-
PDF
- Split View
-
Views
-
Cite
Cite
Parker Magin, Amanda Tapley, Simon Morgan, Joshua S Davis, Patrick McElduff, Lucy Yardley, Kim Henderson, Anthea Dallas, Lawrie McArthur, Katie Mulquiney, Andrew Davey, Paul Little, Neil Spike, Mieke L van Driel, Reducing early career general practitioners’ antibiotic prescribing for respiratory tract infections: a pragmatic prospective non-randomised controlled trial, Family Practice, Volume 35, Issue 1, February 2018, Pages 53–60, https://doi.org/10.1093/fampra/cmx070
Close - Share Icon Share
Abstract
Inappropriate antibiotic prescription and consequent antibacterial resistance is a major threat to healthcare.
To evaluate the efficacy of a multifaceted intervention in reducing early career general practitioners’ (GPs’) antibiotic prescribing for upper respiratory tract infections (URTIs) and acute bronchitis/bronchiolitis.
A pragmatic non-randomized trial employing a non-equivalent control group design nested within an existing cohort study of GP registrars’ (trainees’) clinical practice. The intervention included access to online modules (covering the rationale of current clinical guidelines recommending non-prescription of antibiotics for URTI and bronchitis/bronchiolitis, and communication skills in management of acute bronchitis) followed by a face-to-face educational session. The intervention was delivered to registrars (and their supervisors) in two of Australia’s seventeen regional GP training providers (RTPs). Three other RTPs were the control group. Outcomes were proportion of registrars’ URTI consultations and bronchitis/bronchiolitis consultations prescribed antibiotics. Intention-to-treat analyses employed logistic regression within a Generalised Estimating Equation framework, adjusted for relevant independent variables. The predictors of interest were time; treatment group; and an interaction term for time-by-treatment group. The P value associated with an interaction term determined statistically significant differences in antibiotic prescribing.
Analyses include data of 217 intervention RTPs’ and 311 control RTPs’ registrars. There was no significant reduction in antibiotic prescribing for URTIs. For bronchitis/bronchiolitis, a significant reduction (interaction P value = 0.024) remained true for analysis adjusted for independent variables (P value = 0.040). The adjusted absolute reduction in prescribing was 15.8% (95% CI: 4.2%–27.5%).
A multifaceted intervention reduced antibiotic prescribing for bronchitis/bronchiolitis but not URTIs.
Introduction
Antibiotic over-use is problematic worldwide, contributing to community (1) and individual patient (2) bacterial resistance. Most antibiotic prescribing occurs in general practice (1). General practice prescribing is thus central in addressing harmful antibiotic effects including bacterial resistance and consequent antibiotic failure.
Much antibiotic over-use occurs in treating non-pneumonia respiratory tract infections (RTIs) (1). For two common RTIs there is evidence of very little efficacy (acute bronchitis) (3) or no efficacy (Upper RTI—URTI) (4). This evidence has informed evidence-based guidelines internationally, including authoritative Australian guidelines (5). These strongly recommend against managing acute bronchitis or URTI with antibiotics. Despite this, Australian antibiotic prescribing rates for these conditions (6) are inappropriately high compared to international benchmarks (7).
Interventions to influence GPs’ inappropriate antibiotic prescription in RTIs have targeted established GPs and have had mixed success. There was little evidence for substantive benefit in a 2005 Cochrane review (8), but some subsequent studies have demonstrated reduced antibiotic prescribing (9–12).
GPs’ antibiotic prescribing practices, once established, tend to remain consistent and early career and in-training GPs are important targets to influence antibiotic prescribing (13). We have demonstrated that GP registrars’ (trainees’) antibiotic prescribing for URTI and acute bronchitis/bronchiolitis exceeds quality practice benchmarks (14) and, more worryingly, does not improve during training (15).
We have previously established (via simple pre- and post-questionnaire responses to clinical vignettes) that a multifaceted intervention changes registrars’ ‘intended’ prescribing for acute bronchitis (16). Here we report on changes in registrars’ ‘actual’ antibiotic prescribing for URTI and acute bronchitis/bronchiolitis, compared with a control group, following the same intervention.
Methods
The methodology has been described in detail previously (17).
Setting and participants
The study population was GP registrars in five of seventeen Regional Training Providers (RTPs) in five of Australia’s six states. RTPs are government-funded, not-for-profit, geographically defined general practice education and training organisations.
Registrar participants were in general practice-based training terms. Each of three terms lasts 6 months, full-time equivalent. Registrars operate within an apprenticeship-like model with a supervising GP but with considerable autonomy—including full prescribing rights.
Methodology
We employed a non-equivalent control group design (17) nested within an existing ongoing cohort study—the Registrar Clinical Encounters in Training (ReCEnT) study (18).
A randomised design was not appropriate in this project. Assignment to intervention or control in our study was at the level of RTP. GP education and training in Australia is mainly based on an apprenticeship-like model with the majority of time spent in general practice (with advice and assistance from a senior GP supervisor available to the registrar). Formal education sessions are held in each RTP in large-group teaching away from the practice. Assignment to intervention or control at the level of registrar or of other smaller units within the RTP was impracticable. Registrars of individual RTPs share considerable educational and professional contact (including at the group educational sessions) and assignment at the sub-RTP level would result in contamination, compromising study validity.
Cluster randomization, rather than non-random assignment, at the RTP level was also impractical. There are five RTPs participating in the ReCEnT cohort study. The educational sessions in which our intervention was to be delivered are time-limited (with multiple topics competing for inclusion within the educational session program) and programs are set up to 18 months in advance. The limited number of participating RTPs and the time-constraints and inflexibility of individual RTP program schedules made RTP-level cluster randomization impossible.
The registrars of two intervention RTPs, who were in either the first or second of their three GP training terms, received the intervention as part of their routine educational program. Registrars of three other RTPs formed the control group.
All registrars (in intervention and control groups) were participants in the Registrars Clinical Encounters in Training (ReCEnT) project (18) within which this study was nested. Data from that broader project (see below) were used in our analyses.
Intervention
The educational intervention for registrars comprised
i) two online educational modules specified as pre-reading for the educational session.
ii) a 90 minute face-to-face educational session conducted during routinely scheduled educational workshops
The modules
The modules comprised two of three INternet Training for Reducing AntibiOtic use (INTRO) electronic modules (developed and implemented within the European Union-funded GRACE study) (10,19). These entailed a behaviour change intervention (based on Social Cognitive Theory and developed using extensive qualitative work with GPs within the Person-Based Approach (20)) aimed at changing the outcome expectancies and self-efficacy of clinicians to manage RTIs without prescribing antibiotics (19).
The first module covered the need for reduced antibiotic prescribing and the effect of over-prescription on health care systems, patients and GPs (linking this background with the recommendations of Therapeutics Guidelines: Australia (Antibiotics), 2013 version (5)). This module was adapted for the Australian context by the authors (PM, JD, MVD), who have expertise in this area, in consultation with the INTRO team (LY, PL). The second module modelled communication skills in GP management of acute bronchitis, but these skills were relevant to all respiratory tract infections (10).
The online modules were piloted in a small group of non-participating registrars and medical educators/supervisors and amended according to their feedback before roll out. The face-to-face intervention was designed by a team of experienced medical educators and an infectious disease expert and delivered by the local teams in the two participating RTPs in November–December 2014.
GP registrar workshop sessions
The interactive 90-minute workshop session included how to best implement current Australian guidelines (5) in daily practice. URTIs and acute bronchitis were emphasized as exemplars of infections for which antibiotics are seldom indicated.
The workshop content was constructed by the research team of GP clinician/academics and an infectious diseases physician/researcher. The process was informed by literature in the area and our recent qualitative and quantitative work in registrar antibiotic prescribing (14,21).
We proposed three underlying principles as guiding non-pneumonia RTI management. Firstly, the default therapeutic decision is not to prescribe antibiotics (5). The second principle was that attempts to treat non-pneumonia RTIs on the basis of presumed viral or bacterial aetiology are problematic and do not reflect current understanding of the complex interplay of bacterial and viral pathogens. Rather, RTIs should be diagnosed and treated syndromically (3,4). The third principle was that the clinical science of RTI consultations may be straightforward but managing patient perceptions and expectations may not be: the sophistication of consultation techniques will reflect the consultation’s biopsychosocial rather than biological complexity. We also stressed individual GPs’ responsibility for antibiotic stewardship (given practitioners’ propensity to attribute antibiotic resistance to others’ actions).
GP supervisor workshop sessions
We delivered similar sessions (and module access) for registrars’ supervisors. We also encouraged individual registrar-supervisor dyads to discuss case-studies of RTIs during their weekly one-on-one educational sessions. The rationale for the supervisor element of the intervention was that our previous research (14,21) has suggested that prescribing patterns (role-modelling) of supervisors and the ‘apprenticeship’ model of the registrar-supervisor relationship are drivers of non-rational antibiotic prescribing.
Control
The three control RTPs delivered their usual education through a series of workshops. The content of these varied per RTP and may have included information on rational antibiotic prescribing, but did not include the online modules, nor the session with an infectious disease specialist and the supervisor training.
ReCEnT project
ReCEnT is an ongoing cohort study of registrars’ clinical consultations (18). At approximately the midpoint of each of three GP training terms, each registrar records details of 60 consecutive consultations, included diagnoses/problems managed and medications prescribed (these are subsequently coded by trained data-entry staff).
Outcome factors
Primary outcome factors were prescription/non-prescription of antibiotics for presentations with
a) URTI, and
b) acute bronchitis/bronchiolitis.
Antibiotics were defined according to the Anatomic Therapeutic Chemical (ATC) Classification codes J01: ‘antibacterials for systemic use’. ‘URTI’ and ‘acute bronchitis/bronchiolitis’ were defined by their International Primary Care Classification (ICPC-2) codes: R74 and R78, respectively. Registrars recorded their diagnosis or problem formulation in free-text. Trained data-entry coders then matched the registrars’ descriptions to ICPC-2 codes.
Independent variables
Registrar, patient, practice and consultation variables were included in our analyses.
Registrar variables
Age, gender, full-time or part-time status, country of primary medical graduation (Australian/non-Australian) and training term.
Patient variables
Age, gender, Aboriginal or Torres Strait Islander status, non-English speaking background (NESB) status, the patient being new to the practice, and the patient being new to the registrar.
Practice variables
Size (number of full-time equivalent GPs), rurality (Australian Standard Geographical Classification-Remoteness Area—ASGC-RA classification), socioeconomic status (the practice location’s Socioeconomic Index for Area (SEIFA) Relative Index of Disadvantage decile), and billing policy (does the practice routinely bulk bill: that is, provide consultations with no cost to the patient).
Consultation variables
Number of problems addressed and whether the registrar sought in-consultation advice or information (from their supervisor or other resources, such as specialists, books or electronic resources).
Statistical analyses
Logistic regression models were used to determine if there was a reduction in the prescribing of antibiotics as a result of the intervention. The logistic regression models were fitted within a Generalised Estimating Equation (GEE) framework to adjust for correlation of outcomes within registrars. Separate models were fitted for treatment of URTIs and for acute bronchitis/bronchiolitis. The predictors of interest were time (before/after intervention), treatment group and an interaction term for time-by-treatment group. The P value associated with the interaction term was used to determine if there was a statistically significant difference in change from the pre-intervention period to the post-intervention period in the intervention group compared to the control group.
To examine the potential impact of confounding, multiple regression models were fitted for each of the outcomes without including the treatment and time variables as predictors. Variables with P values less than 0.05 in these multiple regression models were then included in a model with time, treatment and the interaction term.
The primary analyses were on an intention-to-treat basis (of all registrars eligible to attend the education session). Analysis was restricted to data from ReCEnT Round 6 (2012, second semester) or later as these rounds coincided with rounds participated in by any of the registrars who were eligible to attend the workshop. Post-intervention data was from Round 11 (2015, first semester).
In sensitivity analyses we explored the impact of choosing Round 6 as the cut-point for inclusion by further analyses using each of Rounds 7 to 10 as the cut-point. The sensitivity analyses were conducted as registrars do not proceed through training uniformly or necessarily continuously (due to part-time training and leave periods). We also performed sensitivity analyses restricted to registrars who attended the educational sessions.
Analyses were programmed in Stata V13 or SAS 9.4. Statistical significance was set at 0.05.
Power and sample size
We anticipated 130 registrars in the intervention RTPs and 160 registrars in the control RTPs. For URTIs, given a pre-intervention rate of antibiotic prescribing of 22%, and registrars managing an average of four URTI consultations per data collection round (from earlier ReCEnT data (14)), we calculated the study to have approximately 80% power to find an absolute reduction of 10% in antibiotic prescribing for URTIs in the intervention group compared with the control group, taking into account an inflation factor of 1.9.
Results
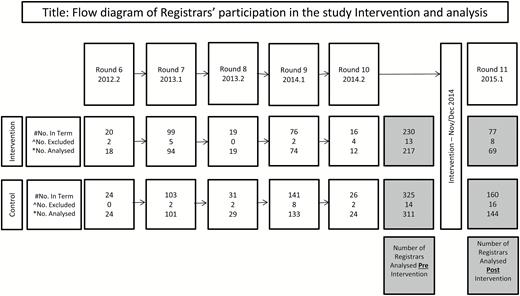
The data of 217 intervention group and 311 control group registrars were included in analyses. The flow diagram of registrars included in the analyses is presented in Figure 1. The response rate in the overall ReCEnT project during the period included in analyses (2012–2015) was 97.7%. Characteristics of registrars and their practices are presented in Table 1.
Flow diagram of Registrars’ participation in the study Intervention and analysis.#Pre-intervention number of registrars enrolled in the Intervention and Control training organizations, respectively, and whose first potential involvement in data collection pre-Intervention is in this Round.^Excluded Registrars—pre-intervention exclusions are registrars who did not complete their data collection or who did not consent to participation. Post-intervention exclusions also include registrars who did not have pre-Intervention data.*The number of individual registrars analysed post-intervention represents registrars who were eligible for the intervention/control and collected data both pre- and post-intervention. Note that an individual registrar could contribute one to three rounds of data pre-Intervention.
Registrar and practice characteristics by intervention group (for two intervention and three control Regional Training Providers, 2012–2015)
| Registrars . | Group . | . | ||
|---|---|---|---|---|
| Characteristic . | Sub group or mean(SD) . | Control (n = 311) . | Intervention (n = 217) . | P . |
| Registrar gender | Male | 107 (34%) | 75 (35%) | 0.97 |
| Female | 204 (66%) | 142 (65%) | ||
| Qualified as a doctor in Australia | No | 40 (13%) | 59 (27%) | <0.001 |
| Yes | 270 (87%) | 157 (73%) | ||
| Registrar workload | Part time | 76 (25%) | 56 (27%) | 0.63 |
| Full time | 229 (75%) | 153 (73%) | ||
| Training term/post | Term 1 | 171 (55%) | 107 (49%) | 0.30 |
| Term 2 | 102 (33%) | 74 (34%) | ||
| Term 3 | 38 (12%) | 36 (17%) | ||
| Registrar age (years) | mean (SD) | 31.3 (5.2) | 33.7 (6.4) | <0.001 |
| Practices | Group | |||
| Characteristic | Sub group or mean(SD) | Control (n = 216) | Intervention (n = 149) | P |
| Rurality | Major city | 131 (61%) | 83 (56%) | 0.57 |
| Inner regional | 43 (20%) | 36 (24%) | ||
| Outer regional/remote | 42 (19%) | 30 (20%) | ||
| Does practice routinely bulk bill | No | 166 (77%) | 120 (81%) | 0.45 |
| Yes | 49 (23%) | 29 (19%) | ||
| Practice size | Small | 76 (36%) | 66 (47%) | 0.035 |
| Large | 136 (64%) | 74 (53%) | ||
| SEIFA Index | median (min, max) | 1010.0 (799.0, 1118.0) | 987.0 (650.0, 1080.0) | <0.001 |
| mean (SD) | 1000.1 (65.0) | 982.3 (51.8) | 0.006 | |
| Registrars . | Group . | . | ||
|---|---|---|---|---|
| Characteristic . | Sub group or mean(SD) . | Control (n = 311) . | Intervention (n = 217) . | P . |
| Registrar gender | Male | 107 (34%) | 75 (35%) | 0.97 |
| Female | 204 (66%) | 142 (65%) | ||
| Qualified as a doctor in Australia | No | 40 (13%) | 59 (27%) | <0.001 |
| Yes | 270 (87%) | 157 (73%) | ||
| Registrar workload | Part time | 76 (25%) | 56 (27%) | 0.63 |
| Full time | 229 (75%) | 153 (73%) | ||
| Training term/post | Term 1 | 171 (55%) | 107 (49%) | 0.30 |
| Term 2 | 102 (33%) | 74 (34%) | ||
| Term 3 | 38 (12%) | 36 (17%) | ||
| Registrar age (years) | mean (SD) | 31.3 (5.2) | 33.7 (6.4) | <0.001 |
| Practices | Group | |||
| Characteristic | Sub group or mean(SD) | Control (n = 216) | Intervention (n = 149) | P |
| Rurality | Major city | 131 (61%) | 83 (56%) | 0.57 |
| Inner regional | 43 (20%) | 36 (24%) | ||
| Outer regional/remote | 42 (19%) | 30 (20%) | ||
| Does practice routinely bulk bill | No | 166 (77%) | 120 (81%) | 0.45 |
| Yes | 49 (23%) | 29 (19%) | ||
| Practice size | Small | 76 (36%) | 66 (47%) | 0.035 |
| Large | 136 (64%) | 74 (53%) | ||
| SEIFA Index | median (min, max) | 1010.0 (799.0, 1118.0) | 987.0 (650.0, 1080.0) | <0.001 |
| mean (SD) | 1000.1 (65.0) | 982.3 (51.8) | 0.006 | |
Registrar and practice characteristics by intervention group (for two intervention and three control Regional Training Providers, 2012–2015)
| Registrars . | Group . | . | ||
|---|---|---|---|---|
| Characteristic . | Sub group or mean(SD) . | Control (n = 311) . | Intervention (n = 217) . | P . |
| Registrar gender | Male | 107 (34%) | 75 (35%) | 0.97 |
| Female | 204 (66%) | 142 (65%) | ||
| Qualified as a doctor in Australia | No | 40 (13%) | 59 (27%) | <0.001 |
| Yes | 270 (87%) | 157 (73%) | ||
| Registrar workload | Part time | 76 (25%) | 56 (27%) | 0.63 |
| Full time | 229 (75%) | 153 (73%) | ||
| Training term/post | Term 1 | 171 (55%) | 107 (49%) | 0.30 |
| Term 2 | 102 (33%) | 74 (34%) | ||
| Term 3 | 38 (12%) | 36 (17%) | ||
| Registrar age (years) | mean (SD) | 31.3 (5.2) | 33.7 (6.4) | <0.001 |
| Practices | Group | |||
| Characteristic | Sub group or mean(SD) | Control (n = 216) | Intervention (n = 149) | P |
| Rurality | Major city | 131 (61%) | 83 (56%) | 0.57 |
| Inner regional | 43 (20%) | 36 (24%) | ||
| Outer regional/remote | 42 (19%) | 30 (20%) | ||
| Does practice routinely bulk bill | No | 166 (77%) | 120 (81%) | 0.45 |
| Yes | 49 (23%) | 29 (19%) | ||
| Practice size | Small | 76 (36%) | 66 (47%) | 0.035 |
| Large | 136 (64%) | 74 (53%) | ||
| SEIFA Index | median (min, max) | 1010.0 (799.0, 1118.0) | 987.0 (650.0, 1080.0) | <0.001 |
| mean (SD) | 1000.1 (65.0) | 982.3 (51.8) | 0.006 | |
| Registrars . | Group . | . | ||
|---|---|---|---|---|
| Characteristic . | Sub group or mean(SD) . | Control (n = 311) . | Intervention (n = 217) . | P . |
| Registrar gender | Male | 107 (34%) | 75 (35%) | 0.97 |
| Female | 204 (66%) | 142 (65%) | ||
| Qualified as a doctor in Australia | No | 40 (13%) | 59 (27%) | <0.001 |
| Yes | 270 (87%) | 157 (73%) | ||
| Registrar workload | Part time | 76 (25%) | 56 (27%) | 0.63 |
| Full time | 229 (75%) | 153 (73%) | ||
| Training term/post | Term 1 | 171 (55%) | 107 (49%) | 0.30 |
| Term 2 | 102 (33%) | 74 (34%) | ||
| Term 3 | 38 (12%) | 36 (17%) | ||
| Registrar age (years) | mean (SD) | 31.3 (5.2) | 33.7 (6.4) | <0.001 |
| Practices | Group | |||
| Characteristic | Sub group or mean(SD) | Control (n = 216) | Intervention (n = 149) | P |
| Rurality | Major city | 131 (61%) | 83 (56%) | 0.57 |
| Inner regional | 43 (20%) | 36 (24%) | ||
| Outer regional/remote | 42 (19%) | 30 (20%) | ||
| Does practice routinely bulk bill | No | 166 (77%) | 120 (81%) | 0.45 |
| Yes | 49 (23%) | 29 (19%) | ||
| Practice size | Small | 76 (36%) | 66 (47%) | 0.035 |
| Large | 136 (64%) | 74 (53%) | ||
| SEIFA Index | median (min, max) | 1010.0 (799.0, 1118.0) | 987.0 (650.0, 1080.0) | <0.001 |
| mean (SD) | 1000.1 (65.0) | 982.3 (51.8) | 0.006 | |
The intervention was delivered between November–December 2014. In the intervention RTPs, there were 89 registrars in Terms 1 and 2, and 67 (75%) attended the workshop. Our systems were unable to monitor rates of accessing the online modules.
Pre- and post-intervention data were collected in 2012–14 and 2015, respectively.
URTI
Numbers and percentages of patients prescribed an antibiotic when seen for an URTI are presented by intervention/control group and ReCEnT round, in Table 2.
Number and percentage of patients treated for acute upper respiratory tract infections & acute bronchitis/bronchiolitis by ReCEnT round and by intervention group
| . | Acute upper respiratory tract infections . | Acute bronchitis/bronchiolitis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control group . | Intervention group . | Control group . | Intervention group . | |||||
| Round . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . |
| 6 | 41/125 | 32.8% | 8/58 | 13.8% | 11/15 | 73.3% | 9/10 | 90.0% |
| 7 | 61/583 | 10.5% | 82/446 | 18.4% | 73/101 | 72.3% | 80/115 | 69.6% |
| 8 | 86/726 | 11.8% | 52/371 | 14.0% | 119/153 | 77.8% | 48/76 | 63.2% |
| 9 | 138/1283 | 10.8% | 98/703 | 13.9% | 192/272 | 70.6% | 150/204 | 73.5% |
| 10 | 109/813 | 13.4% | 69/417 | 16.5% | 121/160 | 75.6% | 64/86 | 74.4% |
| 11 | 79/737 | 10.7% | 47/350 | 13.4% | 122/147 | 83.0% | 63/94 | 67.0% |
| . | Acute upper respiratory tract infections . | Acute bronchitis/bronchiolitis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control group . | Intervention group . | Control group . | Intervention group . | |||||
| Round . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . |
| 6 | 41/125 | 32.8% | 8/58 | 13.8% | 11/15 | 73.3% | 9/10 | 90.0% |
| 7 | 61/583 | 10.5% | 82/446 | 18.4% | 73/101 | 72.3% | 80/115 | 69.6% |
| 8 | 86/726 | 11.8% | 52/371 | 14.0% | 119/153 | 77.8% | 48/76 | 63.2% |
| 9 | 138/1283 | 10.8% | 98/703 | 13.9% | 192/272 | 70.6% | 150/204 | 73.5% |
| 10 | 109/813 | 13.4% | 69/417 | 16.5% | 121/160 | 75.6% | 64/86 | 74.4% |
| 11 | 79/737 | 10.7% | 47/350 | 13.4% | 122/147 | 83.0% | 63/94 | 67.0% |
Number and percentage of patients treated for acute upper respiratory tract infections & acute bronchitis/bronchiolitis by ReCEnT round and by intervention group
| . | Acute upper respiratory tract infections . | Acute bronchitis/bronchiolitis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control group . | Intervention group . | Control group . | Intervention group . | |||||
| Round . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . |
| 6 | 41/125 | 32.8% | 8/58 | 13.8% | 11/15 | 73.3% | 9/10 | 90.0% |
| 7 | 61/583 | 10.5% | 82/446 | 18.4% | 73/101 | 72.3% | 80/115 | 69.6% |
| 8 | 86/726 | 11.8% | 52/371 | 14.0% | 119/153 | 77.8% | 48/76 | 63.2% |
| 9 | 138/1283 | 10.8% | 98/703 | 13.9% | 192/272 | 70.6% | 150/204 | 73.5% |
| 10 | 109/813 | 13.4% | 69/417 | 16.5% | 121/160 | 75.6% | 64/86 | 74.4% |
| 11 | 79/737 | 10.7% | 47/350 | 13.4% | 122/147 | 83.0% | 63/94 | 67.0% |
| . | Acute upper respiratory tract infections . | Acute bronchitis/bronchiolitis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control group . | Intervention group . | Control group . | Intervention group . | |||||
| Round . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . | Treated/Seen . | Percentage . |
| 6 | 41/125 | 32.8% | 8/58 | 13.8% | 11/15 | 73.3% | 9/10 | 90.0% |
| 7 | 61/583 | 10.5% | 82/446 | 18.4% | 73/101 | 72.3% | 80/115 | 69.6% |
| 8 | 86/726 | 11.8% | 52/371 | 14.0% | 119/153 | 77.8% | 48/76 | 63.2% |
| 9 | 138/1283 | 10.8% | 98/703 | 13.9% | 192/272 | 70.6% | 150/204 | 73.5% |
| 10 | 109/813 | 13.4% | 69/417 | 16.5% | 121/160 | 75.6% | 64/86 | 74.4% |
| 11 | 79/737 | 10.7% | 47/350 | 13.4% | 122/147 | 83.0% | 63/94 | 67.0% |
There was no statistically significant difference in change in antibiotic prescription for URTIs in the intervention group compared to the control group post-intervention (interaction P value from GEE = 0.42). This remained true for the analysis adjusted for variables found to be significant in the multiple regression models (P = 0.62). See Table 3.
Adjusted odds ratios for being prescribed an antibiotic for an acute URTI for all variables found to be statistically significantly associated with antibiotic use in the univariate analysis
| Characteristic . | . | Odds ratio (95% CI) . | Adjusted P value . |
|---|---|---|---|
| Period | Post | 0.99 (0.73 to 1.35) | 0.97 |
| Treatment | Intervention | 1.16 (0.89 to 1.53) | 0.28 |
| Period by treatment | Post-intervention | 0.88 (0.54 to 1.44) | 0.62 |
| Patients age group | <5 | Referent | |
| 5 to <15 | 1.70 (1.27 to 2.29) | <0.001 | |
| 15 to <25 | 1.91 (1.41 to 2.59) | <.001 | |
| 25 to <45 | 2.31 (1.80 to 2.97) | <.001 | |
| 45 to <65 | 2.80 (2.14 to 3.66) | <.001 | |
| >=65 | 4.11 (2.93 to 5.75) | <.001 | |
| Assistance sought | No | Referent | |
| Yes | 3.63 (2.60 to 5.05) | <.001 | |
| Is it a new problem | No | Referent | |
| Yes | 0.67 (0.49 to 0.92) | 0.013 | |
| Qualified as a doctor in Australia | No | Referent | |
| Yes | 0.66 (0.47 to 0.91) | 0.012 | |
| Does the practice routinely bulk bill | No | Referent | |
| Yes | 0.66 (0.50 to 0.88) | 0.004 |
| Characteristic . | . | Odds ratio (95% CI) . | Adjusted P value . |
|---|---|---|---|
| Period | Post | 0.99 (0.73 to 1.35) | 0.97 |
| Treatment | Intervention | 1.16 (0.89 to 1.53) | 0.28 |
| Period by treatment | Post-intervention | 0.88 (0.54 to 1.44) | 0.62 |
| Patients age group | <5 | Referent | |
| 5 to <15 | 1.70 (1.27 to 2.29) | <0.001 | |
| 15 to <25 | 1.91 (1.41 to 2.59) | <.001 | |
| 25 to <45 | 2.31 (1.80 to 2.97) | <.001 | |
| 45 to <65 | 2.80 (2.14 to 3.66) | <.001 | |
| >=65 | 4.11 (2.93 to 5.75) | <.001 | |
| Assistance sought | No | Referent | |
| Yes | 3.63 (2.60 to 5.05) | <.001 | |
| Is it a new problem | No | Referent | |
| Yes | 0.67 (0.49 to 0.92) | 0.013 | |
| Qualified as a doctor in Australia | No | Referent | |
| Yes | 0.66 (0.47 to 0.91) | 0.012 | |
| Does the practice routinely bulk bill | No | Referent | |
| Yes | 0.66 (0.50 to 0.88) | 0.004 |
Adjusted odds ratios for being prescribed an antibiotic for an acute URTI for all variables found to be statistically significantly associated with antibiotic use in the univariate analysis
| Characteristic . | . | Odds ratio (95% CI) . | Adjusted P value . |
|---|---|---|---|
| Period | Post | 0.99 (0.73 to 1.35) | 0.97 |
| Treatment | Intervention | 1.16 (0.89 to 1.53) | 0.28 |
| Period by treatment | Post-intervention | 0.88 (0.54 to 1.44) | 0.62 |
| Patients age group | <5 | Referent | |
| 5 to <15 | 1.70 (1.27 to 2.29) | <0.001 | |
| 15 to <25 | 1.91 (1.41 to 2.59) | <.001 | |
| 25 to <45 | 2.31 (1.80 to 2.97) | <.001 | |
| 45 to <65 | 2.80 (2.14 to 3.66) | <.001 | |
| >=65 | 4.11 (2.93 to 5.75) | <.001 | |
| Assistance sought | No | Referent | |
| Yes | 3.63 (2.60 to 5.05) | <.001 | |
| Is it a new problem | No | Referent | |
| Yes | 0.67 (0.49 to 0.92) | 0.013 | |
| Qualified as a doctor in Australia | No | Referent | |
| Yes | 0.66 (0.47 to 0.91) | 0.012 | |
| Does the practice routinely bulk bill | No | Referent | |
| Yes | 0.66 (0.50 to 0.88) | 0.004 |
| Characteristic . | . | Odds ratio (95% CI) . | Adjusted P value . |
|---|---|---|---|
| Period | Post | 0.99 (0.73 to 1.35) | 0.97 |
| Treatment | Intervention | 1.16 (0.89 to 1.53) | 0.28 |
| Period by treatment | Post-intervention | 0.88 (0.54 to 1.44) | 0.62 |
| Patients age group | <5 | Referent | |
| 5 to <15 | 1.70 (1.27 to 2.29) | <0.001 | |
| 15 to <25 | 1.91 (1.41 to 2.59) | <.001 | |
| 25 to <45 | 2.31 (1.80 to 2.97) | <.001 | |
| 45 to <65 | 2.80 (2.14 to 3.66) | <.001 | |
| >=65 | 4.11 (2.93 to 5.75) | <.001 | |
| Assistance sought | No | Referent | |
| Yes | 3.63 (2.60 to 5.05) | <.001 | |
| Is it a new problem | No | Referent | |
| Yes | 0.67 (0.49 to 0.92) | 0.013 | |
| Qualified as a doctor in Australia | No | Referent | |
| Yes | 0.66 (0.47 to 0.91) | 0.012 | |
| Does the practice routinely bulk bill | No | Referent | |
| Yes | 0.66 (0.50 to 0.88) | 0.004 |
Acute bronchitis/bronchiolitis
Numbers and percentages of patients prescribed an antibiotic when seen for acute bronchitis/bronchiolitis are presented by intervention/control group and ReCEnT round, in Table 2.
There was a statistically significant difference in change in antibiotic prescribing for bronchitis/bronchiolitis in the intervention group compared to the control group post-intervention (interaction P value from GEE = 0.024). This remained true for the analysis adjusted for variables found to be significant in the multiple regression models (P = 0.040). See Table 4.
Adjusted odds ratios for being prescribed an antibiotic for an acute bronchitis/bronchiolitis for all variables found to be statistically significantly associated with antibiotic use in the univariate analysis
| Characteristic . | . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Period | Post | 1.43 (0.92 to 2.23) | 0.12 |
| Treatment | Intervention | 1.11 (0.79 to 1.56) | 0.55 |
| Period by treatment | Post-intervention | 0.47 (0.23 to 0.96) | 0.040 |
| Patients age group | <5 | Referent | |
| 15 to <25 | 5.15 (2.86 to 9.28) | <0.001 | |
| 25 to <45 | 5.77 (3.12 to 10.6) | <0.001 | |
| 45 to <65 | 4.09 (2.65 to 6.29) | <0.001 | |
| 5 to <15 | 5.64 (3.71 to 8.57) | <0.001 | |
| ≥65 | 5.67 (3.62 to 8.87) | <0.001 | |
| Is it a new problem | No | Referent | |
| Yes | 2.74 (1.95 to 3.85) | <0.001 | |
| New patient to practice or registrar | No | Referent | |
| New patient to registrar | 1.17 (0.87 to 1.58) | 0.28 |
| Characteristic . | . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Period | Post | 1.43 (0.92 to 2.23) | 0.12 |
| Treatment | Intervention | 1.11 (0.79 to 1.56) | 0.55 |
| Period by treatment | Post-intervention | 0.47 (0.23 to 0.96) | 0.040 |
| Patients age group | <5 | Referent | |
| 15 to <25 | 5.15 (2.86 to 9.28) | <0.001 | |
| 25 to <45 | 5.77 (3.12 to 10.6) | <0.001 | |
| 45 to <65 | 4.09 (2.65 to 6.29) | <0.001 | |
| 5 to <15 | 5.64 (3.71 to 8.57) | <0.001 | |
| ≥65 | 5.67 (3.62 to 8.87) | <0.001 | |
| Is it a new problem | No | Referent | |
| Yes | 2.74 (1.95 to 3.85) | <0.001 | |
| New patient to practice or registrar | No | Referent | |
| New patient to registrar | 1.17 (0.87 to 1.58) | 0.28 |
The variable ‘is new to practice/registrar’ had to be removed from the model due to collinearity.
Adjusted odds ratios for being prescribed an antibiotic for an acute bronchitis/bronchiolitis for all variables found to be statistically significantly associated with antibiotic use in the univariate analysis
| Characteristic . | . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Period | Post | 1.43 (0.92 to 2.23) | 0.12 |
| Treatment | Intervention | 1.11 (0.79 to 1.56) | 0.55 |
| Period by treatment | Post-intervention | 0.47 (0.23 to 0.96) | 0.040 |
| Patients age group | <5 | Referent | |
| 15 to <25 | 5.15 (2.86 to 9.28) | <0.001 | |
| 25 to <45 | 5.77 (3.12 to 10.6) | <0.001 | |
| 45 to <65 | 4.09 (2.65 to 6.29) | <0.001 | |
| 5 to <15 | 5.64 (3.71 to 8.57) | <0.001 | |
| ≥65 | 5.67 (3.62 to 8.87) | <0.001 | |
| Is it a new problem | No | Referent | |
| Yes | 2.74 (1.95 to 3.85) | <0.001 | |
| New patient to practice or registrar | No | Referent | |
| New patient to registrar | 1.17 (0.87 to 1.58) | 0.28 |
| Characteristic . | . | Odds ratio (95% CI) . | P . |
|---|---|---|---|
| Period | Post | 1.43 (0.92 to 2.23) | 0.12 |
| Treatment | Intervention | 1.11 (0.79 to 1.56) | 0.55 |
| Period by treatment | Post-intervention | 0.47 (0.23 to 0.96) | 0.040 |
| Patients age group | <5 | Referent | |
| 15 to <25 | 5.15 (2.86 to 9.28) | <0.001 | |
| 25 to <45 | 5.77 (3.12 to 10.6) | <0.001 | |
| 45 to <65 | 4.09 (2.65 to 6.29) | <0.001 | |
| 5 to <15 | 5.64 (3.71 to 8.57) | <0.001 | |
| ≥65 | 5.67 (3.62 to 8.87) | <0.001 | |
| Is it a new problem | No | Referent | |
| Yes | 2.74 (1.95 to 3.85) | <0.001 | |
| New patient to practice or registrar | No | Referent | |
| New patient to registrar | 1.17 (0.87 to 1.58) | 0.28 |
The variable ‘is new to practice/registrar’ had to be removed from the model due to collinearity.
The absolute reduction in prescribing of the intervention group compared to the control group, adjusted for clustering was 15.1% (95% CIs: 1.3%–28.9%) and also adjusted for independent variables was 15.8% (95% CIs: 4.2%–27.5%).
Sensitivity analyses
The difference in change in antibiotic prescribing for bronchitis/bronchiolitis remained stable as the number of rounds of data collection included in the analysis was reduced (Supplementary Table 1).
In analyses restricted to workshop-attending registrars, there remained no significant difference in antibiotic prescribing for URTIs (P = 0.58), and for acute bronchitis/bronchiolitis the difference remained significant with little impact on the significance of the interaction term (P = 0.045).
Discussion
Our intervention significantly reduced antibiotic prescribing for acute bronchitis/bronchiolitis but not for URTIs. The lack of significant reduction in prescribing for URTIs may partially reflect modest baseline levels of prescribing pre-intervention. In the data collection round immediately prior to the intervention the proportion of URTIs prescribed antibiotics was 16.5% in the intervention group and 13.4% in the control group—appreciably less than the 21.6% found in the early rounds of the ReCEnT project (2010–2012) (14). This may be explained by the trend of reducing antibiotic prescribing for URTIs that has been observed in earlier studies, but contemporary data are not available (22). The proportion of URTIs prescribed antibiotics in the pre-intervention round is within the ‘acceptable range’ (0–20%) of European quality indicators for outpatient antibiotic prescribing for URTIs (7). Thus there may have been limited scope for improvement.
For acute bronchitis/bronchiolitis, the Odds Ratio (OR) of 0.47 for the interaction term (period by treatment) included an increase in prescribing in the control group as well as a reduction in the intervention group (as was so for RCTs of interventions in this area (23–26)). This OR and the absolute reduction in prescribing compared to the control group of 15.1% (15.8%, adjusted for independent variables) represent an effect size greater than the mean effect size (8.7%) reported in studies with either before-and-after measurement or a control group in a 2012 systematic review of interventions for RTIs/antibiotics (11). The unexpected high antibiotic prescribing rate observed in the control group post-intervention may have inflated our effect. We do not have an explanation for this, but this effect is comparable with results of subsequent RCTs (ORs 0.72 (9), 0·53, 0.68 and 0·38 (12)). Nevertheless, these findings should be interpreted with caution.
Strengths and limitations
A strength of the study was that, despite the sophistication of the intervention’s development process (19), this was a pragmatic intervention delivered within existing educational/training programs. The use of online materials and relatively brief large-group training makes it an efficient means of delivering a complex intervention. Some previous interventions have required onerous training time commitments (1 day (9), or 2–3 days (23,24)), or delivery of separate educational sessions to small groups (9,26) or practices (25)). The peer-to-peer interaction inherent in our intervention workshop is an aspect of RTI-antibiotic education previously found to be particularly valued by GPs (27).
The high response rates and detailed recording of consultation data in the ReCEnT study were also strengths, allowing for tight attribution of clinical indication to antibiotic prescription. Some previous studies have employed overall antibiotic prescribing, not linked to indication, as their outcome measure (10,24,25).
The main limitation of the study is the non-randomized design. As discussed in our Methods, a randomized design was not appropriate for this intervention in this setting. In a non-randomized design, the observed differences may be due to differences between groups in confounding variables rather than to the intervention. We have taken these potential confounders into account in our adjusted model. Nevertheless, the attribution of causality in our study cannot be as strong as for a randomized trial despite a robust non-randomized design and analyses.
The face-to-face workshops were delivered by local teams, which may have impacted on effectiveness. However, the workshop schedule, content and materials were developed collaboratively and the messages delivered were clear and consistent. The control groups may have had education on antibiotic prescribing, but this was shown to be ineffective (15). Moreover, this pragmatic approach ensured the educational intervention was implementable in other RTPs after the study. The acceptability of the intervention package was tested in an accompanying qualitative study, which also explored the perceptions of the core messages. A further limitation is that we do not have clinical contextual data: e.g. temperature, comorbidities, sputum colour or measures of clinical severity. But, while consideration of such clinical factors may be included in some guidelines, this is not so for Australian guidelines (5) which are applicable to our study population. Furthermore, assessing severity and identifying these potentially higher-risk subgroups, e.g. by using sputum colour as an indicator, does not confer clinically meaningful benefit from antibiotics in acute bronchitis (28).
Our analyses are based on the provisional contemporaneous diagnoses of trainees and are coded by trained coders. Any imprecision in diagnosis for our outcome diagnoses will be slight and unlikely to affect our results. A diagnosis of (broncho)pneumonia in Australia requires chest radiography, making it unlikely that this would have been mistakenly coded as bronchitis. Prescribing may have been affected by occurrence of pertussis, however pertussis prevalence was very low during the study period and similar in both groups.
We are also unable to determine whether prescriptions written were filled by patients—however, the scope of our study is the prescribing decisions made by registrars rather than subsequent patient behaviours.
Implications for practice
Several aspects of our intervention having produced a significant reduction in antibiotic prescribing for acute bronchitis/bronchiolitis have educational implications. Early career GPs in the Australian GP training program have for the first time in their careers substantive clinical autonomy in prescribing and are still establishing prescribing habits/patterns. There is evidence that antibiotic prescribing patterns once established in general practice remain stable (13) and that junior hospital doctors (that is pre-vocational training doctors) have poor understanding of antibiotic stewardship prior to entering vocational training (29). Thus, vocational training in general practice may be a singularly appropriate time for interventions aimed at influencing antibiotic prescribing. Also, training programs provide a substrate for efficient delivery of an intervention such as ours by providing a forum for the large-group teaching component. There is evidence from the United Kingdom, however, that GP vocational training programs have a paucity of training in antimicrobial resistance and antimicrobial stewardship—much less than other specialty training programs which prepare trainees for lower volume antibiotic prescribing specialties (30). Hence, our educational intervention addresses a pressing need.
The principles which framed our intervention focus on non-prescription of antibiotics as the default, with a syndromic rather than aetiologically based management approach and consultation techniques reflecting the biopsychosocial rather than biological complexity. We also engaged the trainee-supervisor dyad within the apprenticeship model. We suggest that these elements could inform interventions in other settings.
Implications for future research
The study demonstrates the utility of the translational educational research model inherent in our design—a pragmatic trial nested within an ongoing cohort study where data analysis identifies, quantifies and elucidates an evidence-practice gap, informs an intervention to address that evidence-practice gap, and assesses the effectiveness of the intervention.
An area for further enquiry is any ‘carry-over’ effect on prescribing for other RTIs (sore throat, otitis media, and acute sinusitis) from this intervention focussing on URTI and acute bronchitis/bronchiolitis as exemplars of RTIs not requiring antibiotics.
The longer-term durability of changes in prescribing behaviour we have demonstrated is also a suitable focus for follow-up research.
Conclusion
We have demonstrated that a pragmatic complex educational intervention embedded in an established training program can reduce early career GPs’ antibiotic prescribing for acute bronchitis/bronchiolitis. In the context of relatively low baseline rates of antibiotic prescription, the intervention did not significantly reduce antibiotic use for URTIs.
Supplementary Material
Supplementary data are available at Family Practice online.
Declaration
Funding: a Therapeutic Guidelines Limited (TGL)/RACGP Research Grant of the Royal Australian College of General Practitioners. The Registrars Clinical Encounters in Training project in which the study was nested was funded by the participating Regional Training Providers (General Practice Training Valley to Coast, Victorian Metropolitan Alliance, Adelaide to Outback General Practice Training, General Practice Training Tasmania, and Tropical Medical Training). These organizations were funded by the Australian Commonwealth Government.
Ethics approval: Human Research Ethics Committee of the University of Newcastle (Approval number: H-2009-0323).
Conflicts of interest: none.
Trial registration: ANZCTR Trial ID ACTRN12614001209684.
Acknowledgements
The authors would like to acknowledge the registrars, supervisors and practices involved in this study.