-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah N. Bevins, Kerri Pedersen, Mark W. Lutman, Thomas Gidlewski, Thomas J. Deliberto, Consequences Associated with the Recent Range Expansion of Nonnative Feral Swine, BioScience, Volume 64, Issue 4, April 2014, Pages 291–299, https://doi.org/10.1093/biosci/biu015
Close - Share Icon Share
Abstract
Feral swine (Sus scrofa) have been repeatedly introduced to locations around the world. Aided by both an adaptable biology and deliberate introductions by people, the range of invasive feral swine in the United States has expanded from 17 to 38 states over the past 30 years. The swine's generalist diet combined with high population densities can complicate efforts to conserve threatened and endangered species, and losses from crop damage and livestock predation in the United States alone are estimated to be more than $1 billion. In addition, feral swine can be a reservoir for multiple pathogens, some of which are zoonotic. Management responses to mitigate these threats by reducing population numbers face resistance from groups that value feral swine for subsistence or sport hunting, which results in complicated policy actions that are extremely divisive and difficult to implement.
The problems associated with invasive, feral swine populations are not new. Vitousek and colleagues (1996) stated that feral swine may be the single most damaging introduced species in national parks and reserves of the United States. Elton (1958) listed pigs as a threat to island species. Worldwide, their history as an introduced species goes back even further, with societies transporting them to new ranges for thousands of years. Early Polynesian settlers of Hawaii brought pigs with them when they originally settled the islands around 300 or 400 CE (Kirch 1982). In the continental United States, feral swine populations are believed to have arrived in present-day Florida with the Spanish explorers Ponce de Leon and Hernando de Soto in the early 1500s, at which point pigs either escaped or were allowed to roam free (Mayer and Brisbin 2008). Although there are limited data on any potential ecological impacts in these early areas of introduction, feral swine's establishment and their eventual spread were probably not entirely benign.
Feral swine are members of the Suidae family. This family includes 16 extant species, but invasive feral swine are often a combination of escaped or released domestic pigs and undomesticated wild boar (Goedbloed et al. 2013), which are considered to be members of a single species, Sus scrofa. Since S. scrofa contains domestic and nondomestic counterparts, along with multiple additional subspecies, feral swine can have a striking range of phenotypic variation and life-history traits. Therefore, feral swine genetics can run along a continuum, and populations often contain hybrids of the domestic and wild phenotypes. In recent genomewide single-nucleotide polymorphism work on populations of European wild boar, Goedbloed and colleagues (2013) concluded that genetic introgression from domestic pigs is more recent and more common than was expected, given that domestic pig farming in developed countries generally occurs in biosecure facilities. Indeed, this genetic variation is represented by the broad differences in physical appearance (Mayer and Brisbin 2008) that can be seen in feral swine populations (figure 1), even within the same social group (or sounder). This morphological variation includes well-documented variation in coat color and bristle length but also includes differences in skull characteristics, such as nasal length and zygomatic breadth (Genov 1999, Mayer and Brisbin 2008).
Trail camera image taken of a feral swine sounder in Ohio, demonstrating the dramatic phenotypic differences that exist in wild populations, which can contain escaped or released domestic pigs, undomesticated boars, and hybrids of the two. Photograph: Craig Hicks, US Department of Agriculture.
Despite the range of variation seen within this one species, suids do possess traits that are readily generalized, including a rapid reproduction rate and an adaptable biology. Feral swine are omnivores with a generalist diet that is both flexible and opportunistic. In many regions, they primarily subsist on grasses, legumes, and herbs, but they will also readily root for seeds and other plant matter. Female S. scrofa reach breeding age in less than a year and can have more than one litter of 4–10 piglets annually (Choquenot et al. 1996). These traits, combined with limited predation in areas where predator populations have been greatly reduced, can lead to rapid population growth. The historic native range of S. scrofa is believed to have spanned from North Africa; across Europe, Asia, and Japan; and down into present-day Indonesia (Mayer and Brisbin 2008), which speaks to the species’ ability to live in a variety of climates and habitat types. These attributes, along with its propensity to thrive in human-altered landscapes—cropland and areas containing artificial water sources created by damming and irrigation—have helped speed S. scrofa's establishment and spread. The United States has seen a dramatic increase both in feral swine population numbers and in their geographic distribution over the past 30 years. Long-established populations in focal regions of the Southeast have expanded to the point that feral swine populations are now known to exist in a majority of states (Gipson et al. 1998). Europe, Japan, and Australia have also documented a steady increase in feral swine and wild boar populations (Spencer and Hampton 2005, Acevedo et al. 2007, Saito et al. 2012).
In this review, we attempt to summarize the impacts associated with the recent range expansion of feral swine. As an invasive species, feral swine bring with them a unique range of issues, including the ability to substantially affect agricultural production, the capacity to transmit disease to livestock and humans, and the potential to complicate the protection of threatened and endangered species. Management and policy actions designed to mitigate the issues associated with feral swine are complex, in part because increases in feral swine populations are the result of human-mediated introductions. Further complicating this picture is the fact that they are already an established invasive species, which makes eradication infeasible at the scale of the entire continental United States. Successful management actions will require the use of multiple techniques for population control. In addition, robust data on feral swine population numbers and locations are extremely limited, which highlights the need for continued research.
Impacts on agriculture and livestock
There are long-established associations between feral swine and agricultural crop damage. Genov (1981) analyzed the stomach contents of wild boar in Poland and documented the consumption of 131 species of plants and animals. Although feral swine are known to feed on both plants and animals, plant matter made up 91% of the stomach contents in that study. Plant material is considered the species’ primary food source, and mast from beech, oak, and other species have repeatedly been shown to be the foundation for feral swine and wild boar diets in multiple parts of the world (see Barrios-Garcia and Ballari 2012 for a thorough review); however, mast seeding is a seasonal and variable event, which requires feral swine to exploit other food sources, including agricultural crops. Cultivated crops, including potatoes, oats, maize, and rye, accounted for 71% of the plant matter identified by Genov (1981).
Feral swine's reliance on and subsequent destruction of crops in many regions have been thoroughly described (see Campbell and Long 2009 for a thorough review), with feral swine densities being fourfold higher when they are located near cropland (Caley 1993). Feral swine in Queensland, Australia, were documented to have consumed more than 20 metric tons of sugarcane per year (Tisdell 1982). Damage in the United States is often associated with row crop rooting (figure 2), but damage can also be caused by the creation of wallows near water sources on farms and ranches (Campbell and Long 2009). Impacts on forest plantations are also well documented; feral swine often root up seedlings or browse on the roots of recently planted trees. These activities can cause regeneration failure and can also severely limit recruitment in forest ecosystems (Campbell and Long 2009). It is generally agreed that feral swine do not consume large amounts of agricultural crops when natural resources are abundant (Geisser and Reyer 2005, Barrios-Garcia and Ballari 2012); however, the recent explosive feral swine population growth around the world has resulted in high population densities in optimal habitat. New populations have also been established in habitats that were previously classified as of poor quality. Supplemental feeding, additional land being used for agricultural production, and irrigation have allowed populations to flourish where they were previously absent. These conditions have brought about an increase in the scale and dollar value of crop damage (Mengak 2012).
Row crop damage in Kansas from feral swine. Photograph: John Johnson and Jason Kloft, US Department of Agriculture.
Few robust estimates exist on the cost of agriculture losses related to feral swine damage each year, and all of the available figures are broad approximations, but estimates suggest that losses in Australia alone amount to more than $100 million annually (Choquenot et al. 1996). Pimentel (2007) estimated the combined annual costs of damage and control in the United States at $1.5 billion, although these numbers were extrapolated from estimates generated at a much smaller scale. Economic impacts are often associated with crop loss but also include the loss of livestock to feral swine predation, as well as the cost of control measures and repairs incurred by both government agencies and individual landowners. A limited estimate of crop loss, as well as damage to roads, fencing, and trails in California, was estimated at $1.7 million in 1996 (Frederick 1998), but not all of the participants responded, and swine populations are now substantially larger and more widespread. A more recent survey in Texas revealed that feral swine damage averaged $7515 per landowner surveyed, with an additional $2631 spent on repairs (Adams et al. 2005). Impacts on agricultural crops in Georgia were recently estimated to be $57 million per year (Mengak 2012). These region-specific economic impacts make it difficult to accurately assess the monetary losses associated with feral swine at the scale of an entire country; however, these surveys do reflect the challenges that expanding feral swine populations pose to agricultural and livestock producers.
Although the year-round diet of feral swine is primarily composed of plant material, they are opportunists and will also eat other organisms, including earthworms, rodents, turtles, reptiles, and ground-nesting birds. Feral swine in coastal areas are often seen on beaches, where their diet can consist almost entirely of crabs during seasons when other food options are scarce (Baron 1982). This dietary flexibility can lead to clashes with livestock producers. Australian sheep ranchers have long documented lamb predation by feral swine, which can lead to substantial economic losses (Choquenot et al. 1997). In one quantitative assessment, Pavlov and Hone (1982) documented that 18.7% of newborn lambs were preyed on by feral swine during a multiyear study. Damage to pasture and fences is another indirect effect feral swine have on livestock producers (Mengak 2012).
Impacts on protected land and on species of conservation concern
Along with the impacts that feral swine have on agriculture and livestock, their presence on the landscape can also affect both protected lands and conservation efforts for threatened and endangered species. They have been classified as ecosystem engineers, because their rooting behaviors can fundamentally alter habitats (Hone 2002). Vegetation, soil composition, and water quality can all be changed when feral swine are present on the landscape (see Barrios-Garcia and Bellari 2012 for a thorough review).
Feral swine have been linked to substantial levels of sapling mortality in protected forest regions of Malaysia, primarily through rooting and nest building (Ickes et al. 2003, 2005). Aboveground biomass, belowground production, and root zone expansion were all decreased in plots where feral swine grazing and rooting took place (Ford and Grace 1998); although feral swine can have a positive impact on soil chemistry, the negative impacts that they have on plant cover can counteract these effects. It has also been shown that feral swine strongly prefer to browse on certain species of tree saplings and plants, which can lead to species-specific impacts and decreased biodiversity in heavy-use areas (Siemann et al. 2009). In Florida, feral swine are associated with the decline of at least 26 plant and animal species that are now listed as rare, threatened, endangered, or of special concern (USDA 2002).
Negative effects, both direct and indirect, have been noted in animal species, as well. Island species and ground-nesting birds are the best-known examples of species directly affected when pigs were introduced to regions that had no evolutionary history with S. scrofa. Feral swine are believed to have caused the destruction of Hutton's shearwater (Puffinus huttoni) colonies in New Zealand through nest predation and probably contributed to the historic contraction of the shearwater's range (Cuthbert 2002). Prior to a large-scale eradication campaign, feral swine in the Galápagos Islands preyed on Galápagos tortoise (Geochelone elephantopus) eggs and hatchlings, green sea turtles (Chelonia mydas), lava lizards (Microlophus albemarlensis), and Galápagos petrels (Pterodroma phaeopygia). Feral swine probably played a role in the majority of documented extinctions on these islands, as well (Cruz et al. 2005). A similar situation existed off the coast of California, on Santa Cruz Island. Introduced feral swine played either a direct or an indirect role in the decline of 10 different federally listed threatened and endangered species on Santa Cruz Island (Parkes et al. 2010). Feral swine predation has also been shown to negatively affect bobwhite quail and wild turkey nest success in the continental United States (Rollins and Carroll 2001). In the case of northern snake-necked turtles (Chelodina regosa) in Australia, theoretical research suggests that pig predation will lead to localized disappearance of the turtle within 50 years if no management actions are initiated (Fordham et al. 2008).
Despite the well-documented negative impacts that feral swine have on protected areas and on threatened and endangered species, their presence can have positive effects, as well. Cole and colleagues (2012) documented an invasive shrub that actually increased in density when feral swine were excluded from forested plots in Hawaii for more than 15 years. The feral swine presence in California increased plant species richness by reducing competition intensity (Siemann et al. 2009). Soil properties can also be affected, and increases in mineral soil carbon and nitrogen, as well as microbial biomass carbon—all of which could improve growing conditions for plants—have been found in habitats that have experienced recent feral swine expansion (Wirthner et al. 2012). In Brazil, research has shown that feral swine are a preferred source of bushmeat, and this actually releases native fauna from hunting pressure (Desbiez et al. 2011). In addition, feral swine can be part of the diet of threatened and endangered species, such as the Florida panther (Puma concolor coryi).
Pathogen transmission
Feral swine can also carry a multitude of pathogens, and although this is one of the least discussed effects that they have as an invasive species, it is a significant issue. In the United States, feral swine have been documented as actively infected with and having contributed to the transmission of a wide variety of parasites, viruses, and bacteria that can infect humans, species of conservation concern, and domestic livestock (table 1).
Nationwide disease surveillance results in feral swine for select pathogens that pose a risk to humans, domestic animals, and livestock. All results reflect antibody prevalence.
| Disease . | Taxonomic association . | Years conducted . | Seroprevalence (percentage) . | 95% confidence interval . | Description . |
|---|---|---|---|---|---|
| Brucellosis | Brucella spp. | 2006–2012 | 4.3 | 4.0–4.6 | Multiple Brucella species and biovars, some of which can be transmitted to multiple species, including humans, in which they can cause serious disease |
| Influenza A | Multiple strains of influenza A and C | 2010–2012 | 10.8 | 9.9–11.8 | Multiple strains of influenza can circulate in swine, including the 2009 outbreak of a novel H1N1 strain that eventually spread to people worldwide |
| Pseudorabies (as Aujeszky's disease) | Suid herpesvirus I | 2007–2012 | 15.5 | 14.9–16.1 | Endemic swine disease that can be transmitted to other wild and domestic animals, including cattle, sheep, and dogs |
| Trichinella | Nematoda | 2009–2012 | 2.0 | 1.5–2.6 | Parasitic roundworm with a wide range of potential hosts, including humans, who can be exposed through the ingestion of undercooked swine meat |
| Hepatitis E | Hepatitis E virus genotypes 3 and 4 | 2010–2012 | 4.4 | 3.7–5.2 | Can cause brief, acute illness in infected people, with feral swine potentially acting as a viral reservoir and with transmission to humans occurring through the consumption of swine |
| Disease . | Taxonomic association . | Years conducted . | Seroprevalence (percentage) . | 95% confidence interval . | Description . |
|---|---|---|---|---|---|
| Brucellosis | Brucella spp. | 2006–2012 | 4.3 | 4.0–4.6 | Multiple Brucella species and biovars, some of which can be transmitted to multiple species, including humans, in which they can cause serious disease |
| Influenza A | Multiple strains of influenza A and C | 2010–2012 | 10.8 | 9.9–11.8 | Multiple strains of influenza can circulate in swine, including the 2009 outbreak of a novel H1N1 strain that eventually spread to people worldwide |
| Pseudorabies (as Aujeszky's disease) | Suid herpesvirus I | 2007–2012 | 15.5 | 14.9–16.1 | Endemic swine disease that can be transmitted to other wild and domestic animals, including cattle, sheep, and dogs |
| Trichinella | Nematoda | 2009–2012 | 2.0 | 1.5–2.6 | Parasitic roundworm with a wide range of potential hosts, including humans, who can be exposed through the ingestion of undercooked swine meat |
| Hepatitis E | Hepatitis E virus genotypes 3 and 4 | 2010–2012 | 4.4 | 3.7–5.2 | Can cause brief, acute illness in infected people, with feral swine potentially acting as a viral reservoir and with transmission to humans occurring through the consumption of swine |
Nationwide disease surveillance results in feral swine for select pathogens that pose a risk to humans, domestic animals, and livestock. All results reflect antibody prevalence.
| Disease . | Taxonomic association . | Years conducted . | Seroprevalence (percentage) . | 95% confidence interval . | Description . |
|---|---|---|---|---|---|
| Brucellosis | Brucella spp. | 2006–2012 | 4.3 | 4.0–4.6 | Multiple Brucella species and biovars, some of which can be transmitted to multiple species, including humans, in which they can cause serious disease |
| Influenza A | Multiple strains of influenza A and C | 2010–2012 | 10.8 | 9.9–11.8 | Multiple strains of influenza can circulate in swine, including the 2009 outbreak of a novel H1N1 strain that eventually spread to people worldwide |
| Pseudorabies (as Aujeszky's disease) | Suid herpesvirus I | 2007–2012 | 15.5 | 14.9–16.1 | Endemic swine disease that can be transmitted to other wild and domestic animals, including cattle, sheep, and dogs |
| Trichinella | Nematoda | 2009–2012 | 2.0 | 1.5–2.6 | Parasitic roundworm with a wide range of potential hosts, including humans, who can be exposed through the ingestion of undercooked swine meat |
| Hepatitis E | Hepatitis E virus genotypes 3 and 4 | 2010–2012 | 4.4 | 3.7–5.2 | Can cause brief, acute illness in infected people, with feral swine potentially acting as a viral reservoir and with transmission to humans occurring through the consumption of swine |
| Disease . | Taxonomic association . | Years conducted . | Seroprevalence (percentage) . | 95% confidence interval . | Description . |
|---|---|---|---|---|---|
| Brucellosis | Brucella spp. | 2006–2012 | 4.3 | 4.0–4.6 | Multiple Brucella species and biovars, some of which can be transmitted to multiple species, including humans, in which they can cause serious disease |
| Influenza A | Multiple strains of influenza A and C | 2010–2012 | 10.8 | 9.9–11.8 | Multiple strains of influenza can circulate in swine, including the 2009 outbreak of a novel H1N1 strain that eventually spread to people worldwide |
| Pseudorabies (as Aujeszky's disease) | Suid herpesvirus I | 2007–2012 | 15.5 | 14.9–16.1 | Endemic swine disease that can be transmitted to other wild and domestic animals, including cattle, sheep, and dogs |
| Trichinella | Nematoda | 2009–2012 | 2.0 | 1.5–2.6 | Parasitic roundworm with a wide range of potential hosts, including humans, who can be exposed through the ingestion of undercooked swine meat |
| Hepatitis E | Hepatitis E virus genotypes 3 and 4 | 2010–2012 | 4.4 | 3.7–5.2 | Can cause brief, acute illness in infected people, with feral swine potentially acting as a viral reservoir and with transmission to humans occurring through the consumption of swine |
Pseudorabies, also known as Aujeszky's disease, is a herpesvirus endemic to most feral swine populations around the world. Adult swine are often asymptomatic, but the disease can cause high mortality rates in piglets, making it a disease of concern for domestic swine producers. Pseudorabies can also be transmitted to other domestic and wild mammals. A fatal pseudorabies virus infection in endangered Florida panthers was likely associated with the consumption of infected feral swine (Glass et al. 1994), and similar infections have been extensively noted in domestic dogs used to hunt feral swine (Cramer et al. 2011). Nationwide surveillance in the United States conducted by the US Department of Agriculture's (USDA) Animal and Plant Health Inspection Service (AHPIS) Wildlife Services’ National Wildlife Disease Program documented pseudorabies exposure in feral swine in many regions, which suggests a disease that is both entrenched and widespread (table 1). Domestic swine production in the United States was declared pseudorabies free in 2004, after a long-term eradication campaign, but a reintroduction from endemically infected feral swine remains a constant threat.
Feral swine can also be infected with multiple strains of brucellosis. Brucellosis can cause morbidity and mortality in multiple livestock species and in humans. It has also been eradicated from the domestic pig industry in the United States (Guerrier et al. 2011, Jiang et al. 2012). Any reintroduction of the pathogen from feral swine into US domestic swine could severely affect this $34 billion per year industry. Nationwide, feral swine disease surveillance data showed an average Brucella sp. seroprevalence of 4.3% between 2007 and 2012 (table 1). In some regions, seroprevalence can also be substantially higher (Pedersen et al. 2012). In addition, the well-known link between swine and influenza virus evolution and transmission has recently ignited interest in the potential for feral swine to contribute to influenza dynamics. Nationwide surveillance in feral swine has documented influenza exposure, as well (table 1), and two isolates from feral swine collected as part of the National Wildlife Disease Program were determined to be pandemic H1N1 influenza (Clavijo et al. 2013). How those pigs were infected and the role that feral swine might play in the emergence of novel influenza strains remains unknown.
Feral swine have also been directly associated with Trichinella spiralis and hepatitis E transmission to humans. The former is a nematode parasite found in a wide range of mammal species, but human infections are often traced back to undercooked pork products. The illness's clinical symptoms can be severe and can, in some cases, result in death. Outbreaks of trichinosis have been attributed to consumption of contaminated feral swine or wild boar meat (Gottstein et al. 2009). Hepatitis E exposure can occur through multiple routes of infection, but domestic animals are believed to be a primary reservoir for the virus, and seroprevalence in some domestic pig herds can exceed 95%. Transmission to humans has been documented when infected meat has been consumed (Li et al. 2005). The ability of feral swine to shed pathogens into the environment is also a cause for concern. Teague and colleagues (2009) identified feral swine as a significant contributor to Escherichia coli contamination in watersheds. Interest in the role that feral swine may play in foodborne illness has also increased after recent outbreaks of Salmonella spp. in spinach and other leafy greens were traced back to farms in areas with substantial feral swine populations.
In addition to the pathogens that they can actively transmit, feral swine are also exposed to a long list of diseases. Their contribution to the persistence of these pathogens on the landscape is still largely unknown but includes diseases such as tularemia (Hartin et al. 2007), Chagas disease (Pizarro and Stevens 2008), Coxiella burnetii (Meng et al. 2009), and bubonic plague (Smith 1994). Perhaps even more significant is the potential for feral swine to serve as an unmonitored reservoir for diseases that are not currently found in the United States but that would bring with them devastating consequences if they were accidently or intentionally introduced. These foreign animal diseases include pathogens associated with high morbidity and mortality, such as classical swine fever, Japanese encephalitis (Meng et al. 2009), and foot-and-mouth disease.
Expansion and management of feral swine populations
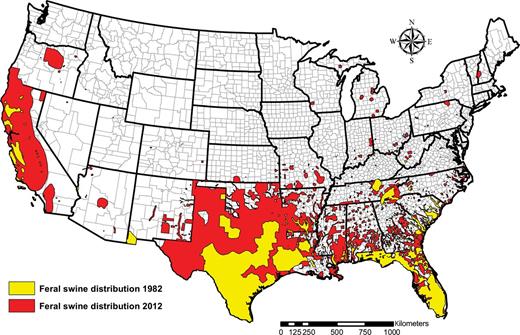
The problems associated with feral swine have increased as their population sizes and geographic range have expanded. For hundreds of years, the feral swine distribution in the United States was primarily limited to Hawaii, the southeastern United States, and California (Mayer and Brisbin 2008). Recently, however, feral swine populations have begun to spread. Seventeen states had documented feral swine populations in 1988. By 2011, that number had jumped to 38 states (figure 3). Feral swine population numbers in the United States have been estimated at 5 million individuals (Pimentel 2007), but this number is an estimate and is based on limited data. In their recent research from Texas, which has the largest feral swine population in the country, Timmons and colleagues (2012) estimated the numbers in that state alone to be between 1.8 and 3.4 million animals, with densities of 1.3–2.4 pigs per square mile in suitable habitat. Even before this more recent expansion, feral swine were the most prevalent and widespread wild, nonnative ungulate in the United States (Schmidt and Gilbert 1978).
In recent years, the distribution of feral swine has expanded dramatically in the United States. This map shows the reported feral swine expansion from 1982 (based on data provided by the Southeastern Cooperative Wildlife Disease Study) and from 2012 (based on data collected by the US Department of Agriculture, Animal and Plant Health Inspection Service, National Wildlife Disease Program).
Most drivers of this continental-scale population growth are anthropogenic, which makes the management of this invasive species extremely difficult. Pigs escape from farms and hunting reserves (Bratton 1975). Supplemental feeding of populations for hunting is also common in many areas. These practices, which contribute to increases in feral swine numbers and density, are not limited to the United States. Populations of wild S. scrofa have recently exploded throughout Europe as well, with much of the increase also attributed to human management practices and purposeful reintroductions, although multiple factors are probably involved, including a lack of natural predators, favorable climate conditions, and changes in crop production (Acevedo et al. 2007). Japan has seen similar expansion related to hunting activities (Saito et al. 2012). Australia has documented feral swine population increases related to human-mediated transport and introduction and also has the dubious distinction of supporting some of the highest numbers in the world: 13–23 million pigs, covering nearly 40% of the continent (Hone and Pech 1990, Choquenot et al. 1996).
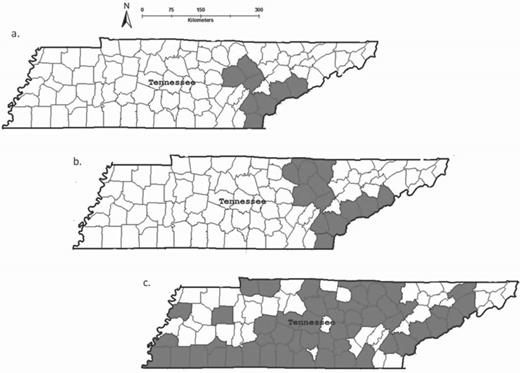
Unexpectedly, expansion in the United States has also occurred when some states created feral swine hunting seasons with the intent to enlist public help in population-control efforts. Enthusiastic public responses often followed and were accompanied by the illegal transport and release of feral swine to new areas in order to create local and, therefore, easily accessible hunting opportunities. In Tennessee, for example, feral swine populations were found in only six counties from the 1950s through the 1980s, with limited range expansion. A statewide, year-round, no-limit hunting program was instituted in 1999 in an attempt to enlist the public in controlling feral swine distribution in the state (Chuck Yoest, Tennessee Wildlife Resource Agency, personal communication, 10 May 2013). Since that time, populations have expanded, and numerous new populations have been established throughout the state. Nearly 70 Tennessee counties had documented pockets of feral swine by 2011 (figure 4), and, correspondingly, the number of reports of damage to property and crops dramatically increased (James D. Freye, USDA APHIS Wildlife Services, personal communication, 4 November 2013).
Feral swine distribution in Tennessee, showing known breeding populations (a) before 1950; (b) in 1988, prior to the open-season hunting program; and (c) in 2012, after the hunting program ceased in 2010. The data were provided by Daryl Ratajczak and Chuck Yoest, of the Tennessee Wildlife Resources Agency.
A similar feral swine expansion was documented in California (Zivin et al. 2000), where pigs were designated a game animal in 1956. In this case, the increase in pig populations not only involved the illegal movement and release of feral swine to create personal hunting opportunities, but it also involved people motivated by the chance to profit from fees charged to hunt on private land. Prior to the 1950s, the feral swine distribution in California was primarily limited to a handful of coastal counties, but, by the 1980s, that range had expanded to include 33 counties (Waithman et al. 1999). Feral swine are now present in 56 of the 58 counties in California. Although this dramatic expansion also involved other factors, including increased forage related to agricultural development, Waithman and colleagues (1999) stated that the most important anthropogenic factor influencing the increase in California feral swine populations was the interest among landowners in establishing or augmenting populations on private land. Zivin and colleagues (2000) went further, stating that any arguments suggesting that private hunting is an effective means of reducing feral swine populations may be ignoring the powerful incentive to establish and maintain viable pig populations on private land. The financial incentives associated with hunting fees led directly to intentional feral swine releases on private land. Conversely, managing feral swine as game animals, as California does, helps generate needed revenue for feral swine management in the state: Over a 5-year period, feral swine tags generated $3.3 million (Kreith 2007).
Bounty programs have also been attempted in some areas as an alternative way to enlist public help in reducing invasive species numbers. This approach has often produced mixed results. One feral swine bounty program attempted on a US military base paid hunters to submit tails from each feral swine they removed. The result was a costly bill, reports of people submitting pig tails procured from meat processers, and no reduction in pig numbers on the military base (Rob Holtfreter, Auburn University, personal communication, 30 October 2013). As with managing feral swine as game animals, bounty programs also have a fundamental problem in that they incentivize the production of the commodity that is being removed. Once again, the programs are intended to harness the enthusiasm for sport hunting by using it to control an invasive pest, but, if the species in question is eradicated, the program participants no longer have the opportunity to collect bounties. In the case of feral swine, the already entrenched interest in hunting leads to the same impasse: The desire to participate in the bounty program contrasts with the desire to not eliminate the species, which, if it were accomplished, would subsequently eliminate the opportunity to hunt (Weeks and Packard 2009). Despite these difficulties, bounty programs can have well-documented benefits, such as an increase in public awareness and engagement in invasive species issues. Feral swine bounty programs are still being implemented in additional states, with results yet to be determined.
Despite these difficulties, in some cases, focused management actions have been successful in the control and eradication of feral swine through plans that did not involve the general public. A 30-year eradication campaign on Santiago Island, in the Galápagos archipelago, resulted in the removal of nearly 20,000 feral swine through a combination of ground hunting and toxicants (Cruz et al. 2005). A similar combination of poisoning, trapping, and hunting was used to substantially reduce feral swine numbers in an area of New South Wales, in Australia (Hone 2002), a country that has also taken the lead in attempting to develop humane feral swine toxicants that have limited impacts on nontarget species. The use of toxicants, however, remains controversial. Feral swine eradication campaigns were also successfully carried out on Santa Cruz Island (Parkes et al. 2010) and in Pinnacles National Monument (McCann and Garcelon 2008), both in California. Oregon and Kansas have been successful at reducing feral swine populations by rapidly responding to initial introductions and by making it illegal to hunt or transport feral swine. These examples of successful feral swine control all share the simultaneous use of multiple management strategies (e.g., hunting, toxicants, trapping, exclusion fencing, policy implementation), and all required sustained efforts and consistent funding over time.
These final two requirements—sustained effort and consistent funding—can be the most difficult to achieve. Managing an already established invasive species, such as feral swine in the United States, can be costly, although allowing the species to persist and to spread without impediment can be costly in its own right (Choquenot et al. 1996, Pimentel 2007). In the case of managing feral swine at the scale of the entire continental United States, complete eradication is not a realistic goal. Rather, control by limiting the growth of existing populations while attempting to reduce expansion along the margins of current distributions are the management actions most often implemented; however, information is limited on how much a large population must be reduced in order to stop population growth or to reverse it. This is partially because there is a lack of reliable estimates on feral swine population sizes and densities, although theoretical work from Texas indicates that 66% of the population would have to be removed annually to restrict population growth in that specific region (Timmons et al. 2012). Currently, approximately 29% of the population is removed each year in Texas through a combination of hunting, removal by landowners, and government control programs. Estimates suggest that, without continued harvest, the feral swine population in Texas would triple in 5 years (Timmons et al. 2012).
Conclusions
Feral swine have been present as a nonnative species in the United States for nearly 500 years, but their populations have recently begun to increase and expand with alarming rapidity. This situation is mirrored by population increases in Europe, Japan, and Australia (Gipson et al. 1998, Spencer and Hampton 2005, Acevedo et al. 2006, 2007). These increases can be attributed to the illegal transport and release of feral swine and to other human-mediated landscape changes, including the expansion of land under agricultural production, the decline in predatory species, and the presence of artificial water sources. The problems associated with large numbers of feral swine on the landscape are multidimensional and encompass issues associated with agricultural destruction, the conservation of threatened and endangered species, and health risks related to disease transmission to humans and livestock. Attempts to control feral swine numbers or to limit their expansion are also multifaceted; the previous attempts have revealed that combinations of approaches are required over a sustained time frame. However, these management actions are divisive and complex. These difficulties are present even when the goal does not include complete eradication. Current approaches, instead, are designed to limit the damages and risks associated with feral swine by preventing continued geographic spread and by reducing population densities. The limited information available on the size and locations of feral swine populations in the United States underscores the need for more research that could then be used to inform successful management strategies.
The efforts of wildlife disease biologists and others associated with US Department of Agriculture Animal and Plant Health Inspection Service Wildlife Services National Wildlife Disease Program are what make research at this scale possible and also provide on-the-ground information that is otherwise difficult to come by. Additional information was generously provided by Daryl Ratajczak and Chuck Yoest at the Tennessee Wildlife Resources Agency. Mention of trade names or commercial products in this work is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.