-
PDF
- Split View
-
Views
-
Cite
Cite
Chin L. Poh, Roberto Chiletti, Diana Zannino, Christian Brizard, Igor E. Konstantinov, Stephen Horton, Johnny Millar, Yves d’Udekem, Ventricular assist device support in patients with single ventricles: the Melbourne experience, Interactive CardioVascular and Thoracic Surgery, Volume 25, Issue 2, August 2017, Pages 310–316, https://doi.org/10.1093/icvts/ivx066
Close - Share Icon Share
Abstract
The capacity and limitations of ventricular assist device (VAD) support in single-ventricle physiology remains poorly understood. We aimed to review our experience in the use of VAD support in the single-ventricle circulation to determine its feasibility in this population.
We reviewed our experience with VAD support in patients with single ventricles over the past 25 years. Fifty-seven patients received 64 runs of VAD support between 1990 and 2015 at a median age of 13 days [interquartile range (IQR) 4.1–99.4 days], of which 55 were supported for post-cardiotomy failure. The majority of patients received short-term VAD support, while 4 patients were either directly supported (1) or transitioned onto the Berlin Heart EXCOR (3).
The median duration of support was 3.5 days (IQR 2.8–5.2 days). Twelve patients suffered significant neurological complications, and thromboembolic events occurred in 8 patients. Twenty-nine of the 55 patients were successfully weaned off support (53%). There were 37 inpatient deaths, with a survival-to-hospital discharge rate of 33% (18 of 55). Of the 4 patients supported after early Fontan failure, 3 died. Having a higher mean arterial blood pressure on initiation of VAD support was the only significant predictor of death (hazards ratio 1.08; 95% confidence interval 1.03–1.14; P = 0.002). Patients who required a second run of support had higher hospital mortality (83% vs 63%; P = 0.84). Of the hospital survivors, 12 patients (63%) remain alive without heart transplantation at median 7.2 years (IQR 3.5–14.0) post VAD support.
VAD support in patients with a single ventricle has a high hospital mortality, with 1 of 3 patients surviving to discharge. Systemic VAD support is likely futile in the setting of early Fontan failure or when re-initiation of support is required.
INTRODUCTION
There is little doubt that we face an urgent need to improve our capacity to mechanically support patients with single-ventricle circulation. Over the last decades, we have pushed increasingly complex patients with single ventricular physiology through surgical palliation. Our success in mechanically supporting these patients, however, has stagnated with an expected survival of around 40% after extracorporeal membrane oxygenation (ECMO) support, far less than what we achieve in patients with biventricular circulation [1, 2]. Our success with these palliative procedures has nonetheless led to an increasing population of patients reaching adulthood. More than 1000 Fontan procedures are done every year in the USA alone, producing an estimated population of 50 000 to 70 000 patients with a Fontan circulation worldwide [3, 4]. It is evident that there will not be enough donor organs to offer transplantation to this population when their circulations fail, and mechanical circulatory support will likely be the option of last resort [5, 6]. However, the progress in that direction has been minimal so far [7, 8].
In Melbourne, over the last 2 decades, we have favoured ventricular assist device (VAD) support of single-ventricular circulation over ECMO whenever feasible, because we believe that patients may experience better outcomes if supported without an oxygenator [9, 10]. However, ECMO remains the favoured strategy of support for patients with single-ventricle circulation worldwide. We decided to review our experience with preferential use of VAD support to identify the feasibility and pitfalls of this approach.
MATERIALS AND METHODS
The design of the study was approved by the Royal Children’s Hospital Ethics Committee. The need for consent was waived because of the retrospective nature of the study. All patients with a single ventricle requiring both short- and long-term VAD support in the Royal Children’s Hospital, Melbourne, were identified and their files were reviewed. VAD support was defined as mechanical circulatory support without an oxygenator. This included short-term VAD support via centrifugal pumps (Biomedicus [Medtronic PLC, Dublin, Ireland] or Jostra Rotaflow [Maquet, Rastatt, Germany]) and long-term VAD support via the Berlin Heart EXCOR (Berlin, Germany).
A total of 57 patients received 64 runs of VAD support from January 1990 to December 2015. Patient characteristics are described in Table 1. The most frequent cardiac morphology was hypoplastic left heart syndrome, being present in 39 of the 57 patients (68%). Other morphologies were double-outlet right ventricle (5; 9%), tricuspid atresia (4; 7%), pulmonary atresia—intact ventricular septum (4; 7%), double-inlet left ventricle (3; 5%) and Shone’s syndrome (1; 2%). Of the group, 41 patients had completed Stage I palliation (Stage I Norwood: 35; Blalock–Taussig shunt: 4; Damus–Kaye–Stansel: 2), 11 were post-bidirectional cavopulmonary shunt completion (BCPS) and 5 were post-Fontan completion. Patients were defined to be supported for acute post-cardiotomy failure when VAD support was initiated within a week post cardiac surgery. This included all but 2 patients who would be described separately.
Patient characteristics of the 57 patients who received VAD support between 1990 and 2015
| . | All patients (n = 57) . | Survivors at discharge (n = 19) . | Non-survivors (n = 38) . |
|---|---|---|---|
| Male | 34 (60%) | 10 (53%) | 24 (63%) |
| Age at VAD support (days) | 13.2 | 14.2 | 12.2 |
| (IQR 4.1–99.4) | (IQR 4.1–84.2) | (IQR 3.0–171.3) | |
| Body surface area (m2) | 0.23 | 0.24 | 0.22 |
| (IQR 0.21–0.30) | (IQR 0.20–0.30) | (IQR 0.21–0.32) | |
| Hypoplastic left heart syndrome | 39 (68%) | 14 (74%) | 25 (66%) |
| Right ventricular dominance | 48 (84%) | 16 (84%) | 32 (84%) |
| Stage at VAD | |||
| Stage I | 41 (72%) | 13 (68%) | 28 (74%) |
| BCPS | 11 (19%) | 4 (21%) | 7 (18%) |
| Fontan | 5 (9%) | 2 (11%) | 3 (8%) |
| Indication for VAD | |||
| To wean off CPB | 26 (46%) | 9 (48%) | 17 (45%) |
| Cardiac arrest | 15 (26%) | 5 (26%) | 10 (26%) |
| Low cardiac output | 15 (26%) | 5 (26%) | 10 (26%) |
| Unknown | 1 | 1 | |
| ECMO pre-VAD | 19 (33%) | 6 (32%) | 13 (34%) |
| Duration of first run of VAD support (days) | 3.5 | 3.0 | 3.6 |
| (IQR 2.8–5.2) | (IQR 2.6–4.0) | (IQR 2.6–5.7) | |
| First mean arterial blood pressure on VAD (median; mmHg) | 50 | 45 | 50 |
| (IQR 45–55) | (IQR 40–50) | (IQR 45–58) | |
| First arterial oxygen saturations on VAD (%) | 78 | 80 | 78 |
| (IQR 71–85) | (IQR 75–84) | (IQR 70–85) | |
| First arterial pH on VAD (median) | 7.35 | 7.34 | 7.36 |
| (IQR 7.26–7.45) | (IQR 7.25–7.44) | (IQR 7.27–7.46) | |
| First arterial lactate on VAD (median) | 4.9 | 4.6 | 5.5 |
| (IQR 2.6–10.9) | (IQR 2.7–8.9) | (IQR 2.6–11.0) | |
| Septicaemia | 17 (38%) | 3 (16%) | 14 (54%) |
| Second run of support | 13 (22%) | 3 (16%) | 10 (26%) |
| . | All patients (n = 57) . | Survivors at discharge (n = 19) . | Non-survivors (n = 38) . |
|---|---|---|---|
| Male | 34 (60%) | 10 (53%) | 24 (63%) |
| Age at VAD support (days) | 13.2 | 14.2 | 12.2 |
| (IQR 4.1–99.4) | (IQR 4.1–84.2) | (IQR 3.0–171.3) | |
| Body surface area (m2) | 0.23 | 0.24 | 0.22 |
| (IQR 0.21–0.30) | (IQR 0.20–0.30) | (IQR 0.21–0.32) | |
| Hypoplastic left heart syndrome | 39 (68%) | 14 (74%) | 25 (66%) |
| Right ventricular dominance | 48 (84%) | 16 (84%) | 32 (84%) |
| Stage at VAD | |||
| Stage I | 41 (72%) | 13 (68%) | 28 (74%) |
| BCPS | 11 (19%) | 4 (21%) | 7 (18%) |
| Fontan | 5 (9%) | 2 (11%) | 3 (8%) |
| Indication for VAD | |||
| To wean off CPB | 26 (46%) | 9 (48%) | 17 (45%) |
| Cardiac arrest | 15 (26%) | 5 (26%) | 10 (26%) |
| Low cardiac output | 15 (26%) | 5 (26%) | 10 (26%) |
| Unknown | 1 | 1 | |
| ECMO pre-VAD | 19 (33%) | 6 (32%) | 13 (34%) |
| Duration of first run of VAD support (days) | 3.5 | 3.0 | 3.6 |
| (IQR 2.8–5.2) | (IQR 2.6–4.0) | (IQR 2.6–5.7) | |
| First mean arterial blood pressure on VAD (median; mmHg) | 50 | 45 | 50 |
| (IQR 45–55) | (IQR 40–50) | (IQR 45–58) | |
| First arterial oxygen saturations on VAD (%) | 78 | 80 | 78 |
| (IQR 71–85) | (IQR 75–84) | (IQR 70–85) | |
| First arterial pH on VAD (median) | 7.35 | 7.34 | 7.36 |
| (IQR 7.26–7.45) | (IQR 7.25–7.44) | (IQR 7.27–7.46) | |
| First arterial lactate on VAD (median) | 4.9 | 4.6 | 5.5 |
| (IQR 2.6–10.9) | (IQR 2.7–8.9) | (IQR 2.6–11.0) | |
| Septicaemia | 17 (38%) | 3 (16%) | 14 (54%) |
| Second run of support | 13 (22%) | 3 (16%) | 10 (26%) |
ECMO: extracorporeal membrane oxygenation; IQR: interquartile range; VAD: ventricular assist device; CPB: cardiopulmonary bypass.
Patient characteristics of the 57 patients who received VAD support between 1990 and 2015
| . | All patients (n = 57) . | Survivors at discharge (n = 19) . | Non-survivors (n = 38) . |
|---|---|---|---|
| Male | 34 (60%) | 10 (53%) | 24 (63%) |
| Age at VAD support (days) | 13.2 | 14.2 | 12.2 |
| (IQR 4.1–99.4) | (IQR 4.1–84.2) | (IQR 3.0–171.3) | |
| Body surface area (m2) | 0.23 | 0.24 | 0.22 |
| (IQR 0.21–0.30) | (IQR 0.20–0.30) | (IQR 0.21–0.32) | |
| Hypoplastic left heart syndrome | 39 (68%) | 14 (74%) | 25 (66%) |
| Right ventricular dominance | 48 (84%) | 16 (84%) | 32 (84%) |
| Stage at VAD | |||
| Stage I | 41 (72%) | 13 (68%) | 28 (74%) |
| BCPS | 11 (19%) | 4 (21%) | 7 (18%) |
| Fontan | 5 (9%) | 2 (11%) | 3 (8%) |
| Indication for VAD | |||
| To wean off CPB | 26 (46%) | 9 (48%) | 17 (45%) |
| Cardiac arrest | 15 (26%) | 5 (26%) | 10 (26%) |
| Low cardiac output | 15 (26%) | 5 (26%) | 10 (26%) |
| Unknown | 1 | 1 | |
| ECMO pre-VAD | 19 (33%) | 6 (32%) | 13 (34%) |
| Duration of first run of VAD support (days) | 3.5 | 3.0 | 3.6 |
| (IQR 2.8–5.2) | (IQR 2.6–4.0) | (IQR 2.6–5.7) | |
| First mean arterial blood pressure on VAD (median; mmHg) | 50 | 45 | 50 |
| (IQR 45–55) | (IQR 40–50) | (IQR 45–58) | |
| First arterial oxygen saturations on VAD (%) | 78 | 80 | 78 |
| (IQR 71–85) | (IQR 75–84) | (IQR 70–85) | |
| First arterial pH on VAD (median) | 7.35 | 7.34 | 7.36 |
| (IQR 7.26–7.45) | (IQR 7.25–7.44) | (IQR 7.27–7.46) | |
| First arterial lactate on VAD (median) | 4.9 | 4.6 | 5.5 |
| (IQR 2.6–10.9) | (IQR 2.7–8.9) | (IQR 2.6–11.0) | |
| Septicaemia | 17 (38%) | 3 (16%) | 14 (54%) |
| Second run of support | 13 (22%) | 3 (16%) | 10 (26%) |
| . | All patients (n = 57) . | Survivors at discharge (n = 19) . | Non-survivors (n = 38) . |
|---|---|---|---|
| Male | 34 (60%) | 10 (53%) | 24 (63%) |
| Age at VAD support (days) | 13.2 | 14.2 | 12.2 |
| (IQR 4.1–99.4) | (IQR 4.1–84.2) | (IQR 3.0–171.3) | |
| Body surface area (m2) | 0.23 | 0.24 | 0.22 |
| (IQR 0.21–0.30) | (IQR 0.20–0.30) | (IQR 0.21–0.32) | |
| Hypoplastic left heart syndrome | 39 (68%) | 14 (74%) | 25 (66%) |
| Right ventricular dominance | 48 (84%) | 16 (84%) | 32 (84%) |
| Stage at VAD | |||
| Stage I | 41 (72%) | 13 (68%) | 28 (74%) |
| BCPS | 11 (19%) | 4 (21%) | 7 (18%) |
| Fontan | 5 (9%) | 2 (11%) | 3 (8%) |
| Indication for VAD | |||
| To wean off CPB | 26 (46%) | 9 (48%) | 17 (45%) |
| Cardiac arrest | 15 (26%) | 5 (26%) | 10 (26%) |
| Low cardiac output | 15 (26%) | 5 (26%) | 10 (26%) |
| Unknown | 1 | 1 | |
| ECMO pre-VAD | 19 (33%) | 6 (32%) | 13 (34%) |
| Duration of first run of VAD support (days) | 3.5 | 3.0 | 3.6 |
| (IQR 2.8–5.2) | (IQR 2.6–4.0) | (IQR 2.6–5.7) | |
| First mean arterial blood pressure on VAD (median; mmHg) | 50 | 45 | 50 |
| (IQR 45–55) | (IQR 40–50) | (IQR 45–58) | |
| First arterial oxygen saturations on VAD (%) | 78 | 80 | 78 |
| (IQR 71–85) | (IQR 75–84) | (IQR 70–85) | |
| First arterial pH on VAD (median) | 7.35 | 7.34 | 7.36 |
| (IQR 7.26–7.45) | (IQR 7.25–7.44) | (IQR 7.27–7.46) | |
| First arterial lactate on VAD (median) | 4.9 | 4.6 | 5.5 |
| (IQR 2.6–10.9) | (IQR 2.7–8.9) | (IQR 2.6–11.0) | |
| Septicaemia | 17 (38%) | 3 (16%) | 14 (54%) |
| Second run of support | 13 (22%) | 3 (16%) | 10 (26%) |
ECMO: extracorporeal membrane oxygenation; IQR: interquartile range; VAD: ventricular assist device; CPB: cardiopulmonary bypass.
Indications for mechanical circulatory support were known for 54 of the 55 patients, and included assistance to wean off cardiopulmonary bypass (26), refractory low cardiac output (15), and post-cardiac arrest (13). Of the 55 patients, 17 were initially supported on ECMO and later bridged to VAD support after a median of 2 days [interquartile range (IQR) 1–4].
Support strategy
In our institution, we have adopted a strategy for both biventricular and single-ventricle circulations where mechanical support by VAD is preferred over ECMO in all cases when oxygenation of the patients is satisfactory. The practice at our institution has been previously described [11]. We observed that resuscitation using VAD support during a cardiac arrest was unsatisfactory, because it did not produce a rapid decline in arterial blood lactate. Accordingly, all but 13 patients were supported on VAD or transitioned from ECMO to VAD electively. Patients with a systemic–pulmonary shunt had flows adjusted to 50% higher than the flow calculated for their weight (125–200 ml/kg/min). All patients were first anticoagulated with unfractionated heparin. Patients who weighed <15 kg were titrated to a target activated clotting time of 150–170 s. Heavier patients were monitored using activated partial thromboplastin time with a target range of 70–90 s. Patients on long-term VAD support were transitioned on to warfarin, in addition to dual antiplatelet therapy. In the event of thromboembolic events, the target International normalised ratio was increased from 2–3 to 2.5–3.5. However, this was not practised in the setting of embolic stroke to avoid the occurrence of haemorrhagic transformation.
Cannulation and modality of support
The cannulation configuration was known for 55 of the 57 patients. The inflow cannula was placed in the right atrium or common atrium for most patients. Two patients had separate cannulation of their superior and inferior vena cava. All but 1 patient had their outflow cannulas in the aorta. The last patient was cannulated in his left innominate artery. The majority of the patients received short-term VAD support with a centrifugal pump (Biomedicus [Medtronic PLC]: 18, Jostra Rotaflow [Maquet]: 38), which is preferred over the roller pump for its reduced haemolysis [12]. Four patients received support with the Berlin Heart EXCOR, 2 for acute post-cardiotomy failure and 2 for late failure. Only 1 patient was directly supported with the Berlin Heart EXCOR, while 3 additional patients were transitioned from a centrifugal pump to the Berlin Heart after a median of 10 days (range 8–13 days).
Variables
Detailed clinical data were reviewed to identify baseline demographics and outcomes post support. Clinical variables including haemodynamics, oxygen saturation and inotropic requirements immediately after initiation of support were reviewed. The first mean arterial blood pressure measured via invasive arterial monitoring was collected. The first laboratory results including arterial blood pH and lactate on support were obtained. The difference between the measured pH and the closest limit of the normal range (7.35 or 7.45) was determined. This was classified into categories (<0.05, 0.05–0.15, >0.15) for analysis. Development of sepsis was considered as a variable in patients who had survived more than 5 days of VAD support. Septicaemia was diagnosed either with positive blood cultures or a confirmed source of infection and clinical evidence of sepsis. Patients are defined as successfully weaned off VAD support if they are alive without re-initiation of support 24 h post de-cannulation.
Statistical analyses
Patient baseline characteristics were summarized using median and IQRs for continuous variables and percentages for categorical variables. The primary end-point was time to inpatient death or hospital discharge. Analysis was performed only for the patients with acute post-cardiotomy failure using the Cox-proportional hazard model to examine the association between variables and time to end-point using the statistical program Stata (Version 14.0, Timberlake Consultants Limited, Surrey, UK). Relevant predictors were further evaluated via the log-rank test to determine the mortality risk in patients with and without the variable. The comparison of categorical and continuous data was performed using the χ2 test and the paired t–test, respectively.
RESULTS
Hospital outcomes of patients supported for acute failure
Median duration of support was 3.2 days (IQR 2.7–4.9; range 25.6). Median pH and lactate of the cohort upon initiation of support were 7.34 (range 6.97–7.61) and 4.9 (range 1.3–22), respectively.
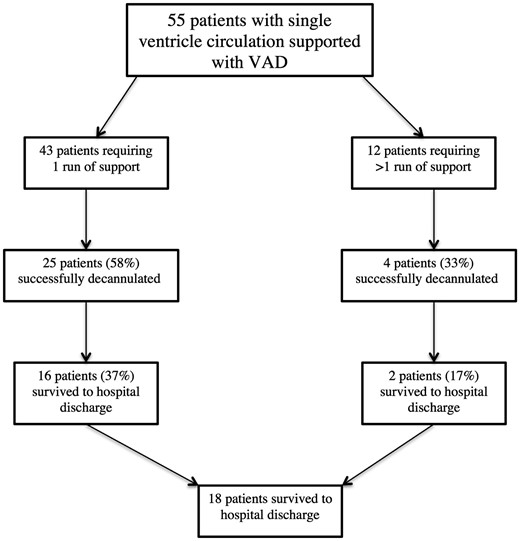
There were 37 inpatient deaths and the causes of death are described in Table 2. Twelve patients required re-initiation of mechanical support after a median of 2.0 days (IQR 1–7.5) post de-cannulation. They received 15 additional runs of support via either VAD (4) or veno-arterial ECMO (11). Indications for re-initiation of support were known for 9 of the 12 patients and included cardiac arrest (3), persistent hypoxaemia (3), low cardiac output (2) and to wean off cardiopulmonary bypass after reoperation (1). Median duration of the second run of support received was 4.0 days (IQR 3.5–6). Of the 55 patients, 29 (53%) were successfully weaned off support after either 1 (25) or 2 (4) runs of support. A total of 18 patients (33%) survived to hospital discharge (Fig. 1). Ten of the 12 patients (83%) requiring re-initiation of support died before hospital discharge.
Diagram describing outcomes of patients supported with ventricular assist device (VAD) for post-cardiotomy failure (n = 55); (%) represent proportion within each arm (1 run or >1 run).
Causes of death for the 37 hospital deaths
| . | Causes . | No. of deaths . |
|---|---|---|
| Died on support or <24 h after withdrawal of support (26) | Withdrawal of care due to poor prognosis | 6 |
| Acute loss of blood pressure | 5 | |
| Low output state/progressive acidosis | 4 | |
| Persistent ventricular failure (unable to wean off | 2 | |
| extracorporeal membrane oxygenation/bypass) | ||
| Neurological complication | 2 | |
| Pulmonary embolism | 1 | |
| Bowel ischaemia secondary to emboli | 1 | |
| Septicaemia | 1 | |
| Intraoperative complication | 1 | |
| Unknown | 3 | |
| Died after successful withdrawal of support (11) | Cardiac arrest | 4 |
| Septicaemia | 1 | |
| Pulmonary embolism | 1 | |
| Heart failure | 1 | |
| Acute acidosis of unknown cause | 1 | |
| Unknown | 3 |
| . | Causes . | No. of deaths . |
|---|---|---|
| Died on support or <24 h after withdrawal of support (26) | Withdrawal of care due to poor prognosis | 6 |
| Acute loss of blood pressure | 5 | |
| Low output state/progressive acidosis | 4 | |
| Persistent ventricular failure (unable to wean off | 2 | |
| extracorporeal membrane oxygenation/bypass) | ||
| Neurological complication | 2 | |
| Pulmonary embolism | 1 | |
| Bowel ischaemia secondary to emboli | 1 | |
| Septicaemia | 1 | |
| Intraoperative complication | 1 | |
| Unknown | 3 | |
| Died after successful withdrawal of support (11) | Cardiac arrest | 4 |
| Septicaemia | 1 | |
| Pulmonary embolism | 1 | |
| Heart failure | 1 | |
| Acute acidosis of unknown cause | 1 | |
| Unknown | 3 |
Causes of death for the 37 hospital deaths
| . | Causes . | No. of deaths . |
|---|---|---|
| Died on support or <24 h after withdrawal of support (26) | Withdrawal of care due to poor prognosis | 6 |
| Acute loss of blood pressure | 5 | |
| Low output state/progressive acidosis | 4 | |
| Persistent ventricular failure (unable to wean off | 2 | |
| extracorporeal membrane oxygenation/bypass) | ||
| Neurological complication | 2 | |
| Pulmonary embolism | 1 | |
| Bowel ischaemia secondary to emboli | 1 | |
| Septicaemia | 1 | |
| Intraoperative complication | 1 | |
| Unknown | 3 | |
| Died after successful withdrawal of support (11) | Cardiac arrest | 4 |
| Septicaemia | 1 | |
| Pulmonary embolism | 1 | |
| Heart failure | 1 | |
| Acute acidosis of unknown cause | 1 | |
| Unknown | 3 |
| . | Causes . | No. of deaths . |
|---|---|---|
| Died on support or <24 h after withdrawal of support (26) | Withdrawal of care due to poor prognosis | 6 |
| Acute loss of blood pressure | 5 | |
| Low output state/progressive acidosis | 4 | |
| Persistent ventricular failure (unable to wean off | 2 | |
| extracorporeal membrane oxygenation/bypass) | ||
| Neurological complication | 2 | |
| Pulmonary embolism | 1 | |
| Bowel ischaemia secondary to emboli | 1 | |
| Septicaemia | 1 | |
| Intraoperative complication | 1 | |
| Unknown | 3 | |
| Died after successful withdrawal of support (11) | Cardiac arrest | 4 |
| Septicaemia | 1 | |
| Pulmonary embolism | 1 | |
| Heart failure | 1 | |
| Acute acidosis of unknown cause | 1 | |
| Unknown | 3 |
Five patients needed further surgical procedures on support. These included re-exploration for persistent bleeding (3) and 2 re-operations on cardiopulmonary bypass including tricuspid valve repair (1) and the creation of Blalock-Taussig shunt (1). Only 1 of the 5 patients survived to discharge. Overall survival from support to hospital discharge was 33% (19 of 57). Twenty-one of the 37 inpatient deaths took place before successful wean off support. There were 6 deaths that occurred after active withdrawal of care in view of their poor prognosis. Twelve patients suffered significant neurological complications, of which 3 survived to hospital discharge. Patients who received ECMO support before or after VAD support had more neurological events than patients who had not received ECMO support (6 of 21 vs 6 of 34; 29% vs 18%, P = 0.34). Thromboembolic events occurred in 9 patients, comprising circuit thrombosis (6), pulmonary embolism (3) and intra-abdominal emboli (1). The incidence of thromboembolism was similarly higher in patients who received additional ECMO support compared with those who never received ECMO (5 of 21 vs 4 of 34 (24% vs 12%, P = 0.24). Eight of the 9 patients with thromboembolic complications died before hospital discharge.
By univariate analysis, having a higher mean arterial blood pressure on initiation of VAD support was the only significant predictor of death [HR 1.08 95% confidence interval (CI) 1.03 – 1.14; P = 0.002] (Table 3). This was independent of their risk of neurological complications. Patients who suffered neurological events had lower mean arterial blood pressure than those who were event-free (43.9 ± 4.37 vs 53.3 ± 11.7, P = 0.04). Risk of hospital death was 81% (13 of 16) in patients with sepsis and 44% (12 of 27) without septicaemia (P = 0.07). None of the other variables, including oxygen saturations, lactate, pH or the need for inotropy was predictive of a worse outcome. Having a cardiac arrest before institution of support was not associated with an increased risk of death (63% vs 71%, P = 0.70). Patients who were supported in the past decade received a longer duration of support than those supported earlier (6.7 ± 5.7 vs 3.8 ± 2.3 days, P = 0.02). Survival outcomes trended towards improvement with more recent support (HR 0.60 95% CI 0.31–1.14, P = 0.12).
Univariate analysis of predictors of hospital mortality for patients who received VAD support for acute failure (n = 55)
| Variable . | . | Total no. of patients (55) . | Total deaths (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Pre-VAD variables | |||||
| Diagnosis | Hypoplastic left heart syndrome | 38 | 25 (66%) | 0.91 (0.45–1.80) | 0.78 |
| Non-hypoplastic left heart syndrome | 17 | 12 (71%) | 1 | ||
| Age at support | 1.03 (0.90–1.18) | 0.68 | |||
| Body surface area (m2) | 0.94 (0.15–5.81) | 0.95 | |||
| Stage of palliation | First stage | 41 | 28 (68%) | 1 | 0.87 |
| BCPS | 10 | 6 (64%) | 0.79 (0.33–1.91) | ||
| Fontan | 4 | 3 (60%) | 0.95 (0.29–3.14) | ||
| Systemic–pulmonary flow | Yes | 41 | 28 (68%) | 1.19 (0.56–2.53) | 0.64 |
| No | 14 | 9 (64%) | 1 | ||
| Indication | To assist wean off CPB | 26 | 17 (65%) | 1 | 0.95 |
| Low cardiac output | 13 | 9 (69%) | 0.97 (0.45–2.13) | ||
| Cardiac arrest | 15 | 10 (67%) | 1.12 (0.50–2.52) | ||
| Unknown | 1 | 1 (100%) | |||
| Support initiated post-staged palliation surgery | Yes | 49 | 33 (67%) | 1.25 (0.44–3.53) | 0.67 |
| No | 6 | 4 (67%) | 1 | ||
| Preceding cardiac arrest | Yes | 19 | 12 (63%) | 0.88 (0.44–1.75) | 0.71 |
| No | 34 | 24 (71%) | 1 | ||
| Unknown | 2 | 1 (50%) | |||
| ECMO pre-VAD | Yes | 17 | 12 (71%) | 0.97 (0.49–1.93) | 0.93 |
| No | 38 | 25 (66%) | |||
| ECMO pre- or post-VAD | Yes | 21 | 15 (71%) | 0.99 (0.51–1.90) | 0.97 |
| No | 34 | 22 (65%) | |||
| Variables on VAD | |||||
| Time of VAD support | 1990–2005 | 27 | 19 | 0.60 (0.31 – 1.14) | 0.12 |
| 2005–2015 | 28 | 18 | |||
| Duration of first run of VAD support | 1 (1.00–1.00) | 0.39 | |||
| Total duration of support (VAD + ECMO) | 1 (1.00–1.01) | 0.73 | |||
| First mean arterial blood pressure | 1.08 (1.03–1.14) | 0.002 | |||
| First peripheral arterial oxygen saturation | 0.98 (0.94–1.02) | 0.30 | |||
| Inotropes required on VAD support | Yes | 26 | 17 | 0.99 (0.91–1.07) | 0.74 |
| No | 5 | 4 | |||
| Unknown | 24 | 16 | |||
| Septicaemiaa | Yes | 16 | 13 (81%) | 1.98 (0.90–4.39) | 0.09 |
| No | 27 | 12 (44%) | |||
| First arterial lactate | 1.02 (0.96–1.08) | 0.52 | |||
| First arterial pH deviationb | <0.05 | 27 | 20 (74%) | 1 | 0.48 |
| 0.05–0.15 | 13 | 8 (62%) | 0.79 (0.35–1.79) | ||
| >0.15 | 10 | 5 (50%) | 0.57 (0.21–1.51) | ||
| Second run of support | Yes | 12 | 10 (83%) | 1.08 (0.52–2.24) | 0.84 |
| No | 43 | 27 (63%) | |||
| Neurological complications | Yes | 12 | 9 (75%) | 1.15 (0.54–2.44) | 0.72 |
| No | 43 | 28 (65%) | 1 | ||
| Thromboembolic event | Yes | 9 | 8 | 1.55 (0.71–3.40) | 0.29 |
| No | 46 | 29 | 1 | ||
| Variable . | . | Total no. of patients (55) . | Total deaths (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Pre-VAD variables | |||||
| Diagnosis | Hypoplastic left heart syndrome | 38 | 25 (66%) | 0.91 (0.45–1.80) | 0.78 |
| Non-hypoplastic left heart syndrome | 17 | 12 (71%) | 1 | ||
| Age at support | 1.03 (0.90–1.18) | 0.68 | |||
| Body surface area (m2) | 0.94 (0.15–5.81) | 0.95 | |||
| Stage of palliation | First stage | 41 | 28 (68%) | 1 | 0.87 |
| BCPS | 10 | 6 (64%) | 0.79 (0.33–1.91) | ||
| Fontan | 4 | 3 (60%) | 0.95 (0.29–3.14) | ||
| Systemic–pulmonary flow | Yes | 41 | 28 (68%) | 1.19 (0.56–2.53) | 0.64 |
| No | 14 | 9 (64%) | 1 | ||
| Indication | To assist wean off CPB | 26 | 17 (65%) | 1 | 0.95 |
| Low cardiac output | 13 | 9 (69%) | 0.97 (0.45–2.13) | ||
| Cardiac arrest | 15 | 10 (67%) | 1.12 (0.50–2.52) | ||
| Unknown | 1 | 1 (100%) | |||
| Support initiated post-staged palliation surgery | Yes | 49 | 33 (67%) | 1.25 (0.44–3.53) | 0.67 |
| No | 6 | 4 (67%) | 1 | ||
| Preceding cardiac arrest | Yes | 19 | 12 (63%) | 0.88 (0.44–1.75) | 0.71 |
| No | 34 | 24 (71%) | 1 | ||
| Unknown | 2 | 1 (50%) | |||
| ECMO pre-VAD | Yes | 17 | 12 (71%) | 0.97 (0.49–1.93) | 0.93 |
| No | 38 | 25 (66%) | |||
| ECMO pre- or post-VAD | Yes | 21 | 15 (71%) | 0.99 (0.51–1.90) | 0.97 |
| No | 34 | 22 (65%) | |||
| Variables on VAD | |||||
| Time of VAD support | 1990–2005 | 27 | 19 | 0.60 (0.31 – 1.14) | 0.12 |
| 2005–2015 | 28 | 18 | |||
| Duration of first run of VAD support | 1 (1.00–1.00) | 0.39 | |||
| Total duration of support (VAD + ECMO) | 1 (1.00–1.01) | 0.73 | |||
| First mean arterial blood pressure | 1.08 (1.03–1.14) | 0.002 | |||
| First peripheral arterial oxygen saturation | 0.98 (0.94–1.02) | 0.30 | |||
| Inotropes required on VAD support | Yes | 26 | 17 | 0.99 (0.91–1.07) | 0.74 |
| No | 5 | 4 | |||
| Unknown | 24 | 16 | |||
| Septicaemiaa | Yes | 16 | 13 (81%) | 1.98 (0.90–4.39) | 0.09 |
| No | 27 | 12 (44%) | |||
| First arterial lactate | 1.02 (0.96–1.08) | 0.52 | |||
| First arterial pH deviationb | <0.05 | 27 | 20 (74%) | 1 | 0.48 |
| 0.05–0.15 | 13 | 8 (62%) | 0.79 (0.35–1.79) | ||
| >0.15 | 10 | 5 (50%) | 0.57 (0.21–1.51) | ||
| Second run of support | Yes | 12 | 10 (83%) | 1.08 (0.52–2.24) | 0.84 |
| No | 43 | 27 (63%) | |||
| Neurological complications | Yes | 12 | 9 (75%) | 1.15 (0.54–2.44) | 0.72 |
| No | 43 | 28 (65%) | 1 | ||
| Thromboembolic event | Yes | 9 | 8 | 1.55 (0.71–3.40) | 0.29 |
| No | 46 | 29 | 1 | ||
Sepsis in patients surviving >5 days of VAD support.
Arterial pH deviation: difference of arterial pH in first blood sample post initiation of VAD and its closest limit of the normal range (7.35–7.45). BCPS: post-bidirectional cavopulmonary shunt completion; ECMO: extracorporeal membrane oxygenation; VAD: ventricular assist device.
Univariate analysis of predictors of hospital mortality for patients who received VAD support for acute failure (n = 55)
| Variable . | . | Total no. of patients (55) . | Total deaths (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Pre-VAD variables | |||||
| Diagnosis | Hypoplastic left heart syndrome | 38 | 25 (66%) | 0.91 (0.45–1.80) | 0.78 |
| Non-hypoplastic left heart syndrome | 17 | 12 (71%) | 1 | ||
| Age at support | 1.03 (0.90–1.18) | 0.68 | |||
| Body surface area (m2) | 0.94 (0.15–5.81) | 0.95 | |||
| Stage of palliation | First stage | 41 | 28 (68%) | 1 | 0.87 |
| BCPS | 10 | 6 (64%) | 0.79 (0.33–1.91) | ||
| Fontan | 4 | 3 (60%) | 0.95 (0.29–3.14) | ||
| Systemic–pulmonary flow | Yes | 41 | 28 (68%) | 1.19 (0.56–2.53) | 0.64 |
| No | 14 | 9 (64%) | 1 | ||
| Indication | To assist wean off CPB | 26 | 17 (65%) | 1 | 0.95 |
| Low cardiac output | 13 | 9 (69%) | 0.97 (0.45–2.13) | ||
| Cardiac arrest | 15 | 10 (67%) | 1.12 (0.50–2.52) | ||
| Unknown | 1 | 1 (100%) | |||
| Support initiated post-staged palliation surgery | Yes | 49 | 33 (67%) | 1.25 (0.44–3.53) | 0.67 |
| No | 6 | 4 (67%) | 1 | ||
| Preceding cardiac arrest | Yes | 19 | 12 (63%) | 0.88 (0.44–1.75) | 0.71 |
| No | 34 | 24 (71%) | 1 | ||
| Unknown | 2 | 1 (50%) | |||
| ECMO pre-VAD | Yes | 17 | 12 (71%) | 0.97 (0.49–1.93) | 0.93 |
| No | 38 | 25 (66%) | |||
| ECMO pre- or post-VAD | Yes | 21 | 15 (71%) | 0.99 (0.51–1.90) | 0.97 |
| No | 34 | 22 (65%) | |||
| Variables on VAD | |||||
| Time of VAD support | 1990–2005 | 27 | 19 | 0.60 (0.31 – 1.14) | 0.12 |
| 2005–2015 | 28 | 18 | |||
| Duration of first run of VAD support | 1 (1.00–1.00) | 0.39 | |||
| Total duration of support (VAD + ECMO) | 1 (1.00–1.01) | 0.73 | |||
| First mean arterial blood pressure | 1.08 (1.03–1.14) | 0.002 | |||
| First peripheral arterial oxygen saturation | 0.98 (0.94–1.02) | 0.30 | |||
| Inotropes required on VAD support | Yes | 26 | 17 | 0.99 (0.91–1.07) | 0.74 |
| No | 5 | 4 | |||
| Unknown | 24 | 16 | |||
| Septicaemiaa | Yes | 16 | 13 (81%) | 1.98 (0.90–4.39) | 0.09 |
| No | 27 | 12 (44%) | |||
| First arterial lactate | 1.02 (0.96–1.08) | 0.52 | |||
| First arterial pH deviationb | <0.05 | 27 | 20 (74%) | 1 | 0.48 |
| 0.05–0.15 | 13 | 8 (62%) | 0.79 (0.35–1.79) | ||
| >0.15 | 10 | 5 (50%) | 0.57 (0.21–1.51) | ||
| Second run of support | Yes | 12 | 10 (83%) | 1.08 (0.52–2.24) | 0.84 |
| No | 43 | 27 (63%) | |||
| Neurological complications | Yes | 12 | 9 (75%) | 1.15 (0.54–2.44) | 0.72 |
| No | 43 | 28 (65%) | 1 | ||
| Thromboembolic event | Yes | 9 | 8 | 1.55 (0.71–3.40) | 0.29 |
| No | 46 | 29 | 1 | ||
| Variable . | . | Total no. of patients (55) . | Total deaths (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|---|---|
| Pre-VAD variables | |||||
| Diagnosis | Hypoplastic left heart syndrome | 38 | 25 (66%) | 0.91 (0.45–1.80) | 0.78 |
| Non-hypoplastic left heart syndrome | 17 | 12 (71%) | 1 | ||
| Age at support | 1.03 (0.90–1.18) | 0.68 | |||
| Body surface area (m2) | 0.94 (0.15–5.81) | 0.95 | |||
| Stage of palliation | First stage | 41 | 28 (68%) | 1 | 0.87 |
| BCPS | 10 | 6 (64%) | 0.79 (0.33–1.91) | ||
| Fontan | 4 | 3 (60%) | 0.95 (0.29–3.14) | ||
| Systemic–pulmonary flow | Yes | 41 | 28 (68%) | 1.19 (0.56–2.53) | 0.64 |
| No | 14 | 9 (64%) | 1 | ||
| Indication | To assist wean off CPB | 26 | 17 (65%) | 1 | 0.95 |
| Low cardiac output | 13 | 9 (69%) | 0.97 (0.45–2.13) | ||
| Cardiac arrest | 15 | 10 (67%) | 1.12 (0.50–2.52) | ||
| Unknown | 1 | 1 (100%) | |||
| Support initiated post-staged palliation surgery | Yes | 49 | 33 (67%) | 1.25 (0.44–3.53) | 0.67 |
| No | 6 | 4 (67%) | 1 | ||
| Preceding cardiac arrest | Yes | 19 | 12 (63%) | 0.88 (0.44–1.75) | 0.71 |
| No | 34 | 24 (71%) | 1 | ||
| Unknown | 2 | 1 (50%) | |||
| ECMO pre-VAD | Yes | 17 | 12 (71%) | 0.97 (0.49–1.93) | 0.93 |
| No | 38 | 25 (66%) | |||
| ECMO pre- or post-VAD | Yes | 21 | 15 (71%) | 0.99 (0.51–1.90) | 0.97 |
| No | 34 | 22 (65%) | |||
| Variables on VAD | |||||
| Time of VAD support | 1990–2005 | 27 | 19 | 0.60 (0.31 – 1.14) | 0.12 |
| 2005–2015 | 28 | 18 | |||
| Duration of first run of VAD support | 1 (1.00–1.00) | 0.39 | |||
| Total duration of support (VAD + ECMO) | 1 (1.00–1.01) | 0.73 | |||
| First mean arterial blood pressure | 1.08 (1.03–1.14) | 0.002 | |||
| First peripheral arterial oxygen saturation | 0.98 (0.94–1.02) | 0.30 | |||
| Inotropes required on VAD support | Yes | 26 | 17 | 0.99 (0.91–1.07) | 0.74 |
| No | 5 | 4 | |||
| Unknown | 24 | 16 | |||
| Septicaemiaa | Yes | 16 | 13 (81%) | 1.98 (0.90–4.39) | 0.09 |
| No | 27 | 12 (44%) | |||
| First arterial lactate | 1.02 (0.96–1.08) | 0.52 | |||
| First arterial pH deviationb | <0.05 | 27 | 20 (74%) | 1 | 0.48 |
| 0.05–0.15 | 13 | 8 (62%) | 0.79 (0.35–1.79) | ||
| >0.15 | 10 | 5 (50%) | 0.57 (0.21–1.51) | ||
| Second run of support | Yes | 12 | 10 (83%) | 1.08 (0.52–2.24) | 0.84 |
| No | 43 | 27 (63%) | |||
| Neurological complications | Yes | 12 | 9 (75%) | 1.15 (0.54–2.44) | 0.72 |
| No | 43 | 28 (65%) | 1 | ||
| Thromboembolic event | Yes | 9 | 8 | 1.55 (0.71–3.40) | 0.29 |
| No | 46 | 29 | 1 | ||
Sepsis in patients surviving >5 days of VAD support.
Arterial pH deviation: difference of arterial pH in first blood sample post initiation of VAD and its closest limit of the normal range (7.35–7.45). BCPS: post-bidirectional cavopulmonary shunt completion; ECMO: extracorporeal membrane oxygenation; VAD: ventricular assist device.
Outcomes of patients post first-stage palliation
Forty-one of the 55 patients with single-ventricle physiology (72%) were supported after initial Stage 1 palliation. Thirty-six of these 41 patients had hypoplastic left heart syndrome (32) or a hypoplastic left heart variant (4), all requiring a Norwood surgery. All but 1 patient were supported directly from bypass (18) or within 48 h of the initial operation (17). The remaining patient was supported 27 days after reoperation.
Nine of the 41 patients required a second run of mechanical circulatory support post de-cannulation via VAD (4) or ECMO (6). Need for second run of support was associated with an increased risk of death before hospital discharge, with only 1 of the 9 patients surviving to discharge [8 of 9 vs 20 of 32 (89% vs 63%); P = 0.23]. A total of 13 patients (32%) of 41 survived to hospital discharge. Of the survivors, there were 3 late deaths and 2 patients who underwent heart transplantation during follow-up.
Outcomes of patients post-BCPS
There were 10 patients who were supported for acute failure at the BCPS stage. Of this group, 8 required support after staged surgery to assist weaning off cardiopulmonary bypass (6) or as resuscitation post-cardiac arrest (2). The last 2 patients required support after atrioventricular valve repair 6 and 15 months after staged palliation.
In the majority of the group (9 of 10), the inflow cannula was placed in the right or common atrium, with 1 patient having an additional drainage cannula in the left atrium. The last patient was cannulated in both superior and inferior vena cava. There were 6 inpatient deaths within the group (60%), 5 of whom were supported for early failure post-BCPS. Two of the 4 hospital survivors died at 1 and 3 years post hospital discharge.
Outcomes of patients post-Fontan
There were 4 patients who were supported with Fontan physiology, with 3 requiring support for early Fontan failure. Two of these 3 patients were supported with the Berlin Heart EXCOR for 16 and 17 days, respectively. One died from multi-organ failure, while the other patient died from intra-abdominal embolic events. One patient was supported a decade post-Fontan completion after reoperation for subaortic stenosis. She received 6 days of VAD support before urgent transplantation, despite ongoing Gram-negative septicaemia, and died 1 day post-transplantation. Only 1 of the 4 patients survived to hospital discharge and is alive today, albeit in a vegetative state from severe neurological injury on support.
Hospital outcomes of the patients supported for late failure
There were 2 patients who received support for chronic failure of their circulations. The first patient presented with a failing BCPS and required urgent VAD support after a cardiac arrest. He was supported with a centrifugal pump for 8 days before transitioning to support with the Berlin Heart EXCOR. He died of complications secondary to severe ischaemic brain injury after 1 month of support.
The second patient presented with failing Fontan physiology 1 year post-Fontan completion. He suffered a cardiac arrest during an episode of massive haemoptysis and needed urgent ECMO support. Berlin Heart cannulae were implanted 4 days later through which he was supported using the Jostra Rotaflow pump for 2 weeks, before transitioning to the Berlin Heart EXCOR. He was supported for a further 5 months before successful cardiac transplantation.
Long-term outcomes
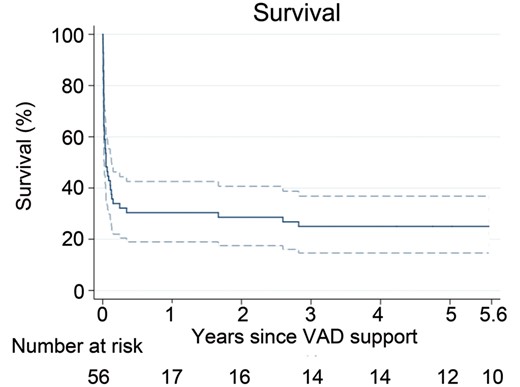
There were a total of 18 patients who survived to hospital discharge. After a median follow-up period of 7.2 years (IQR 3.5–14.0), there were an additional 5 late deaths over a median of 31 months (IQR 20–34) post-VAD support. Kaplan–Meier survival at 5 years post VAD support was 25% (95% CI 15–37%; Fig. 2). Two additional patients underwent transplantation during the follow-up period, both at 15 years post initial VAD support. Estimated freedom from death or transplantation was 25% (95% CI 15–37%) at 5 years and 22% (95% CI 12–34%) at 8 years post support. Twelve of the original 57 patients (21%) were alive without transplantation at last follow-up. Of the late survivors, 10 patients were neurologically intact.
Kaplan–Meier survival curve of long-term survival of single-ventricle patients who received VAD support. (1 patient excluded from the analysis due to unknown follow-up time).
DISCUSSION
Projection estimates state that the population living with a single ventricle will double within the next 20 years [4]. These patients face the risk of progressive failure of their circulation with time. As such, the current limitations of mechanical circulatory support in this population need to be recognized. In view of our unit’s preferential use of VAD over ECMO, we believed a review of our experience would offer insight in the use of VAD as an option for short-term support in this difficult cohort. Our series confirmed the challenges of the use of VAD support in this group, with only one-third surviving to discharge. However, our results were comparable to the survival from existing studies in this group [13, 14].
Modality of support
Over the last 27 years in Melbourne, we have favoured mechanical circulatory support with VAD rather than ECMO whenever possible, as we believed that it posed a lower risk of thromboembolic complications and would be better tolerated [9, 10]. Studies comparing the 2 modalities have been scarce, but a reduction of neurological complications with VAD instead of ECMO has been described [13, 15]. We routinely screen for neurological injury whenever there is clinical suspicion of possible insult. However, the incidence of neurological complications likely remains underestimated in patients who die shortly after initiation of support. Due to our unified strategy of favouring VAD whenever possible, this review did not permit us to compare the outcomes of VAD versus ECMO.
Predictors of poor outcome after VAD support
Only a fraction of patients (21%) had long-term survival without transplantation. One-third of the deaths occurred after successful wean off VAD support. The discrepancy in the number of patients de-cannulated and those who survive to discharge is often observed with mechanical circulatory support in younger patients and confirms the vulnerable state of patients after weaning off support [2, 16].
Our review confirms that a second run of support is usually futile, as almost all of the patients ultimately die. And finally, suffering a neurologic event on support signals a very unfavourable prognosis.
Support early after first stage palliation
Only one-third of the patients survived to hospital discharge, and it could be questioned whether it is worth supporting these children. However, the long-term outcomes of hospital survivors were comparable to other patients with hypoplastic left heart syndrome. Therefore, continued support for this group of patients is warranted until the better candidates can be delineated. Long-term VAD support has been dismal in this population and is not currently indicated.
Support after BCPS
Again, our success with short-term VAD support in patients with a BCPS was limited. Others have described similar difficulty with the support of patients at this stage of palliation [8, 17, 18]. This may be related to the large amount of pulmonary collateral blood flow [19]. We now believe that early failure likely signals an intolerance of the cavopulmonary circulation. The North American experience had reported the survival of 7 of the 12 patients supported with the Excor device, but it was unclear whether support took place early post-BCPS [8]. Unless there are surgical reinterventions to optimize their circulation, we believe early failure should be treated by the takedown of the BCPS, with the creation of a systemic–pulmonary shunt.
Support after Fontan
We have encountered some success in the support of late failure of the Fontan circulation (one patient described in this series and another supported in our parent adult institution [20]). However, like others, our experience with the use of the Berlin Heart Excor device in patients with single ventricle has been poor. The only patient who survived had isolated ventricular dysfunction, which we believe may be the only valid indication for systemic VAD support. In the patients who were supported for early failure after Fontan completion, outcomes were consistently dismal.
Technical characteristics of VAD support in patients with single-ventricle physiology
We were able to achieve satisfactory oxygen saturations (baseline saturations >85%) in patients with systemic–pulmonary shunts, provided that VAD flow was maintained >150% of their estimated cardiac output. One would wonder whether the oxygen saturations of a patient post-BCPS would benefit from short-term VAD support, with preferential drainage of inferior vena cava over superior vena cava. We have consistently improved oxygen saturations on initiation of VAD, even in patients with a BCPS. We attribute this to an improved circulation driven by the negative suction pressures in the atrium, improving venous return that ultimately increases pulmonary flow.
We believe that Fontan patients who fail in the setting of elevated systemic venous pressures cannot be supported with a systemic VAD, with the ‘pulling' of blood through the pulmonary circulation. We attribute this to the increase in cardiac output, which overrides the benefit of reducing atrial pressure. We now suspect that in this scenario, right-sided ventricular support may be necessary.
CONCLUSION
In critically ill patients with a single ventricle, VAD support may provide a valuable window of opportunity for recovery or transplantation. However, the use of systemic VAD support is likely to be futile in the setting of failure early after BCPS or Fontan surgery or with deterioration after the first run of support.
Funding
This work was supported by the Health Professional Scholarship from the National Heart Foundation of Australia to Chin L. Poh. Yves d'Udekem is a NHMRC Clinician Practitioner Fellow (1082186). The Victorian Government’s Operational Infrastructure Support Program supported this research project.
Conflict of interest: Yves d'Udekem as a consultant for MSD and Actelion.
REFERENCES
Author notes
†Presented at the 30th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 1–5 October 2016.