-
PDF
- Split View
-
Views
-
Cite
Cite
T Truong, D K Gardner, Antioxidants improve IVF outcome and subsequent embryo development in the mouse, Human Reproduction, Volume 32, Issue 12, December 2017, Pages 2404–2413, https://doi.org/10.1093/humrep/dex330
Close - Share Icon Share
Abstract
What is the effect of a combination of three antioxidants (Acetyl-L-Carnitine, N-Acetyl-L-Cysteine and α-Lipoic Acid), present in IVF medium during mouse oocyte and sperm collection, on fertilization and subsequent IVF embryo development?
A combination of antioxidants resulted in faster developmental times from the 2-cell stage through to expanded blastocyst stage, accompanied by a significant increase in blastocyst cell number and a reduction of intracellular hydrogen peroxide (H2O2) levels.
The antioxidant combination Acetyl-L-Carnitine, N-Acetyl-L-Cysteine and α-Lipoic Acid, when present in embryo culture media, has a significant beneficial effect on in vitro fertilized mouse pronucleate oocyte development, especially under oxidative stress.
IVF was conducted with combined antioxidants supplemented in IVF medium that was used for mouse oocyte collection and fertilization (oocyte IVF medium, 4 h exposure) and sperm collection and preparation (sperm IVF medium, 1 h exposure).
IVF was conducted under 20% oxygen, in the presence or absence of a combination of antioxidants (10 μM Acetyl-L-Carnitine, 10 μM N-Acetyl-L-Cysteine, 5 μM α-Lipoic Acid) and resultant embryos cultured with and without antioxidants under 20% oxygen. Subsequently, the effects of antioxidants on either oocytes or sperm was evaluated. Embryo development was analysed through time-lapse microscopy followed by differential nuclear staining to determine cell allocation in the blastocyst. Intracellular levels of H2O2 were assessed using an aryl boronate probe after 4 h of incubation with antioxidants. Controls were gametes and embryos that had no antioxidants in the medium. In a separate series of experiments, pronucleate oocytes were collected in handling medium with and without antioxidants for 20 min and subsequent cell numbers analysed.
Antioxidant treatment during both IVF and culture resulted in significantly faster development times to two cell cleavage (P < 0.01), which continued through to the expanded blastocyst stage (P < 0.05). Resultant blastocysts had a significant increase in both trophectoderm (TE) cell numbers, inner cell mass (ICM) and total cell numbers (P < 0.001). The addition of antioxidants to IVF medium or embryo culture media exclusively also resulted in a significant increase in both blastocyst TE and ICM numbers leading to an increase in total cell numbers (P < 0.001). Antioxidant supplementation of either oocyte IVF medium alone, or in both oocyte and sperm IVF medium, lead to significantly faster times to two cell cleavage, which continued through to the expanded blastocyst stage. Blastocyst cell number in both these groups had significantly higher TE cell numbers resulting in an increase in total cell numbers. In contrast, there were no differences in embryo developmental rates and blastocyst cell number when antioxidants were present only in the sperm IVF medium. Levels of H2O2 were significantly reduced in pronucleate oocytes that were cultured in the presence of antioxidants (P < 0.001) compared to control, untreated embryos. Similarly, pronucleate oocytes treated with the combined antioxidants during pronucleate oocyte collection resulted in significantly increased blastocyst ICM numbers compared with controls (P < 0.05).
Embryo development was only examined in the mouse.
These findings suggest that supplementation of antioxidants to the IVF medium, as well as to embryo culture media, may further assist in maintaining the viability of human embryos in ART, conceivably through the reduction of oxidative stress.
This work was funded by a research grant from Vitrolife AB (Sweden). The authors have no conflict of interest to declare.
Introduction
Mammalian preimplantation embryos are particularly sensitive to their environment, which can impact their developmental potential (Lane and Gardner, 2005; Gardner and Kelley, 2017). Harmful reactive oxygen species (ROS) are generated throughout human ART, especially during the collection, manipulation and culture of gametes (Agarwal and Said, 2003). While low ROS levels, produced in vivo by gametes and embryos, are necessary for proper regulation of gamete function and development (Harvey et al., 2002; Sunderam et al., 2014), ROS induced in vitro, especially under atmospheric culture conditions, have been associated with poorer embryo quality and viability (Truong et al., 2016). ROS such as H2O2 have been found in higher concentrations in fragmented human embryos and have been linked to apoptosis (Yang et al., 1998) and delayed embryo development resulting from cell membrane and DNA damage (Guerin et al., 2001). The damaging effect of ROS on oocytes leads to impaired reproductive ability (Heike et al., 2005; Ruder et al., 2008) while its negative influences on sperm motility and sperm–oocyte fusion affect the ability of gametes to fertilize (Aitken and Baker, 2006). This detrimental effect on gamete quality and interaction may explain why the process of in vitro fertilization (IVF) adds to the cumulative stress associated with ART (Wale and Gardner, 2016).
Embryo culture performed at lower oxygen (typically 5%) more closely resembles the oviduct and uterine environment of several mammalian species (~2–8%) (Fischer and Bavister, 1993), with increased embryo development and viability shown in mice and livestock studies (Tervit et al., 1972; Harlow and Quinn, 1979; Batt et al., 1991; Thompson, 2000). Furthermore, human embryos cultured at reduced oxygen levels have improved quality and development correlating with increased live birth rates (Meintjes et al., 2009). In contrast, under atmospheric culture conditions (~20% oxygen) embryo cleavage division rates are delayed (Wale and Gardner, 2010) with adverse effects on embryo gene expression (Rinaudo and Schultz, 2004; Gardner and Lane, 2005), proteome (Katz-Jaffe et al., 2006), metabolism (Lane and Gardner, 2005; Wale and Gardner, 2012) and the epigenome (Li et al., 2016; Gaspar et al., 2015; Ghosh et al., 2017). Despite these harmful effects it is estimated that 75% of IVF cycles worldwide still utilize atmospheric oxygen for a part, or all, of IVF and embryo culture (Christianson et al., 2014).
The female reproductive tract is not only characterized by low levels of oxygen but also possesses antioxidant systems to alleviate oxidative damage (Gupta et al., 2010). While antioxidants are found in the reproductive tract and seminal fluid (Sikka, 2001), routine ART procedures, such as gamete preparation, by default eliminate these protective compounds. High ROS levels are formed during sperm preparation for ART (typically performed at atmospheric oxygen) (Sikka, 2001) especially when the samples are immature sperm (Gil-Guzman et al., 2001), and when samples are free of seminal plasma (Sikka, 2001) and thus devoid of endogenous seminal fluid antioxidants.
Antioxidants, such as L-Carnitine (LC), present in the epididymal fluid, support sperm motility, maturation and fertilization, and protect against oxidative damage by reducing apoptosis (Brooks, 1979; Lenzi et al., 2004; Dokmeci, 2005). LC has been demonstrated to protect sperm plasma membrane integrity (Lenzi et al., 2004), mitigate sperm DNA damage and thus increase normal chromatin quality and improve sperm motility (Brooks, 1979; Aliabadi et al., 2013). Furthermore, addition of LC to mouse in vitro maturation (IVM) medium has been shown to improve hatching blastocyst rates and ICM numbers (Dunning and Robker, 2012). In addition, in vitro cultured follicles treated with LC yield a higher number of MII oocytes, accompanied by increased fertilization and blastocyst development rates (Dunning and Robker, 2012), and the presence of LC in embryo culture media is associated with decreased DNA damage (Abdelrazik et al., 2009). The mechanism of LC may be through the regulation and transport of long chain fatty acids into mitochondria for β-oxidation and ATP production (Somfai et al., 2011) and through its antioxidant actions by reducing the levels of ROS (Emiliani et al., 2000).
α-Lipoic Acid (ALA) has been shown to protect mouse embryos against oxidative stress (Linck et al., 2007) and has been found to improve sperm motility and reduce DNA damage (Selvakumar et al., 2006; Ibrahim et al., 2008). As ALA regulates mitochondrial function and assists in the production of ATP for energy production (Palaniappan and Dai, 2007; Plotnikov et al., 2007), it is conceivable that the actions of ALA may contribute to the increase in sperm motility. Supplementation of ALA to IVM medium was also found to improve the maturation rate of cloned goat oocytes (Zhang et al., 2013). The subsequent enhanced developmental competence was mediated through the reduction of cellular apoptosis by the inhibition of apoptotic activator genes (Zhang et al., 2013). In addition, ALA has been shown to stimulate expression of antioxidant genes involved in defending against oxidative stress (Luberda, 2005; Zhang et al., 2013).
Glutathione (GSH) is key to sperm head decondensation following fertilization (de Matos et al., 1996), and as cysteine is the rate limiting factor in GSH synthesis (Anderson and Meister, 1983; de Matos et al., 1995; de Matos and Furnus, 2000), its presence consequently impacts fertilization and embryo development. Pretreatment of oocytes with cysteine analogues, including N-Acetyl-L-Cysteine (NAC), results in increased fertilization rates of mouse oocytes (Takeo et al., 2015). The enhanced fertilization efficiency may be mediated by cysteine reducing disulphide bonds and inducing expansion in the zona pellucida (ZP) of mouse oocytes, with the morphological changes allowing for greater fertilizing ability/activity (Takeo et al., 2015). Furthermore, cysteine supplemented to bovine IVM medium improves bovine blastocyst development through the up regulation of GSH synthesis (Caamano et al., 1998; Furnus et al., 2008). Thus, the improved embryo quality may be a result of cysteine affecting GSH levels, therefore protecting the cell from oxidative stress through an increased antioxidant capacity.
Limited studies have been conducted on the effects of combinatorial antioxidants in IVF medium on embryo development (Kim et al., 2006; Saikhun et al., 2008; Kang et al., 2015). Recently, the antioxidant combination Acetyl-L-Carnitine, N-Acetyl-L-Cysteine and α-Lipoic Acid in embryo culture media was shown to significantly improve mouse embryo development and viability, both at atmospheric (20%) and 5% oxygen (although the effect was less at physiological oxygen) (Truong et al., 2016). Further, these antioxidants were able to maintain intracellular GSH levels. However, as gametes are particularly susceptible to oxidative damage, supplementation of media used for both oocytes and sperm during IVF may be similarly beneficial, particularly as both sperm and oocytes are exposed to atmospheric oxygen during their collection, preparation and ICSI. Additionally, cleavage stage embryos are often exposed to atmospheric oxygen at fertilization check and at grading (if not cultured in time-lapse incubators). Consequently, we have evaluated the effects of a combination of antioxidants during oocyte and sperm collection and subsequent IVF on embryo development.
Materials and Methods
Pronucleate oocyte (2PN) collection
F1 virgin hybrid female mice (C57BL/6 × CBA) at 3–4 weeks old were injected intraperitoneally with 5 IU of pregnant mare's serum gonadotrophin (PMSG) (Folligon, Intervet, UK). Following administration of 5 IU hCG (Chorulon, Intervet, UK) 48 h later, female mice were mated with 12-week-old F1 male mice. The presence of a vaginal plug the following morning was indicative of successful mating. Pronucleate oocytes (2PN) were collected 22 h post-hCG, in pre-warmed handling medium G-MOPS PLUS (G-MOPS PLUS, Vitrolife AB, Sweden). GMOPS containing 300 IU/ml hyaluronidase (bovine testes type IV, Sigma Aldrich, NSW), but lacking human serum albumin (HSA), was used to denude attached cumulus cells. Prior to allocation for culture, all embryos were washed twice in G-MOPS PLUS medium and once in G1 medium containing 5 mg/ml HSA (G1-Plus, Vitrolife). Forty-eight hours later embryos were transferred to G2 medium containing 5 mg/ml HSA (G2-Plus, Vitrolife). Mice were housed under a 12 h light-12 h dark photoperiod with food and water available ad libitum. All mice experimentation was approved by The University of Melbourne, Animal Ethics Committee (AEC #1313046.1).
In vitro fertilization
All stages of IVF were conducted in 20% oxygen in G-IVF medium (G-IVF, Vitrolife) in the presence or absence of antioxidants (10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid) (Sigma Aldrich, USA). Oocytes were collected from 3- to 4-week-old F1 virgin hybrid female mice (C57BL/6 × CBA) stimulated with 5 IU PMSG, followed 48 h later with 5 IU hCG. Oocytes were collected 15 h post-hCG in pre-equilibrated oocyte IVF medium (G-IVF containing 10 mg/ml HSA) and transferred to 45 μl drops of oocyte IVF medium for culture under paraffin oil (Ovoil, Vitrolife) in 6% CO2 in air at 37°C. Sperm from 8- to 12-week-old F1 hybrid male mice (C57BL/6 × CBA) were collected by puncturing the cauda epididymides and vas deferens with a needle while submerged in 500 μl of sperm IVF medium. Sperm swim-up and capacitation was carried out for 1 h in sperm IVF medium and sperm concentration was determined using a Makler counting chamber, following which 1–2 million motile sperm per ml was added to each cumulus oocyte complex and fertilization allowed to take place over a 4 h period. Following fertilization and prior to allocation for culture, all embryos were washed once in both IVF medium and G1. For each biological replicate oocytes from all female mice were pooled and sperm from one male mouse was used for fertilization. The subsequent fertilized oocytes were then randomly allocated to treatment groups to account for variations in oocyte/sperm quality.
Treatments
Antioxidants in IVF and culture media
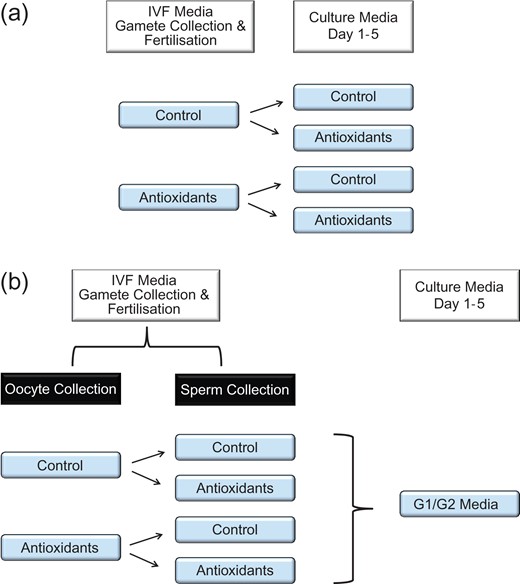
All stages of IVF were conducted in the presence or absence of antioxidants (10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid). The resulting embryos were then cultured in G1 and G2 media in the EmbryoScope™ (Vitrolife) individually in 25 μl volumes, with or without antioxidants, creating four groups (Fig. 1a). Embryo development was analysed by time-lapse microscopy followed by differential nuclear staining as outlined below.
Experimental design. 1a. Experimental design for the analysis of antioxidants in IVF and/or culture media. Gamete collection and fertilization were conducted in the presence or absence of antioxidants (10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid). The resulting embryos were then cultured in media with or without antioxidants, thereby creating four groups. 1b. Experimental design for the analysis of antioxidants in either oocyte and/or sperm media for IVF. Sperm from F1 male mice were collected in sperm IVF medium with and without antioxidants. Similarly, oocytes were collected from F1 female mice in oocyte IVF medium with and without antioxidants, thereby creating four groups. Resultant embryos were then cultured in media without antioxidants.
Antioxidants in oocyte and sperm IVF media
Sperm from F1 male mice were collected in sperm IVF medium with and without antioxidants (10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid). Similarly, oocytes were collected from F1 female mice in oocyte IVF medium with and without antioxidants to create four groups (Fig. 1b). IVF was conducted under paraffin oil in 6% CO2 in air at 37°C and the resulting embryos were then cultured in the EmbryoScope™ individually in 25 μl volumes of G1 and G2 media without antioxidants and development and differential nuclear staining analysed.
Antioxidants in handling media
Pronucleate oocytes were collected in pre-warmed handling medium (G-MOPS PLUS) with and without antioxidants (10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid). Pronucleate oocytes were kept in the handling medium for 20 min on a warming stage at 37°C, at atmospheric oxygen, to mimic embryo handling times, before being transferred to a culture dish in an incubator. In this experiment, embryos were cultured in groups of 10 in 20 μl drops of media with no antioxidants, in G1 medium for 48 h and then for a further 48 h in G2 medium.
Analysis of morphokinetics
Embryos were cultured individually in 25 μl drops of G1 medium for 48 h and then for a further 48 h in G2 medium under paraffin oil in 6% CO2 in air at 37°C, in an EmbryoScope™ time-lapse imaging incubator. Time-lapse images were acquired at 15 min intervals throughout the culture period. Timing of morphokinetic events, including syngamy (2PN fading), cleavage division from 2- to 8-cell stages, compaction (beginning of junction formation), morulae formation (reduction in embryo volume and merging of individual cells), cavitation (appearance of the blastocoel) and blastocyst (blastocoel takes up half or more of the volume of the embryo), expanded blastocyst (zona is thinning and the entire volume of the embryo is filled with the blastocoel) and hatching blastocyst stages (Gardner and Lane, 2014), were recorded as hours post syngamy. Blastocysts at the end of the culture period 96 h after fertilization were differentially stained to determine the allocation of cells to the trophectoderm (TE) and inner cell mass (ICM) as outlined below.
Determination of blastocyst cell numbers
Blastocysts were differentially stained using a modified staining protocol as previously described (Hardy et al., 1989). Briefly, after pronase treatment to remove the zona, TE nuclei were labelled with propidium iodide following complement-mediated lysis, leaving the ICM nuclei unlabelled. All nuclei were stained with bisbenzimide. Blastocysts were mounted in glycerol and imaged under a fluorescence microscope (Nikon Eclipse TS100) and cells counted using ImageJ software.
Determination of intracellular hydrogen peroxide levels
Fluorometric analysis was carried out measuring intracellular levels of H2O2 in pronucleate oocytes using peroxyfluor1 (PF1) (Centre for Nanoscale BioPhotonics, Adelaide), an aryl boronate probe which has high specificity for H2O2 and fluoresces upon reaction with it (Baena and Lendl, 2004). Pronucleate oocytes were incubated in 20 μl drops of G1 medium with or without antioxidants, under paraffin oil in 6% CO2 in air at 37°C for 4 h. Pronucleate oocytes from both control and antioxidant treatment groups were treated with 10 μM PF1 for 1 h under the same culture conditions. Pronucleate oocytes were rinsed thoroughly in G1 medium, placed in individual 2 μl drops of GMOPS-PLUS made on glass bottom dishes (Fluorodish, Coherent Scientific, Australia), and overlayed with paraffin oil. Fluorescence was quantitated under a fluorescence microscope (Nikon Eclipse TS100). Results were calculated by subtracting the values of blank (embryos with no PF1 treatment) and basal culture media levels from the fluorescence recorded using ImageJ. All media and oil used were pre-equilibrated overnight at 6% CO2, in air at 37°C.
Statistical analysis
Cell number data and time-lapse analysis for all treatments were compared to the control and were subjected to a one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test or were compared to controls using Student's t-test. All groups were tested for normality prior to analysis using Bartlett's test. Differences were considered biologically significant at a P-value of 0.05. All analyses were performed using GraphPad Prism.
Results
Morphokinetic events and blastocyst cell numbers of embryos cultured with antioxidants during IVF and culture
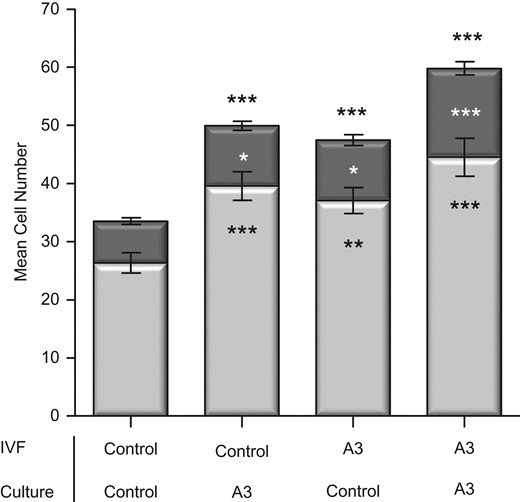
The presence of antioxidants throughout IVF had no effect on fertilization rate (84.7%) or percentage blastocyst formation (93.4%) from control values (Supplementary Table SI). Addition of antioxidants exclusively to embryo culture media resulted in a significant increase in TE (P < 0.001) and ICM (P < 0.05) cell numbers leading to an increase in total blastocyst cell numbers (P < 0.001) (Fig. 2). Similarly, antioxidants present solely in the IVF medium resulted in a significant increase in TE (P < 0.01), ICM (P < 0.05) and consequently total blastocyst cell numbers (P < 0.001). The presence of antioxidants in both the IVF medium and embryo culture media also lead to a significant increase in TE (P < 0.001), ICM (P < 0.001) and total (P < 0.001) cell numbers. The presence of antioxidants during both IVF and culture was also associated with significantly faster developmental times compared to control embryos with no antioxidants in either IVF or culture (Table I). This was observed as early as two cell cleavage (P < 0.01), and continued through to the expanded blastocyst stage. Timing for the duration interval between cc3 (t5-t4) cleavage stages was also significant (P < 0.05).
Time of cleavage events of IVF embryos cultured with or without antioxidants.
| . | IVF . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Culture . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 14.66 ± 0.22 | 14.66 ± 0.18 | 14.39 ± 0.15 | 14.36 ± 0.20 |
| Developmental event (h post syngamy) | Syn-t2 | 1.67 ± 0.15 | 1.49 ± 0.04 | 1.48 ± 0.04 | 1.47 ± 0.06 |
| 1st cleavage (t2) | 16.33 ± 0.22 | 16.15 ± 0.18 | 15.87 ± 0.15 | 15.83 ± 0.20* | |
| cc2 (t3-t2) | 20.19 ± 0.22 | 20.73 ± 0.19 | 20.18 ± 0.17 | 20.24 ± 0.20 | |
| 2nd cleavage (t3) | 36.52 ± 0.30 | 36.88 ± 0.29 | 36.05 ± 0.22 | 36.06 ± 0.30 | |
| s2 (t4-t3) | 0.95 ± 0.15 | 1.13 ± 0.15 | 1.07 ± 0.13 | 1.15 ± 0.12 | |
| t4 | 37.47 ± 0.31 | 38.01 ± 0.30 | 37.11 ± 0.26 | 37.19 ± 0.32 | |
| cc3 (t5-t4) | 16.59 ± 0.63 | 15.54 ± 0.35 | 15.98 ± 0.39 | 14.85 ± 0.44* | |
| 3rd cleavage (t5) | 54.06 ± 0.81 | 53.50 ± 0.52 | 53.12 ± 0.43 | 51.81 ± 0.56** | |
| t6 | 55.19 ± 0.87 | 54.40 ± 0.54 | 54.08 ± 0.46 | 52.70 ± 0.61** | |
| 4th cleavage (t7) | 56.42 ± 0.96 | 55.48 ± 0.61 | 55.20 ± 0.52 | 53.75 ± 0.52** | |
| t8 | 57.51 ± 0.99 | 56.63 ± 0.65 | 56.15 ± 0.55 | 54.68 ± 0.71** | |
| s3 (t8-t5) | 3.45 ± 0.26 | 3.18 ± 0.23 | 2.81 ± 0.22 | 2.87 ± 0.23 | |
| Cavitation | 80.03 ± 0.96 | 79.75 ± 0.66 | 79.45 ± 0.68 | 77.22 ± 0.79* | |
| Blastocyst | 83.48 ± 1.09 | 82.34 ± 0.71 | 82.72 ± 0.73 | 80.11 ± 0.83* | |
| Expanded blastocyst | 85.23 ± 1.04 | 85.04 ± 0.72 | 85.67 ± 0.81 | 82.39 ± 0.79* | |
| Hatching | 86.83 ± 1.32 | 88.14 ± 1.04 | 87.13 ± 1.02 | 84.83 ± 1.02 |
| . | IVF . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Culture . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 14.66 ± 0.22 | 14.66 ± 0.18 | 14.39 ± 0.15 | 14.36 ± 0.20 |
| Developmental event (h post syngamy) | Syn-t2 | 1.67 ± 0.15 | 1.49 ± 0.04 | 1.48 ± 0.04 | 1.47 ± 0.06 |
| 1st cleavage (t2) | 16.33 ± 0.22 | 16.15 ± 0.18 | 15.87 ± 0.15 | 15.83 ± 0.20* | |
| cc2 (t3-t2) | 20.19 ± 0.22 | 20.73 ± 0.19 | 20.18 ± 0.17 | 20.24 ± 0.20 | |
| 2nd cleavage (t3) | 36.52 ± 0.30 | 36.88 ± 0.29 | 36.05 ± 0.22 | 36.06 ± 0.30 | |
| s2 (t4-t3) | 0.95 ± 0.15 | 1.13 ± 0.15 | 1.07 ± 0.13 | 1.15 ± 0.12 | |
| t4 | 37.47 ± 0.31 | 38.01 ± 0.30 | 37.11 ± 0.26 | 37.19 ± 0.32 | |
| cc3 (t5-t4) | 16.59 ± 0.63 | 15.54 ± 0.35 | 15.98 ± 0.39 | 14.85 ± 0.44* | |
| 3rd cleavage (t5) | 54.06 ± 0.81 | 53.50 ± 0.52 | 53.12 ± 0.43 | 51.81 ± 0.56** | |
| t6 | 55.19 ± 0.87 | 54.40 ± 0.54 | 54.08 ± 0.46 | 52.70 ± 0.61** | |
| 4th cleavage (t7) | 56.42 ± 0.96 | 55.48 ± 0.61 | 55.20 ± 0.52 | 53.75 ± 0.52** | |
| t8 | 57.51 ± 0.99 | 56.63 ± 0.65 | 56.15 ± 0.55 | 54.68 ± 0.71** | |
| s3 (t8-t5) | 3.45 ± 0.26 | 3.18 ± 0.23 | 2.81 ± 0.22 | 2.87 ± 0.23 | |
| Cavitation | 80.03 ± 0.96 | 79.75 ± 0.66 | 79.45 ± 0.68 | 77.22 ± 0.79* | |
| Blastocyst | 83.48 ± 1.09 | 82.34 ± 0.71 | 82.72 ± 0.73 | 80.11 ± 0.83* | |
| Expanded blastocyst | 85.23 ± 1.04 | 85.04 ± 0.72 | 85.67 ± 0.81 | 82.39 ± 0.79* | |
| Hatching | 86.83 ± 1.32 | 88.14 ± 1.04 | 87.13 ± 1.02 | 84.83 ± 1.02 |
Cell cleavage and development times (h) of mouse embryos cultured individually under 20% oxygen in IVF media supplemented with combination of antioxidants (A3) comprising of 10 μM Acetyl-L-Carnitine, 10 μM N-Acetyl-L-Cysteine and 5 μM α-Lipoic Acid, or in control media without antioxidants during IVF (gamete collection and fertilization) and/or culture. t2 represents the time from syngamy to cleavage to 2 cell. t3, t4, t5, t6, t7, t8, represent the time to cleavage to 3 cell, 4 cell, 5 cell, 6 cell, 7 cell and 8 cell, respectively. syn-t2 = duration from syngamy to two cell; cc2 = the duration of the second cell cycle; s2 = duration of second synchrony; cc3 = third cell cycle; s3 = duration of third synchrony. Data are expressed as mean ± SEM of cleavage events (h post syngamy), or duration between cleavage events (h). n = at least 60 embryos per treatment from six independent biological replicates. Asterisks denote significant differences from the control. *P < 0.05, **P < 0.01.
Time of cleavage events of IVF embryos cultured with or without antioxidants.
| . | IVF . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Culture . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 14.66 ± 0.22 | 14.66 ± 0.18 | 14.39 ± 0.15 | 14.36 ± 0.20 |
| Developmental event (h post syngamy) | Syn-t2 | 1.67 ± 0.15 | 1.49 ± 0.04 | 1.48 ± 0.04 | 1.47 ± 0.06 |
| 1st cleavage (t2) | 16.33 ± 0.22 | 16.15 ± 0.18 | 15.87 ± 0.15 | 15.83 ± 0.20* | |
| cc2 (t3-t2) | 20.19 ± 0.22 | 20.73 ± 0.19 | 20.18 ± 0.17 | 20.24 ± 0.20 | |
| 2nd cleavage (t3) | 36.52 ± 0.30 | 36.88 ± 0.29 | 36.05 ± 0.22 | 36.06 ± 0.30 | |
| s2 (t4-t3) | 0.95 ± 0.15 | 1.13 ± 0.15 | 1.07 ± 0.13 | 1.15 ± 0.12 | |
| t4 | 37.47 ± 0.31 | 38.01 ± 0.30 | 37.11 ± 0.26 | 37.19 ± 0.32 | |
| cc3 (t5-t4) | 16.59 ± 0.63 | 15.54 ± 0.35 | 15.98 ± 0.39 | 14.85 ± 0.44* | |
| 3rd cleavage (t5) | 54.06 ± 0.81 | 53.50 ± 0.52 | 53.12 ± 0.43 | 51.81 ± 0.56** | |
| t6 | 55.19 ± 0.87 | 54.40 ± 0.54 | 54.08 ± 0.46 | 52.70 ± 0.61** | |
| 4th cleavage (t7) | 56.42 ± 0.96 | 55.48 ± 0.61 | 55.20 ± 0.52 | 53.75 ± 0.52** | |
| t8 | 57.51 ± 0.99 | 56.63 ± 0.65 | 56.15 ± 0.55 | 54.68 ± 0.71** | |
| s3 (t8-t5) | 3.45 ± 0.26 | 3.18 ± 0.23 | 2.81 ± 0.22 | 2.87 ± 0.23 | |
| Cavitation | 80.03 ± 0.96 | 79.75 ± 0.66 | 79.45 ± 0.68 | 77.22 ± 0.79* | |
| Blastocyst | 83.48 ± 1.09 | 82.34 ± 0.71 | 82.72 ± 0.73 | 80.11 ± 0.83* | |
| Expanded blastocyst | 85.23 ± 1.04 | 85.04 ± 0.72 | 85.67 ± 0.81 | 82.39 ± 0.79* | |
| Hatching | 86.83 ± 1.32 | 88.14 ± 1.04 | 87.13 ± 1.02 | 84.83 ± 1.02 |
| . | IVF . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Culture . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 14.66 ± 0.22 | 14.66 ± 0.18 | 14.39 ± 0.15 | 14.36 ± 0.20 |
| Developmental event (h post syngamy) | Syn-t2 | 1.67 ± 0.15 | 1.49 ± 0.04 | 1.48 ± 0.04 | 1.47 ± 0.06 |
| 1st cleavage (t2) | 16.33 ± 0.22 | 16.15 ± 0.18 | 15.87 ± 0.15 | 15.83 ± 0.20* | |
| cc2 (t3-t2) | 20.19 ± 0.22 | 20.73 ± 0.19 | 20.18 ± 0.17 | 20.24 ± 0.20 | |
| 2nd cleavage (t3) | 36.52 ± 0.30 | 36.88 ± 0.29 | 36.05 ± 0.22 | 36.06 ± 0.30 | |
| s2 (t4-t3) | 0.95 ± 0.15 | 1.13 ± 0.15 | 1.07 ± 0.13 | 1.15 ± 0.12 | |
| t4 | 37.47 ± 0.31 | 38.01 ± 0.30 | 37.11 ± 0.26 | 37.19 ± 0.32 | |
| cc3 (t5-t4) | 16.59 ± 0.63 | 15.54 ± 0.35 | 15.98 ± 0.39 | 14.85 ± 0.44* | |
| 3rd cleavage (t5) | 54.06 ± 0.81 | 53.50 ± 0.52 | 53.12 ± 0.43 | 51.81 ± 0.56** | |
| t6 | 55.19 ± 0.87 | 54.40 ± 0.54 | 54.08 ± 0.46 | 52.70 ± 0.61** | |
| 4th cleavage (t7) | 56.42 ± 0.96 | 55.48 ± 0.61 | 55.20 ± 0.52 | 53.75 ± 0.52** | |
| t8 | 57.51 ± 0.99 | 56.63 ± 0.65 | 56.15 ± 0.55 | 54.68 ± 0.71** | |
| s3 (t8-t5) | 3.45 ± 0.26 | 3.18 ± 0.23 | 2.81 ± 0.22 | 2.87 ± 0.23 | |
| Cavitation | 80.03 ± 0.96 | 79.75 ± 0.66 | 79.45 ± 0.68 | 77.22 ± 0.79* | |
| Blastocyst | 83.48 ± 1.09 | 82.34 ± 0.71 | 82.72 ± 0.73 | 80.11 ± 0.83* | |
| Expanded blastocyst | 85.23 ± 1.04 | 85.04 ± 0.72 | 85.67 ± 0.81 | 82.39 ± 0.79* | |
| Hatching | 86.83 ± 1.32 | 88.14 ± 1.04 | 87.13 ± 1.02 | 84.83 ± 1.02 |
Cell cleavage and development times (h) of mouse embryos cultured individually under 20% oxygen in IVF media supplemented with combination of antioxidants (A3) comprising of 10 μM Acetyl-L-Carnitine, 10 μM N-Acetyl-L-Cysteine and 5 μM α-Lipoic Acid, or in control media without antioxidants during IVF (gamete collection and fertilization) and/or culture. t2 represents the time from syngamy to cleavage to 2 cell. t3, t4, t5, t6, t7, t8, represent the time to cleavage to 3 cell, 4 cell, 5 cell, 6 cell, 7 cell and 8 cell, respectively. syn-t2 = duration from syngamy to two cell; cc2 = the duration of the second cell cycle; s2 = duration of second synchrony; cc3 = third cell cycle; s3 = duration of third synchrony. Data are expressed as mean ± SEM of cleavage events (h post syngamy), or duration between cleavage events (h). n = at least 60 embryos per treatment from six independent biological replicates. Asterisks denote significant differences from the control. *P < 0.05, **P < 0.01.
Impact of antioxidants present throughout IVF (gamete collection and fertilization) and/or culture on blastocyst cell number. Cell lineage allocation of IVF embryos cultured individually at 20% oxygen. Antioxidant combination (A3) comprises 10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid. Controls were gamete and/or culture media with no antioxidants. Light and dark bar portions represent the average trophectoderm (TE) and inner cell mass (ICM) cells respectively. Data are expressed as mean ± SEM. n = at least 60 embryos per treatment, from six independent biological replicates. Asterisks denote significant differences from the control, where asterisks above the bar represent significantly different to the total cell number and asterisks within the bars represents significantly different to the TE or ICM *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of antioxidants in gamete IVF medium on embryo development and blastocyst cell number
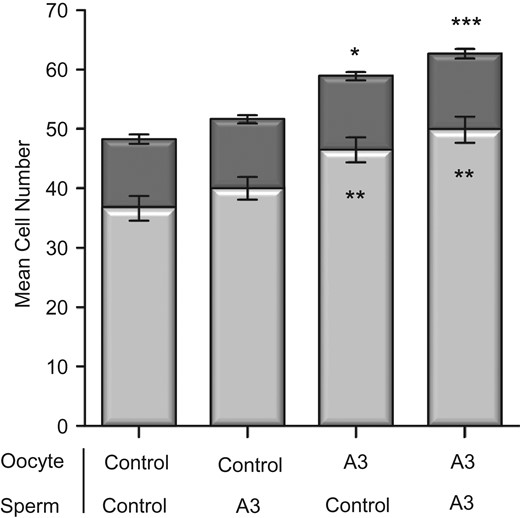
IVF was conducted with antioxidants present or absent in oocyte and/or sperm IVF medium with subsequent embryos cultured in medium with no antioxidants in order to isolate the effects of antioxidants at IVF. The presence of antioxidants had no effect on fertilization rate or percentage blastocyst formation from control values, being 82.3% and 94.9%, respectively (Supplementary Table SII). There were no differences in blastocyst total cell numbers in embryos that had antioxidants only in the sperm IVF medium when compared to control embryos that had no antioxidants in both oocyte and sperm IVF medium (Fig. 3). Embryos that had antioxidants solely in oocyte IVF medium had increased TE cell numbers (P < 0.01) resulting in an increase in total blastocyst cell numbers (P < 0.05) compared to controls. Similarly, embryos developing from oocytes and sperm IVF medium containing antioxidants had higher TE cell numbers (P < 0.01) contributing to the highest increase in total blastocyst cell numbers (P < 0.001). Analysis of developmental kinetic events revealed that embryos that had antioxidants only in the sperm IVF medium had no differences in developmental times compared to embryos that saw no antioxidants (Table II). Embryos that had antioxidants solely in oocyte IVF medium developed significantly faster to the 2-cell cleavage stage (P < 0.01) which continued through to expanded blastocysts (P < 0.001). Similarly, when antioxidants were added to both oocyte and sperm IVF medium, resultant embryos had significantly faster times to the 2-cell cleavage stage (P < 0.05) and this continued through to hatching blastocysts (P < 0.05) compared to embryos generated in the absence of antioxidants (Table II).
Time of cleavage events of IVF embryos cultured with antioxidants in gamete collection medium.
| . | Oocyte . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Sperm . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 13.70 ± 0.11 | 13.82 ± 0.10 | 13.30 ± 0.09 | 13.24 ± 0.07 |
| Developmental event (h post syngamy) | Syn-t2 | 1.51 ± 0.04 | 1.49 ± 0.03 | 1.49 ± 0.04 | 1.56 ± 0.03 |
| 1st cleavage (t2) | 15.21 ± 0.11 | 15.31 ± 0.10 | 14.79 ± 0.09** | 14.80 ± 0.07* | |
| cc2 (t3-t2) | 20.73 ± 0.16 | 20.42 ± 0.18 | 20.20 ± 0.13* | 20.18 ± 0.13* | |
| 2nd cleavage (t3) | 35.94 ± 0.20 | 35.73 ± 0.20 | 34.99 ± 0.14*** | 34.98 ± 0.14*** | |
| s2 (t4-t3) | 1.03 ± 0.10 | 1.34 ± 0.14 | 0.94 ± 0.10 | 0.92 ± 0.14 | |
| t4 | 36.97 ± 0.23 | 37.07 ± 0.27 | 35.93 ± 0.17** | 35.90 ± 0.17** | |
| cc3 (t5-t4) | 15.13 ± 0.29 | 15.06 ± 0.29 | 14.78 ± 0.28 | 14.71 ± 0.23 | |
| 3rd cleavage (t5) | 52.09 ± 0.41 | 52.01 ± 0.37 | 50.70 ± 0.30* | 50.61 ± 0.30* | |
| t6 | 53.05 ± 0.41 | 53.13 ± 0.41 | 51.53 ± 0.31* | 51.41 ± 0.32** | |
| 4th cleavage (t7) | 54.11 ± 0.43 | 54.25 ± 0.43 | 52.44 ± 0.33** | 52.30 ± 0.34** | |
| t8 | 55.14 ± 0.45 | 55.31 ± 0.45 | 53.43 ± 0.36** | 53.29 ± 0.35** | |
| S3 (t8-t5) | 3.04 ± 0.16 | 3.30 ± 0.18 | 2.82 ± 0.18 | 2.48 ± 0.13 | |
| Cavitation | 78.63 ± 0.48 | 78.54 ± 0.53 | 75.80 ± 0.54*** | 76.35 ± 0.46** | |
| Blastocyst | 81.71 ± 0.55 | 81.68 ± 0.55 | 78.72 ± 0.53*** | 78.93 ± 0.44*** | |
| Expanded blastocyst | 84.38 ± 0.61 | 84.47 ± 0.66 | 81.23 ± 0.56*** | 81.78 ± 0.58* | |
| Hatching | 89.10 ± 0.84 | 87.23 ± 0.89 | 86.08 ± 1.05 | 85.95 ± 0.94* |
| . | Oocyte . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Sperm . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 13.70 ± 0.11 | 13.82 ± 0.10 | 13.30 ± 0.09 | 13.24 ± 0.07 |
| Developmental event (h post syngamy) | Syn-t2 | 1.51 ± 0.04 | 1.49 ± 0.03 | 1.49 ± 0.04 | 1.56 ± 0.03 |
| 1st cleavage (t2) | 15.21 ± 0.11 | 15.31 ± 0.10 | 14.79 ± 0.09** | 14.80 ± 0.07* | |
| cc2 (t3-t2) | 20.73 ± 0.16 | 20.42 ± 0.18 | 20.20 ± 0.13* | 20.18 ± 0.13* | |
| 2nd cleavage (t3) | 35.94 ± 0.20 | 35.73 ± 0.20 | 34.99 ± 0.14*** | 34.98 ± 0.14*** | |
| s2 (t4-t3) | 1.03 ± 0.10 | 1.34 ± 0.14 | 0.94 ± 0.10 | 0.92 ± 0.14 | |
| t4 | 36.97 ± 0.23 | 37.07 ± 0.27 | 35.93 ± 0.17** | 35.90 ± 0.17** | |
| cc3 (t5-t4) | 15.13 ± 0.29 | 15.06 ± 0.29 | 14.78 ± 0.28 | 14.71 ± 0.23 | |
| 3rd cleavage (t5) | 52.09 ± 0.41 | 52.01 ± 0.37 | 50.70 ± 0.30* | 50.61 ± 0.30* | |
| t6 | 53.05 ± 0.41 | 53.13 ± 0.41 | 51.53 ± 0.31* | 51.41 ± 0.32** | |
| 4th cleavage (t7) | 54.11 ± 0.43 | 54.25 ± 0.43 | 52.44 ± 0.33** | 52.30 ± 0.34** | |
| t8 | 55.14 ± 0.45 | 55.31 ± 0.45 | 53.43 ± 0.36** | 53.29 ± 0.35** | |
| S3 (t8-t5) | 3.04 ± 0.16 | 3.30 ± 0.18 | 2.82 ± 0.18 | 2.48 ± 0.13 | |
| Cavitation | 78.63 ± 0.48 | 78.54 ± 0.53 | 75.80 ± 0.54*** | 76.35 ± 0.46** | |
| Blastocyst | 81.71 ± 0.55 | 81.68 ± 0.55 | 78.72 ± 0.53*** | 78.93 ± 0.44*** | |
| Expanded blastocyst | 84.38 ± 0.61 | 84.47 ± 0.66 | 81.23 ± 0.56*** | 81.78 ± 0.58* | |
| Hatching | 89.10 ± 0.84 | 87.23 ± 0.89 | 86.08 ± 1.05 | 85.95 ± 0.94* |
Cell cleavage and development times of mouse embryos cultured individually under 20% oxygen during IVF in the presence of a combination of antioxidants (A3) comprising of 10 μM Acetyl-L-Carnitine, 10 μM N-Acetyl-L-Cysteine and 5 μM α-Lipoic Acid, or in control media without antioxidants, in oocyte IVF media (oocyte collection and fertilization) and/or sperm IVF media (sperm collection and preparation). t2 represents the time from syngamy to cleavage to 2 cell. t3, t4, t5, t6, t7, t8, represent the time to cleavage to 3 cell, 4 cell, 5 cell, 6 cell, 7 cell and 8 cell, respectively. syn-t2 = duration from syngamy to two cell; cc2 = duration of the second cell cycle; s2 = duration of second synchrony; cc3 = duration of the third cell cycle; s3 = duration of third synchrony. Data are expressed as mean ± SEM of cleavage events (h post syngamy), or duration between cleavage events (h). n = at least 80 embryos per treatment from six biological replicates. Asterisks denote significant differences from the control. *P < 0.05, **P < 0.01, ***P < 0.001.
Time of cleavage events of IVF embryos cultured with antioxidants in gamete collection medium.
| . | Oocyte . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Sperm . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 13.70 ± 0.11 | 13.82 ± 0.10 | 13.30 ± 0.09 | 13.24 ± 0.07 |
| Developmental event (h post syngamy) | Syn-t2 | 1.51 ± 0.04 | 1.49 ± 0.03 | 1.49 ± 0.04 | 1.56 ± 0.03 |
| 1st cleavage (t2) | 15.21 ± 0.11 | 15.31 ± 0.10 | 14.79 ± 0.09** | 14.80 ± 0.07* | |
| cc2 (t3-t2) | 20.73 ± 0.16 | 20.42 ± 0.18 | 20.20 ± 0.13* | 20.18 ± 0.13* | |
| 2nd cleavage (t3) | 35.94 ± 0.20 | 35.73 ± 0.20 | 34.99 ± 0.14*** | 34.98 ± 0.14*** | |
| s2 (t4-t3) | 1.03 ± 0.10 | 1.34 ± 0.14 | 0.94 ± 0.10 | 0.92 ± 0.14 | |
| t4 | 36.97 ± 0.23 | 37.07 ± 0.27 | 35.93 ± 0.17** | 35.90 ± 0.17** | |
| cc3 (t5-t4) | 15.13 ± 0.29 | 15.06 ± 0.29 | 14.78 ± 0.28 | 14.71 ± 0.23 | |
| 3rd cleavage (t5) | 52.09 ± 0.41 | 52.01 ± 0.37 | 50.70 ± 0.30* | 50.61 ± 0.30* | |
| t6 | 53.05 ± 0.41 | 53.13 ± 0.41 | 51.53 ± 0.31* | 51.41 ± 0.32** | |
| 4th cleavage (t7) | 54.11 ± 0.43 | 54.25 ± 0.43 | 52.44 ± 0.33** | 52.30 ± 0.34** | |
| t8 | 55.14 ± 0.45 | 55.31 ± 0.45 | 53.43 ± 0.36** | 53.29 ± 0.35** | |
| S3 (t8-t5) | 3.04 ± 0.16 | 3.30 ± 0.18 | 2.82 ± 0.18 | 2.48 ± 0.13 | |
| Cavitation | 78.63 ± 0.48 | 78.54 ± 0.53 | 75.80 ± 0.54*** | 76.35 ± 0.46** | |
| Blastocyst | 81.71 ± 0.55 | 81.68 ± 0.55 | 78.72 ± 0.53*** | 78.93 ± 0.44*** | |
| Expanded blastocyst | 84.38 ± 0.61 | 84.47 ± 0.66 | 81.23 ± 0.56*** | 81.78 ± 0.58* | |
| Hatching | 89.10 ± 0.84 | 87.23 ± 0.89 | 86.08 ± 1.05 | 85.95 ± 0.94* |
| . | Oocyte . | Control . | Control . | A3 . | A3 . |
|---|---|---|---|---|---|
| Sperm . | Control . | A3 . | Control . | A3 . | |
| h post hCG | Syngamy | 13.70 ± 0.11 | 13.82 ± 0.10 | 13.30 ± 0.09 | 13.24 ± 0.07 |
| Developmental event (h post syngamy) | Syn-t2 | 1.51 ± 0.04 | 1.49 ± 0.03 | 1.49 ± 0.04 | 1.56 ± 0.03 |
| 1st cleavage (t2) | 15.21 ± 0.11 | 15.31 ± 0.10 | 14.79 ± 0.09** | 14.80 ± 0.07* | |
| cc2 (t3-t2) | 20.73 ± 0.16 | 20.42 ± 0.18 | 20.20 ± 0.13* | 20.18 ± 0.13* | |
| 2nd cleavage (t3) | 35.94 ± 0.20 | 35.73 ± 0.20 | 34.99 ± 0.14*** | 34.98 ± 0.14*** | |
| s2 (t4-t3) | 1.03 ± 0.10 | 1.34 ± 0.14 | 0.94 ± 0.10 | 0.92 ± 0.14 | |
| t4 | 36.97 ± 0.23 | 37.07 ± 0.27 | 35.93 ± 0.17** | 35.90 ± 0.17** | |
| cc3 (t5-t4) | 15.13 ± 0.29 | 15.06 ± 0.29 | 14.78 ± 0.28 | 14.71 ± 0.23 | |
| 3rd cleavage (t5) | 52.09 ± 0.41 | 52.01 ± 0.37 | 50.70 ± 0.30* | 50.61 ± 0.30* | |
| t6 | 53.05 ± 0.41 | 53.13 ± 0.41 | 51.53 ± 0.31* | 51.41 ± 0.32** | |
| 4th cleavage (t7) | 54.11 ± 0.43 | 54.25 ± 0.43 | 52.44 ± 0.33** | 52.30 ± 0.34** | |
| t8 | 55.14 ± 0.45 | 55.31 ± 0.45 | 53.43 ± 0.36** | 53.29 ± 0.35** | |
| S3 (t8-t5) | 3.04 ± 0.16 | 3.30 ± 0.18 | 2.82 ± 0.18 | 2.48 ± 0.13 | |
| Cavitation | 78.63 ± 0.48 | 78.54 ± 0.53 | 75.80 ± 0.54*** | 76.35 ± 0.46** | |
| Blastocyst | 81.71 ± 0.55 | 81.68 ± 0.55 | 78.72 ± 0.53*** | 78.93 ± 0.44*** | |
| Expanded blastocyst | 84.38 ± 0.61 | 84.47 ± 0.66 | 81.23 ± 0.56*** | 81.78 ± 0.58* | |
| Hatching | 89.10 ± 0.84 | 87.23 ± 0.89 | 86.08 ± 1.05 | 85.95 ± 0.94* |
Cell cleavage and development times of mouse embryos cultured individually under 20% oxygen during IVF in the presence of a combination of antioxidants (A3) comprising of 10 μM Acetyl-L-Carnitine, 10 μM N-Acetyl-L-Cysteine and 5 μM α-Lipoic Acid, or in control media without antioxidants, in oocyte IVF media (oocyte collection and fertilization) and/or sperm IVF media (sperm collection and preparation). t2 represents the time from syngamy to cleavage to 2 cell. t3, t4, t5, t6, t7, t8, represent the time to cleavage to 3 cell, 4 cell, 5 cell, 6 cell, 7 cell and 8 cell, respectively. syn-t2 = duration from syngamy to two cell; cc2 = duration of the second cell cycle; s2 = duration of second synchrony; cc3 = duration of the third cell cycle; s3 = duration of third synchrony. Data are expressed as mean ± SEM of cleavage events (h post syngamy), or duration between cleavage events (h). n = at least 80 embryos per treatment from six biological replicates. Asterisks denote significant differences from the control. *P < 0.05, **P < 0.01, ***P < 0.001.
Impact of antioxidant supplementation of either oocyte or sperm IVF media on blastocyst cell number and cell lineage allocation of IVF embryos cultured individually at 20% oxygen. Antioxidant combination (A3) is comprised of 10 μM Acetyl-L-Carnitine, 10 μM N-Acetyl-L-Cysteine and 5 μM α-Lipoic Acid. Antioxidants were in oocyte IVF media for oocyte collection and fertilization and/or sperm IVF media for sperm collection and preparation. Controls had no antioxidants in oocyte and sperm IVF media. No antioxidants were present in culture media. Light and dark bar portions represent the average trophectoderm (TE) and inner cell mass (ICM) cells respectively. Data are expressed as mean ± SEM. n = at least 80 embryos per treatment from six independent biological replicates. Asterisks denote significantly different from the control. Asterisks above the bar represent significantly different to the control total cell number. Asterisks within the bars represent significantly different from the control TE. *P < 0.05, **P < 0.01, ***P < 0.001.
Antioxidants in culture media reduce intracellular H2O2
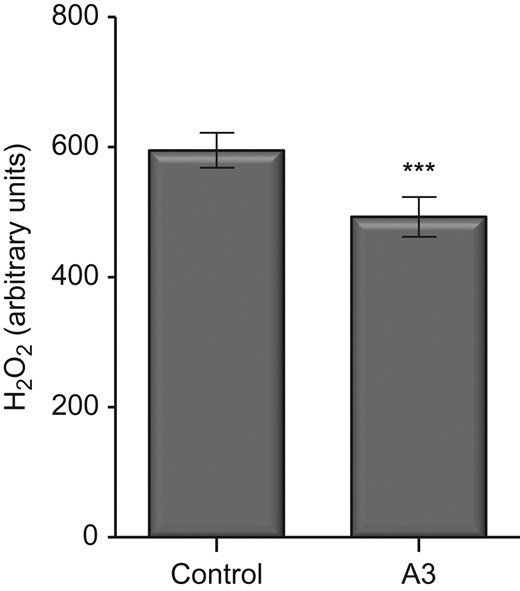
Levels of H2O2 were significantly decreased in pronucleate oocytes that were incubated in the presence of antioxidants for 4 h (P < 0.001) compared to control embryos (Fig. 4).
Effect of antioxidants on intracellular H2O2 levels of 2PN mouse embryos, determined through fluorescence labelling with 10 μM PF1. Fluorescence values are shown in arbitrary units. Data are expressed as mean ± SEM. Control were media with no antioxidants, A3 comprises of combined antioxidants (10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid), n = at least 164 embryos from 10 independent biological replicates. Treatment mean was compared with control using Student's t-test. ***P < 0.001.
Antioxidants present for 2PN collection affect blastocyst cell number
Pronucleate oocytes treated with the combined antioxidants for 20 min during collection displayed significantly increased blastocyst ICM numbers (16.18 ± 1.07 versus 19.39 ± 1.08, P < 0.05) compared with controls. However, there was no effect of treatment on blastocyst formation at the end of the culture period, 96 h after fertilization.
Discussion
Currently, with the exception of lipoic acid, present in G1 and G2 media, antioxidants are not listed as components of media routinely used in human IVF. Previously we showed that a triple antioxidant combination consisting of 10 μM Acetyl-L-Carnitine/10 μM N-Acetyl-L-Cysteine/5 μM α-Lipoic Acid, in embryo culture media imparts significant benefit on pronucleate oocyte development under both 5% and 20% oxygen, with the benefit being significantly greater at 20% (Truong et al., 2016). Here we show that the same combination of antioxidants in the culture media of IVF derived embryos resulted in a significant increase in blastocyst total cell number. Findings from the present study also show that the presence of antioxidants supplemented to the IVF medium during gamete collection and fertilization (IVF) alone was sufficient to improve embryo development, with beneficial effects occurring in the early cleavage stages of embryo development and continuing through to blastocyst development. Antioxidant supplementation throughout both IVF and culture also improved subsequent embryo development with significantly faster developmental times from syngamy to the 2-cell cleavage stage through to post compaction, accompanied by the greatest increase in blastocyst total cell number. These data suggest that there is a synergistic benefit on embryo development when antioxidants are present during both IVF and the culture period.
As gametes are particularly susceptible to oxidative damage, the effects of the three antioxidants may be attributed to their ability to reduce oxidative stress and may alleviate the delayed development of embryos cultured at 20% oxygen that culminates in lower blastocyst cell numbers (Wale and Gardner, 2010). The recycling of intracellular GSH (Packer et al., 1995) by ALA can replenish antioxidants within the sperm microenvironment (Lewis et al., 1997). As high levels of ROS are generated at fertilization in mice (Nasr-Esfahani et al., 1990), ALA supplementation may promote fertilization by decreasing the occurrence of oxidative stress. In addition, ALA in the IVF medium may improve sperm motility as observed in other studies (Ibrahim et al., 2008) plausibly through the regulation of mitochondrial function and energy production (Palaniappan and Dai, 2007; Plotnikov et al., 2007). Inclusion of LC may supply an essential co-factor to the oocyte and embryo to utilize fatty acids (Somfai et al., 2011) and increase the number of MII oocytes, cleavage rates and mitochondrial activity as seen in porcine studies (Somfai et al., 2011; Cooper et al., 2006), all which decrease oxidative stress resulting in improved developmental potential of oocytes and subsequently increase embryo development.
Interestingly our data show that antioxidant supplementation to the oocyte IVF medium alone was able to significantly increase blastocyst total cell numbers compared to embryos that had antioxidants solely in sperm IVF medium. It may be that LC in the oocyte IVF medium sustains GSH stores by counteracting H2O2, while ALA recycles intracellular GSH (Packer et al., 1995) and NAC affects the concentration of GSH in oocytes through substrate availability. In addition, NAC and LC may prevent meiotic oocyte damage as seen in bovine studies (Giorgi et al., 2016). Acting in combination the antioxidants could enhance the developmental potential of oocytes resulting in increased embryo development.
While there were no apparent improvements on embryo development (fertilization rate, blastocyst cell number and developmental rates) following antioxidant supplementation of sperm IVF medium alone, other assessments such as sperm motility and membrane integrity, as well as metabolic function were not conducted. In addition, the duration of sperm exposure to antioxidant treatment was shorter than for oocytes due to the IVF protocol used. It is also important to acknowledge that the sperm used were from young fertile male mice and are therefore not representative of infertile men. Importantly, although we did not detect a benefit of antioxidants on sperm, there was nonetheless a synergistic, beneficial effect when used together with oocytes that had antioxidants in the oocyte IVF medium. This result is comparable to our previous data where antioxidants throughout both IVF and the culture period resulted in the greatest increase in embryo development and highlights the need for antioxidants at all stages of IVF and culture.
Our data also show that other stages of ART benefit from the inclusion of antioxidants, with pronucleate oocytes exhibiting an increase in blastocyst ICM number when supplemented with antioxidants in the handling medium during collection. As exposure to atmospheric oxygen during oocyte collection is largely unavoidable, these findings emphasize the impact of the preimplantation embryo environment, and show that even a brief exposure to antioxidants can maintain developmental potential, and conversely their absence is associated with impaired development.
To investigate whether the effects of antioxidants on embryo development was related to changes in oxidative capacity we analysed the intracellular H2O2 levels in pronucleate oocytes cultured in the presence or absence of antioxidants using PF1, a novel probe specific for H2O2, as opposed to DCFDA, a fluorogenic marker for non-specific ROS. Pronucleate oocytes cultured for 4 h in the absence of antioxidants had significantly higher levels of H2O2 compared to embryos with antioxidants, indicating that the antioxidant combination reduced the levels of H2O2 within embryos, reflecting reduced oxidative stress during culture. This supports studies in sheep embryos that show LC supplementation of IVM reduced oxidative stress induced by H2O2, decreased ROS and increased intracellular GSH (Mishra et al., 2016). Similarly, the addition of LC during porcine IVM reduced oocyte ROS and increased GSH production, resulting in improved nuclear maturation with fewer apoptotic cells in blastocysts following parthenogenic activation (Cooper et al., 2006). The resultant improvement on embryo development seen in the present study may be attributed to the actions of LC sustaining GSH stores during early embryo development by counteracting H2O2 and thus reducing oxidative stress. As high GSH levels in embryos are correlated with improved embryo quality (Takahashi et al., 1993; de Matos et al., 1996), intracellular concentrations of H2O2 may be used as an indirect measure to assess embryo quality and to evaluate developmental competence. It is important to note that although the three antioxidants reduced the levels of H2O2 within embryos, they did not eliminate H2O2 completely. This is important given low levels of ROS are necessary for proper cell signalling and regulation of gamete function and development (Harvey et al., 2002; Sunderam et al., 2014; Lees et al., 2017).
In conclusion, the presence of antioxidants during IVF and throughout embryo culture imparts significant beneficial effects on embryo development. The presence of antioxidants during IVF resulted in significantly faster rates of embryo development and subsequent increases in cell numbers, along with a reduction in H2O2 levels. These findings indicate that supplementation of media with antioxidants for all stages of the IVF procedures and culture could be beneficial in human ART and may maintain the viability of human embryos. This is of clinical significance not only for the high number of clinics who still utilize 20% oxygen in their embryo culture system (Christianson et al., 2014) but also in all clinical procedures when there are transient exposures to atmospheric oxygen, such as gamete preparation, ICSI, embryo biopsy and cryopreservation. Clinical trials implementing the combined antioxidants in culture media are therefore currently being undertaken.
Supplementary data
Supplementary data are available at Human Reproduction online.
Acknowledgements
The authors would like to thank Professor Andrew Abell and Dr Malcom Purdy of the ARC Centre for Nanoscale BioPhotonics, University of Adelaide for the generous supply of the PF1 probe, and to Dr. Alexandra Harvey for her expert comments on the manuscript.
Authors’ roles
D.K.G. and T.T.T. conceptualized the study design. T.T.T. performed embryo cultures, statistical analysis and contributed to the manuscript. D.K.G. provided input and critical analysis of the data, and contributed to the writing of the manuscript.
Funding
This work was supported by Vitrolife AB, Sweden.
Conflict of interest
None declared.