-
PDF
- Split View
-
Views
-
Cite
Cite
Andrés J. M. Ferreri, Maurilio Ponzoni, Massimo Guidoboni, Antonio Giordano Resti, Letterio S. Politi, Sergio Cortelazzo, Judit Demeter, Francesco Zallio, Angelo Palmas, Giuliana Muti, Giuseppina P. Dognini, Elisa Pasini, Antonia Anna Lettini, Federico Sacchetti, Carlo De Conciliis, Claudio Doglioni, Riccardo Dolcetti, Bacteria-Eradicating Therapy With Doxycycline in Ocular Adnexal MALT Lymphoma: A Multicenter Prospective Trial, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 19, 4 October 2006, Pages 1375–1382, https://doi.org/10.1093/jnci/djj373
Close - Share Icon Share
Abstract
Background: An association between ocular adnexal MALT lymphoma (OAL) and Chlamydia psittaci ( Cp ) infection has been proposed, and recent reports suggest that doxycycline treatment causes tumor regression in patients with Cp -related OAL. The effectiveness of doxycycline treatment in Cp -negative OAL has not been tested. Methods: In a prospective trial, 27 OAL patients (15 newly diagnosed and 12 having experienced relapse) were given a 3-week course of doxycycline therapy. Objective lymphoma response was assessed by computerized tomography scans or magnetic resonance imaging at 1, 3, and 6 months after the conclusion of therapy and every 6 months during follow-up. Cp infection in patients was determined by touchdown enzyme time-release polymerase chain reaction (TETR-PCR). Statistical tests were two-sided. Results: Eleven patients were Cp DNA–positive and 16 were Cp DNA negative. Doxycycline was well tolerated. At a median follow-up of 14 months, lymphoma regression was complete in six patients, and a partial response (≥50% reduction of all measurable lesions) was observed in seven patients (overall response rate [complete and partial responses] = 48%). Lymphoma regression was observed in both Cp DNA–positive patients (seven of 11 experienced regression) and Cp DNA–negative patients (six of 16 experienced regression) (64% versus 38%; P = .25, Fisher's exact test). The three patients with regional lymphadenopathies and three of the five patients with bilateral disease achieved objective response. In relapsed patients, response was observed both in previously irradiated and nonirradiated patients. The 2-year failure-free survival rate among the doxycycline-treated patients was 66% (95% confidence interval = 54 to 78), and 20 of the 27 patients were progression free. Conclusions: Doxycycline is a fast, safe, and active therapy for Cp DNA–positive OAL that was effective even in patients with multiple failures involving previously irradiated areas or regional lymphadenopathies. The responses observed in PCR-negative OAL may suggest a need for development of more sensitive methods for Cp detection and investigation of the potential role of other doxycycline-sensitive bacteria.
Ocular adnexal lymphoma of the MALT type (OAL) is one of the most common forms of extragastric marginal zone B-cell lymphoma. It is an indolent malignancy that can originate in the lachrymal gland, conjunctiva, eyelids, or orbital soft tissues. Although OAL is rarely lethal and is sometimes manageable with watchful observation alone ( 1 ) , it frequently needs treatment because it can cause symptoms that compromise the patient's quality of life.
Conventional treatment for OAL consists of radiotherapy for patients with localized disease and alkylating-based chemotherapy for those in whom extraorbital lesions are also present. Radiotherapy is associated with local control rates ranging from 85% to 100% and a 10-year survival rate of 75% ( 2 , 3 ) . In 10%–15% of patients, relapses occur, and new disease sites can be within or, more commonly, outside the radiation field. Ocular side effects of radiation therapy are mild but common ( 2 , 3 ) . Experience with alkylating-based chemotherapy is more limited. Treatment with chlorambucil has been associated with a 79% complete remission rate, a 12% relapse rate, and a 5-year relapse-free survival rate of 62% ( 4 ) . Despite the use of more lines of active therapies, patients with OAL usually experience repeated relapses, underscoring the need for new therapeutic approaches—preferably ones based on etiopathogenic mechanisms.
A strong association between OAL and Chlamydia psittaci (Cp) infection has been reported ( 5 ) . This association raises the possibility that treatment with antibiotics may constitute an effective therapy for patients with OAL as it did for patients with gastric MALT lymphomas, for which Helicobacter pylori eradication is now a well-established therapy. Accordingly, we initiated a multicenter prospective trial to assess the activity of a 3-week regimen of doxycycline treatment in OAL. In the pilot phase of the trial, among nine patients with Cp -related OAL, four showed complete or partial (≥50% tumor regression) response and three had a minimal response (<50% tumor regression) ( 6 ) . Subsequently, two reports on small retrospective series of OAL patients treated with doxycycline reported contradictory response rates (0% and 100% overall response, respectively) ( 7 , 8 ) . However, in these series, patients were not assessed for chlamydial infection before therapy, raising the question of the activity of doxycycline in Cp -negative or -unknown OAL. Moreover, recent reports have demonstrated that the prevalence of the Chlamydia–OAL association varies greatly among different geographic areas, even within the same country ( 9 ) . Therefore, the effect of antibiotic therapy in apparently Chlamydia-negative OAL patients needs to be prospectively investigated and compared with outcomes of the therapy in patients with Cp -related OAL.
Here, we report the final results of what is, to our knowledge, the first multicenter prospective trial focused exclusively on OAL. In this trial, the antilymphoma activity of the antibiotic doxycycline was assessed in 27 consecutive patients with newly diagnosed or relapsed OAL. Nine of these patients were included in the previously reported pilot phase of the trial ( 6 ) . All patients were assessed for Cp infection before therapy and treated with doxycycline independently of Cp DNA status. Therapeutic results were analyzed on the basis of site and extension of disease, previous treatments, and whether there was evidence of Cp infection.
P ATIENTS AND M ETHODS
Study Group
Selection criteria for the trial were a histologic diagnosis of marginal zone B-cell lymphoma of the MALT type ( 10 ) ; uni- or bilateral involvement of the ocular adnexa (lachrymal gland, conjunctiva, eyelid, or orbital soft tissue) at diagnosis or relapse, with or without regional lymphadenopathies; at least one measurable lesion; age ≥ 18 years old; and no administration of cytostatics, steroids, antibiotics, immunotherapy, or radiotherapy in the period from the start of antibiotic therapy to lymphoma progression. Patients with allergy to tetracycline or who had been diagnosed with myasthenia gravis or systemic lupus erythematosus were excluded from the trial. We enrolled 27 patients, 15 newly diagnosed and 12 with relapsed disease. Tumor samples from all patients were analyzed by polymerase chain reaction (PCR) for the presence Cp DNA (see below).
The pilot phase of the trial was limited to patients with Cp -positive lymphoma. Preliminary results of the first nine patients have been previously reported ( 6 ) ; the current study includes results of more extended follow-up of this subgroup. In January 2004, the protocol was amended, and, in the second phase of the trial defined by the revised protocol, patients were enrolled regardless of whether they tested positive for Cp DNA. Written informed consent was obtained from each patient. This trial conformed to the tenets of the Declaration of Helsinki and was approved by the institutional review boards of the participating institutions: San Raffaele H Scientific Institute, Milan, Italy; Centro di Riferimento Oncologico, Aviano, Italy; Ospedale Riuniti di Bergamo, Bergamo, Italy; Semmelweis University, Budapest, Hungary; Istituto Nazionale dei Tumori, Milan, Italy; Ospedale San Francesco, Nuoro, Italy; IRCCS Niguarda Cà Granda, Milan, Italy.
Before the start of doxycycline therapy, lymphoma stage was assessed by physical examination, ultrasonography of the neck, total-body computerized tomography scan, gastroscopy with multiple biopsies (biopsies were performed to assess gastric involvement by lymphoma and H. pylori infection), and bilateral bone marrow biopsy. H. pylori –positive patients were treated for 7 days with erythromycin 500 mg twice a day, omeprazole 20 mg twice a day, and tinidazole 500 mg twice a day. Stage of disease was defined according to the Ann Arbor staging system ( 11 ) .
Trial Design
Before the start of therapy, tumor samples from all patients were assessed for the presence of Cp DNA by touchdown enzyme time-release (TETR) PCR. In 20 patients (13 patients at diagnosis and seven at relapse), TETR-PCR to detect Cp was also performed on DNA from peripheral blood mononuclear cells (PBMCs) before the start of antibiotic therapy. In relapsed patients, the period between the diagnosis of lymphoma and PBMC sampling varied between 19 and 165 months (median = 79). In some patients whose PBMCs were tested for Cp infection, Cp eradication was assessed by TETR-PCR of DNA from PBMCs obtained at 1 month after completion of antibiotic therapy and at 1 year of follow-up.
Eligible patients (n = 27, of whom nine were reported on previously) were treated with doxycycline (100 mg, twice a day) for 3 weeks. The drug was administered in the form of oral tablets (Bassado, 100 mg tablets; Pharmacia, Rome, Italy). The study had two primary endpoints: objective lymphoma response and the presence or absence of Cp DNA in PBMC, as a measure of Cp eradication.
Objective lymphoma response was assessed in all patients 1, 3, and 6 months after conclusion of antibiotic therapy and every 6 months during follow-up (follow-up period varied from 3 to 45 months). Follow-up for the analysis reported here was complete as of March 1, 2006.
Detection of Cp DNA
The presence or absence of Cp DNA in formalin-fixed, paraffin-embedded tumor samples (diagnostic samples) was determined using multiplex TETR-PCR that was designed to simultaneously detect Chlamydia trachomatis , Chlamydia pneumoniae, and Cp DNA, as previously reported ( 2 ) . Two to three 5-μm sections were cut from formalin-fixed, paraffin-embedded specimens from lymphoma and control tissue samples, deparaffinated with two xylene washes (10 minutes at room temperature), and rehydrated through decreasing concentrations of ethanol (100%, 96%, 80%, 50%, distilled water). The tissue sections were then placed in 100 μL digestion buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 50 mM EDTA, 1% Triton X-100) containing 2 mg/mL proteinase K and incubated at 50 °C for 3 days. The proteinase K–digested mixture was then purified by silica gel spin cartridge (Talent S.r.l, Trieste, Italy), according to the manufacturer's instructions. DNA was extracted from PBMC samples following a standard phenol–chloroform extraction technique after proteinase K digestion. Ten microliters of template DNA was added to a PCR tube containing 40 μL of PCR mixture overlaid with one drop of mineral oil. Blank reactions containing 50 μL of PCR mixture were interspersed every three to four samples to monitor possible contamination of PCR reagents by Chlamydiae DNA and rule out false-positive results. DNA equivalent to <1 infection-forming unit per PCR tube of Chlamydia trachomatis L2, Chlamydia pneumoniae TW-183, or Chlamydia psittaci ORNI were included as positive controls. Amplified DNA corresponds to a sequence beginning in the 16S rRNA gene and ending at the beginning of the 16S-23S spacer region in the ribosomal genes. Primer pairs specific for C. trachomatis and C. pneumoniae are located entirely within the 16S rRNA gene; for Cp , one primer is located in the 16S rRNA gene and one in the 16S-23S spacer region.
PCR products were separated by electrophoresis in 2% agarose gels and stained with ethidium bromide. DNA fragment size was quantified by image analysis (Image Station 440CF, NEN Life Science Products, Boston, MA). Three amplifications were performed per tissue and PBMC sample. Case patient samples were considered positive for chlamydial infection when Chlamydiae DNA was amplified in at least two independent experiments. Specificity of the amplified fragments was confirmed by direct sequencing of both sense and antisense strands of purified PCR products using an ABI PRISM 310 Genetic Analyzer (Perkin Elmer, Foster City, CA). Sequence specificity was assessed by BLAST search ( http://www.ncbi.nlm.nih.gov/blast ) and heterogeneity of Chlamydiae sequences across the different samples was investigated by using MultAlin software to align them ( http://prodes.toulouse.inra.fr/multalin/multalin.html ) ( 12 ) . In lymphoma samples negative to TETR-PCR, amplification of β-globin was carried out as previously described ( 13 ) .
Objective Response Assessment
Computerized tomography scans (two patients), magnetic resonance imaging (25 patients) of the orbits, and any other radiologic procedures that showed evidence of lymphoma before antibiotic therapy (ultrasonography of the neck in three patients) were used to measure lesions. Magnetic resonance imaging included axial and coronal T1-weighted (W) spin-echo (SE) and T2W SE sequences with 3-mm-thick slices. When possible, diffusion-weighted imaging (DWI) oriented on both axial and coronal planes with 3-mm-thick slices and “b factor” = 0 and 700 was obtained; isotropic (Trace) images and ADC maps were also calculated. T1W SE with spectral suppression of fat signal oriented on the axial, coronal, and parasagittal planes with 3-mm-thick slices was acquired after intravenous administration of 0.2 mL/kg of paramagnetic contrast agent. Maximum lesion size in two dimensions from images obtained after gadolinium injection was considered for response definition. Objective response was defined according to the World Health Organization criteria ( 14 ) : complete remission was defined as the disappearance of all clinical evidence of the disease, partial response was defined as a more than 50% reduction in size of all measurable lesions, stable disease was defined as regression of any measurable lesion by ≤50% (minimal response) or no change for the measurable lesions, and progressive disease was defined as the appearance of any new lesion or an increase in the size of a tumor of ≥25% at a previously involved site.
Statistical Methods
Clinical characteristics of subgroups of patients, divided according to whether they showed signs of Cp infection in tumors (positive versus negative PCR results for Cp DNA) and according to response rates after doxycycline, were compared using the Fisher's exact test for categorical variables. Response rates were analyzed according to whether or not Cp infection was indicated by PCR of tumor biopsies, primary site of disease (conjunctiva versus intraorbital), and previous lines of treatment (none versus ≥1). Independent associations among studied variables and objective response were assessed by logistic regression. Failure-free survival was calculated from the first day of doxycycline administration to relapse (lymphoma recurrence after initial response), progression, death, or to the last date of follow-up (March 1, 2006), whereas overall survival was calculated from the date of the start of doxycycline therapy to death or to the last date of follow-up. Failure-free survival curves were generated by the Kaplan–Meier method (with relapse and progression as events) and compared using the log-rank test. All statistical tests were two-sided. Analyses were carried out using the Statistica 4.0 statistical package for Windows (Statsoft Inc, Tulsa, OK).
R ESULTS
Patient Characteristics
Every eligible patient seen at the participating centers was enrolled in the trial. Fifteen of the 27 enrolled patients were newly diagnosed, and 12 had experienced relapse. Biopsies of all enrolled patients were assessed for the presence of Cp DNA, and all patients were treated with doxycycline. Cp DNA was detected in the lymphomatous tissue obtained by biopsy in 11 (41%) patients, whereas 16 patients were negative for Cp DNA ( Table 1 ). Of the 15 newly diagnosed patients, four were positive for Cp DNA by PCR of tumor samples; of the 12 patients with relapse, seven were positive for Cp DNA by PCR. None of the patients was positive for C. trachomatis or C. pneumoniae DNA (data not shown). The median age of the patients at the time of antibiotic therapy was 56 years (range = 29–87 years). None of the patients had an Eastern Cooperative Oncology Group performance status greater than 1, and none exhibited increased serum levels of lactate dehydrogenase or systemic symptoms. Five patients had H. pylori –associated chronic gastritis detected by gastroscopy performed during staging immediately after lymphoma diagnosis; in all of them H. pylori was eradicated after triple antibiotic combination therapy. This treatment was initiated 32–67 months before initiation of doxycycline treatment; the period elapsed between anti– H. pylori therapy and doxycycline is long enough to exclude any interference of the triple antibiotic therapy with doxycycline activity assessment. Eleven (41%) patients reported prolonged contact with household animals, and 11 had a previous history of chronic conjunctivitis. None of the differences between Cp -positive and Cp -negative patients (in patients' characteristics and extent of disease) were statistically significant ( Table 1 ).
Patient characteristics at the time of enrollment *
| Characteristics . | Patients with Cp DNA positive lymphomas (n = 11) † . | Patients with Cp DNA negative lymphomas (n = 16) . | P‡ . |
|---|---|---|---|
| Median age (range) | 66 (48–87) | 53 (29–83) | .10 |
| Males, n (%) | 3 (27) | 5 (31) | .99 |
| ECOG PS >1, n | 0 | 0 | .99 |
| Lymphoma in conjunctiva, n (%) | 5 (45) | 9 (56) | |
| Lymphoma in lachrymal gland or orbit, n (%) | 6 (55) | 7 (44) | .70 |
| Bilateral orbit involvement, n (%) | 3 (27) | 2 (13) | .35 |
| Extraorbital disease | |||
| Concomitant lymphadenopathies, n (%) | 3 (27) | 0 (0) | .05 |
| Extranodal disease, n (%) | 0 (0) | 0 (0) | .99 |
| Systemic symptoms, n (%) § | 0 (0) | 0 (0) | .99 |
| LDH ratio >1, n (%) ‖ | 0 (0) | 0 (0) | .99 |
| Gastric Helicobacter pylori infection, n (%) ¶ | 3 (27) | 2 (13) | .35 |
| Hepatitis C virus infection, n (%) | 2 (18) | 0 (0) | .16 |
| Prolonged contact with household animals, n (%) | 5 (45) | 6 (38) | .69 |
| History of chronic conjunctivitis, n (%) | 6 (55) | 5 (31) | .26 |
| Doxycycline administered at diagnosis, n (%) | 4 (36) | 11 (69) | |
| Doxycycline administered at relapse, n (%) | 7 (64) | 5 (31) | .13 |
| Proportion of patients with Cp -positive PBMC (%) # | 4/7 (57%) | 0/13 (0%) | .007 |
| Characteristics . | Patients with Cp DNA positive lymphomas (n = 11) † . | Patients with Cp DNA negative lymphomas (n = 16) . | P‡ . |
|---|---|---|---|
| Median age (range) | 66 (48–87) | 53 (29–83) | .10 |
| Males, n (%) | 3 (27) | 5 (31) | .99 |
| ECOG PS >1, n | 0 | 0 | .99 |
| Lymphoma in conjunctiva, n (%) | 5 (45) | 9 (56) | |
| Lymphoma in lachrymal gland or orbit, n (%) | 6 (55) | 7 (44) | .70 |
| Bilateral orbit involvement, n (%) | 3 (27) | 2 (13) | .35 |
| Extraorbital disease | |||
| Concomitant lymphadenopathies, n (%) | 3 (27) | 0 (0) | .05 |
| Extranodal disease, n (%) | 0 (0) | 0 (0) | .99 |
| Systemic symptoms, n (%) § | 0 (0) | 0 (0) | .99 |
| LDH ratio >1, n (%) ‖ | 0 (0) | 0 (0) | .99 |
| Gastric Helicobacter pylori infection, n (%) ¶ | 3 (27) | 2 (13) | .35 |
| Hepatitis C virus infection, n (%) | 2 (18) | 0 (0) | .16 |
| Prolonged contact with household animals, n (%) | 5 (45) | 6 (38) | .69 |
| History of chronic conjunctivitis, n (%) | 6 (55) | 5 (31) | .26 |
| Doxycycline administered at diagnosis, n (%) | 4 (36) | 11 (69) | |
| Doxycycline administered at relapse, n (%) | 7 (64) | 5 (31) | .13 |
| Proportion of patients with Cp -positive PBMC (%) # | 4/7 (57%) | 0/13 (0%) | .007 |
Cp = Chlamydia psittaci ; ECOG PS = performance status according to the Eastern Cooperative Oncology Group score; LDH = serum levels of lactic dehydrogenase; PBMC = peripheral blood mononuclear cells.
Includes the nine previously reported patients.
Fisher's exact test for categorical variables.
Fever of no evident cause, night sweats, and weight loss of more than 10% of body weight in the last 6 months are considered systemic symptoms.
Ratio of individual serum level of lactic dehydrogenase and upper normal value.
Was determined by gastroscopy with multiple biopsies.
Fraction of patients (among those tested) with Cp DNA present in PBMCs before initiation of treatment, as determined by PCR.
Patient characteristics at the time of enrollment *
| Characteristics . | Patients with Cp DNA positive lymphomas (n = 11) † . | Patients with Cp DNA negative lymphomas (n = 16) . | P‡ . |
|---|---|---|---|
| Median age (range) | 66 (48–87) | 53 (29–83) | .10 |
| Males, n (%) | 3 (27) | 5 (31) | .99 |
| ECOG PS >1, n | 0 | 0 | .99 |
| Lymphoma in conjunctiva, n (%) | 5 (45) | 9 (56) | |
| Lymphoma in lachrymal gland or orbit, n (%) | 6 (55) | 7 (44) | .70 |
| Bilateral orbit involvement, n (%) | 3 (27) | 2 (13) | .35 |
| Extraorbital disease | |||
| Concomitant lymphadenopathies, n (%) | 3 (27) | 0 (0) | .05 |
| Extranodal disease, n (%) | 0 (0) | 0 (0) | .99 |
| Systemic symptoms, n (%) § | 0 (0) | 0 (0) | .99 |
| LDH ratio >1, n (%) ‖ | 0 (0) | 0 (0) | .99 |
| Gastric Helicobacter pylori infection, n (%) ¶ | 3 (27) | 2 (13) | .35 |
| Hepatitis C virus infection, n (%) | 2 (18) | 0 (0) | .16 |
| Prolonged contact with household animals, n (%) | 5 (45) | 6 (38) | .69 |
| History of chronic conjunctivitis, n (%) | 6 (55) | 5 (31) | .26 |
| Doxycycline administered at diagnosis, n (%) | 4 (36) | 11 (69) | |
| Doxycycline administered at relapse, n (%) | 7 (64) | 5 (31) | .13 |
| Proportion of patients with Cp -positive PBMC (%) # | 4/7 (57%) | 0/13 (0%) | .007 |
| Characteristics . | Patients with Cp DNA positive lymphomas (n = 11) † . | Patients with Cp DNA negative lymphomas (n = 16) . | P‡ . |
|---|---|---|---|
| Median age (range) | 66 (48–87) | 53 (29–83) | .10 |
| Males, n (%) | 3 (27) | 5 (31) | .99 |
| ECOG PS >1, n | 0 | 0 | .99 |
| Lymphoma in conjunctiva, n (%) | 5 (45) | 9 (56) | |
| Lymphoma in lachrymal gland or orbit, n (%) | 6 (55) | 7 (44) | .70 |
| Bilateral orbit involvement, n (%) | 3 (27) | 2 (13) | .35 |
| Extraorbital disease | |||
| Concomitant lymphadenopathies, n (%) | 3 (27) | 0 (0) | .05 |
| Extranodal disease, n (%) | 0 (0) | 0 (0) | .99 |
| Systemic symptoms, n (%) § | 0 (0) | 0 (0) | .99 |
| LDH ratio >1, n (%) ‖ | 0 (0) | 0 (0) | .99 |
| Gastric Helicobacter pylori infection, n (%) ¶ | 3 (27) | 2 (13) | .35 |
| Hepatitis C virus infection, n (%) | 2 (18) | 0 (0) | .16 |
| Prolonged contact with household animals, n (%) | 5 (45) | 6 (38) | .69 |
| History of chronic conjunctivitis, n (%) | 6 (55) | 5 (31) | .26 |
| Doxycycline administered at diagnosis, n (%) | 4 (36) | 11 (69) | |
| Doxycycline administered at relapse, n (%) | 7 (64) | 5 (31) | .13 |
| Proportion of patients with Cp -positive PBMC (%) # | 4/7 (57%) | 0/13 (0%) | .007 |
Cp = Chlamydia psittaci ; ECOG PS = performance status according to the Eastern Cooperative Oncology Group score; LDH = serum levels of lactic dehydrogenase; PBMC = peripheral blood mononuclear cells.
Includes the nine previously reported patients.
Fisher's exact test for categorical variables.
Fever of no evident cause, night sweats, and weight loss of more than 10% of body weight in the last 6 months are considered systemic symptoms.
Ratio of individual serum level of lactic dehydrogenase and upper normal value.
Was determined by gastroscopy with multiple biopsies.
Fraction of patients (among those tested) with Cp DNA present in PBMCs before initiation of treatment, as determined by PCR.
Tolerability
All patients completed the antibiotic therapy, but one elderly patient took doxycycline irregularly. No symptomatic toxicity or changes in biochemical parameters (serum levels of markers of hepatic and renal function) were observed in any patients.
Eradication of Cp
Before initiating doxycycline treatment, we withdrew PBMCs from enrolled patients and tested for the presence of Cp DNA by PCR. DNA from PBMCs was not available from seven patients due to their geographic inaccessibility. Four patients had Cp DNA–positive PBMCs, all of whom had lymphomas that also tested positive for Cp DNA by PCR ( Table 1 ). One month after the end of doxycycline treatment PBMCs were obtained again, and PCR testing of PBMCs for the presence of Cp DNA was repeated. At this time, Cp DNA was not detected in the PBMCs of any of the 20 patients (data not shown). The same testing was repeated 1 year after follow-up and none of the patients tested positive, indicating that the eradication was long lasting.
Objective Lymphoma Response
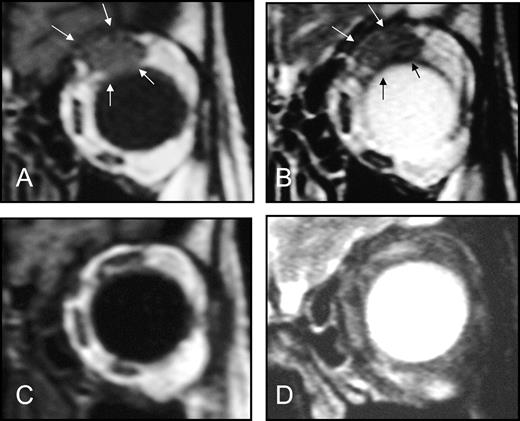
After a median posttreatment follow-up of 14 months (range 3–45 months), complete lymphoma remission was observed in six patients and a partial response was observed in seven patients, for an overall response rate of 48% ( Table 2 ). Three patients had a minimal response, i.e., less than 50% regression in any measurable lesion, at follow-up times of 7, 11, and 18 months. An example of complete remission in an 85-year-old woman who had experienced a relapse after treatment with chlorambucil and irradiation of the left orbit is shown in Fig. 1 . A neoplastic lesion in the left orbit that had infiltrated the superior rectum muscle was detected before doxycycline treatment by coronal T1W ( Fig. 1, A ) and T2W ( Fig. 1, B ) magnetic resonance images, and a complete remission of the lesion was confirmed in a magnetic resonance examination performed 3 months after conclusion of doxycycline therapy ( Fig. 1, C and D ). Nine patients had stable disease at a median follow-up of 7 months (range 3–13 months), whereas two patients experienced progressive disease at 3 and 6 months.
Effect of 3-week doxycycline therapy in an 85-year-old woman with ocular adnexal MALT lymphoma that was negative for Chlamydia psittaci DNA. The patient had experienced a relapse after chlorambucil and left orbit irradiation. Magnetic resonance imaging performed 2 weeks before doxycycline therapy showing in coronal T1-weighted ( A ) and T2-weighted ( B ) images (3-mm-thick slices) a neoplastic lesion in the left orbit that had infiltrated the superior rectum muscle ( bordered by arrows ). Coronal T1W ( C ) and T2W ( D ) magnetic resonance examination (3-mm-thick slices) performed 3 months after conclusion of doxycycline treatment showing a complete remission of the neoplastic lesion in the left orbit.
Activity of doxycycline according to PCR-detected C p DNA status and extension and site of disease *
| Patient subgroup . | n . | CR (%) . | P† . | PR (%) . | ORR (%) . | P‡ . | MR (%) . | SD (%) . | PD (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Entire series | 27 | 6 (22) | 7 (26) | 13 (48) § | 3 (11) | 9 (33) | 2 (7) | ||
| Cp DNA positive | 11 | 4 (36) | 3 (27) | 7 (64) | 1 (9) | 2 (18) | 1 (9) | ||
| Cp DNA negative | 16 | 2 (13) | .18 | 4 (25) | 6 (38) | .25 | 2 (13) | 7 (44) | 1 (6) |
| Newly diagnosed disease | 15 | 2 (13) | 3 (20) | 5 (33) | 2 (13) | 7 (47) | 1 (7) | ||
| Relapsed disease | 12 | 4 (33) | .36 | 4 (33) | 8 (67) | .13 | 1 (8) | 2 (17) | 1 (8) |
| OAL in conjunctiva | 14 | 2 (14) | 4 (29) | 6 (43) | 2 (14) | 5 (36) | 1 (7) | ||
| OAL in lachrymal gland/orbit | 13 | 4 (31) | .38 | 3 (23) | 7 (54) | .71 | 1 (8) | 4 (31) | 1 (8) |
| Reported series ‖ | 9 | 4 (44) | 2 (22) | 6 (66) | 1 (11) | 1 (11) | 1 (11) | ||
| Following series ¶ | 18 | 2 (11) | .14 | 5 (28) | 7 (39) | .24 | 3 (17) | 7 (39) | 1 (6) |
| Patient subgroup . | n . | CR (%) . | P† . | PR (%) . | ORR (%) . | P‡ . | MR (%) . | SD (%) . | PD (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Entire series | 27 | 6 (22) | 7 (26) | 13 (48) § | 3 (11) | 9 (33) | 2 (7) | ||
| Cp DNA positive | 11 | 4 (36) | 3 (27) | 7 (64) | 1 (9) | 2 (18) | 1 (9) | ||
| Cp DNA negative | 16 | 2 (13) | .18 | 4 (25) | 6 (38) | .25 | 2 (13) | 7 (44) | 1 (6) |
| Newly diagnosed disease | 15 | 2 (13) | 3 (20) | 5 (33) | 2 (13) | 7 (47) | 1 (7) | ||
| Relapsed disease | 12 | 4 (33) | .36 | 4 (33) | 8 (67) | .13 | 1 (8) | 2 (17) | 1 (8) |
| OAL in conjunctiva | 14 | 2 (14) | 4 (29) | 6 (43) | 2 (14) | 5 (36) | 1 (7) | ||
| OAL in lachrymal gland/orbit | 13 | 4 (31) | .38 | 3 (23) | 7 (54) | .71 | 1 (8) | 4 (31) | 1 (8) |
| Reported series ‖ | 9 | 4 (44) | 2 (22) | 6 (66) | 1 (11) | 1 (11) | 1 (11) | ||
| Following series ¶ | 18 | 2 (11) | .14 | 5 (28) | 7 (39) | .24 | 3 (17) | 7 (39) | 1 (6) |
PCR = polymerase chain reaction; Cp = Chlamydia psittaci ; CR = complete remission; PR = partial response; ORR = overall response rate; MR = minimal response; SD = stable disease; PD = progressive disease; OAL = ocular adnexal MALT lymphoma.
Comparison of complete remission rates (Fisher's exact test for categorical variables).
Comparison of overall response rates (Fisher's exact test for categorical variables).
95% confidence interval = 30% to 66%.
This subgroup is constituted by the first nine patients, who have been previously reported. With a longer follow-up with respect to the previous report (median: 26 and 12 months, respectively), two patients with PR achieved a CR and two patients with MR achieved a PR.
Subgroup of the 18 unpublished patients (median follow-up: 9 months).
Activity of doxycycline according to PCR-detected C p DNA status and extension and site of disease *
| Patient subgroup . | n . | CR (%) . | P† . | PR (%) . | ORR (%) . | P‡ . | MR (%) . | SD (%) . | PD (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Entire series | 27 | 6 (22) | 7 (26) | 13 (48) § | 3 (11) | 9 (33) | 2 (7) | ||
| Cp DNA positive | 11 | 4 (36) | 3 (27) | 7 (64) | 1 (9) | 2 (18) | 1 (9) | ||
| Cp DNA negative | 16 | 2 (13) | .18 | 4 (25) | 6 (38) | .25 | 2 (13) | 7 (44) | 1 (6) |
| Newly diagnosed disease | 15 | 2 (13) | 3 (20) | 5 (33) | 2 (13) | 7 (47) | 1 (7) | ||
| Relapsed disease | 12 | 4 (33) | .36 | 4 (33) | 8 (67) | .13 | 1 (8) | 2 (17) | 1 (8) |
| OAL in conjunctiva | 14 | 2 (14) | 4 (29) | 6 (43) | 2 (14) | 5 (36) | 1 (7) | ||
| OAL in lachrymal gland/orbit | 13 | 4 (31) | .38 | 3 (23) | 7 (54) | .71 | 1 (8) | 4 (31) | 1 (8) |
| Reported series ‖ | 9 | 4 (44) | 2 (22) | 6 (66) | 1 (11) | 1 (11) | 1 (11) | ||
| Following series ¶ | 18 | 2 (11) | .14 | 5 (28) | 7 (39) | .24 | 3 (17) | 7 (39) | 1 (6) |
| Patient subgroup . | n . | CR (%) . | P† . | PR (%) . | ORR (%) . | P‡ . | MR (%) . | SD (%) . | PD (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Entire series | 27 | 6 (22) | 7 (26) | 13 (48) § | 3 (11) | 9 (33) | 2 (7) | ||
| Cp DNA positive | 11 | 4 (36) | 3 (27) | 7 (64) | 1 (9) | 2 (18) | 1 (9) | ||
| Cp DNA negative | 16 | 2 (13) | .18 | 4 (25) | 6 (38) | .25 | 2 (13) | 7 (44) | 1 (6) |
| Newly diagnosed disease | 15 | 2 (13) | 3 (20) | 5 (33) | 2 (13) | 7 (47) | 1 (7) | ||
| Relapsed disease | 12 | 4 (33) | .36 | 4 (33) | 8 (67) | .13 | 1 (8) | 2 (17) | 1 (8) |
| OAL in conjunctiva | 14 | 2 (14) | 4 (29) | 6 (43) | 2 (14) | 5 (36) | 1 (7) | ||
| OAL in lachrymal gland/orbit | 13 | 4 (31) | .38 | 3 (23) | 7 (54) | .71 | 1 (8) | 4 (31) | 1 (8) |
| Reported series ‖ | 9 | 4 (44) | 2 (22) | 6 (66) | 1 (11) | 1 (11) | 1 (11) | ||
| Following series ¶ | 18 | 2 (11) | .14 | 5 (28) | 7 (39) | .24 | 3 (17) | 7 (39) | 1 (6) |
PCR = polymerase chain reaction; Cp = Chlamydia psittaci ; CR = complete remission; PR = partial response; ORR = overall response rate; MR = minimal response; SD = stable disease; PD = progressive disease; OAL = ocular adnexal MALT lymphoma.
Comparison of complete remission rates (Fisher's exact test for categorical variables).
Comparison of overall response rates (Fisher's exact test for categorical variables).
95% confidence interval = 30% to 66%.
This subgroup is constituted by the first nine patients, who have been previously reported. With a longer follow-up with respect to the previous report (median: 26 and 12 months, respectively), two patients with PR achieved a CR and two patients with MR achieved a PR.
Subgroup of the 18 unpublished patients (median follow-up: 9 months).
In some patients, response was slow and gradual; the median time to the best response in the 16 patients with any response was 6 months (range = 3–36 months). Four patients achieved complete remission within 3 months from end of doxycycline treatment, whereas five patients achieved the best response only after 1 year of follow-up.
Three patients had regional lymphadenopathies and five patients had bilateral involvement of the ocular adnexa. All three patients with regional lymphadenopathies and three of the five with bilateral involvement of the adnexa achieved objective response (four complete remissions and two partial responses), that lasted 3+, 13+, 16+, 22+, 26+, and 37 months. When administered at relapse, doxycycline was also associated with lymphoma regression in previously irradiated areas ( Fig. 1 ). In fact, objective responses were observed in three of the five previously irradiated patients and in five of the seven nonirradiated patients.
Lymphoma regression after doxycycline treatment was observed both in patients whose lymphomas were Cp DNA positive and in those whose lymphomas were Cp DNA negative ( Table 2 ). Although complete remission was more common among patients whose lymphomas were positive for Cp DNA, six of the 16 patients whose lymphomas were Cp DNA negative displayed an objective response (complete or partial remission [ Table 2 ]). Lymphoma regression was observed when doxycycline was used either as first-line treatment or as salvage therapy (i.e., in patients with relapsed disease), with a slightly higher rate of response in those with relapsed disease. Tumor regression was less common in patients with conjunctival lymphomas in comparison with patients with intraorbital lesions, but the difference was not statistically significant ( Table 2 ).
Duration of Response and Survival
At the time of analysis, 13 of the 16 patients who responded to doxycycline with at least a minimal response and seven of the 11 unresponsive patients were failure free, with a median failure-free survival in all patients of 11+ months (range 0–45) and a 2-year failure-free survival for the entire patient series of 66% (95% confidence interval = 54 to 78 [ Fig. 2 ]). Failures (relapses and progressive disease) were approximately equally distributed among subgroups of patients divided according to whether their biopsies tested positive for Cp DNA (four of 11 Cp -positive patients and three of 16 Cp -negative patients), site of disease (three of 14 patients with conjunctival lymphoma and four of 13 patients with intraorbital lymphoma), and previous treatment (three of 15 patients treated at diagnosis and four of 12 patients treated at relapse). Six of the seven failures occurred within the first year of follow-up and involved the primary site of disease; the remaining failure, which occurred in an hepatitis C virus–positive patient, 37 months after treatment, was accompanied by bilateral lesions in the kidneys and lungs and multiple subcutaneous nodules. Relapses were asymptomatic and indolent. Five relapsed patients were treated with radiotherapy or chemotherapy according to previous treatments, and complete remission was achieved in three of them. All patients were alive at the time of analysis (March 15, 2006), with a median overall follow-up of 21 months (range = 4–204 months).
Failure-free survival curve for the 27 patients treated with doxycycline. Failure-free survival rates at 12, 24, and 36 months were 81% (95% confidence interval [CI] = 72% to 90%), 66% (95% CI = 54% to 78%), and 66% (95% CI = 54% to 78%), respectively. The numbers of patients at risk at 12, 24, and 36 months were 12, 4, and 2, respectively.
D ISCUSSION
Doxycycline administration was associated with an overall response rate of 64% and a 2-year failure-free survival of 67% in patients with Cp -associated OAL. Tumor remission was observed even in patients with multiple failures, i.e., those with involvement of regional lymph nodes and those with lesions that arose in previously irradiated areas. Objective lymphoma regression was also observed in patients in whom Cp infection was not detected. This study therefore raises questions about the possible role of other doxycycline-sensitive microorganisms in the pathogenesis of OAL.
The heterogeneity of the studied series, that is the inclusion of patients at diagnosis and at relapse, and the follow-up shorter than 9 months in eight (30%) of the 27 patients constitute the main limitations of this study. However, the latter issue may result in underestimation of response rates, which renders these results even more encouraging. In fact, a sizeable fraction of OAL patients experienced slow and gradual tumor regression after doxycycline treatment. It is noteworthy that a slow and gradual response has also been observed in patients with gastric MALT lymphoma who were treated with H. pylori –eradicating antibiotics ( 15 ) . In five (31%) of the 16 responsive patients in our study, the best response was achieved only after 1 year of follow-up. This finding suggests that patients with OAL who are treated with antibiotics should be followed for a long period to assess response before concluding that antibiotics are ineffective and starting alternative therapies. Delay of salvage treatment in patients with indolent lymphoma and residual disease generates a number of ethical and medical dilemmas. However, based on our results, we recommend watchful waiting and the withholding of salvage therapy in patients with OAL who have residual disease after doxycycline treatment until lymphoma progression. This recommendation is supported by the fact that the progressed disease that we observed after doxycycline treatment was usually indolent and localized, particularly in conjunctival lymphomas. The single exception to this pattern in our series was a hepatitis C virus–positive patient with a systemic dissemination at relapse. The subgroup of hepatitis C virus–positive patients could constitute an exception to our recommendation because this underlying infection is associated with more aggressive and disseminated OAL ( 16 ) .
Doxycycline treatment induced lymphoma regression even in those relapsing patients in whom there was involvement of regional lymph nodes. By contrast, in patients with gastric MALT lymphoma who are treated with antibiotics, the detection of perivisceral lymphadenopathies is considered a negative predictor of response ( 17 , 18 ) . Our observations suggest that the role of doxycycline in OAL patients with disseminated disease needs to be investigated further. More important, doxycycline induced durable objective remission in OAL patients who had previously experienced relapse within irradiated areas, making alkylating-based chemotherapy and its accompanying toxicity unnecessary.
Three of the 11 patients with Cp -associated OAL did not respond to doxycycline, underscoring the importance of identifying predictors of response. As is the case for gastric MALT lymphomas, analysis by fluorescence in situ hybridization of chromosomal translocations may be a useful tool to identify those patients with OAL whose disease is refractory to antibiotic therapy. The chromosomal translocation t(11;18)(q21;q21) is a reliable lymphoma response marker ( 19 – 21 ) that is present in 13%–35% of MALT lymphomas ( 22 ) , but it has not been described in OAL ( 22 ) . The translocations t(14;18)(q32;q21) and t(3;14)(p14.1;q32) have been reported to be present in up to 20% of OAL cases ( 23 , 24 ) , but their predictive value remains to be determined.
Six (38%) of the 16 Cp -negative OAL patients experienced lymphoma regression after doxycycline treatment. The failure to detect Cp in these patients could be misleading: the negative PCR results could be explained by Cp DNA levels that were below the threshold of PCR detection, possibly due to the frequent use of broad-spectrum local antibiotics before biopsy. Strain-specific sequence variation is another factor that might prevent amplification of the target DNA. If, however, the PCR results are accurate indicators of the presence of Cp , then responses of Cp -negative patients may suggest that OAL is related to infection by other doxycycline-sensitive bacteria. Some evidence for this possibility comes from preliminary studies that suggest that the association between OAL and Cp varies widely according to geographic area ( 9 ) . In the original Italian study ( 5 ) and in a subsequent study in Korea ( 25 ) , 70%–80% of OAL patients were shown to test positive for Cp . By contrast, an absence or very low prevalence of correlation between OAL and Cp infection has been reported in patient series from the United States ( 26 , 27 ) , Cuba ( 28 ) , Japan ( 29 ) , Netherlands ( 30 ) , and France ( 31 ) . Even if these geographic variations in the rates of Cp infection in OAL patients could be explained by differences in sensitivity or specificity of the detection methods used, it is worth noting that variations among different geographic areas have been reported for other associations between lymphomas and infectious agents, such as hepatitis C virus ( 32 ) and Borrelia burgdorferi ( 33 ) . A conclusive demonstration of geographic variations in the association between Cp and OAL will stimulate further studies to better define the roles of epidemiologic, environmental, and genetic factors as well as the involvement of other infectious agents in the etiology of this disease.
Seven of the patients in our study experienced failure, three after an initial response. Failure rates were not dependent on whether Cp was detected by PCR, site of disease, or previous treatment. Most occurred within the first year of follow-up and involved the primary site of disease. This clinical finding in patients with OAL is similar to that reported for patients with gastric MALT lymphomas, 10% of whom experience relapse, most often with no evidence of H. pylori reinfection ( 15 , 34 ) . To investigate H. pylori reinfection in gastric lymphoma is usually simple, requiring endoscopic biopsies, breath test, and/or detection of Cag-A in feces. By contrast detection of Cp DNA in patients with relapsed OAL can require a surgical biopsy of orbit, especially in cases in which Cp DNA cannot be detected in PBMC samples. Moreover, although it is well documented that H. pylori reinfection is rare, the modalities by which Chlamydia infection is spreading among humans are less well known. It is possible that patients with OAL are chronically exposed to Chlamydiae and suffer repeated reinfections that may favor lymphoma relapse. If so, it would be useful to assess the efficacy of periodic antibiotic retreatment in patients with Cp -related OAL. It has been suggested ( 35 ) that a similar strategy, in the form of lifelong maintenance administration of tetracycline, could be used to treat patients with immunoproliferative small intestinal disease that is caused by Campylobacter jejuni .
The objective responses observed in patients with Cp -negative OAL may suggest that doxycycline should be used in every OAL patient independently of Cp infection assessment. The activity of doxycycline in OAL patients, without a previous molecular assessment for chlamydial infection, has been tested in two small, retrospective series. In an Austrian study ( 8 ) , none of 11 treated patients experienced lymphoma response at a median follow-up of 9 months. However, the inclusion in that study of some patients previously treated with doxycycline at other institutions and the short median follow-up time may have introduced interpretation biases. Conversely, in an American study ( 7 ) , three patients with OAL have been treated with antibiotic therapy without a previous assessment for chlamydial infection, two of them with doxycycline. Two had complete remissions and one a partial response lasting for 18–42 months ( 7 ) . It is difficult to draw firm conclusions from these small studies in which molecular assessment for Cp infection was lacking. We suggest that, despite the responses we observed in patients with Cp -negative OAL, eradicating antibiotic therapy should always be preceded by Cp infection assessment to avoid confounding conclusions, at least until ample, worldwide cumulative experience is available.
In conclusion, our prospective trial revealed that doxycycline is a fast, safe, and active treatment for OAL, both at initial diagnosis and at relapse. Its excellent safety profile and its activity in elderly and heavily pretreated patients as well as in patients with relapses involving regional lymph nodes or previously irradiated areas make doxycycline a valid therapeutic alternative to conventional chemotherapy and radiotherapy against Chlamydia-related OAL. The finding of objective lymphoma regression in Chlamydia-negative patients suggest the need for the development of more sensitive detection methods and the study of potential associations with other doxycycline-sensitive bacteria. These encouraging findings on the benefits of doxycycline therapy need to be confirmed by other studies before this approach is used outside the context of a controlled trial. Under the sponsorship of the International Extranodal Lymphoma Study Group (IELSG), an international prospective trial with centralized molecular analysis has recently been activated ( 36 ) . The main goals of this trial, named IELSG #27, are to explore the role of antibiotic therapy in OAL, to correlate therapeutic activity with molecular genetic findings, to screen other infectious agents potentially associated with OAL, and to define geographic variations in the distribution of these microorganisms. This prospective examination of bacteria-eradicating therapy may result in safer and more efficient therapeutic options for patients affected by these indolent malignancies.
A. J. M. Ferreri and M. Ponzoni contributed equally to this article.
This work was supported in part by a grant from the Italian Association for Cancer Research (to C. Doglioni and R. Dolcetti). The authors indicated no potential conflicts of interest.
The authors had sole responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decission to submit the manuscript for publication, and the writing of the manuscript.
Preliminary results were presented at the Ninth Annual Meeting of the European Hematology Association, Stockholm, Sweden, on June 2, 2005. Results were also published in abstract form as: Ferreri AJM, Ponzoni M, Guidoboni M, Lettini A, Sacchetti F, Giordano Resti A, et al. Activity of Chlamydia psittaci -eradicating antibiotic therapy in MALT-type lymphomas of the ocular adnexa (OAL): a polymerase chain reaction (PCR)-based prospective study [abstract 270]. Haematologica 2005;6(Suppl 2).
References
Matsuo T, Yoshino T. Long-term follow-up results of observation or radiation for conjunctival malignant lymphoma.
Uno T, Isobe K, Shikama N, Nishikawa A, Oguchi M, Ueno N, et al. Radiotherapy for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: a multiinstitutional, retrospective review of 50 patients.
Sasai K, Yamabe H, Dodo Y, Kashii S, Nagata Y, Hiraoka M. Non-Hodgkin's lymphoma of the ocular adnexa.
Simon GJ, Cheung N, McKelvie P, Fox R, McNab AA. Oral chlorambucil for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue of the orbit.
Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas.
Ferreri AJ, Ponzoni M, Guidoboni M, De Conciliis C, Resti AG, Mazzi B, et al. Regression of ocular adnexal lymphoma after Chlamydia psittaci-eradicating antibiotic therapy.
Abramson DH, Rollins I, Coleman M. Periocular mucosa-associated lymphoid/low grade lymphomas: treatment with antibiotics.
Grünberger B, Hauff W, Lukas J, Wohrer S, Zielinski CC, Streubel B, et al. “Blind” antibiotic treatment targeting Chlamydia is not effective in patients with MALT lymphoma of the ocular adnexa.
Chanudet E, Zhou Y, Bacon C, Wotherspoon A, Muller-Hermelink H, Adam P, et al. Chlamydia psittaci is variably associated with ocular MALT lymphoma in different geographical regions.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997.
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification.
Corpet F. Multiple sequence alignment with hierarchical clustering.
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase.
Zubrod CG, Scheidermann MA, Frei E. Appraisal of methods for the study of chemotherapy in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide.
Zucca E, Roggero E, Delchier JC, Smith PJ, Copie-Bergmann C, Traullé C, et al. Interim evaluation of gastric MALT lymphoma response to antibiotics in the ongoing LY03 randomised cooperative trial of observation vs. chlorambucil after anti-Helicobacter therapy [abstract].
Ferreri AJM, Viale E, Guidoboni M, Giordano Resti A, De Conciliis C, Politi L, et al. Clinical implications of hepatitis C virus infection in MALT-type lymphoma of the ocular adnexa.
Sackmann M, Morgner A, Rudolph B, Neubauer A, Thiede C, Schulz H, et al. Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging. MALT Lymphoma Study Group.
Ruskone-Fourmestraux A, Lavergne A, Aegerter PH, Megraud F, Palazzo L, de Mascarel A, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment.
Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma.
Liu H, Ye H, Dogan A, Ranaldi R, Hamoudi RA, Bearzi I, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10.
Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy.
Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas.
Du MQ, Peng H, Liu H, Hamoudi RA, Diss TC, Willis TG, et al. BCL10 gene mutation in lymphoma.
Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma.
You C, Ryu M, Huh J, Park JO, Kang H, Ahn H, et al. Ocular adnexal lymphoma is highly associated with Clamydia pstittaci [abstract].
Rosado MF, Byrne JG, Ding F, Fields KA, Ruiz P, Dubovy SR, et al. Ocular adnexal lymphoma: a clinicopathological study of a large cohort of patients with no evidence for an association with Chlamydia psittaci.
Vargas RL, Fallone E, Felgar RE, Friedberg JW, Arbini AA, Andersen AA, et al. Is there an association between ocular adnexal lymphoma and infection with Chlamydia psittaci? The University of Rochester experience.
Gracia E, Mazzucchelli L, Frosch L, Bertoni F, Zucca E, Cavalli F. Low prevalence of Chlamydia psittaci infection in ocular adnexal lymphoma from Cuban patients [abstract].
Daibata M, Nemoto Y, Togitani K, Fukushima A, Ueno H, Ouchi K, et al. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients.
Mulder MMS, Heddema ER, Pannekoek Y, Faridpooya K, Oud MECM, Schilder-Tol E, et al. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from the Netherlands.
De Cremoux P, Subtil A, Ferreri AJ, Vincent-Salomon A, Ponzoni M, Chaoui D, et al. Low prevalence of Chlamydia psittaci infection in French patients with ocular adnexal lymphomas.
Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis.
Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma.
Horstmann M, Erttmann R, Winkler K. Relapse of MALT lymphoma associated with Helicobacter pylori after antibiotic treatment.
Celik AF, Pamuk GE, Pamuk ON, Uzunismail H, Oktay E, Dogusoy G. Should we suppress the antigenic stimulus in IPSID for lifelong?
Ferreri AJM, Radford J. A clinico-pathological phase II study with translational elements to investigate the possible infective causes of MALT lymphoma of the ocular adnexa with particular reference to Chlamydia species and the effects of treatment with tetracycline. Available at: http://www.ielsg.org/trialsonfr.html . [Last accessed: May 9,


![Failure-free survival curve for the 27 patients treated with doxycycline. Failure-free survival rates at 12, 24, and 36 months were 81% (95% confidence interval [CI] = 72% to 90%), 66% (95% CI = 54% to 78%), and 66% (95% CI = 54% to 78%), respectively. The numbers of patients at risk at 12, 24, and 36 months were 12, 4, and 2, respectively.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/98/19/10.1093_jnci_djj373/2/m_jncidjj373f02_lw.jpeg?Expires=1716568836&Signature=jJ5L01WjU4lCB8QOvvLN7r6WCFZ65LDBw6wVhf0qkuvWuu6poq0p4QsyseDQ8BnlQf81KDDUxq78~z93URuJ8ch85OisJyTaoiphyLEEDfqXNqZMk4DU7UejvAfNo31MfrRwZWmeZNWeEqbcTqJEhVqqSOOk-5HcNS26p0~ccRnd53oytQ~r668bzW6lmvXhHW7QuZqFWyPLBLvNRWfckkB0rktcvjipTYmz5-~58JhyPLo6kCgSDD~YGIeWnxVsCSElVuw7wIyqQjFZ974S10ArK2DefKGXMx2CHDVEH0UcL3V6NWQKcPRbFOdgF~ZMrG3uCIYgoJwezdu3clwP-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
