al. 2014). Sweet alyssum has a high sucrose:hextose ratio which is very attractive to hoverflies; however, more eggs were laid when hoverflies had access to coriander but larvae took longer to complete their development on coriander than on sweet alyssum.
For the anthocorid pirate bug, Orius sauteri (Poppius), a generalist predator of aphids, thrips, and whiteflies, the presence of black-eyed Susan Rudbeckia hirta L. in tomato greenhouses resulted in significantly higher densities of the predator than with other greenhouse insectary plants (Nagai and Hikawa 2012). When prey populations were low, the pirate bug was sustained on the black-eyed Susan and migrated to the tomato plants. Flowering alyssum plants have also shown promise for supporting populations of Orius laevigatus (Fieber) for thrips control in strawberry and could have potential for use in other crops (Fig. 2) (Bennison et al. 2011).
Supplementary Food
The presence of noncrop plants can disrupt planting and harvesting in the limited space in greenhouses; therefore, methods for providing supplementary food to BCAs are being developed (Jonsson et al. 2008, Messelink et al. 2014). Different types of food and methods of delivery have been shown to enhance BCA populations: flour moth eggs or brine shrimp cysts (Bonte and de Clercq 2008) and pollen (van Rijn et al. 2002, Weintraub et al. 2009) have received much attention in recent years. Artificial diets that are liquid based are somewhat problematic because of the sticky residue that remains on plants. Powdered factitious diets have been successful with the generalist predatory bug, Geocoris varius (Uhler) (Igarashi et al. 2013).
The provisioning of supplemental food is not without drawbacks. Some pests, such as thrips, also feed on pollen and their populations may be enhanced and IGP may occur (Shakaya et al. 2009). The development of a nutritious, easily applied factitious diets would be a significant development for the enhancement of BCAs in organic greenhouses.
Semiochemicals
Any chemical substance or mixture produced by an organism that conveys information to another is broadly termed semiochemical; thus, attractants such as allomones, kairomones, pheromone, and repellents are all forms of semiochemicals. Pheromone can be used for monitoring, mass trapping, or mating disruption. Additionally, secondary plant volatiles can be used as: plant traps, in push-pull strategies, or synthesized and used as attractants or lures (Harari et al. 2016). Today, there are a number of commercial lures that are used in association with various types of traps. Since the lures are naturally produced, they can be very sensitive and species specific (Sampson and Kirk 2013) and are compatible with many management practices.
Pesticides and Biopesticides
The list of amendments, fertilizers, and pesticides qualified for use in organic agriculture varies among countries or regions. In North America, the Organic Materials Review Institute has developed a list for Canada and the United States (Anonymous 2014a). In Europe, there are efforts underway to produce harmonized organic agriculture regulations among all of the countries by the end of 2017 (Anonymous 2014b). In South America, there is no single accreditation; however, growers exporting to the European Union must be certified by Naturland, BCS Oeko-Garantie, or the Institute fur Marktoekologie (IMO), and to North American it must be certified by The Organic Crop Improvement Association (OCIA) or Farm Verified Organic (FVO) (Garibay and Ugas 2009). In general, to qualify for use in organic crops, the active ingredient of a pesticide or biopesticides must be a derivative of a living organism (microbe, fermentation product thereof, or plant-based) and the formulation must have no added synthetic ingredients (Sieber et al. 2014). Additionally, nonsynthesized minerals (e.g., copper oxide, calcium hydroxide) are generally acceptable. Application does not require the use of special equipment; however, it is necessary to consider that these materials are often less persistent and degrade more quickly within the greenhouse environment than synthetic chemical insecticides. Therefore, these materials are commonly applied during environmental conditions favorable for their efficacy. For example, entomopathogenic fungi are best sprayed during periods of high relative humidity rather than periods of high UV radiation or at very high temperatures.
According to organic farming principles, biopesticides may be used as a curative tool for pest control. One group of active substances are secondary metabolites produced by plants, commonly known as botanical insecticides, such as antifeedants (Chandler et al. 2014). For example, neem oil extracted from the seeds of Azadirachta indica Adrien-Henri de Jussieu is currently the most widely used botanical compound to control insects in organic agriculture (including aphids on vegetable crops) in those countries where neem products are approved. The insecticide spinosad, based on secondary metabolites synthetized by soil actinomycetes, is approved for use in organic agriculture to control thrips, as well as Lepidoptera and Diptera. Spinosad is a mixture of two macrolide compounds from the soil bacterium, Saccharopolyspora spinosa Mertz & Yao, through fermentation (Mertz and Yao 1990). In European countries, approved spinosad products are obtained directly from microbial production, whereas in the United States and Switzerland, purified toxin is used (Zehnder et al. 2007). Other types of biopesticides are based on potassium salts of fatty acids (insecticidal soaps) which can provide some control of aphids and other soft-bodied arthropods by contact action, although thorough plant coverage is needed for optimal effect.
Management Scenarios for Key Arthropod Pests
Most crops can be infested by a wide range of pests that can cause severe economic damage. Mites have historically been a difficult pest group to control, and resistance to conventional acaricides has been reported beginning in the late 1950s. In the second half of the 20th century, outbreaks of aphids, whiteflies, mealybugs, and thrips have been the cause of major yield losses in greenhouse crops as a result of their direct feeding damage and/or the indirect effects of the viruses they can transmit to crops.
Acari
Tetranychidae
One of the most important mite pests of greenhouse crops is the two-spotted spider mite, Tetranychus urticae Koch because it infests many plant families and is cosmopolitan in distribution. This mite reproduces quickly and produces profuse webbing on the leaves and stems that can protect all life stages from pesticides and most predators (Gerson and Weintraub 2012). Resistance to conventional acaricides was reported more than 60 years ago, and there are no effective organic pesticides. The primary means of spider mite management is through the use of specialist predators such as Phytoseiulus persimilis Athias-Henriot, a fast-moving, voracious long-legged predator that can defeat the webbing (Gerson and Weintraub 2007). However, P. persimilis is efficacious only at high tetranychid densities and leaves when populations are low. In northern Europe, P. persimilis can coexist with other predatory mites such as Amblyseius andersoni (Chant), and attempts have been made to release multiple acarine predators including, Neoseiulus californicus (McGregor) (Fig. 3) and Amblyseius swirskii Athias-Henriot (see next paragraph). In contrast, in Chile, P. persimilis did not improve spider mite control in strawberries (Albendin et al. 2015). Recently there has been some success in managing spider mites with vermicompost in bush beans, Phaseolus vulgaris L., and eggplant, Solanum melongena L. (Arancon et al. 2007).
Neoseiulus californicus feeding on immature spider mite. Photo credit: Eric Palevsky.
Tarsonemidae
Two important tarsonemid pests are the broad mite, Polyphagotarsonemus latus (Banks) (Fig. 4) and the cyclamen mite Phytonemus pallidus (Banks). The former tends to occur more on vegetable crops and the latter on ornamental crops although the cyclamen mite is also known as the strawberry mite due to the extensive damage it can cause on strawberry. Both of these mites only feed on tender young leaves and meristematic tissue thereby causing plant deformity and stunting. In addition, both of these mites can be quickly and efficiently transported throughout a greenhouse by phoresy (by attaching themselves to another organism), especially on whiteflies (Zhang 2003). Fortunately, a number of phytoseiid predators are also of small size and can therefore physically reach and prey on tarsonemid mites to provide effective control. Predators include Neoseiulus cucumeris (Oudemans) (Fig. 5), N. californicus, N. fallacis (Garman), and A. swirskii (Zhang 2003, Gerson and Weintraub 2007, Weintraub et al. 2010). A. swirskii is a mite that originated from the east Mediterranean region and is considered a generalist predator feeding on many different small insects such as thrips larvae and mites as well as on pollen by piercing and draining their contents using its mouthparts. N. cucumeris is an important predator that feeds on small insects such as thrips larvae, mites, and pollen in northern Europe.
Female Polyphagotarsonemus latus and two eggs on Capsicum annuum. Photo credit: Phyllis G. Weintraub
Egg-bearing Neoseiulus cucumeris Photo credit: Phyllis G. Weintraub.
Eriophyidae
An additional group of important plant parasitic mites is the russet or gall mites. Although this group contains a large number of species, the only important greenhouse eriophyid is the tomato russet mite, Aculops lycopersici (Massee) which is host limited. This very small mite completes a generation in 1 wk and large populations rapidly develop. The pest can be treated with predatory mites including, N. cucumeris, A. fallacis, and A. swirskii when ‘hot spots’ appear (Brodeur et al. 1997, Park et al. 2010). However, the sustained biological control on tomato has not been achieved due to the toxic nature and variety of glandular trichomes (van Houten et al. 2013). Recent research has shown that Amblydromalus limonicus (Garman & McGregor) has good potential for control of tomato russet mite (van Houten et al. 2017). Table 2 lists commonly available predators for general mite control.
Commonly available biological control agents for pest management with recommendations summarized from numerous commercial and research sources for the purpose of general comparisons
| Target pest . | BCA type . | BCA species . | Release rate1 . | Species attacked . | Comments . |
|---|---|---|---|---|---|
| Herbivorous mites | Predators | Amblyseius (Neoseiulus) cucumeris | 100/m2 | Aculops lycopersici, Polyphagotarsonemus latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present |
| Amblyseius fallacis | 100/m2 | A. lycopersici | Good for treating ‘hot spots’, will not establish on tomato plants | ||
| Amblyseius swirskii | 100/m2 | A. lycopersici, P. latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present | ||
| Galendromus occidentalis | 100/m2 | Tetranychus spp. P. latus, Phytonemus pallidus | High temperature, low R.H., efficacious at low spider mite populations | ||
| Macrolophus pygmaeus | 1–5/ m2 | Tetranychus spp. | For solanaceous crops | ||
| Neoseiulus californicus | 100/m2 | Many species | Tolerant of high temperatures | ||
| Phytoseiulus persimilis | 100/m2 | Only Tetranychus spp. | Not efficacious at low spider mite density, not tolerant of high temperature | ||
| Stethorus punctillum | 100/m2 | Tetranychus spp. | Moderate to high temperatures | ||
| Thrips | Predators | Neoseiulus cucumeris | 25–100/m2 | All species | Feeds on first and second instar thrips, also consumes pollen |
| Gaeolaelaps aculeifer | 100–500/m2 | Soil-dwelling arthropods | Requires moist soil, temperatures > 15°C. Feed on prepupae and pupae in ground | ||
| Orius laevigatus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey, consumes pollen, moth eggs, etc. | ||
| Orius insidiosus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey consumes pollen and other arthropods | ||
| Steinernema feltiae, S. carpocapsae | 500,000/m2 | Mainly controls ground-dwelling stages. Requires soil temperature > 10°C | |||
| Aphid | Parasitoids | Aphelinus abdominalis | 1–4/m2 | Wide range of species | High temperature tolerant |
| Aphidius colemani | 1–4/m2 | Small-sized aphids, e.g., Muzus persicae, Aphis gossypii | Banker plant friendly | ||
| Aphidius ervi | 1–4/m2 | Large-sized aphids, e.g., Macrosiphum euphorbiae, Aulacorthum solani | Often used with A. colemani. Active at cool temperatures | ||
| Aphidius matricariae | 1–4/m2 | Green peach aphid | Active at cool temperatures | ||
| Predators | Aphidoletes aphidimyza | 1–10/m2 | All species | Moderate temperature and RH | |
| Adalia bipunctata | 8–50/m2 | Wide range of species | Best at high aphid populations | ||
| Chrysoperla carnea | 10–50 eggs/m2 | Larvae feed primarily on aphids | Best at moderate RH (<75%) | ||
| Chrysoperla ryfilabris | 50 eggs/m2 | Larae feed primarily on aphids | Tolerates high RH (>75%) | ||
| Hippodamia convergens | 22/m2 | All species, generalist predator | Moderate temperatures | ||
| Whitefly | Parasitoids | Encarsia formosa | 1–10/m2 | Trialeurodes vaporarorium, Bemisia tabaci | Moderate temperatures |
| Eretmocerus mundus, E. eremicus | 1–9/m2 | T. vaporarorium, B. tabaci | Attacks second to fourth nymphal stages | ||
| Predators | Delphastus catalinae | 1–50/m2 | Many species | Highly efficient in moderate to high temperatures | |
| A. swirskii | 25–100/m2 | T. vaporarorium, B. tabaci eggs and larvae | Tolerant of high temperature | ||
| M. pygmaeus, M. caliginosus | 1–5/m2 | T. vaporarorium, B. tabaci | For solanaceous crops, slow development at low temperatures or low pest populations | ||
| Nesidiocoris tenuis | 1–5/m2 | Generalist predator | For solanaceous crops, slow development at low temperatures or low pest populations |
| Target pest . | BCA type . | BCA species . | Release rate1 . | Species attacked . | Comments . |
|---|---|---|---|---|---|
| Herbivorous mites | Predators | Amblyseius (Neoseiulus) cucumeris | 100/m2 | Aculops lycopersici, Polyphagotarsonemus latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present |
| Amblyseius fallacis | 100/m2 | A. lycopersici | Good for treating ‘hot spots’, will not establish on tomato plants | ||
| Amblyseius swirskii | 100/m2 | A. lycopersici, P. latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present | ||
| Galendromus occidentalis | 100/m2 | Tetranychus spp. P. latus, Phytonemus pallidus | High temperature, low R.H., efficacious at low spider mite populations | ||
| Macrolophus pygmaeus | 1–5/ m2 | Tetranychus spp. | For solanaceous crops | ||
| Neoseiulus californicus | 100/m2 | Many species | Tolerant of high temperatures | ||
| Phytoseiulus persimilis | 100/m2 | Only Tetranychus spp. | Not efficacious at low spider mite density, not tolerant of high temperature | ||
| Stethorus punctillum | 100/m2 | Tetranychus spp. | Moderate to high temperatures | ||
| Thrips | Predators | Neoseiulus cucumeris | 25–100/m2 | All species | Feeds on first and second instar thrips, also consumes pollen |
| Gaeolaelaps aculeifer | 100–500/m2 | Soil-dwelling arthropods | Requires moist soil, temperatures > 15°C. Feed on prepupae and pupae in ground | ||
| Orius laevigatus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey, consumes pollen, moth eggs, etc. | ||
| Orius insidiosus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey consumes pollen and other arthropods | ||
| Steinernema feltiae, S. carpocapsae | 500,000/m2 | Mainly controls ground-dwelling stages. Requires soil temperature > 10°C | |||
| Aphid | Parasitoids | Aphelinus abdominalis | 1–4/m2 | Wide range of species | High temperature tolerant |
| Aphidius colemani | 1–4/m2 | Small-sized aphids, e.g., Muzus persicae, Aphis gossypii | Banker plant friendly | ||
| Aphidius ervi | 1–4/m2 | Large-sized aphids, e.g., Macrosiphum euphorbiae, Aulacorthum solani | Often used with A. colemani. Active at cool temperatures | ||
| Aphidius matricariae | 1–4/m2 | Green peach aphid | Active at cool temperatures | ||
| Predators | Aphidoletes aphidimyza | 1–10/m2 | All species | Moderate temperature and RH | |
| Adalia bipunctata | 8–50/m2 | Wide range of species | Best at high aphid populations | ||
| Chrysoperla carnea | 10–50 eggs/m2 | Larvae feed primarily on aphids | Best at moderate RH (<75%) | ||
| Chrysoperla ryfilabris | 50 eggs/m2 | Larae feed primarily on aphids | Tolerates high RH (>75%) | ||
| Hippodamia convergens | 22/m2 | All species, generalist predator | Moderate temperatures | ||
| Whitefly | Parasitoids | Encarsia formosa | 1–10/m2 | Trialeurodes vaporarorium, Bemisia tabaci | Moderate temperatures |
| Eretmocerus mundus, E. eremicus | 1–9/m2 | T. vaporarorium, B. tabaci | Attacks second to fourth nymphal stages | ||
| Predators | Delphastus catalinae | 1–50/m2 | Many species | Highly efficient in moderate to high temperatures | |
| A. swirskii | 25–100/m2 | T. vaporarorium, B. tabaci eggs and larvae | Tolerant of high temperature | ||
| M. pygmaeus, M. caliginosus | 1–5/m2 | T. vaporarorium, B. tabaci | For solanaceous crops, slow development at low temperatures or low pest populations | ||
| Nesidiocoris tenuis | 1–5/m2 | Generalist predator | For solanaceous crops, slow development at low temperatures or low pest populations |
1Release rates vary from prophylactic/low pest populations to curative/high pest populations/hot spot.
Commonly available biological control agents for pest management with recommendations summarized from numerous commercial and research sources for the purpose of general comparisons
| Target pest . | BCA type . | BCA species . | Release rate1 . | Species attacked . | Comments . |
|---|---|---|---|---|---|
| Herbivorous mites | Predators | Amblyseius (Neoseiulus) cucumeris | 100/m2 | Aculops lycopersici, Polyphagotarsonemus latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present |
| Amblyseius fallacis | 100/m2 | A. lycopersici | Good for treating ‘hot spots’, will not establish on tomato plants | ||
| Amblyseius swirskii | 100/m2 | A. lycopersici, P. latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present | ||
| Galendromus occidentalis | 100/m2 | Tetranychus spp. P. latus, Phytonemus pallidus | High temperature, low R.H., efficacious at low spider mite populations | ||
| Macrolophus pygmaeus | 1–5/ m2 | Tetranychus spp. | For solanaceous crops | ||
| Neoseiulus californicus | 100/m2 | Many species | Tolerant of high temperatures | ||
| Phytoseiulus persimilis | 100/m2 | Only Tetranychus spp. | Not efficacious at low spider mite density, not tolerant of high temperature | ||
| Stethorus punctillum | 100/m2 | Tetranychus spp. | Moderate to high temperatures | ||
| Thrips | Predators | Neoseiulus cucumeris | 25–100/m2 | All species | Feeds on first and second instar thrips, also consumes pollen |
| Gaeolaelaps aculeifer | 100–500/m2 | Soil-dwelling arthropods | Requires moist soil, temperatures > 15°C. Feed on prepupae and pupae in ground | ||
| Orius laevigatus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey, consumes pollen, moth eggs, etc. | ||
| Orius insidiosus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey consumes pollen and other arthropods | ||
| Steinernema feltiae, S. carpocapsae | 500,000/m2 | Mainly controls ground-dwelling stages. Requires soil temperature > 10°C | |||
| Aphid | Parasitoids | Aphelinus abdominalis | 1–4/m2 | Wide range of species | High temperature tolerant |
| Aphidius colemani | 1–4/m2 | Small-sized aphids, e.g., Muzus persicae, Aphis gossypii | Banker plant friendly | ||
| Aphidius ervi | 1–4/m2 | Large-sized aphids, e.g., Macrosiphum euphorbiae, Aulacorthum solani | Often used with A. colemani. Active at cool temperatures | ||
| Aphidius matricariae | 1–4/m2 | Green peach aphid | Active at cool temperatures | ||
| Predators | Aphidoletes aphidimyza | 1–10/m2 | All species | Moderate temperature and RH | |
| Adalia bipunctata | 8–50/m2 | Wide range of species | Best at high aphid populations | ||
| Chrysoperla carnea | 10–50 eggs/m2 | Larvae feed primarily on aphids | Best at moderate RH (<75%) | ||
| Chrysoperla ryfilabris | 50 eggs/m2 | Larae feed primarily on aphids | Tolerates high RH (>75%) | ||
| Hippodamia convergens | 22/m2 | All species, generalist predator | Moderate temperatures | ||
| Whitefly | Parasitoids | Encarsia formosa | 1–10/m2 | Trialeurodes vaporarorium, Bemisia tabaci | Moderate temperatures |
| Eretmocerus mundus, E. eremicus | 1–9/m2 | T. vaporarorium, B. tabaci | Attacks second to fourth nymphal stages | ||
| Predators | Delphastus catalinae | 1–50/m2 | Many species | Highly efficient in moderate to high temperatures | |
| A. swirskii | 25–100/m2 | T. vaporarorium, B. tabaci eggs and larvae | Tolerant of high temperature | ||
| M. pygmaeus, M. caliginosus | 1–5/m2 | T. vaporarorium, B. tabaci | For solanaceous crops, slow development at low temperatures or low pest populations | ||
| Nesidiocoris tenuis | 1–5/m2 | Generalist predator | For solanaceous crops, slow development at low temperatures or low pest populations |
| Target pest . | BCA type . | BCA species . | Release rate1 . | Species attacked . | Comments . |
|---|---|---|---|---|---|
| Herbivorous mites | Predators | Amblyseius (Neoseiulus) cucumeris | 100/m2 | Aculops lycopersici, Polyphagotarsonemus latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present |
| Amblyseius fallacis | 100/m2 | A. lycopersici | Good for treating ‘hot spots’, will not establish on tomato plants | ||
| Amblyseius swirskii | 100/m2 | A. lycopersici, P. latus | Will not establish on tomato plants, but will readily establish on sweet peppers if prey or pollen present or on cucumber if prey is present | ||
| Galendromus occidentalis | 100/m2 | Tetranychus spp. P. latus, Phytonemus pallidus | High temperature, low R.H., efficacious at low spider mite populations | ||
| Macrolophus pygmaeus | 1–5/ m2 | Tetranychus spp. | For solanaceous crops | ||
| Neoseiulus californicus | 100/m2 | Many species | Tolerant of high temperatures | ||
| Phytoseiulus persimilis | 100/m2 | Only Tetranychus spp. | Not efficacious at low spider mite density, not tolerant of high temperature | ||
| Stethorus punctillum | 100/m2 | Tetranychus spp. | Moderate to high temperatures | ||
| Thrips | Predators | Neoseiulus cucumeris | 25–100/m2 | All species | Feeds on first and second instar thrips, also consumes pollen |
| Gaeolaelaps aculeifer | 100–500/m2 | Soil-dwelling arthropods | Requires moist soil, temperatures > 15°C. Feed on prepupae and pupae in ground | ||
| Orius laevigatus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey, consumes pollen, moth eggs, etc. | ||
| Orius insidiosus | 1–10/m2 | All species | Feed on nymphs and adults. In absence of prey consumes pollen and other arthropods | ||
| Steinernema feltiae, S. carpocapsae | 500,000/m2 | Mainly controls ground-dwelling stages. Requires soil temperature > 10°C | |||
| Aphid | Parasitoids | Aphelinus abdominalis | 1–4/m2 | Wide range of species | High temperature tolerant |
| Aphidius colemani | 1–4/m2 | Small-sized aphids, e.g., Muzus persicae, Aphis gossypii | Banker plant friendly | ||
| Aphidius ervi | 1–4/m2 | Large-sized aphids, e.g., Macrosiphum euphorbiae, Aulacorthum solani | Often used with A. colemani. Active at cool temperatures | ||
| Aphidius matricariae | 1–4/m2 | Green peach aphid | Active at cool temperatures | ||
| Predators | Aphidoletes aphidimyza | 1–10/m2 | All species | Moderate temperature and RH | |
| Adalia bipunctata | 8–50/m2 | Wide range of species | Best at high aphid populations | ||
| Chrysoperla carnea | 10–50 eggs/m2 | Larvae feed primarily on aphids | Best at moderate RH (<75%) | ||
| Chrysoperla ryfilabris | 50 eggs/m2 | Larae feed primarily on aphids | Tolerates high RH (>75%) | ||
| Hippodamia convergens | 22/m2 | All species, generalist predator | Moderate temperatures | ||
| Whitefly | Parasitoids | Encarsia formosa | 1–10/m2 | Trialeurodes vaporarorium, Bemisia tabaci | Moderate temperatures |
| Eretmocerus mundus, E. eremicus | 1–9/m2 | T. vaporarorium, B. tabaci | Attacks second to fourth nymphal stages | ||
| Predators | Delphastus catalinae | 1–50/m2 | Many species | Highly efficient in moderate to high temperatures | |
| A. swirskii | 25–100/m2 | T. vaporarorium, B. tabaci eggs and larvae | Tolerant of high temperature | ||
| M. pygmaeus, M. caliginosus | 1–5/m2 | T. vaporarorium, B. tabaci | For solanaceous crops, slow development at low temperatures or low pest populations | ||
| Nesidiocoris tenuis | 1–5/m2 | Generalist predator | For solanaceous crops, slow development at low temperatures or low pest populations |
1Release rates vary from prophylactic/low pest populations to curative/high pest populations/hot spot.
Thysanoptera
Thripidae
Thrips species represent a diverse collection of lifestyles including fungivorous, phytophagous, and predatory. The plant-feeding species are believed to have evolved from fungus-feeding thrips that to this day represent ca. 50% of the known species. Greenhouse pest species are in the family Thripidae. The life cycle includes eggs that are laid in the plant tissue that hatch to produce two larval instars, a prepupal stage, and one pupal stage that develops into the adult. In the main pestiferous species, development and reproductive rate are strongly influenced by temperature with a generation time span from egg to adult of about 9–17 d under warm conditions (25 to 30°C) depending on host crop. The main pest species are polyvoltine, moving between available host plants as long as there are suitable climate conditions.
Only a few species of thrips are responsible for significant losses in yield and quality on protected crops. All of the following thrips species discussed here are major pests causing direct damage as well as being competent vectors of tospoviruses (e.g., tomato spotted wilt virus and impatiens necrotic spot virus) except for Scirtothrips dorsalis Hood (Chen and Chiu 1996, Kliot et al. 2016). Frankliniella occidentalis (Pergande), known as the western flower thrips, originated from the western United States and has spread worldwide. F. occidentalis has a very broad host-plant range, is strongly thigmotactic, and is often found deep in flowers and under the calyx of solanaceous plants. Thrips tabaci Lindeman, onion thrips, is of Mediterranean origin and is now cosmopolitan in distribution. Although onion and leek are preferred host plants, onion thrips can infest a wide range of vegetable, ornamental, and weed species and their host range includes plants from about 25 families. In addition to F. occidentalis, T. tabaci is considered a major pest of greenhouse crops such as cucumber and sweet pepper. T. tabaci causes a typical silvering feeding pattern on foliage and flowers. Thrips palmi Karny, melon thrips, originated in southeast Asia and has spread throughout the southern hemisphere. Melon thrips is a polyphagous species and is considered an important pest on ca. 20 plant families but is best known for its preference to Cucurbitaceae and Solanaceae species such as sweet pepper, cucumber, and eggplant in greenhouses. Damage usually consists of a silvery-bronzed appearance on leaves and fruit. T. palmi are also known to feed on stems and flowers (among the petals and developing ovary). Shoots of damaged plants become stunted and fruit scarred and deformed. S. dorsalis, chilli thrips, also originated in southern Asia and has spread into the northern hemisphere as far north as Israel and the southern United States.
There are a number of BCAs commercially available for thrips management, Table 2. Predatory phytoseiid adults and nymphs can feed only on the first or second stage thrips immatures but cannot attack adults due to their small size (see Mites above). After release, the predatory mites aggregate on high-density patches of F. occidentalis immatures where they lay eggs. Predatory mites have the ability to suppress thrips populations, especially if applied early in the season as a preventative measure. However, once F. occidentalis have established and developed large populations curative releases are not successful.
The minute pirate bugs, Orius spp., are generalist anthocorid predators that consume small arthropods as well as pollen. As with other Hemiptera, their mouthparts are of the piercing sucking type, with the rostrum inserted into the prey’s body to remove fluids. Species of Orius are commonly associated with flowers of herbaceous plants, where they feed extensively on thrips, and therefore, a number of Orius species are considered important BCAs in greenhouses. Both adults and nymphs feed on F. occidentalis adults and larvae, with adults consuming five to 20 thrips a day (van Lenteren, 2003). Due to their variable diet, Orius spp. are used preventively in the greenhouse on flowering crops (e.g., on sweet pepper) where they feed on pollen before the thrips begin to infest and then persist in the greenhouse long after thrips populations have been reduced. Orius spp. are known to feed on other predators, including phytoseiid mites, when prey populations are low (Shakaya et al. 2009).
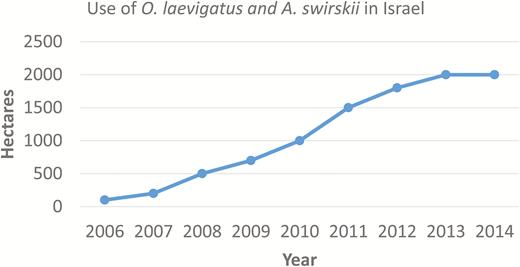
There are five commercially available Orius species the most widely used being O. laevigatus (used in Europe, North Africa, Asia, and Israel) (Fig. 2), O. insidiosus (Say) (used in Europe exclusive of the United Kingdom, and mainly North America and Latin America), and O. majusculus (Reuter) (used mainly in Europe). The combined use of two predators, O. laevigatus and A. swirskii, against thrips in recent years has increased steadily in Israel and this trend; is representative of the situation worldwide (Fig. 6).
Use of a predatory Hemiptera (Orius laevigatus) and mite (Amblyseius swirskii) for thrips management in organic greenhouses in Israel (Pollination Services Yad Mordechai, Israel marketing evaluation).
Hemiptera
Aphididae
Aphids are small, soft-bodied insects that constitute one of the major pests in protected crops. In greenhouse environments, aphids are parthenogenetic and viviparous, building up large populations in a short period. These hemimetabolous insects develop multigenerational colonies on plants that confer an advantage to natural enemies such as some parasitoids and predators because searching time is reduced. There are two types of adult morphs: wingless (apterous) and winged (alate), depending on several factors mainly temperature, crowding, and nutritional conditions of the host plant.
Only a few polyphagous and cosmopolitan aphids are the primary species responsible for losses in yield and quality on protected vegetable crops. M. persicae is common on greenhouse pepper, eggplant, and lettuce, and A. gossypii frequently infests cucurbits, pepper, and eggplant. Macrosiphum euphorbiae Thomas, the potato aphid, is a common pest in protected vegetable crops such as tomato, pepper, and other solanaceous plants and on lettuce. These three aphid species can act as vectors for persistent and nonpersistent viruses in addition to causing direct plant damage. A. solani causes direct damage by feeding on pepper, due to their highly toxic saliva that may produce deformation and yellow discoloration of the leaves resulting in complete plant defoliation and deformed fruits. In recent decades, Nasonovia ribisnigri (Mosley) has become a major pest of protected lettuce, Lactuca sativa L. crops. N. ribisnigri deposits their offspring near the young terminal growth of lettuce and colonizes the inner leaves inside the developing heads, causing leaf distortion and vigor reduction in seedlings. At harvest, N. ribisnigri presents a significant cosmetic problem because the presence of living aphids on the leaves reduces the market value or makes lettuce unmarketable (Palumbo 2000).
Pepper and cabbage, Brassica oleracea L., plants grown in soil amended with 20% vermicompost have shown lower numbers of M. persicae adults and nymphs and lower number of alates alighting on them (Arancon et al. 2005, Arancon et al. 2007, Little et al. 2011). Similar results were observed for M. persicae infesting plants fertilized with animal manure; however, the opposite effects were observed for the specialist cabbage aphid, Brevicoryne brassicae (L.) (Stafford et al. 2012).
Physical barriers such as nets can be used around vents in solid structure greenhouses and act as exclusion barriers between the plant and the pest and in consequence are an effective tool to limit the entrance and dispersal of aphids on protected vegetable crops. In addition, the use of UV-blocking plastic materials, reflective and colored mulches applied on the soil can reduce numbers of aphids landing on and colonizing or spreading viruses within greenhouses (Avilla et al. 1997, Diaz et al. 2006, Messelink et al. 2014).
Approved practices for biological control of aphids in organic greenhouses are based on routine preventive releases of parasitoids, followed by predators or entomopathogens (International Federation of Organic Agriculture Movements 2014). Aphid parasitoids usually consume all or most of the aphid body, killing its host at the final nymphal stage and then pupae. At this stage, the dead aphid takes on a beige, brown, or black color and swollen appearance, commonly known as a mummy, which is easily recognizable within an aphid colony (Fig. 1B). Free-living adult wasps emerge from the pupae and begin a new generation of parasitoids. The adult wasps require nectar, pollen, or honeydew as food. If banker plants are used to augment numbers of parasitoids, and to act as a preventative measure, timing of their introduction into the greenhouse is crucial to achieve successful control of aphid populations. In a recent study (in Argentina), the introduction of a banker plant system was compared with inoculative releases of A. colemani in Eruca sativa Miller (arugula) and C. annum greenhouses. The banker plant strategy was the most efficient method to control M. persicae on arugula; however, no significant differences between these two strategies were found on pepper crops (Andorno and López 2014). Pineda and Marcos-Garcia (2008) introduced aphid-infested barley as banker plants in pepper greenhouses and concluded that they were also effective in attracting natural populations of aphidophagous hoverflies to control aphids.
The main parasitoids of aphids are A. colemani (attacks A. gossypii and M. persicae), A. ervi, and A. abdominalis (attack M. euphorbiae and A. solani). Other common wasp species parasitizing aphids in protected crops are Lysiphlebus testaceipes (Cresson) and Praon volucre (Haliday). Mummies of P. volucre are easily recognizable from other parasitoids species because the dead aphid remains attached on a white silk cocoon, while the parasitoid is developing within it. Parasitoid mixes containing up to six species of parasitoids (A. colemani, Aphidius matricariae Haliday, A. ervi, A. abdominalis, P. vulucre, and Ephedrus cerasicola Stary) are now commercially available, and these mixes can be very useful in a wide range of crops where several aphid species can occur and thus need a range of parasitoid species for control (Bennison et al. 2012, Dassonville et al. 2015).
It should be noted that the efficacy of any parasitoid species could vary depending on a number of factors. For example, and focusing only on A. colemani, Prado et al. (2015) showed that stressors on different trophic levels could affect fitness and efficacy of the parasitoid. Biotic factors such as crop morphology, crop quality, production of volatile organic compounds, pest density, presence of other natural enemies, including hyperparasitoids and abiotic factors such as temperature, humidity, and lighting in the greenhouse must be considered. Recently, even the type of grain used in banker plants for A. colemani has been shown to affect the parasitoid efficacy (McClure and Frank 2015).
Ladybirds, lacewings, hoverflies, and the gall midge, Aphidoletes aphidimyza (Rondani), represent the main aphid predators. Predatory insects differ from parasitoids in that they catch and feed on their prey, and their juvenile stages require several to many prey individuals to attain maturity. Several species of ladybirds are commonly found naturally in protected crops, such as Adalia bipunctata (L.), Coccinella septempunctata (L.), and Hippodamia spp. Lacewings are generalist predators feeding on various pests affecting greenhouse vegetables crops; however, they have a preference for aphids as was observed for Crysoperla carnea (Stephens) larvae when aphids and thrips were provided simultaneously (Shrestha and Enkegaard 2014). Hoverfly larvae also feed on aphids by puncturing the cuticle and imbibing their content, whereas adults are diurnal flower visitors feeding on nectar and pollen (van Rijn et al. 2002, Biesmeijer et al. 2006, Hickman et al. 2010). Table 2 lists commonly available parasitoids and predators for general aphid management.
Entomopathogenic fungi attack the integument of a variety of sucking insects, including aphids. Species of fungi-infecting aphids belong mainly to the Entomophthorales and Hypocreales. Entomophthorales are obligate pathogens and have a narrow host range of arthropods causing death of aphids by the invasion of different tissues and organs. Hypocreales are generalist pathogens that also cause fungal diseases on aphids, but they combine a parasitic phase with a saprophytic phase colonizing the body after the death, which is mediated by toxin production. Within the Entomophthorales, Pandora neoaphidis (Remaudière & Hennebert) Humber is the most abundant naturally occurring aphid-pathogenic fungus and frequently cause epizootics in several protected crops in Argentina (Scorsetti et al. 2010). In addition, other species of Entomophthorales such as Zoophthora radicans (Brefeld) Batko, Conidiobolus obscurus (Hall & Dunn) Remaudière & Keller, and Entomophtora planchoniana Cornu were found killing M. persicae and N. ribisnigri in lettuce greenhouses in Argentina (Scorsetti et al. 2007). Several species of Hypocreales such as Beauveria bassiana (Balsamo-Crivelli) Vuillemin, Paecylomices sp., Metarhizium anisopliae (Metchnikoff) Sorokin and Lecanicillium lecanii R. Zare & W. Gams were observed infecting aphids (Goettel and Glare 2010, Samson et al. 1988).
As was mentioned previously, aphids produce large quantities of sugar-rich honeydew, which is very attractive for ants. Ants actively milk/stroke (Offenberg 2001), herd/move (Way 1954, Ho and Khoo 1997), and protect (Way 1954, Banks and Macaulay 1967, Mansour et al. 2012) honeydew-producing aphids in a symbiotic relationship. These behaviors can be highly disruptive to biological control because ants fend off predators and parasitoid as well as translocate the aphids to other parts of the plant. However, ants can be managed in organic crops with baits, pheromone disruptors, or insecticides. Boron compounds are often used as an abrasive but can be incorporated into baits. Borate (disodium octaborate tetrahydrate), boric acid, or sodium borate in sugar water can disrupt activity for tens of meters from the trap through trophallaxis (Greenberg et al. 2006, Sola et al. 2013). Additionally, ants can be managed by disruption of trail pheromone (Suckling et al. 2008). Finally, pyrethrum, diatomaceous earth, and silicon dioxide could be applied for management.
Aleyrodidae
The lifecycle of a whitefly is comprised of an egg, four nymphal stages, and winged adults. The first instar, termed ‘crawler’, has well-developed legs and walks short distance in search of suitable settling sites. The crawler is the only active nymphal stage and once settled then molts. The next three sessile nymphal instars feed on the phloem tissue available within reach of their stylets throughout their development. Unlike the gradual metamorphosis of other Hemiptera, whitefly metamorphosis is intermediate. During the latter part of the fourth instar, colloquially referred to as the ‘red-eyed nymph’ and technically a puparium, the four wings and antennae of the adults develop inside the pupal case. Two whiteflies specie have been the focus of many studies due to the extensive damage they cause to protected crops. These are the sweetpotato whitefly, B. tabaci and the greenhouse whitefly, Trialeurodes vaporariorum Westwood. These two species can be easily distinguished as the nymphs of T. vaporariorum have long hair-like projections while B. tabaci does not, and B. tabaci adults hold their wings in a roof-like manner over their body whereas T. vaporariorum holds its wings more horizontally at rest (Gerling et al. 2016).
There are a number of BCAs commercially available for whitefly management, Table 2. A. swirskii is a predatory phytoseiid mite (see Thrips above). The first published study on its biology showed that A. swirskii can be maintained on a diet of B. tabaci eggs and young larvae; however, it was not proven successful as a BCA for B. tabaci in the greenhouses of Spain until early 2000s (Calvo et al. 2015). A. swirskii are able to establish on many vegetable crops before pests are present because they feed on pollen. Since A. swirskii is adapted to warmer and humid subtropical climates, it may be less effective in cooler dry conditions. Currently, A. swirskii is marketed in more than 20 countries worldwide including Europe, Africa, North America, Latin America, and Asia.
The predatory mirid bug, Macrolophus pygmaeus, originated in the Mediterranean region. It is a highly polyphagous predator feeding on several soft-bodied pests such as whiteflies, aphids, mites, thrips, and moth eggs with a preference for whitefly eggs and nymphs. The predator has long legs which allow movement even on hairy leaves. The life cycle includes eggs that are oviposited deep in the plant tissue, five nymphal stages, and the adult. To ensure the predator’s population establishment in the greenhouse, it is important to make releases early in the season. However, populations of the predator need to be carefully managed since once prey populations are low they can feed on the crop to be protected causing premature fruit and flower drop and direct damage to fruit (Castane et al. 2011).
The two primary aphelinid parasitoid wasps used for whitefly management in greenhouses are Encarsia formosa Gahan, a solitary endoparasitoid (which lays its eggs and develops inside the body of the host) and Eretmocerus mundus (Mercet) (Fig. 7) (which lays eggs under the nymph with subsequent larvae penetrating and developing in the whitefly nymph). E. mundus is indigenous to the Mediterranean Basin and is one of the most effective natural enemies of B. tabaci, although it is also known to parasitize and control T. vaporariorum in greenhouses. Both species can be released as parasitized whiteflies affixed to cards, and E. mundus can also be spread as loose parasitized pupae mixed in sawdust. Eretmocerus eremicus Rose & Zolnerowich is used instead of E. mundus because in some European countries, including the United Kingdom, E. mundus is not approved for release.
Pseudococcidae
There are a few polyphagous mealybug species with worldwide distribution that cause damage to greenhouse crops, yet these species are not generally considered key pests in organic greenhouses. In South America, Phencoccus solenopsis Tinsley was first recorded in northern Argentina on sweet pepper (Granara de Willink 2003) and 2 yr later in tomato in northern Brazil (Culik and Gullan 2005) but does not cause economic damage. In northern Europe, mealybugs are not generally pests in organic greenhouses although they occasionally occur in tomatoes. The species discussed below do cause economic damage in organic herbs and peppers in parts of Israel.
The citrus mealybug, Planococcus citri (Risso), is highly polyphagous and considered the most cosmopolitan and destructive species of the family (Blumberg and van Driesche 2001). In recent years, the citrus mealybug has infested organic herbs: including: tarragon, Artemisia dracunculus L., basil, Ocimum basilicum L., mint, Mentha sp. and arugula, Eruca sativa Miller in the Israeli regions of Judea and Samaria/West bank. Phenacoccus solenopsis Tinsley, is also a polyphagous species from all zoogeographic regions of the world. In Israel P. solenopsis infests organic sweet pepper in the Shfela region of south-central Israel. Phenacoccus solani Ferris, is commonly found in the New World, expanding from the Mediterranean into Israel in 2003 (Ben-Dov 2005). No males have been found, so it has been assumed to reproduce parthenogenetically. In recent years, P. solani has increased its presence and while it causes damage in organically grown tarragon, mint, and sweet peppers, it is easily controlled through the management of ants (see Aphididae above).
Biological control of mealybugs is difficult due to their cryptic behavior, clumped distribution pattern, protective wax secretion, and protection by ants (Buckley and Gullan 1991, Franco et al. 2009, Daane et al. 2012, Hayon et al. 2016). As with other pest species, the international plant trade has been a major contributor to the invasive status of many multivoltine mealybug species, which are able to develop on a wide range of host plants (Franco et al. 2009, Hayon et al. 2016).
In Israel, additional BCAs are used against mealybugs such as the encyrtid parasitoids Leptomastix algirica Trjapitzin for the management of Ph. solenopsis and Ph. solani mealybugs and Anagyrus sp. near pseudococci against Pl. citri.
Lepidoptera
Gelechiidae
Tuta absoluta (Meyrick), the tomato leaf miner, is native to South America. The moth invaded Europe through Spain in 2006 and since then has spread to the Mediterranean Basin, the Middle East, and Europe. T. absoluta is a major pest of tomato, although there is evidence that it can complete its life cycle on other Solanaceae plants (Desneux et al. 2010). The pest status of T. absoluta changed from being a local South American pest to being a key pest that threatens the world tomato industry (Desneux et al. 2011). T. absoluta established in greenhouses in western Europe regardless of the cold winters typical of this region. The supercooling point of the adult pest is −17.8°C and −18.2°C for the larvae and at 0°C, 10% of the larvae may survive for more than 3 wk and adults may survive more than 1 mo (van Damme et al. 2015). There is no evidence of an obligatory diapause (Potting et al. 2013), and if food is available, the pest may still be active in winter. Maintaining greenhouses free of tomato long enough to eradicate the pest is not commercially feasible in northwestern Europe.
Since T. absoluta was detected in the Mediterranean Basin, a few indigenous predators and parasitoids have been reported. Predators include the zoophytophagous predators Nesidiocoris tenuis (Reuter) and M. pygmaeus (Rambur) whose nymphs prey on T. absoluta eggs and adults consume eggs and larvae (Arnó et al. 2009, Urbaneja et al. 2009). Egg parasitoids as Trichogramma spp., are also known especially T. pretiosum Riley and T. achaeae (Nagaraja & Nagarkatti). Several parasitoids species of various families were recorded from T. absoluta larvae in the Mediterranean basin (Sánchez et al. 2009, Urbaneja et al. 2012).
Microbial agents, such as the entomophathogenic bacteria Bacillus thuringiensis (Bt), are used in greenhouses against T. absoluta first instar larvae. In some cases, weekly repeated applications have resulted in good control of the pest (Molla et al. 2011). The combination of Bt with B. bassiana has also demonstrated better results than when either was applied alone (Torres-Gregorio et al. 2009). T. absoluta larvae and pupae were also found to be susceptible to three species of entomopathogenic nematodes (Batalla-Carrera et al. 2010). These microbial agents may be used in combination with other control agents in IPM programs against the pest.
The female sex pheromone is often used for mass trapping of males although when used alone the method was not effective enough in reducing moth population and damage to the crop (Cocco et al. 2012). The pheromone has also been used for mating disruption, but studies in protected crops revealed mixed results (Cocco et al. 2013). The failure to achieve effective control of the pest using the pheromone can be attributed to the efficacy of the formulated pheromone, the initial pest population level, isolation of plots from invading males and gravid females (Cardé and Minks, 1995), and the moth mating behavior (Lee et al. 2014, Harari et al. 2015). In T. absoluta, the males and females mate more than once. Male sensitivity to the pheromone assures efficacy in mate finding and remating and female remating tendency increases the chances of encountering high-quality males.
Trials using the above mentioned methods, natural enemies, and semiochemicals are currently being studied to manage T. absoluta (Arno et al. 2009, Mollá et al. 2011). Reduced crop damage was observed a few years after the first invasion of the pest to the new region but is likely attributed to increased grower awareness of the pest biology and behavior. Additionally, damage reduction has been the result of augmentation of opportunistic native natural enemies (Mollá et al. 2011) and applications of microbial agents such as foliar-applied Bt.
Noctuidae
Lacanobia oleracea (L), the bright-line brown-eye moth, can be a major pest of organic sweet pepper, due to larval feeding on leaves and internally within fruit. The pest can be difficult to control with foliar Bt products because only early instars are sensitive and Bt must be consumed by larvae prior to entering the fruit.
If a greenhouse is properly closed, larger lepidopterans pests should not gain access. However, there are several polyphagous predator species that contribute to the control of lepidopteran eggs and larvae, such as, O. laevigatus, N. tenuis, C. carnea, and Nabis spp. Microhymenopteran parasitoids such as Cotesia spp., Hyposoter didymator (Thungberg), and Chelonus oculator (Linnaeus) are also effective. Microbial insecticides based on B. thuringiensis var. aizawai, on nucleopolyhedrosus virus and certain formulations of spinosad have been approved for use in organic certified crops.
Conclusions
Growers of most agricultural and horticultural crops use IPM strategies, which is now required of all conventional European Union farmers under the Sustainable Use Directive (SUD). Within this framework, organic pest management relies heavily on the use of preventative control measures because there are few registered organic pesticides. These preventative measures include soil amendments such as vermicompost which is rarely used in conventional greenhouse management. Preventative measures can extend to establishing BCAs in the form of open banker plants before pests establish. Therefore, biological control is extensively relied upon as a primary pest management technique.
Organic farming, although accounting for only a small total percentage of production, is increasing both in area farmed and in consumer demand; since 1990 there has been double-digit growth according to the USDA (Greene 2015). The primary reason for consumers preferring organic produce is the perception that it is healthier, lacking chemicals used in conventional food (Padel and Foster 2005, Hughner et al., 2007). Furthermore, there is research supporting the adaptation of organic agriculture as a means of mitigating climate change; organic soils have reduced N2O emissions and higher carbon sequestration in crop land (Scialabba and Muller-Lindenlauf 2010). These researchers also noted that organic growers can realize incomes higher than conventional growers in countries that promote climate-friendly farming practices.
Further research in organic greenhouse production remains and includes the following proposed research areas:
Ventilation in tropical and subtropical climates needs improvement to reduce plant pathogens and could be addressed with innovative cladding material that will still exclude most arthropod pests.
Predator and parasitoid populations could be enhanced through the use of supplemental food while eliminating the need for secondary plants.
The search for more efficacious predators and parasitoids needs to continue for
a) Pests that currently have no commercial BCAs,
b) Species that poorly establish in greenhouses, and
c) Species that can tolerate a range of environmental situations.
4. Elucidation of new and selective pesticides that can be registered for organic production, with improved and innovative delivery systems.
Acknowledgments
We thank the following people for donating photographs: Alex Protasov and Eric Palevsky with the Agricultural Research Organization, and Dovik Oppenheim from Kibbutz Dafna.
References Cited
Author notes
(deceased 18 April 2017)