-
PDF
- Split View
-
Views
-
Cite
Cite
Marie-Liesse Asselin-Labat, Mark Shackleton, John Stingl, François Vaillant, Natasha C. Forrest, Connie J. Eaves, Jane E. Visvader, Geoffrey J. Lindeman, Steroid Hormone Receptor Status of Mouse Mammary Stem Cells, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 14, 19 July 2006, Pages 1011–1014, https://doi.org/10.1093/jnci/djj267
Close - Share Icon Share
Abstract
The estrogen receptor α (ERα), progesterone receptor (PR), and erbB2 (Her2 in humans) are important prognostic markers of human breast cancer, and they are variably expressed in different subtypes of breast cancer. The basal subtype, for example, is negative for ERα, PR, and Her2 by immunohistochemistry. We investigated the expression of these signaling molecules in enriched populations of mouse mammary stem cells and luminal cells that were isolated according to their differential expression of CD24 and the α6β1-integrin complex. We found that the basal population, which is enriched in mouse mammary stem cells, did not express ERα, PR, or ErbB2/Her2 but did express epidermal growth factor receptor (EGFR)/ErbB1, whereas the subset of cells enriched for luminal cells expressed ERα (37% of cells) and PR (40% of cells) but not ErbB2/Her2 or EGFR/ErbB1. Ovariectomy confirmed the importance of estrogen signaling to luminal cell proliferation but had no effect on the size of the mouse mammary stem cell-enriched population. Thus, mouse mammary stem cells were negative for ERα, PR, and ErbB2 and appeared to share common properties with poor-prognosis basal breast cancer.
Recent gene-profiling studies have provided a basis for classifying human breast tumors into the following five subtypes: luminal A, luminal B, ErbB2/Her2, basal, and a normal breast-like subtype ( 1 , 2 ) . Estrogen receptor α (ERα) is a critical element in the definition of the luminal A and B subclasses, with the luminal B subtype (which expresses lower levels of ERα transcripts) representing the more aggressive and less effectively treated subtype of the two. The basal subtype of breast cancer does not express the ERα, progesterone receptor (PR), or ErbB2/Her2 but generally does express cytokeratin 5 or 6 and epidermal growth factor receptor (EGFR)/ErbB1 ( 3 ) . Basal cancers are associated with a poor prognosis and commonly develop in BRCA1 mutation carriers ( 4 , 5 ) . Such classifications have stimulated great interest in identifying the cells in which these phenotypically distinct tumor types arise. One possibility is that normal progenitors already restricted in their differentiation potential may be transformed directly by acquiring mutations that endow them with self-renewal properties (akin to stem cells). Other possibilities are that normal stem cells may be initially targeted and then additional genetic changes responsible for full malignancy arise in later progenitors or that all changes could be confined to stem cells but result in more profound effects in later cell types ( 6 , 7 ) . These possibilities prompted us to investigate the expression of ERα, PR, ErbB2/Her2, and EGFR/ErbB1 in normal mouse mammary stem cells.
We have recently described ( 8 , 9 ) methods for the prospective and differential isolation of enriched populations of mouse mammary stem cells and their derivative colony-forming cell progeny, the latter cells being in the luminal population. These isolation methods involve the removal of hematopoietic and endothelial cells and the selection of different mammary cell subsets by their surface expression level of CD24 (heat-stable antigen) and either component of the α6β1-integrin complex (i.e., CD49f and CD29, respectively) ( Fig. 1, A and B ). Mammary epithelial cells that express high levels of CD24 (CD24 + ) and lower levels of CD29 or CD49f (CD29 lo and CD49f lo , respectively) have features of luminal cells, as assessed by immunostaining and gene expression studies, and are enriched in mouse mammary stem cell derivative colony-forming cell progeny. Similar studies of cells expressing slightly lower levels of CD24 (termed CD24 + in this report) and the highest levels of CD29 or CD49f (CD29 hi and CD49f hi , respectively) indicate that these express markers of the basally located myoepithelial cells and are highly enriched in their content of mouse mammary stem cells.
Hormone receptor status in the mouse mammary stem cell (MaSC)–enriched population. A ) Estrogen receptor α (ERα) mRNA expression in the MaSC-enriched population. Left ) Flow cytometric expression profile of CD24 and CD49f (heat-stable antigen and α6-integrin, respectively) in mammary cells depleted of hematopoietic and endothelial cells from C57BL/6 mice showing the populations of isolated luminal (LUM; CD49f lo CD24 + ) and MaSC-enriched (CD49f hi CD24 + ) cell subsets, as assessed by the relative intensity of staining as previously described ( 9 ) . Right ) Relative levels of ERα mRNA in these subpopulations, measured by quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) ( n = 3 experiments), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference used to normalize the levels of RNA. The 5′ to 3′ sequences of the primer pairs used were as follows: GAPDH forward, 5′-CCCATCACCATCTTCCAGGAG-3′, and GAPDH reverse, 5′-CTTCTCCATGGTGGTGAAGACG-3′; and ERα forward, 5′-CTGTCGGCTGCGCAAGTGTT-3′, and ERα reverse, 5′-CATCTCTCTGACGCTTGTGCT-3′. Relative level of ERα in LUM cells was 2.68 (95% confidence interval [CI] = 0.51 to 4.85) and in MaSCs was 0.15 (95% CI = 0 to 0.42). Error bars are 95% CIs. B ) Flow cytometric expression profiles for CD24 and CD29 in mammary cells depleted of hematopoietic and endothelial cells. The gating strategy that was used to isolate luminal (CD29 lo CD24 + ) and MaSC-enriched (CD29 hi CD24 + ) cell subsets as previously described is shown ( 8 ) . C ) Representative immunostaining of ERα, progesterone receptor (PR, A isoform), ErbB2/Her2, epidermal growth factor receptor (EGFR)/ErbB1, the p53 family member p63, and the cell cycle inhibitor p21 in the doubly sorted subpopulations shown in panel B. ERα inset ) Isotype control. ErbB2 inset ) Positive control cells from a mammary tumor arising in a mouse mammary tumor virus-neu (ErbB2) transgenic mouse. Real-time RT-PCR confirmed low levels of ErbB2 transcript in the two populations (data not shown). Freshly sorted cells were cytospun onto a slide, fixed with 4% paraformaldehyde at room temperature for 10 minutes, and then stained with the following antibodies: ERα (Santa Cruz, Richmond, CA), PR (hPRa7, a gift from C. Clarke, Westmead Millennium Institute, Westmead, New South Wales, Australia) ( 18 ) , ErbB2 (Calbiochem, Darmstadt, Germany), EGFR (Cell Signalling, Beverly, MA), p63 (BD Pharmingen, Bedford, MA), cytokeratin (K) 14 (Covance, Berkeley, CA), K18 (Progen Biotechnik, Heidelberg, Germany), p21 (Santa Cruz), p27 (Santa Cruz), or proliferating cell nuclear antigen (PCNA) (DAKO, Glostrup, Denmark). Slides were then incubated with either biotinylated goat anti-rabbit or goat anti-mouse immunoglobulin G (Vector, Burlingame, CA). A streptavidin-biotin peroxidase detection system was used with 3,3′-diaminobenzidine as substrate (DAKO). Positive reaction gives a brown staining. Scale bars = 25 μm. D ) Histograms showing the mean percentages ( error bars as 95% confidence intervals) of positively stained cells, with the indicated antibodies, in the luminal (CD29 lo ) and MaSC-enriched (CD29 hi ) CD24 + populations shown in panel B, as well as for K14, K18, p27, and PCNA. Mammary cell suspensions were prepared from six to eight female FVB/NJ mice (8 to 10 weeks old) for each experiment. A minimum of 1000 cells in 10 randomly selected fields was counted under a ×40 objective, with each field containing approximately 100 cells. Data are from two (ErbB2, EGFR, p63, K14, K18, p21, p27, and PCNA staining) or three (ER and PR staining) experiments. The paired t test value comparing the CD29 lo CD24 + (luminal) and CD29 hi CD24 + (MaSC-enriched) populations for each marker was used to determine statistical significance as follows: ERα, P = .01; PR, P = .01; EGFR, P = .04; p63, P = .04; K14, P = .06; K18, P = .003; p21, P = .04; p27, P = .08. None of 1000 cells were ERα or PR positive in the CD29 hi CD24 + population.
We determined the expression of ERα in the mouse mammary stem cell–enriched and luminal cell populations at the mRNA and protein levels. Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analyses of RNA extracts from the two populations defined by CD49f and CD24 showed that ERα transcripts were confined to the luminal population ( Fig. 1, A ). Consistent with these findings, immunostaining of double-sorted CD24 + cells distinguished by their expression of CD29 ( Fig. 1, B ) confirmed that a substantial proportion (37%, 95% confidence interval [CI] = 23% to 51%) of the luminal CD29 lo cells express ERα protein, whereas the mouse mammary stem cell–enriched populations do not (less than 0.01%) ( Fig. 1, C and D ). We therefore conclude that mouse mammary stem cells are ERα negative.
We then evaluated these same subpopulations for their expression of other markers of important prognostic significance in breast cancer ( 10 ) , including the PR, the tyrosine kinase receptors ErbB2/Her2 and EGFR/ErbB1, the cell cycle inhibitors p21 and p27, and the proliferation marker proliferating cell nuclear antigen (PCNA). Very few cells in the mouse mammary stem cell–enriched population (less than 0.01%) expressed detectable levels of PR, as compared with the luminal population (40%, 95% CI = 24% to 55%), similar to that observed for ERα ( Fig. 1, C and D ). In addition, few cells in the mouse mammary stem cell–enriched population expressed p21 (2%, 95% CI = 1.8% to 2.6%). In contrast, a substantial proportion of this subpopulation expressed PCNA (21%, 95% CI = 15% to 27%), EGFR/ErbB1 (49%, 95% CI = 37% to 61%) and p27 (39%, 95% CI = 36% to 43%), as well as several expected markers of myoepithelial cells (p63 and keratin 14; 63%, 95% CI = 58% to 67%, and 95%, 95% CI = 93% to 96%, respectively) but not keratin 18, a feature of luminal cells. In contrast, the ERα-positive, keratin 18-positive luminal cell population contained a high proportion of cells that were positive for p21 (67%, 95% CI = 50% to 83%) and p27 (60%, 95% CI = 58% to 62%), with 20% (95% CI = 10% to 31%) positive for PCNA and considerably fewer cells that expressed the basal markers. The high association between the expression of ERα, p21, and p27 and the luminal cell population is consistent with data that ERα-positive cells in the mammary gland are noncycling ( 11 ) . The presence of some PCNA-positive cells in both populations is consistent with previous findings indicating that both mouse mammary stem cells and their derivative colony-forming cell progeny are proliferating in vivo ( 9 ) . ErbB2/Her2 staining was negligible in both cell populations, in contrast to the high levels in mammary tumor cells from ErbB2 transgenic mice ( Fig. 1, C , inset). This finding is consistent with previous reports of low levels of ErbB2/Her2 in rodent ( 12 , 13 ) and human ( 14 ) mammary epithelium, as assessed by immunohistochemistry. Thus, the mouse mammary stem cell–enriched subpopulation has an ERα-negative, PR-negative, and ErbB2/Her2-negative phenotype and expresses abundant p63, cytokeratin 14, and EGFR, all of which are hallmarks of the basal subclass of human breast cancers ( 3 , 15 ) .
To determine the functional consequences of estrogen signaling on mammary cell subpopulations, we performed bilateral ovariectomies on adult (8-week-old) virgin female mice and then analyzed the number of CD29 lo CD24 + (luminal) cells and CD29 hi CD24 + (basal or myoepithelial) cells present in their mammary glands 3 weeks later. Ovariectomy produced a marked and selective reduction in the number of CD29 lo CD24 + (luminal) cells, as compared with age-matched nonovariectomized control mice ( Fig. 2 ), but the number of CD29 hi CD24 + (basal or myoepithelial) cells remained unchanged. Thus, consistent with their steroid hormone receptor phenotype (ERα + , PR + ), the size of the luminal population was highly sensitive to hormonal deprivation, in contrast to the ERα − , PR − mouse mammary stem cell–enriched population, which was unaffected by hormonal deprivation.
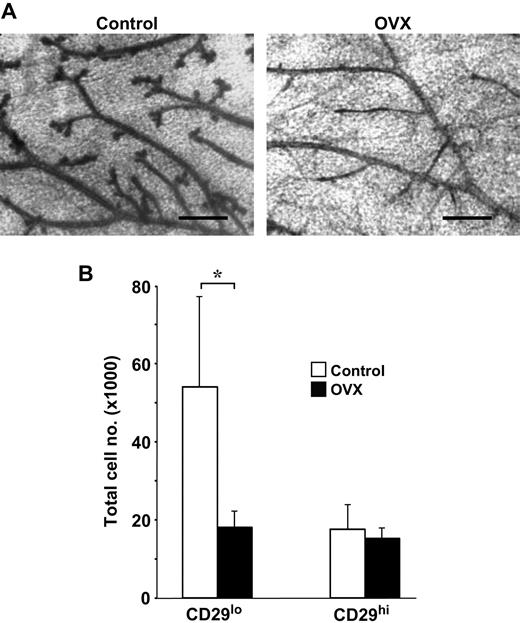
Effect of ovariectomy on the luminal (CD29 lo CD24 + ) and mouse mammary stem cell (MaSC)–enriched (CD29 hi CD24 + ) subpopulations. Virgin female FVB/NJ mice were ovariectomized (OVX) at 8 weeks of age and the cells in the thoracic and inguinal mammary glands collected 3 weeks later for analysis, as described previously ( 8 ) . A ) Representative whole mounts of mammary glands from normal and OVX mice, as indicated. The ductal structures in the OVX mice were less complex, exhibiting fewer side branches. Scale bars = 400 μm. B ) Histogram showing the absolute numbers of cells per mouse for each population in normal and OVX mice. Results represent the means (error bars as 95% confidence intervals) of two experiments (three or four mice per group per experiment). * P = .02 (two-sided t test). For ovariectomy, mice were anesthetized with ketamine at 12 mg/mL and xylazil at 1.6 mg/mL at a dose of 10 μL/g. All experiments were approved by the relevant Animal Research Ethics Committees of the Melbourne Health Research Directorate and the University of British Columbia and carried out in accordance with institutional guidelines.
The phenotype of the mouse mammary stem cell–enriched population thus recapitulates numerous characteristics of the basal phenotype of human breast cancer. However, ovariectomy affords some protection against breast tumorigenesis in BRCA1 mutation carriers ( 16 , 17 ) , although in our study, we detected no effect of ovariectomy on the size of the basal population, which is highly enriched in mouse mammary stem cells. Our study is potentially limited by the lack of known markers that would further enrich for mammary stem cells or provide a better definition of the progenitor cells that they generate. Markers that identify human breast stem cells are also currently not known, and it is possible that there will be species-specific differences. However, the key signaling molecules that we have described are shared between mice and humans. Additional experiments will therefore be required to determine how ovariectomy may have this differential effect. One possibility is that paracrine signaling from adjacent ERα-positive luminal cells influences stem cell behavior. Alternatively, further delineation of the hierarchy of stem and progenitor cells within the mammary gland may reveal as yet unidentified intermediates with basal-like features but with biologically relevant levels of ER and PR expression.
Supported by the Victorian Breast Cancer Research Consortium Inc. Australia, the National Health and Medical Research Council, Australia, the National Breast Cancer Foundation, Australia (J. E. Visvader, G. J. Lindeman), the INSERM/NHMRC agreement (M.-L. Asselin-Labat), the Canadian Stem Cell Network and Genome BC/Canada (C. J. Eaves), and Canadian Breast Cancer Foundation, BC/Yukon Chapter, and the Natural Sciences and Engineering Research Council of Canada (J. Stingl).
The granting agencies played no role in the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, or the writing of the manuscript.
M. Shackleton and J. Stingl contributed equally to this work. C. Eaves, J. Visvader, and G. Lindeman contributed equally to this work.
We thank Catherine Smith for expert technical assistance, Gordon Smyth for discussions, and Christine Clarke for providing PR antibody.
Present address: J. Stingl, Mammary Apoptosis and Development Group, Department of Pathology, University of Cambridge, Cambridge, United Kingdom.
References
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnson H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications.
Sotiriou C, Neo S-Y, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma.
Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer.
Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells.
Wang JC, Dick JE. Cancer stem cells: lessons from leukemia.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells.
Esteva FJ, Hortobagyi GN. Prognostic molecular markers in early breast cancer.
Clarke RB, Howell A, Potten CS, Anderson E. P27(KIP1) expression indicates that steroid receptor-positive cells are a non-proliferating, differentiated subpopulation of the normal human breast epithelium.
Dati C, Maggiora P, Puech C, De Bortoli M, Escot C. Expression of the erbB-2 proto-oncogene during differentiation of the mammary gland in the rat.
Schroeder JA, Lee DC. Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland.
Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues.
Matos I, Dufloth R, Alvarenga M, Zeferino LC, Schmitt F. p63, cytokeratin 5, and P-cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas.
Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation.
Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations.


![Hormone receptor status in the mouse mammary stem cell (MaSC)–enriched population. A ) Estrogen receptor α (ERα) mRNA expression in the MaSC-enriched population. Left ) Flow cytometric expression profile of CD24 and CD49f (heat-stable antigen and α6-integrin, respectively) in mammary cells depleted of hematopoietic and endothelial cells from C57BL/6 mice showing the populations of isolated luminal (LUM; CD49f lo CD24 + ) and MaSC-enriched (CD49f hi CD24 + ) cell subsets, as assessed by the relative intensity of staining as previously described ( 9 ) . Right ) Relative levels of ERα mRNA in these subpopulations, measured by quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) ( n = 3 experiments), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference used to normalize the levels of RNA. The 5′ to 3′ sequences of the primer pairs used were as follows: GAPDH forward, 5′-CCCATCACCATCTTCCAGGAG-3′, and GAPDH reverse, 5′-CTTCTCCATGGTGGTGAAGACG-3′; and ERα forward, 5′-CTGTCGGCTGCGCAAGTGTT-3′, and ERα reverse, 5′-CATCTCTCTGACGCTTGTGCT-3′. Relative level of ERα in LUM cells was 2.68 (95% confidence interval [CI] = 0.51 to 4.85) and in MaSCs was 0.15 (95% CI = 0 to 0.42). Error bars are 95% CIs. B ) Flow cytometric expression profiles for CD24 and CD29 in mammary cells depleted of hematopoietic and endothelial cells. The gating strategy that was used to isolate luminal (CD29 lo CD24 + ) and MaSC-enriched (CD29 hi CD24 + ) cell subsets as previously described is shown ( 8 ) . C ) Representative immunostaining of ERα, progesterone receptor (PR, A isoform), ErbB2/Her2, epidermal growth factor receptor (EGFR)/ErbB1, the p53 family member p63, and the cell cycle inhibitor p21 in the doubly sorted subpopulations shown in panel B. ERα inset ) Isotype control. ErbB2 inset ) Positive control cells from a mammary tumor arising in a mouse mammary tumor virus-neu (ErbB2) transgenic mouse. Real-time RT-PCR confirmed low levels of ErbB2 transcript in the two populations (data not shown). Freshly sorted cells were cytospun onto a slide, fixed with 4% paraformaldehyde at room temperature for 10 minutes, and then stained with the following antibodies: ERα (Santa Cruz, Richmond, CA), PR (hPRa7, a gift from C. Clarke, Westmead Millennium Institute, Westmead, New South Wales, Australia) ( 18 ) , ErbB2 (Calbiochem, Darmstadt, Germany), EGFR (Cell Signalling, Beverly, MA), p63 (BD Pharmingen, Bedford, MA), cytokeratin (K) 14 (Covance, Berkeley, CA), K18 (Progen Biotechnik, Heidelberg, Germany), p21 (Santa Cruz), p27 (Santa Cruz), or proliferating cell nuclear antigen (PCNA) (DAKO, Glostrup, Denmark). Slides were then incubated with either biotinylated goat anti-rabbit or goat anti-mouse immunoglobulin G (Vector, Burlingame, CA). A streptavidin-biotin peroxidase detection system was used with 3,3′-diaminobenzidine as substrate (DAKO). Positive reaction gives a brown staining. Scale bars = 25 μm. D ) Histograms showing the mean percentages ( error bars as 95% confidence intervals) of positively stained cells, with the indicated antibodies, in the luminal (CD29 lo ) and MaSC-enriched (CD29 hi ) CD24 + populations shown in panel B, as well as for K14, K18, p27, and PCNA. Mammary cell suspensions were prepared from six to eight female FVB/NJ mice (8 to 10 weeks old) for each experiment. A minimum of 1000 cells in 10 randomly selected fields was counted under a ×40 objective, with each field containing approximately 100 cells. Data are from two (ErbB2, EGFR, p63, K14, K18, p21, p27, and PCNA staining) or three (ER and PR staining) experiments. The paired t test value comparing the CD29 lo CD24 + (luminal) and CD29 hi CD24 + (MaSC-enriched) populations for each marker was used to determine statistical significance as follows: ERα, P = .01; PR, P = .01; EGFR, P = .04; p63, P = .04; K14, P = .06; K18, P = .003; p21, P = .04; p27, P = .08. None of 1000 cells were ERα or PR positive in the CD29 hi CD24 + population.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/98/14/10.1093_jnci_djj267/2/m_jncidjj267f01_4c.jpeg?Expires=1716583608&Signature=lBgvN4XqthhI~ROv~452nYhrQrlul7CkchPJqMZxJWhIgv-B2rkCZzwzpNqOlFP3wzfw7H5EFcAtgFqw6oN0sgDppLK7pVkK2lPasKPpoVO1~1d94vTGls6Bh4zqXlK1-WhYywW~NF4V5TdfVwFcSQhQVYSv~RUdT~Mo~nTNF3JT6lo3TvFVyCh7Ww2fS6huJCqBcRij-61kMe0~Kt6x~tTrld6bKFdSX~brsgqezeu3g-JPX3kjdFlgKBluXwTNT1SbdXVMH~2xoamC5Nhs4~AgEqfjO4klykJaliXtinB8Rgq-WKhle9kgj0ftlSVS3vRJfMIoWkvdCchGbea0Tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)