-
PDF
- Split View
-
Views
-
Cite
Cite
Jason Q. D. Goodger, Robert E. Sharp, Ellen L. Marsh, Daniel P. Schachtman, Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques, Journal of Experimental Botany, Volume 56, Issue 419, September 2005, Pages 2389–2400, https://doi.org/10.1093/jxb/eri231
Close - Share Icon Share
Abstract
Many different techniques have been used for xylem sap collection, but few direct comparisons of techniques have been conducted and few comparisons have been based on comprehensive analyses of xylem sap. Moreover, the suitability of extraction techniques for use on plants grown under water-stress conditions has not been addressed. Xylem sap was extracted from both well-watered and water-stressed Zea mays plants using three different techniques. The main aim was to determine how the extraction method altered the correlations between sap constituents and stomatal conductance in order to determine which relationships change with extraction technique. A ‘root pressure’ technique was the simplest method of extracting large volumes of sap, but the low sap delivery rates altered the composition of sap. Two pressurization techniques that varied in the position from which sap was collected were tested. The pressurization techniques allowed for the control of delivery rates that influence sap constituent concentrations. The position from which xylem sap was collected on the plant was also found to be important. All three techniques produced consistent correlations between ABA and chloride delivery rates and changes in stomatal conductance, suggesting that each technique could be applied to identify certain putative xylem-borne signals.
This paper is available online free of all access charges (see http://jxb.oupjournals.org/open_access.html for further details)
The online version of this article has been published under an Open Access model. Users are entitled to use, reproduce, disseminate, or display the Open Access version of this article for non-commercial purposes provided that: the original authorship is properly and fully attributed; the Journal and the Society for Experimental Biology are attributed as the original place of publication with the correct citation details given; if an article is subsequently reproduced or disseminated not in its entirety but only in part or as a derivative work this must be clearly indicated. For commercial re-use, please contact: journals.permissions@oupjournals.org
Introduction
Soil water deficit is an important environmental constraint to plant growth and development. Plants typically respond to soil water deficits by closing stomata, slowing leaf expansion, curling leaves, and senescing older leaves. Such stress responses are initiated by chemical and hydraulic signals. Chemical signals result from perturbations in compounds produced by water-stressed roots and received by shoots via the xylem stream (Blackman and Davies, 1985; Gowing et al., 1990; Schurr et al., 1992; Jackson, 1997). Hydraulic signals are produced when shoot water status decreases as a consequence of limited water uptake by roots (Kramer, 1969; Chazen and Neumann, 1994; Comstock and Mencuccini, 1998). In many plant species chemical signals are produced before hydraulic signals and afford plants a form of ‘early warning’ of soil water deficits (Davies et al., 2002). This earlier release of chemical signals may enhance plant water-stress tolerance and has therefore made chemical signalling an attractive target for both agronomic (Davies et al., 2000) and genetic manipulation (Sauter et al., 2001).
Despite work on the identification and characterization of the chemical signals involved in plant water-stress responses, the identity of the chemical signal(s) remains contentious. The production of abscisic acid (ABA) by the roots and its transport to the leaves in the xylem stream has been shown to play a dominant role in the chemical signalling of soil water availability (Zhang and Davies, 1991; Zhu and Zhang, 1997; Sauter et al., 2001). However, a number of studies have also reported unidentified chemical signalling components in xylem sap (Munns, 1992; Munns et al., 1993; Holbrook et al., 2002). Similarly, a diminished or interactive role for ABA has been suggested in a more complex signalling pathway involving conjugated ABA (Dodd and Davies, 1996; Hansen and Dörffling, 1999), pH (Davies and Zhang, 1991; Schurr et al., 1992; Bacon et al., 1998; Wilkinson, 1999), inorganic ions (Schurr et al., 1992) or cytokinins (Bradford, 1983; Blackman and Davies, 1985; Bano et al., 1993, 1994). Alternatively, organic acids such as malate have been suggested to be involved in chemical signalling and stomatal control (Patonnier et al., 1999).
A varied array of techniques has been devised in an attempt to overcome the difficulties inherent in extracting xylem sap (Schurr, 1998), but many of these techniques produce only small volumes of sap (de Carrasco et al., 1994; Schill et al., 1996; Radomiljac et al., 1998; Patonnier et al., 1999; Malone et al., 2002). Techniques that generate much greater volumes of xylem sap often involve the detachment of the shoot, leaving only a short stub of hypocotyl or mesocotyl tissue from which sap exuding out of the roots is collected. Sap is slowly expelled from such stubs by ‘root pressure’ (Noodén et al., 1989). Alternatively, a mostly non-invasive technique that was introduced by Passioura, 1980; Passioura and Tanner, 1985, and modified by Gollan et al., 1992, uses pneumatic pressure applied to roots to force sap exudation from a cut leaf petiole or mid-rib.
Many authors have highlighted the potential problems of particular sap extraction techniques (Schurr, 1998; Tiekstra et al., 2000; Jackson, 2002), but no study has compared extraction methods from well-watered and water-stressed plants using comprehensive data on xylem sap composition. Some sap extraction technique comparisons have been conducted using flooded versus well-drained plants, but those studies only quantified osmolality, calcium, and ABA in the xylem sap (Jokhan et al., 1999; Tiekstra et al., 2000). Because of the importance of root-to-shoot signalling studies for drought tolerance (Davies et al., 2002), it is very important to know how extraction methods potentially affect sap composition. It is also important to provide a comprehensive data set on sap composition, because sap constituents may change independently of each other. A lack of comprehensive analyses may be due in part to the difficulty of xylem sap extraction and the limited sap volumes obtained by some extraction techniques. Two studies on water-stressed plants have provided relatively comprehensive xylem sap composition data sets, but neither study determined how sampling technique altered sap composition (Gollan et al., 1992; Bahrun et al., 2002).
The aim of this study was to extract xylem sap from both well-watered and water-stressed maize plants using three different techniques. A comprehensive analysis of sap composition was then conducted. Two commonly used techniques for sap collection from the mesocotyl of maize were chosen to provide sufficient sap volumes to enable comprehensive analyses of sap constituents from a suitable number of replicate plants simultaneously. In addition, sap was collected from a leaf to compare the sap composition from roots and how it is modified by leaves. The sap composition data were then correlated with changes in stomatal conductance to determine if the observed relationships were altered by the technique of extraction. The method of principal component analysis was also used to study relationships between xylem sap constituents.
Materials and methods
Plant material
Single seeds of corn (Zea mays L. cv. FR697) were sown into large pots (115 mm diameter × 410 mm height, 4.26 dm3 capacity) containing Metro-Mix 702 soil (The Scotts Company, Marysville, USA). Pots were weighed to ensure equivalent soil volumes. Plants were watered daily with an automated watering system until the point at which water dripped from the bottom of the pots. Miracid Professional 21-7-7 Acid Special (The Scotts Company, Marysville, USA) was dissolved in the water to supply nutrients to each pot at a level of 7 mol m−3 ammoniacal and urea nitrogen. In addition, 70 cm3 of 35 mol m−3 ferrous sulphate (QC Corporation, Cape Girardeau, USA) was added by hand to each pot 9 d and 16 d after sowing (DAS). Seeds were sown 8 cm below the surface to allow the development of a long mesocotyl which facilitated sealing of plants into pressure chambers. The elongated mesocotyl that results from deeply sowing maize seeds provides a convenient point for the collection of xylem sap that is flowing from roots, prevents the formation of nodal roots, and facilitates sealing of the mesocotyl into pressure vessels for sap extraction. During the early stages of maize development, plants are unlikely to be adversely affected by a lack of nodal roots (Jeschke et al., 1997). Pots were placed in a dark chamber with a day/night temperature of 26/18 °C. Six DAS the top 7 cm of soil was removed to expose the mesocotyl. Plants were staked to support the mesocotyl and were transferred to a controlled-environment chamber with a day/night temperature of 26/18 °C, RH of 45%, under a photoperiod of 16 h and a light intensity of 815 μmol m−2 s−1. Water was withheld from half of the pots from 17 DAS. Sap was extracted from both well-watered and water-stressed plants 7 d after water was withheld (24 DAS).
Measurement of soil moisture and plant parameters
Soil moisture content (v/v) determinations were carried out daily using a Theta soil-moisture PR-1 profile probe (Delta-T Devices, Cambridge, UK). Prior to seed sowing, a PR-1 access tube was placed in each of 12 pots to enable PR-1 probe insertion without disturbing the plant roots. A replica access tube was placed in the remaining pots. Six plants growing in pots containing PR-1 access tubes were randomly assigned to each treatment. The PR-1 probe was used to determine the soil moisture content at depths of approximately 5, 15, and 25 cm below the soil surface simultaneously.
Ten plants from each treatment were randomly selected for daily leaf measurements at the commencement of water withholding. The length of the expanding sixth leaf from each selected plant was measured at the same time every day and used to determine leaf extension rate (LER; mm d−1). Daily leaf conductance (gs) was measured on the abaxial surface of leaf four (avoiding major veins) for each plant 8 h into the photoperiod using an AP4 steady-state porometer (Delta-T Devices, Cambridge, UK). On the day of sap harvest (24 DAS), the xylem pressure potential of leaf four from each plant was determined 8 h into the photoperiod using a Scholander-type pressure chamber (Soil Moisture Equipment Corp., Santa Barbara, CA, USA). This time-point was chosen because it was the mid-point in xylem sap sampling. The predawn xylem pressure potential of a separate cohort of eight plants from each treatment was also determined 24 DAS.
Whole-plant transpiration for both well-watered and water-stressed plants was determined 24 DAS to estimate the flow rate of sap through the mesocotyl in whole plants. The weight loss over 6 h during the day was determined for 10 plants of the same size (including pots) from each treatment and averaged. The base and surface of pots were covered with plastic to stop evaporation directly from the soil surface. The 6 h period corresponded with the time of sap extraction and the whole-plant transpiration data were used to estimate average sap flow rates through the mesocotyl during this period for use in extraction techniques.
Xylem sap extraction techniques
Three different techniques were used to extract xylem sap from Z. mays plants. Exact volumes of exudates from each technique were determined by weighing. The volume delivery rates were calculated by multiplying the concentration by the flow rate (volume collected over a defined period of time). All sap samples were frozen immediately after sampling and stored at −80 °C until analysed.
In the first technique (abbreviated to Rmctyl), 12 plants from each treatment were detopped by severing the mesocotyl 0.5 cm below the shoot and xylem sap was collected from the cut surface of the mesocotyl under ‘root pressure’ (Schurr and Schulze, 1995). Immediately after detopping, each mesocotyl stump was rinsed with deionized H2O and blotted with absorbent tissue to remove contaminants from cut cells. After discarding approximately 50 mm3 of sap, each cut surface was blotted again and silicon tubing was fitted over the stump. Sap flowing from the tubing was collected in preweighed vials for 45 min.
In the second technique (abbreviated to Pmctyl), 12 plants from each treatment were detopped 0.5 cm below the shoot and each pot containing the roots and exposed mesocotyl was placed in custom-built pressure vessels (CHPT Manufacturing, Delaware, USA). Pots were sealed into vessels using silicon gaskets (Sylgard 170, Dow Corning, Midland, USA) made to fit around the mesocotyl. Three pressure vessels were connected in a series to a pressure controller that operated a cylinder of compressed air. The mesocotyl stumps were rinsed, the initial sap discarded and tubing fitted as in Rmctyl. The rate of exudation from the cut mesocotyl stump was increased by application of pressure to the root (to approximately 0.3 MPa) and sap was collected for 7.5 min. The applied pressure created sap exudation fluxes that were comparable with the estimated rate of whole plant transpiration over the period of extraction.
In the third technique (abbreviated to Pleaf), 12 intact plants from each treatment were sealed in the pressure vessels around the mesocotyl as in Pmctyl, but without detopping the shoots. To facilitate sap extraction, a small wedge of mid-rib (3 mm wide × 3 mm deep) was excised from the abaxial surface of the mid-rib half way along leaf four. The cut region was rinsed with de-ionized H2O and blotted with absorbent tissue to remove contaminants from damaged cells. Pressure was slowly applied to the roots via each pressure vessel until sap exuded from each cut. After discarding approximately the first 50 mm3, sap was collected for 20 min.
Sap analyses
The pH of sap samples was measured with a microelectrode (Model MI-410 Combination pH electrode, Microelectrodes, Inc., Bedford, USA) interfaced with a pH meter (Model 430, Corning, New York, USA) and pH values were converted to [H+]. Sap samples were analysed for sucrose using a test kit (Boehringer Mannheim/R-Biopharm, Darmstadt, Germany) and for ABA using a competitive ELISA (Agdia Phytodetek, Elkhart, USA), with (±) cis-trans ABA (Sigma, St Louis, USA) as the standard. Anions, cations, and organic acids in the xylem sap were determined by isocratic ion chromatography using an ICS-1000 Ion Chromatography System (Dionex Corporation, Sunnyvale, USA). Anions were determined on a Dionex IonPac AS14A analytical column (4×250 mm, 8.0 mM Na2CO3/1.0 mM NaHCO3 eluent), cations were determined on a Dionex IonPac CS12A analytical column (4×250 mm, 20 mM methanesulphonic acid eluent), and organic acids were determined on a Dionex IonPac ICE-AS6 analytical column (9×250 mm, 0.4 mM heptafluorobutyric acid eluent).
Statistical analyses
Statistical analyses on sap constituents and measured plant parameters were conducted using SPSS for Windows (Version 11.5.0; SPSS Inc., Chicago, USA). Sap constituent levels were analysed on both a concentration and a delivery rate basis. Two-way ANOVA (using treatment and technique as factors) or one-way ANOVA were conducted on the data after Shapiro-Wilk's tests for normality and Levene's tests for homogeneity of variance. Two-way ANOVA results were further analysed with Tukey post hoc tests. Data were transformed, where necessary, with log, square, or Box-Cox transformations. Box-Cox transformations were performed using Rundom Box-Cox freeware (Rundom BC version 1.0, http://pjadw.tripod.com/bc.htm). Relationships among normally distributed sap constituent levels were explored with Pearson's correlations.
Principal Components Analyses (PCA) were performed on the sap constituent data (volume delivery rate basis) for each extraction technique separately. Since PCA analysis is intended to reveal common principles in the data (Tausz et al., 1998, 2001), both treatments for each technique were pooled and the data were transformed where necessary to fit a normal distribution. Sulphate and ammonium were excluded from the analyses due to an inability to transform the data to fit a normal distribution. PCA extraction from the correlation matrix was accepted when eigen values were greater than one (Kaiser criterion). Component scores and factor loadings were calculated after Varimax orthogonal rotation with Kaiser normalization. The rotated component scores for components one and two were used to derive 2-dimensional scatter plots of sap constituents.
Results
Characterizing water stress
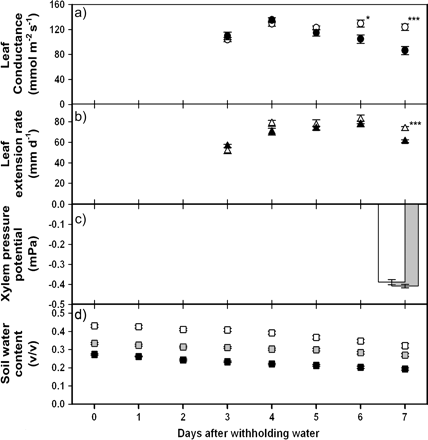
In order to characterize the level of water stress imposed, leaf conductance, leaf extension rate (LER), soil moisture content, and leaf water potential were measured. By days 6 and 7 after withholding water from the water-stressed treatment, both leaf conductance and LER were significantly lower than for the well-watered plants, indicating a physiological response to the drying soil conditions (Fig. 1a, b). At this early stage of water stress, xylem pressure potential remained the same between the treatments 8 h into the photoperiod (Fig. 1c) and also at predawn on the day of sap harvest (data not shown). Under well-watered conditions soil moisture content remained relatively constant over time at approximately 42%, 35%, and 28% for depths 20–30 cm, 10–20 cm, and 0–10 cm below the soil surface, respectively. By contrast, soil moisture in the water-stressed pots declined over time after water was withheld (Fig. 1d). This controlled drought system was developed to study putative root signals that may modulate growth and stomatal conductance in response to drought without the complication of additional hydraulic signals attributable to changes in xylem pressure potential.
Leaf and soil parameters for well-watered (WW) and water-stressed (WS) Zea mays plants. Water was withheld in the WS treatment from day zero onwards and xylem sap was extracted from both WW and WS plants on day 7. (a) Leaf 4 conductance measurements for WW (white symbols) and WS (black symbols) plants. Values are the mean ±SE of 10 plants for each treatment each day. Leaf 4 was not large enough for porometry until 3 d after water was withheld. (b) Leaf 6 extension rate for WW (white symbols) and WS (black symbols) plants. Values are the mean ±SE of 10 plants for each treatment each day. Leaf 6 did not emerge until 2 d after water was withheld. (c) Leaf 4 xylem pressure potential measurements for WW (white bar) and WS (grey bar) plants 7 d after water was withheld. Values are the mean ±SE of 10 plants for each treatment. (d) Volumetric soil water content for pots in which WS plants were grown. Measurements were made using a Theta profile probe at three depths simultaneously each day. Black, grey, and white symbols represent depths of approximately 5, 15, and 25 cm below the soil surface, respectively. Values are presented as the means ±SE of six pots. Significant differences between treatments for (a) and (b) are indicated as * P <0.05; *** P <0.001.
Whole plant water loss was measured over the period of sap extraction. Water loss per plant was used to provide an estimate of the flow rate of xylem sap through the mesocotyl. The mean (±SE) whole plant water loss was 1.5±0.1 mm3 s−1 for the well-watered plants and 1.4±0.1 mm3 s−1 for the water-stressed plants at 24 DAS. The lowest whole plant water loss rate for each treatment was approximately 1.0 mm3 s−1. To minimize artefacts that may be caused by over-pressurizing plants using the Pmctyl technique, pneumatic pressure was applied at 0.3 MPa to induce sap to flow at a mean rate of 1.1 mm3 s−1 for plants from both treatments (Table 1). The measured decreases in leaf conductance and whole plant water loss were quantitatively different and this may be due to differences between measurements based on a small region of leaf four versus the entire shoot or the different time scales involved with each method.
Leaf conductance (gs) and xylem sap constituents for well-watered and water-stressed Zea mays plants
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| gs | mmol m−2 s−1 | 119±4 a | 131±5 a | 129±5 a | 96±5 a | 95±5 a | 104±6 a | ||||
| Sap flow | mm3 s−1 | 0.49±0.06 a | 1.1±0.1 b | 0.44±0.05 a | 0.28±0.02 a | 1.1±0.1 b | 0.40±0.03 c | ||||
| pH | – | 5.3±0.0 a | 5.2±0.0 a | 5.0±0.0 b | 5.2±0.1 a | 5.3±0.1 a | 5.2±0.0 a | ||||
| Proton | mmol m−3 | 5.7±0.5 a | 6.5±0.6 a | 10.7±0.7 b | 6.9±0.8 a | 5.8±0.6 a | 6.2±0.2 a | ||||
| Abscisic acid | μmol m−3 | 4.2±0.5 a | 4.2±0.6 a | 11.5±2.1 b | 17.5±1.9 a | 10.4±1.2 b | 19.5±1.5 a | ||||
| Sucrose | mol m−3 | 0.10±0.01 a | 0.15±0.02 b | 0.08±0.01 a | 0.09±0.01 a | 0.25±0.03 b | 0.19±0.03 b | ||||
| Citric acid | mol m−3 | 0.63±0.03 a | 0.44±0.04 b | 0.25±0.02 c | 0.70±0.03 a | 0.31±0.03 b | 0.22±0.01 b | ||||
| malic acid | mol m−3 | 1.1±0.2 a | 1.1±0.2 a | 0.54±0.09 b | 3.9±0.6 a | 2.6±0.6 ab | 1.3±0.2 b | ||||
| Succinic acid | mol m−3 | 0.16±0.02 a | 0.16±0.03 ab | 0.09±0.01 b | 0.44±0.07 a | 0.32±0.07 ab | 0.15±0.02 b | ||||
| Sodium | mol m−3 | 0.16±0.03 a | 0.16±0.02 a | 0.10±0.03 a | 0.15±0.02 a | 0.14±0.03 a | 0.16±0.08 a | ||||
| Ammonium | mol m−3 | 5.2±0.2 a | 3.8±0.5 b | 1.9±0.2 c | 0.85±0.14 a | 0.42±0.11 b | 0.15±0.04 b | ||||
| Potassium | mol m−3 | 20±1 a | 17±1 b | 8.7±0.5 c | 23±1 a | 19±2 a | 7.8±0.4 b | ||||
| magnesium | mol m−3 | 4.2±0.1 a | 2.9±0.2 b | 1.3±0.1 c | 6.1±0.2 a | 3.0±0.3 b | 1.7±0.1 c | ||||
| Calcium | mol m−3 | 2.1±0.1 a | 1.6±0.1 b | 0.89±0.10 c | 3.5±0.2 a | 1.8±0.2 b | 1.2±0.1 c | ||||
| Chloride | mol m−3 | 27±2 a | 22±2 b | 12±1 c | 13±1 a | 11±1 a | 2.6±0.3 b | ||||
| Nitrate | mol m−3 | 6.0±0.7 a | 5.3±0.7 a | 2.4±0.4 b | 12±2 a | 5.1±1.5 b | 1.7±0.4 b | ||||
| Phosphate | mol m−3 | 5.5±0.2 a | 4.0±0.3 b | 2.6±0.2 c | 6.2±0.6 a | 3.9±0.6 b | 2.2±0.2 c | ||||
| Sulphate | mol m−3 | 2.2±0.1 a | 1.6±0.1 b | 0.98±0.07 c | 6.0±0.4 a | 3.5±0.4 b | 2.2±0.1 c | ||||
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| gs | mmol m−2 s−1 | 119±4 a | 131±5 a | 129±5 a | 96±5 a | 95±5 a | 104±6 a | ||||
| Sap flow | mm3 s−1 | 0.49±0.06 a | 1.1±0.1 b | 0.44±0.05 a | 0.28±0.02 a | 1.1±0.1 b | 0.40±0.03 c | ||||
| pH | – | 5.3±0.0 a | 5.2±0.0 a | 5.0±0.0 b | 5.2±0.1 a | 5.3±0.1 a | 5.2±0.0 a | ||||
| Proton | mmol m−3 | 5.7±0.5 a | 6.5±0.6 a | 10.7±0.7 b | 6.9±0.8 a | 5.8±0.6 a | 6.2±0.2 a | ||||
| Abscisic acid | μmol m−3 | 4.2±0.5 a | 4.2±0.6 a | 11.5±2.1 b | 17.5±1.9 a | 10.4±1.2 b | 19.5±1.5 a | ||||
| Sucrose | mol m−3 | 0.10±0.01 a | 0.15±0.02 b | 0.08±0.01 a | 0.09±0.01 a | 0.25±0.03 b | 0.19±0.03 b | ||||
| Citric acid | mol m−3 | 0.63±0.03 a | 0.44±0.04 b | 0.25±0.02 c | 0.70±0.03 a | 0.31±0.03 b | 0.22±0.01 b | ||||
| malic acid | mol m−3 | 1.1±0.2 a | 1.1±0.2 a | 0.54±0.09 b | 3.9±0.6 a | 2.6±0.6 ab | 1.3±0.2 b | ||||
| Succinic acid | mol m−3 | 0.16±0.02 a | 0.16±0.03 ab | 0.09±0.01 b | 0.44±0.07 a | 0.32±0.07 ab | 0.15±0.02 b | ||||
| Sodium | mol m−3 | 0.16±0.03 a | 0.16±0.02 a | 0.10±0.03 a | 0.15±0.02 a | 0.14±0.03 a | 0.16±0.08 a | ||||
| Ammonium | mol m−3 | 5.2±0.2 a | 3.8±0.5 b | 1.9±0.2 c | 0.85±0.14 a | 0.42±0.11 b | 0.15±0.04 b | ||||
| Potassium | mol m−3 | 20±1 a | 17±1 b | 8.7±0.5 c | 23±1 a | 19±2 a | 7.8±0.4 b | ||||
| magnesium | mol m−3 | 4.2±0.1 a | 2.9±0.2 b | 1.3±0.1 c | 6.1±0.2 a | 3.0±0.3 b | 1.7±0.1 c | ||||
| Calcium | mol m−3 | 2.1±0.1 a | 1.6±0.1 b | 0.89±0.10 c | 3.5±0.2 a | 1.8±0.2 b | 1.2±0.1 c | ||||
| Chloride | mol m−3 | 27±2 a | 22±2 b | 12±1 c | 13±1 a | 11±1 a | 2.6±0.3 b | ||||
| Nitrate | mol m−3 | 6.0±0.7 a | 5.3±0.7 a | 2.4±0.4 b | 12±2 a | 5.1±1.5 b | 1.7±0.4 b | ||||
| Phosphate | mol m−3 | 5.5±0.2 a | 4.0±0.3 b | 2.6±0.2 c | 6.2±0.6 a | 3.9±0.6 b | 2.2±0.2 c | ||||
| Sulphate | mol m−3 | 2.2±0.1 a | 1.6±0.1 b | 0.98±0.07 c | 6.0±0.4 a | 3.5±0.4 b | 2.2±0.1 c | ||||
Xylem sap was extracted using three different techniques: Rmctyl, non-pressurized, mesocotyl; Pmctyl, pressurized, mesocotyl; Pleaf, pressurized, leaf mid-rib technique. Values presented as the mean ±SE molar concentration of 12 independent plants for each treatment extracted by each technique. Different letters represent values that were significantly different (P <0.05) between techniques within each treatment.
Leaf conductance (gs) and xylem sap constituents for well-watered and water-stressed Zea mays plants
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| gs | mmol m−2 s−1 | 119±4 a | 131±5 a | 129±5 a | 96±5 a | 95±5 a | 104±6 a | ||||
| Sap flow | mm3 s−1 | 0.49±0.06 a | 1.1±0.1 b | 0.44±0.05 a | 0.28±0.02 a | 1.1±0.1 b | 0.40±0.03 c | ||||
| pH | – | 5.3±0.0 a | 5.2±0.0 a | 5.0±0.0 b | 5.2±0.1 a | 5.3±0.1 a | 5.2±0.0 a | ||||
| Proton | mmol m−3 | 5.7±0.5 a | 6.5±0.6 a | 10.7±0.7 b | 6.9±0.8 a | 5.8±0.6 a | 6.2±0.2 a | ||||
| Abscisic acid | μmol m−3 | 4.2±0.5 a | 4.2±0.6 a | 11.5±2.1 b | 17.5±1.9 a | 10.4±1.2 b | 19.5±1.5 a | ||||
| Sucrose | mol m−3 | 0.10±0.01 a | 0.15±0.02 b | 0.08±0.01 a | 0.09±0.01 a | 0.25±0.03 b | 0.19±0.03 b | ||||
| Citric acid | mol m−3 | 0.63±0.03 a | 0.44±0.04 b | 0.25±0.02 c | 0.70±0.03 a | 0.31±0.03 b | 0.22±0.01 b | ||||
| malic acid | mol m−3 | 1.1±0.2 a | 1.1±0.2 a | 0.54±0.09 b | 3.9±0.6 a | 2.6±0.6 ab | 1.3±0.2 b | ||||
| Succinic acid | mol m−3 | 0.16±0.02 a | 0.16±0.03 ab | 0.09±0.01 b | 0.44±0.07 a | 0.32±0.07 ab | 0.15±0.02 b | ||||
| Sodium | mol m−3 | 0.16±0.03 a | 0.16±0.02 a | 0.10±0.03 a | 0.15±0.02 a | 0.14±0.03 a | 0.16±0.08 a | ||||
| Ammonium | mol m−3 | 5.2±0.2 a | 3.8±0.5 b | 1.9±0.2 c | 0.85±0.14 a | 0.42±0.11 b | 0.15±0.04 b | ||||
| Potassium | mol m−3 | 20±1 a | 17±1 b | 8.7±0.5 c | 23±1 a | 19±2 a | 7.8±0.4 b | ||||
| magnesium | mol m−3 | 4.2±0.1 a | 2.9±0.2 b | 1.3±0.1 c | 6.1±0.2 a | 3.0±0.3 b | 1.7±0.1 c | ||||
| Calcium | mol m−3 | 2.1±0.1 a | 1.6±0.1 b | 0.89±0.10 c | 3.5±0.2 a | 1.8±0.2 b | 1.2±0.1 c | ||||
| Chloride | mol m−3 | 27±2 a | 22±2 b | 12±1 c | 13±1 a | 11±1 a | 2.6±0.3 b | ||||
| Nitrate | mol m−3 | 6.0±0.7 a | 5.3±0.7 a | 2.4±0.4 b | 12±2 a | 5.1±1.5 b | 1.7±0.4 b | ||||
| Phosphate | mol m−3 | 5.5±0.2 a | 4.0±0.3 b | 2.6±0.2 c | 6.2±0.6 a | 3.9±0.6 b | 2.2±0.2 c | ||||
| Sulphate | mol m−3 | 2.2±0.1 a | 1.6±0.1 b | 0.98±0.07 c | 6.0±0.4 a | 3.5±0.4 b | 2.2±0.1 c | ||||
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| gs | mmol m−2 s−1 | 119±4 a | 131±5 a | 129±5 a | 96±5 a | 95±5 a | 104±6 a | ||||
| Sap flow | mm3 s−1 | 0.49±0.06 a | 1.1±0.1 b | 0.44±0.05 a | 0.28±0.02 a | 1.1±0.1 b | 0.40±0.03 c | ||||
| pH | – | 5.3±0.0 a | 5.2±0.0 a | 5.0±0.0 b | 5.2±0.1 a | 5.3±0.1 a | 5.2±0.0 a | ||||
| Proton | mmol m−3 | 5.7±0.5 a | 6.5±0.6 a | 10.7±0.7 b | 6.9±0.8 a | 5.8±0.6 a | 6.2±0.2 a | ||||
| Abscisic acid | μmol m−3 | 4.2±0.5 a | 4.2±0.6 a | 11.5±2.1 b | 17.5±1.9 a | 10.4±1.2 b | 19.5±1.5 a | ||||
| Sucrose | mol m−3 | 0.10±0.01 a | 0.15±0.02 b | 0.08±0.01 a | 0.09±0.01 a | 0.25±0.03 b | 0.19±0.03 b | ||||
| Citric acid | mol m−3 | 0.63±0.03 a | 0.44±0.04 b | 0.25±0.02 c | 0.70±0.03 a | 0.31±0.03 b | 0.22±0.01 b | ||||
| malic acid | mol m−3 | 1.1±0.2 a | 1.1±0.2 a | 0.54±0.09 b | 3.9±0.6 a | 2.6±0.6 ab | 1.3±0.2 b | ||||
| Succinic acid | mol m−3 | 0.16±0.02 a | 0.16±0.03 ab | 0.09±0.01 b | 0.44±0.07 a | 0.32±0.07 ab | 0.15±0.02 b | ||||
| Sodium | mol m−3 | 0.16±0.03 a | 0.16±0.02 a | 0.10±0.03 a | 0.15±0.02 a | 0.14±0.03 a | 0.16±0.08 a | ||||
| Ammonium | mol m−3 | 5.2±0.2 a | 3.8±0.5 b | 1.9±0.2 c | 0.85±0.14 a | 0.42±0.11 b | 0.15±0.04 b | ||||
| Potassium | mol m−3 | 20±1 a | 17±1 b | 8.7±0.5 c | 23±1 a | 19±2 a | 7.8±0.4 b | ||||
| magnesium | mol m−3 | 4.2±0.1 a | 2.9±0.2 b | 1.3±0.1 c | 6.1±0.2 a | 3.0±0.3 b | 1.7±0.1 c | ||||
| Calcium | mol m−3 | 2.1±0.1 a | 1.6±0.1 b | 0.89±0.10 c | 3.5±0.2 a | 1.8±0.2 b | 1.2±0.1 c | ||||
| Chloride | mol m−3 | 27±2 a | 22±2 b | 12±1 c | 13±1 a | 11±1 a | 2.6±0.3 b | ||||
| Nitrate | mol m−3 | 6.0±0.7 a | 5.3±0.7 a | 2.4±0.4 b | 12±2 a | 5.1±1.5 b | 1.7±0.4 b | ||||
| Phosphate | mol m−3 | 5.5±0.2 a | 4.0±0.3 b | 2.6±0.2 c | 6.2±0.6 a | 3.9±0.6 b | 2.2±0.2 c | ||||
| Sulphate | mol m−3 | 2.2±0.1 a | 1.6±0.1 b | 0.98±0.07 c | 6.0±0.4 a | 3.5±0.4 b | 2.2±0.1 c | ||||
Xylem sap was extracted using three different techniques: Rmctyl, non-pressurized, mesocotyl; Pmctyl, pressurized, mesocotyl; Pleaf, pressurized, leaf mid-rib technique. Values presented as the mean ±SE molar concentration of 12 independent plants for each treatment extracted by each technique. Different letters represent values that were significantly different (P <0.05) between techniques within each treatment.
In the case of the Pleaf technique 0.5 MPa of pressure was used because it was the minimum pressure required to extract sap from leaves. Sap flows from leaves were lower (0.4 mm3 s−1) than flows from the mesocotyl because of the additional resistance imposed by the shoot. Since it is difficult to estimate in planta flow rates in the leaf mid-rib, the flow rates between leaf and mesocotyl should not be directly compared.
Comparison of extraction techniques based on constituent concentration
The stomatal conductance of the plants was not significantly different between extraction techniques (Table 1), but was significantly lower in the water-stressed plants. In general, the concentrations of anions, cations, and organic acids in the sap were significantly different between the three extraction techniques, with Rmctyl>Pmctyl>Pleaf. Compared with the Rmctyl technique, the anion, cation, and organic acid concentrations were, on average, 30% and 50% lower with the Pmctyl technique and 60% and 70% lower with the Pleaf technique under well-watered and water-stressed conditions, respectively (Table 1). Interestingly, ABA concentration did not follow this pattern, with sap collected from leaves containing significantly higher concentrations than sap collected from the mesocotyl under well-watered conditions. Under water-stressed conditions ABA concentrations were higher in sap from leaves and Rmctyl compared with sap from Pmctyl (Table 1). Citric acid, ammonium, potassium, magnesium, chloride, calcium, phosphate, and sulphate concentrations were significantly different between extraction techniques under well-watered conditions. With the exception of potassium and chloride, similar trends were found in water-stressed plants.
The extraction techniques produced different sap flow rates with rates being similar between Rmctyl and Pleaf and 2–4 times higher with the Pmctyl technique (Table 1). In addition, sap flow from the water-stressed plants using the Rmctyl technique was approximately half the rate measured for the well-watered plants using the same technique (Table 1). In many other experiments it was also found that sap flow rates are reduced by water stress when sap is collected using natural root pressure (data not shown). Since it has been suggested that sap flow rates can be important for accurately estimating xylem sap constituents (Else et al., 1994; Tiekstra et al., 2000), sap composition was presented on a volume delivery rate basis.
Delivery rates of constituents with treatment and extraction technique
The largest change that occurred between data sets when concentrations were expressed as delivery rates was in the sap collected from the mesocotyl. On a concentration basis Rmctyl values were generally higher than Pmctyl, but when the data were expressed on a delivery rate basis Pmctyl showed higher delivery rates than Rmctyl for many constituents (Tables 1, 2). For xylem sap collected from the mesocotyl there appears to be a relationship between sap flow and concentrations in sap (lower sap flow produces higher concentrations), an observation consistent with previous findings (Munns and Passioura, 1984). This relationship was not observed when sap flows and concentrations were compared between mesocotyl and leaf collection.
Delivery rate of xylem sap constituents for well-watered and water-stressed Zea mays plants
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| Proton | pmol s−1 | 2.7±0.3 a | 7.1±0.8 b | 4.8±0.7 b | 1.9±0.3 a | 5.8±0.5 b | 2.5±0.2 a | ||||
| Abscisic acid | fmol s−1 | 2.1±0.4 a | 4.7±0.7 b | 4.8±0.9 b | 4.5±0.4 a | 11.4±1.9 b | 7.7±0.7 b | ||||
| Sucrose | nmol s−1 | 0.048±0.006 a | 0.161±0.021 b | 0.035±0.006 a | 0.025±0.004 a | 0.294±0.058 b | 0.076±0.009 c | ||||
| Citric acid | nmol s−1 | 0.31±0.03 a | 0.47±0.03 b | 0.11± 0.01 c | 0.19±0.02 a | 0.33±0.04 b | 0.09±0.01 c | ||||
| Malic acid | nmol s−1 | 0.52±0.09 a | 1.2±0.2 b | 0.24±0.05 c | 1.1±0.2 a/b | 3.0±0.7 a | 0.54±0.10 b | ||||
| Succinic acid | nmol s−1 | 0.081±0.012 a | 0.17±0.03 b | 0.039±0.007 c | 0.12±0.02 a | 0.38±0.09 b | 0.061±0.010 a | ||||
| Sodium | nmol s−1 | 0.081±0.018 a | 0.17±0.03 b | 0.039±0.008 a | 0.044±0.008 a | 0.16±0.04 b | 0.064±0.031 ab | ||||
| Ammonium | nmol s−1 | 2.6±0.3 a | 4.1±0.6 a | 0.86±0.13 b | 0.24±0.05 a | 0.38±0.08 a | 0.059±0.016 b | ||||
| Potassium | nmol s−1 | 9.8±1.0 a | 18±1 b | 3.8±0.5 c | 6.4±0.6 a | 20±2 b | 3.1±0.3 c | ||||
| Magnesium | nmol s−1 | 2.1±0.3 a | 3.1±0.2 b | 0.55±0.08 c | 1.7±0.1 a | 3.0±0.3 b | 0.69±0.05 c | ||||
| Calcium | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.38±0.05 c | 0.95±0.08 a | 1.8±0.2 b | 0.48±0.04 c | ||||
| Chloride | nmol s−1 | 13±1 a | 24±2 b | 5.0±0.6 c | 3.5±0.3 a | 11±1 b | 1.1±0.1 c | ||||
| Nitrate | nmol s−1 | 3.0±0.5 a | 5.9±1.0 a | 1.0±0.2 b | 3.3±0.5 a | 4.1±0.8 a | 0.69±0.18 b | ||||
| Phosphate | nmol s−1 | 2.7±0.3 a | 4.3±0.4 b | 1.1±0.2 c | 1.7±0.2 a | 4.4±0.8 b | 0.90±0.11 c | ||||
| Sulphate | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.42±0.05 c | 1.6±0.1 a | 3.8±0.6 b | 0.88±0.07 c | ||||
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| Proton | pmol s−1 | 2.7±0.3 a | 7.1±0.8 b | 4.8±0.7 b | 1.9±0.3 a | 5.8±0.5 b | 2.5±0.2 a | ||||
| Abscisic acid | fmol s−1 | 2.1±0.4 a | 4.7±0.7 b | 4.8±0.9 b | 4.5±0.4 a | 11.4±1.9 b | 7.7±0.7 b | ||||
| Sucrose | nmol s−1 | 0.048±0.006 a | 0.161±0.021 b | 0.035±0.006 a | 0.025±0.004 a | 0.294±0.058 b | 0.076±0.009 c | ||||
| Citric acid | nmol s−1 | 0.31±0.03 a | 0.47±0.03 b | 0.11± 0.01 c | 0.19±0.02 a | 0.33±0.04 b | 0.09±0.01 c | ||||
| Malic acid | nmol s−1 | 0.52±0.09 a | 1.2±0.2 b | 0.24±0.05 c | 1.1±0.2 a/b | 3.0±0.7 a | 0.54±0.10 b | ||||
| Succinic acid | nmol s−1 | 0.081±0.012 a | 0.17±0.03 b | 0.039±0.007 c | 0.12±0.02 a | 0.38±0.09 b | 0.061±0.010 a | ||||
| Sodium | nmol s−1 | 0.081±0.018 a | 0.17±0.03 b | 0.039±0.008 a | 0.044±0.008 a | 0.16±0.04 b | 0.064±0.031 ab | ||||
| Ammonium | nmol s−1 | 2.6±0.3 a | 4.1±0.6 a | 0.86±0.13 b | 0.24±0.05 a | 0.38±0.08 a | 0.059±0.016 b | ||||
| Potassium | nmol s−1 | 9.8±1.0 a | 18±1 b | 3.8±0.5 c | 6.4±0.6 a | 20±2 b | 3.1±0.3 c | ||||
| Magnesium | nmol s−1 | 2.1±0.3 a | 3.1±0.2 b | 0.55±0.08 c | 1.7±0.1 a | 3.0±0.3 b | 0.69±0.05 c | ||||
| Calcium | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.38±0.05 c | 0.95±0.08 a | 1.8±0.2 b | 0.48±0.04 c | ||||
| Chloride | nmol s−1 | 13±1 a | 24±2 b | 5.0±0.6 c | 3.5±0.3 a | 11±1 b | 1.1±0.1 c | ||||
| Nitrate | nmol s−1 | 3.0±0.5 a | 5.9±1.0 a | 1.0±0.2 b | 3.3±0.5 a | 4.1±0.8 a | 0.69±0.18 b | ||||
| Phosphate | nmol s−1 | 2.7±0.3 a | 4.3±0.4 b | 1.1±0.2 c | 1.7±0.2 a | 4.4±0.8 b | 0.90±0.11 c | ||||
| Sulphate | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.42±0.05 c | 1.6±0.1 a | 3.8±0.6 b | 0.88±0.07 c | ||||
Values are presented as the mean rate ±SE of 12 independent plants for each treatment extracted by each technique. Different letters represent values that were significantly different (P <0.05) between techniques within each treatment.
Delivery rate of xylem sap constituents for well-watered and water-stressed Zea mays plants
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| Proton | pmol s−1 | 2.7±0.3 a | 7.1±0.8 b | 4.8±0.7 b | 1.9±0.3 a | 5.8±0.5 b | 2.5±0.2 a | ||||
| Abscisic acid | fmol s−1 | 2.1±0.4 a | 4.7±0.7 b | 4.8±0.9 b | 4.5±0.4 a | 11.4±1.9 b | 7.7±0.7 b | ||||
| Sucrose | nmol s−1 | 0.048±0.006 a | 0.161±0.021 b | 0.035±0.006 a | 0.025±0.004 a | 0.294±0.058 b | 0.076±0.009 c | ||||
| Citric acid | nmol s−1 | 0.31±0.03 a | 0.47±0.03 b | 0.11± 0.01 c | 0.19±0.02 a | 0.33±0.04 b | 0.09±0.01 c | ||||
| Malic acid | nmol s−1 | 0.52±0.09 a | 1.2±0.2 b | 0.24±0.05 c | 1.1±0.2 a/b | 3.0±0.7 a | 0.54±0.10 b | ||||
| Succinic acid | nmol s−1 | 0.081±0.012 a | 0.17±0.03 b | 0.039±0.007 c | 0.12±0.02 a | 0.38±0.09 b | 0.061±0.010 a | ||||
| Sodium | nmol s−1 | 0.081±0.018 a | 0.17±0.03 b | 0.039±0.008 a | 0.044±0.008 a | 0.16±0.04 b | 0.064±0.031 ab | ||||
| Ammonium | nmol s−1 | 2.6±0.3 a | 4.1±0.6 a | 0.86±0.13 b | 0.24±0.05 a | 0.38±0.08 a | 0.059±0.016 b | ||||
| Potassium | nmol s−1 | 9.8±1.0 a | 18±1 b | 3.8±0.5 c | 6.4±0.6 a | 20±2 b | 3.1±0.3 c | ||||
| Magnesium | nmol s−1 | 2.1±0.3 a | 3.1±0.2 b | 0.55±0.08 c | 1.7±0.1 a | 3.0±0.3 b | 0.69±0.05 c | ||||
| Calcium | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.38±0.05 c | 0.95±0.08 a | 1.8±0.2 b | 0.48±0.04 c | ||||
| Chloride | nmol s−1 | 13±1 a | 24±2 b | 5.0±0.6 c | 3.5±0.3 a | 11±1 b | 1.1±0.1 c | ||||
| Nitrate | nmol s−1 | 3.0±0.5 a | 5.9±1.0 a | 1.0±0.2 b | 3.3±0.5 a | 4.1±0.8 a | 0.69±0.18 b | ||||
| Phosphate | nmol s−1 | 2.7±0.3 a | 4.3±0.4 b | 1.1±0.2 c | 1.7±0.2 a | 4.4±0.8 b | 0.90±0.11 c | ||||
| Sulphate | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.42±0.05 c | 1.6±0.1 a | 3.8±0.6 b | 0.88±0.07 c | ||||
Parameter . | Unit . | Well-watered . | . | . | Water-stressed . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Rmctyl . | Pmctyl . | Pleaf . | Rmctyl . | Pmctyl . | Pleaf . | ||||
| Proton | pmol s−1 | 2.7±0.3 a | 7.1±0.8 b | 4.8±0.7 b | 1.9±0.3 a | 5.8±0.5 b | 2.5±0.2 a | ||||
| Abscisic acid | fmol s−1 | 2.1±0.4 a | 4.7±0.7 b | 4.8±0.9 b | 4.5±0.4 a | 11.4±1.9 b | 7.7±0.7 b | ||||
| Sucrose | nmol s−1 | 0.048±0.006 a | 0.161±0.021 b | 0.035±0.006 a | 0.025±0.004 a | 0.294±0.058 b | 0.076±0.009 c | ||||
| Citric acid | nmol s−1 | 0.31±0.03 a | 0.47±0.03 b | 0.11± 0.01 c | 0.19±0.02 a | 0.33±0.04 b | 0.09±0.01 c | ||||
| Malic acid | nmol s−1 | 0.52±0.09 a | 1.2±0.2 b | 0.24±0.05 c | 1.1±0.2 a/b | 3.0±0.7 a | 0.54±0.10 b | ||||
| Succinic acid | nmol s−1 | 0.081±0.012 a | 0.17±0.03 b | 0.039±0.007 c | 0.12±0.02 a | 0.38±0.09 b | 0.061±0.010 a | ||||
| Sodium | nmol s−1 | 0.081±0.018 a | 0.17±0.03 b | 0.039±0.008 a | 0.044±0.008 a | 0.16±0.04 b | 0.064±0.031 ab | ||||
| Ammonium | nmol s−1 | 2.6±0.3 a | 4.1±0.6 a | 0.86±0.13 b | 0.24±0.05 a | 0.38±0.08 a | 0.059±0.016 b | ||||
| Potassium | nmol s−1 | 9.8±1.0 a | 18±1 b | 3.8±0.5 c | 6.4±0.6 a | 20±2 b | 3.1±0.3 c | ||||
| Magnesium | nmol s−1 | 2.1±0.3 a | 3.1±0.2 b | 0.55±0.08 c | 1.7±0.1 a | 3.0±0.3 b | 0.69±0.05 c | ||||
| Calcium | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.38±0.05 c | 0.95±0.08 a | 1.8±0.2 b | 0.48±0.04 c | ||||
| Chloride | nmol s−1 | 13±1 a | 24±2 b | 5.0±0.6 c | 3.5±0.3 a | 11±1 b | 1.1±0.1 c | ||||
| Nitrate | nmol s−1 | 3.0±0.5 a | 5.9±1.0 a | 1.0±0.2 b | 3.3±0.5 a | 4.1±0.8 a | 0.69±0.18 b | ||||
| Phosphate | nmol s−1 | 2.7±0.3 a | 4.3±0.4 b | 1.1±0.2 c | 1.7±0.2 a | 4.4±0.8 b | 0.90±0.11 c | ||||
| Sulphate | nmol s−1 | 1.0±0.1 a | 1.7±0.1 b | 0.42±0.05 c | 1.6±0.1 a | 3.8±0.6 b | 0.88±0.07 c | ||||
Values are presented as the mean rate ±SE of 12 independent plants for each treatment extracted by each technique. Different letters represent values that were significantly different (P <0.05) between techniques within each treatment.
It was examined whether the compositional changes in xylem sap observed between the treatments were influenced by extraction technique. On a concentration basis, few consistent patterns between the techniques could be detected for plants grown under either treatment. However, when data were presented on a delivery rate basis (Table 2), more consistencies became apparent. For example, consistent patterns across treatments could not be observed for concentrations of ABA, citric acid, ammonium, potassium, chloride, and nitrate (Table 1), but could be observed when data were presented on a delivery rate basis (Table 2; Fig. 3).
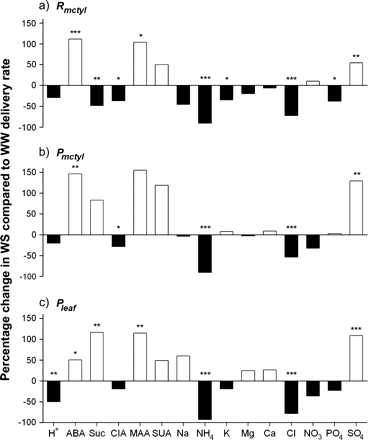
On a delivery rate basis, ABA and sulphate in xylem sap were significantly increased for water-stressed plants, irrespective of extraction technique, and ammonium and chloride were significantly decreased (Fig. 2). The delivery rate of protons in the sap extracted from water-stressed plants using the Pleaf technique was significantly lower than that extracted from well-watered plants. Malic acid showed a significant increase in water-stressed plants using the Rmctyl or Pleaf technique, whereas citric acid showed a significant decrease in the water-stressed plants using either the Rmctyl or Pmctyl techniques (Fig. 2).
Percentage change in sap constituent delivery rate for water-stressed (WS) compared with well-watered (WW) Zea mays plants. Xylem sap was extracted using (a) Rmctyl, the non-pressurized, mesocotyl technique; (b) Pmctyl, the pressurized, mesocotyl technique, and (c) Pleaf, the pressurized, leaf mid-rib technique. White and black bars represent increases and decreases in sap constituent delivery rate under water-stress, respectively. ABA, abscisic acid; Suc, sucrose; CIA, citric acid; MAA, malic acid; SUA, succinic acid; H+, proton; Na, sodium; K, potassium; NH4, ammonium; Mg, magnesium; Ca, calcium; NO3, nitrate; Cl, chloride; SO4, sulphate; PO4, phosphate. One-way ANOVA was performed on the mean delivery rate data and significant differences between treatments for each technique are indicated as * P <0.05; ** P <0.010; *** P <0.001.
The trends across extraction techniques were not consistent for sucrose, sodium, potassium, and nitrate. Sucrose significantly decreased in the water-stressed plants with the Rmctyl technique, but increased significantly in the Pleaf technique (Fig. 2). Preliminary studies suggest that the sucrose in sap from pressurized plants was due to discarding an insufficient volume of the initial exudates. Potassium delivery rates were significantly decreased in sap extracted with the Rmctyl technique, but did not change in sap extracted with the other techniques. Phosphate delivery rates were significantly lower in water-stressed plants extracted using the Rmctyl technique only.
Constituent delivery rate and relationship to leaf conductance
To examine if extraction technique influenced the relationships observed between xylem sap constituents and stomatal conductance, Pearson correlations were performed between the delivery rate of the sap constituents and leaf conductance. To derive these correlations the data from well-watered and water-stressed plants were combined (Table 3). Table 3 shows that, with all extraction techniques, significant negative correlations were observed between leaf conductance and ABA and significant positive correlations were detected between leaf conductance and chloride (Table 3).
Results of correlations between leaf conductance (gs) and delivery rate of xylem sap constituents
Constituent . | Rmctyl . | Pmctyl . | Pleaf . |
|---|---|---|---|
| Proton | +0.41* | +0.36 | +0.42* |
| Abscisic acid | −0.42* | −0.55** | −0.47* |
| Sucrose | +0.19 | −0.33 | n/n |
| Citric acid | +0.33 | +0.26 | +0.20 |
| Malic acid | −0.47* | −0.38 | −0.16 |
| Succinic acid | −0.39 | −0.31 | −0.06 |
| Sodium | +0.25 | +0.39 | +0.08 |
| Ammonium | n/n | n/n | +0.68*** |
| Potassium | +0.39 | −0.14 | +0.21 |
| Magnesium | +0.27 | +0.12 | −0.25 |
| Calcium | +0.23 | −0.06 | −0.21 |
| Chloride | +0.53** | +0.59** | +0.59** |
| Nitrate | +0.15 | +0.43* | +0.27 |
| Phosphate | +0.29 | −0.12 | +0.22 |
| Sulphate | −0.34 | −0.52** | −0.37 |
Constituent . | Rmctyl . | Pmctyl . | Pleaf . |
|---|---|---|---|
| Proton | +0.41* | +0.36 | +0.42* |
| Abscisic acid | −0.42* | −0.55** | −0.47* |
| Sucrose | +0.19 | −0.33 | n/n |
| Citric acid | +0.33 | +0.26 | +0.20 |
| Malic acid | −0.47* | −0.38 | −0.16 |
| Succinic acid | −0.39 | −0.31 | −0.06 |
| Sodium | +0.25 | +0.39 | +0.08 |
| Ammonium | n/n | n/n | +0.68*** |
| Potassium | +0.39 | −0.14 | +0.21 |
| Magnesium | +0.27 | +0.12 | −0.25 |
| Calcium | +0.23 | −0.06 | −0.21 |
| Chloride | +0.53** | +0.59** | +0.59** |
| Nitrate | +0.15 | +0.43* | +0.27 |
| Phosphate | +0.29 | −0.12 | +0.22 |
| Sulphate | −0.34 | −0.52** | −0.37 |
The sap constituent delivery rates were correlated with gs for each extraction technique after combining the normalized data from both treatments as detailed in the Materials and methods. Significant Pearson's correlation coefficients are in bold type with significance level denoted by * P <0.05; ** P <0.010; *** P <0.001. Constituents with non-normal distributions (n/n) were excluded from the analysis.
Results of correlations between leaf conductance (gs) and delivery rate of xylem sap constituents
Constituent . | Rmctyl . | Pmctyl . | Pleaf . |
|---|---|---|---|
| Proton | +0.41* | +0.36 | +0.42* |
| Abscisic acid | −0.42* | −0.55** | −0.47* |
| Sucrose | +0.19 | −0.33 | n/n |
| Citric acid | +0.33 | +0.26 | +0.20 |
| Malic acid | −0.47* | −0.38 | −0.16 |
| Succinic acid | −0.39 | −0.31 | −0.06 |
| Sodium | +0.25 | +0.39 | +0.08 |
| Ammonium | n/n | n/n | +0.68*** |
| Potassium | +0.39 | −0.14 | +0.21 |
| Magnesium | +0.27 | +0.12 | −0.25 |
| Calcium | +0.23 | −0.06 | −0.21 |
| Chloride | +0.53** | +0.59** | +0.59** |
| Nitrate | +0.15 | +0.43* | +0.27 |
| Phosphate | +0.29 | −0.12 | +0.22 |
| Sulphate | −0.34 | −0.52** | −0.37 |
Constituent . | Rmctyl . | Pmctyl . | Pleaf . |
|---|---|---|---|
| Proton | +0.41* | +0.36 | +0.42* |
| Abscisic acid | −0.42* | −0.55** | −0.47* |
| Sucrose | +0.19 | −0.33 | n/n |
| Citric acid | +0.33 | +0.26 | +0.20 |
| Malic acid | −0.47* | −0.38 | −0.16 |
| Succinic acid | −0.39 | −0.31 | −0.06 |
| Sodium | +0.25 | +0.39 | +0.08 |
| Ammonium | n/n | n/n | +0.68*** |
| Potassium | +0.39 | −0.14 | +0.21 |
| Magnesium | +0.27 | +0.12 | −0.25 |
| Calcium | +0.23 | −0.06 | −0.21 |
| Chloride | +0.53** | +0.59** | +0.59** |
| Nitrate | +0.15 | +0.43* | +0.27 |
| Phosphate | +0.29 | −0.12 | +0.22 |
| Sulphate | −0.34 | −0.52** | −0.37 |
The sap constituent delivery rates were correlated with gs for each extraction technique after combining the normalized data from both treatments as detailed in the Materials and methods. Significant Pearson's correlation coefficients are in bold type with significance level denoted by * P <0.05; ** P <0.010; *** P <0.001. Constituents with non-normal distributions (n/n) were excluded from the analysis.
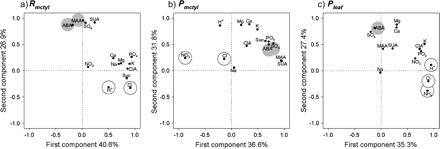
To help visualize possible relationships among the delivery rate of sap constituents and between the three extraction techniques, Principal Components Analysis (PCA) was used to divide the total variance of the sap constituents into a limited number of uncorrelated new variables. In PCA, the first principal component (PC) summarizes most of the variability present in the original data relative to all remaining PCs. Subsequent PCs explain most of the variability not summarized by, and uncorrelated with, previous PCs (Jolliffe, 2002). For clarity and ease of visualization, the first two PCs were chosen to illustrate similarities and differences between the extraction techniques (Fig. 3). The first and second PCs accounted for 67.5%, 68.4%, and 62.7% of the variability in the delivery rate of sap constituents for the Rmctyl, Pmctyl, and Pleaf techniques, respectively. The sap constituents associated with the derived PCs differed between the mesocotyl techniques and the Pleaf technique. This result may have been expected since sampling was done from a completely different part of the plant.
Distribution of Zea mays xylem sap constituents as a function of components 1 and 2 resulting from Principal Components Analyses performed on the matrix of correlations among the constituents for each extraction technique. Sap constituents were analysed on a delivery rate basis for each technique after combining the normalized data from both treatments. Xylem sap was extracted using (a) Rmctyl, the non-pressurized, mesocotyl technique; (b) Pmctyl, the pressurized, mesocotyl technique, and (c) Pleaf, the pressurized, leaf mid-rib technique. See Fig. 2 caption for abbreviations. Significant positive and negative correlations between sap constituents and leaf conductance (from Table 3) are designated by white and grey circles, respectively.
Despite the observed differences between sampling techniques, some consistent relationships were observed. For example, a close relationship was found between ABA and sulphate. Similarly, close associations between calcium and magnesium were observed in all extraction methods. The two organic acids, malic and succinic, were also related in all extraction techniques. For the Rmctyl and Pleaf techniques, ABA and sulphate were marked by high positive loadings with the second PC, but in the Pmctyl they were positively correlated with the first PC. For the case of constituents that were positively correlated to stomatal conductance in Table 3, the PCA grouped together ammonium, chloride, and protons in the case of the Pleaf technique and chloride and protons with the Rmctyl technique. The two variables that are positively related to stomatal conductance for the Pmctyl technique were nitrate and chloride, but these do not appear to be closely related according to the PCA.
Discussion
An important physiological response of plants to soil drying is a decrease in leaf conductance to water. This response to water stress may be regulated by chemical signals borne in the xylem stream (Davies and Zhang, 1991) or by changes in leaf water status (Zhang et al., 1987; Gowing et al., 1990). Xylem-borne signals provide shoots with a measure of water availability as sensed by roots and this root-to-shoot signal enables plants to regulate leaf conductance accordingly (Loveys, 1984; Davies et al., 2002). In fact, much evidence supports the regulation of stomatal behaviour under conditions of water stress by xylem-borne chemical signals (Blackman and Davies, 1985; Gollan et al., 1986; Zhang et al., 1987; Trejo and Davies, 1991; Davies et al., 1994, 2002; Holbrook et al., 2002). The identification of chemical signals in xylem sap is complicated by problems associated with the different techniques of xylem sap extraction. Previous studies have not comprehensively determined how the xylem sap composition varies with extraction techniques (Schurr, 1998). Therefore, Z. mays plants were used as a model to examine different techniques of xylem sap extraction and to investigate the utility of each technique in identifying potential chemical regulators of stomatal behaviour under water stress.
In this study, xylem sap collected using three different techniques was examined because it has been shown that the technique of extraction can alter sap constituent concentrations (Berger et al., 1994). Such comparisons between xylem sap extraction methods have not been reported on water-stressed plants. These results show that there were significant concentration differences between the different extraction techniques with the Rmctyl technique having the highest constituent concentrations. Xylem sap collected with the Rmctyl technique flowed at about one-third the rate of sap collected from the mesocotyl with pressure. The low volume delivery rates were likely to have caused this concentrating effect (Else et al., 1995; Schurr and Schulze, 1995) because the removal of the shoot stops transpirational water movement and results in a lower delivery rate of water through the detached root system (Schurr, 1998). The sap constituents become concentrated relative to the situation in an intact plant (Else et al., 1995; Schurr and Schulze, 1995; Schurr, 1998). This concentrating effect of sap constituents of the Rmctyl technique appears more pronounced under conditions of water stress, possibly due to reduced water availability (Tables 1, 2). In addition to the concentrating effect, some maize lines do not exude sap with the Rmctyl technique (JQD Goodger, DP Schachtman, unpublished observations).
To ensure that the composition of xylem sap collected from detopped plants with the Pmctyl technique accurately reflected what was flowing in the intact plants, pressure was applied to induce sap flow at a rate that approximated the average whole plant water loss over the extraction period. The choice was made to match the lower range of whole plant water loss to minimize possible artefacts associated with over-pressurizing, because pressures that induce flows faster than what is measured in an intact transpiring plant can lead to dilution of the concentration of sap constituents such as nitrate and ABA (Jackson, 1993; Else et al., 1995) or total osmolality (Schurr and Schulze, 1995). The pressure required to exude sap at the lower range of whole plant transpiration was relatively similar to the opposite of the measured xylem pressure potential 8 h into the photoperiod (approximately −0.4 MPa). The pressurization of roots to a positive value equal to the negative of leaf water potential or xylem pressure potential has been promoted as a good guide for approximating sap flow rates (Neuman and Smit, 1991; Shashidhar et al., 1996; Liang and Zhang, 1997). However others (Tiekstra et al., 2000) found that when roots were pressurized to leaf water potential in tomato and maize, sap flows were up to twice as fast as whole plant transpiration in both well-drained and flooded plants. The reason for the different flow rates of xylem sap between the studies at a given pressure may be due to different soil, temperature or lighting conditions used in the studies as well as differences in plant age and size. Nevertheless, these results suggest that xylem pressure potential may be a satisfactory basis for selecting the pneumatic pressures applied to collect xylem sap from detopped maize plants. However, the rate of whole plant water loss should be verified under each experimental condition to be confident of accurately estimating sap flow rates with pressure.
Presentation of data in the form of the volume delivery rates of compounds in the sap appears to be important, especially when comparing sap extracted from well-watered and water-stressed plants, because a decrease in the rate of water loss from water-stressed plants can concentrate a given sap constituent. This is particularly important to consider when using the Rmctyl technique and for severely water-stressed plants. For this reason others have defined a quantitative characteristic of a xylem-borne signal as a change in the rate at which a constituent is delivered in the sap rather than a change in concentration (Neumann and Stein, 1984; Schurr, 1998; Tiekstra et al., 2000; Jackson, 2002). The relationship between sap constituent delivery rates and leaf conductance in the different treatments was generally consistent for many constituents, irrespective of extraction technique, provided that data were presented as delivery rates. This analysis confirms that delivery rates are a preferred form of presenting sap constituents.
These results suggest that delivery rates are useful for identifying correlations between sap constituents and stomatal conductance, but they do not provide mechanistic insights into how a particular constituent alters stomatal conductance. For example, in the case of ABA, delivery rates have been shown to influence stomatal aperture in epidermal strips. However, ABA-metabolism in the leaves of some plant species modifies concentrations so that delivery rates of ABA in intact leaves are less important than the apoplastic concentrations (Gowing et al., 1990; Trejo et al., 1995). ABA arriving from roots at the early stages of water deficit reduces stomatal conductance due to increased apoplastic ABA concentrations and not increased guard cell symplastic concentrations in Vicia faba (Zhang and Outlaw, 2001). It is relevant in this context to note that the correlations found between stomatal conductance and ABA in this study were significant for both delivery rates and concentrations (not shown).
The comparison of the mesocotyl collection techniques with the Pleaf collection technique examined if sap constituents varied due to collection position. The concentration and delivery rates of many constituents differed when sap was sampled from the mesocotyl compared with the leaf. These results are in agreement with a previous study that showed that calcium concentrations and total osmolarity decrease as xylem sap is transported upwards through the shoot (Tiekstra et al., 2000). In contrast to the other sap constituents, ABA was found in higher quantities in sap sampled from the leaves. This result differs with a previous finding where ABA has been shown to decrease as sap moved from the shoot base to a leaf nearer the shoot apex in Ricinus communis (Jokhan et al., 1999). Leaf extraction is a good way of assessing what is arriving at various positions in the canopy; however, this technique does not give an accurate measurement of what is emerging from the roots since depletion and/or enrichment can occur in transit from the shoot base to leaves higher in the canopy (Jackson, 1994). One potential problem with the leaf sampling methods used in this study was that sap was expressed exclusively from the mid-rib of leaves, and it has been reported that sap in major veins, minor veins, and the symplast of leaves can have very different solute concentrations (Jachetta et al., 1986). Although some have suggested that measurements of putative signals should be made as near as possible to their source at the root and again close to the target sites at the shoot (Tiekstra et al., 2000), many consistent trends in correlations were found between xylem sap sourced from roots or leaves and changes in stomatal conductance.
The results presented in this report support previous findings that have shown that ABA is involved in the chemical signalling of water stress and acts to decrease stomatal conductance in maize (Zhang and Davies, 1991; Zhu and Zhang, 1997). However, the comprehensive analysis that was carried out on xylem sap constituents also suggests the possible involvement of other sap constituents with stomatal behaviour. It appears that xylem sap malic acid may be associated with stomatal closure. This organic acid has previously been shown to be involved directly in the regulation of stomatal closure (Hedrich et al., 1994; Patonnier et al., 1999) and indirectly through sap pH increases via possible calcium-malate and magnesium–malate complexes in the xylem sap (Schell, 1997). Another sap component associated with stomatal conductance in two extraction methods was proton concentration, which is a well-known factor influencing stomatal behaviour (Wilkinson, 1999). However, in these experiments it was found that, although proton concentration was positively correlated with stomatal conductance, the magnitude of pH changes in the sap was much smaller than others have observed (see references in Wilkinson and Davies, 2002). Another factor correlated with stomatal conductance, consistent in all extraction techniques, was chloride. Chloride has not previously been suggested to be important in root-to-shoot signalling, but is a factor in guard cell function (Schroeder et al., 2001). Less consistently significant relationships between extraction techniques were found for sulphate and nitrate. Nitrate has been suggested to be involved in chemical signalling in field-grown maize subjected to water stress (Bahrun et al., 2002). It is notable that a strong positive correlation was found between ammonium in leaf sap and stomatal conductance, and qualitatively there also appeared to be this same relationship with the other techniques. In these studies, ammonium concentrations and delivery rates were drastically decreased by drought. Although there is little evidence for a role of ammonium as a signalling molecule (Coruzzi and Bush, 2001), this study's data confirm that ammonium is transported via the xylem in maize (Schjoerring et al., 2002). Decreased ammonium concentrations may be a result of increased assimilation in roots or may be due to decreased ammonium availability in the drying soil. Since the measured pH changes due to water deficit were so small, it is unlikely that ammonium concentrations in sap influenced pH. This study has provided support for previously known signals in the xylem sap and the suggestion of additional signals. Further work will be needed to determine if chloride, malate, ammonium, and sulphate directly influence stomatal conductance or are merely correlated.
The results of this comprehensive analysis are in contrast to a previous analysis of xylem sap composition and stomatal conductance under drought. In that study none of the xylem sap components from sunflower were correlated directly with decreased stomatal conductance induced by soil water deficit (Gollan et al., 1992). The lack of significant correlations found in the study on sunflower was attributed to variation in individual plant responses because of variation in the ionic composition of the sap (Schurr et al., 1992). Only when individual plant sensitivity to ABA was analysed could correlations to nitrate, calcium, and pH be identified (Schurr et al., 1992). Although nitrate was, in some cases, correlated with stomatal conductance in this study, the concentrations measured in sap were very low compared with other reports (Schurr et al., 1992) due to nitrogen being supplied as ammonium and urea.
The Rmctyl technique has the advantage of being a relatively simple means of extracting large volumes of sap, but it does not provide a quantitative estimate of sap constituent delivery rates. Although the Rmctyl technique was easy to use, it failed to extract sap from certain maize genotypes that had been used for other studies, which makes the method unreliable. The reliability of the Rmctyl method is also likely to be reduced when plants are subjected to greater degrees of water stress and, in such studies, pressurization is likely to be required to extract sap. In general, pressurization techniques have the advantage of enabling the accurate estimation of the delivery rates of most sap constituents if pressure is applied to match whole plant transpiration. Pressurization also enables the extraction of sufficient volumes of sap from leaves to determine whether sap constituent modification occurs en route from the roots to the leaves.
In conclusion, it was found that all methods tested provided adequate amounts of sap for comprehensive analyses with the maize genotype used in this study. It was also found that ABA and chloride were consistently correlated with changes in stomatal conductance across all methods, whereas nitrate, sulphate, ammonium, malic acid, and protons were significantly correlated only using particular extraction method(s) or at one collection point. While it cannot be concluded that one technique should not be used for xylem sap collection, the higher concentration of substances in sap collected using the Rmctyl method, the lack of control over exudation rates, and the unreliable nature of that technique suggest that pressurization methods should be used in preference to root pressure methods when pressurization vessels are available.
Abbreviations: ABA, abscisic acid; CIA, citric acid; DAS, days after sowing seed; gs, stomatal conductance to water; LER, leaf extension rate; MAA, malic acid; PC, principal component; PCA, principal component analysis; Pleaf, xylem sap extracted from a leaf mid-rib with applied external pressure; Pmctyl, xylem sap extracted from the mesocotyl with applied external pressure; Rmctyl, xylem sap extracted from the mesocotyl under ‘root pressure’; SUA, succinic acid.
This research was funded by the NSF Genome Program (DBI- DBI-0211842). Thanks to Christine Ehret for assistance with manuscript editing.
References
Bacon MA, Wilkinson S, Davies WJ.
Bahrun A, Jensen CR, Asch F, Mogensen VO.
Bano A, Dörffling K, Bettin D, Hahn H.
Bano A, Hansen H, Dörffling K, Hahn H.
Berger A, Oren R, Schulze E-D.
Blackman PG, Davies WJ.
Bradford KJ.
Chazen O, Neumann PM.
Comstock JP, Mencuccini M.
Coruzzi G, Bush DR.
Davies WJ, Bacon MA, Thompson SD, Sobeih W, Rodríguez LG.
Davies WJ, Tardieu F, Trejo CL.
Davies WJ, Wilkinson S, Loveys BR.
Davies WJ, Zhang J.
de Carrasco CG, Guzman M, Lorente FA, Urrestarazu M.
Dodd IC, Davies WJ.
Else MA, Davies WJ, Whitford PN, Hall KC, Jackson MB.
Else MA, Hall KC, Arnold GM, Davies WJ, Jackson MB.
Gollan T, Passioura JB, Munns R.
Gollan T, Schurr U, Schulze E-D.
Gowing DJG, Davies WJ, Jones HG.
Hansen H, Dörffling K.
Hedrich R, Marten I, Lohse G, Dietrich P, Winter H, Lohaus G, Heldt H-W.
Holbrook NM, Shashidhar VR, James RA, Munns R.
Jachetta JJ, Appleby AP, Boersma L.
Jackson MB.
Jackson MB.
Jackson MB.
Jackson MB.
Jeschke WD, Holobradá M, Hartung W.
Jokhan AD, Harink RJ, Jackson MB.
Liang J, Zhang J.
Loveys BR.
Malone M, Herron M, Morales M-A.
Munns R.
Munns R, Passioura JB.
Munns R, Passioura JB, Milborrow BV, James RA, Close TJ.
Neuman DS, Smit BA.
Neumann PM, Stein Z.
Noodén LD, Singh S, Letham S.
Passioura JB.
Passioura JB, Tanner CB.
Patonnier M, Peltier J, Marigo G.
Radomiljac AM, McComb JA, Pate JS, Tennakoon KU.
Sauter A, Davies WJ, Hartung W.
Schell J.
Schill V, Hartung W, Orthen B, Weisenseel MH.
Schjoerring JK, Husted S, Mack G, Mattsson M.
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D.
Schurr U.
Schurr U, Gollan T, Schulze E-D.
Schurr U, Schulze E-D.
Shashidhar VR, Prasad TG, Sudharshan L.
Tausz M, Bytnerowicz A, Arbaugh MJ, Wonisch A, Grill D.
Tausz M, Soledad Jiménez M, Grille D.
Tiekstra AE, Else MA, Jackson MB.
Trejo CL, Clephan AL, Davies WJ.
Trejo CL, Davies WJ.
Wilkinson S, Davies WJ.
Zhang J, Davies WJ.
Zhang J, Schurr U, Davies WJ.
Zhang SQ, Outlaw Jr WH.




Comments