-
PDF
- Split View
-
Views
-
Cite
Cite
José A Heredia-Guerrero, Antonio Heredia, Eva Domínguez, Roberto Cingolani, Ilker S Bayer, Athanassia Athanassiou, José J Benítez, Cutin from agro-waste as a raw material for the production of bioplastics, Journal of Experimental Botany, Volume 68, Issue 19, 9 November 2017, Pages 5401–5410, https://doi.org/10.1093/jxb/erx272
Close - Share Icon Share
Abstract
Cutin is the main component of plant cuticles constituting the framework that supports the rest of the cuticle components. This biopolymer is composed of esterified bi- and trifunctional fatty acids. Despite its ubiquity in terrestrial plants, it has been underutilized as raw material due to its insolubility and lack of melting point. However, in recent years, a few technologies have been developed to obtain cutin monomers from several agro-wastes at an industrial scale. This review is focused on the description of cutin properties, biodegradability, chemical composition, processability, abundance, and the state of art of the fabrication of cutin-based materials in order to evaluate whether this biopolymer can be considered a source for the production of renewable materials.
Introduction
One of the most important challenges for agriculture in the coming future is to feed the growing world population (FAO, 2017). Indeed, by 2050, the population is estimated to reach close to 10 billion people, almost 3 billion more than the current population (UN, 2015). Hence, a significant increase in the productivity of farming systems will be required (McCalla, 2001). Accordingly, the need for resources for pre- and post-harvesting agriculture activities will also need to increase, as well as the production of specific materials for them. These products are mainly made of petroleum-based synthetic polymers due to their wide range of physical properties, facile processing, and low cost (Mülhaupt, 2013; Heredia-Guerrero and Athanassiou, 2016). The use of plastic in agriculture can be divided in two sectors: cultivation (mulching, greenhouses, plastic reservoirs, and irrigation systems) and food packaging (silage, containers, protective films, etc.). The global demand for plastics in agriculture, mainly polyolefin, polyethylene (PE), polypropylene (PP), ethylene-vinyl acetate (EVA), poly(vinyl chloride) (PVC) and, less frequently, polycarbonate (PC) and poly(methyl methacrylate) (PMMA), reached ∼11 million tons in 2015 (value calculated from data provided by PlasticsEurope, 2016). However, dwindling fossil resources, global warming, and littering problems after the plastics’ life cycle are encouraging the development of fully biodegradable bioplastics and green composites from renewable biomass sources (Klemm et al., 2005; La Mantia and Morreale, 2011; Abba et al., 2013; Bayer et al., 2014; Vilela et al., 2014; Heredia-Guerrero and Athanassiou, 2016). Plant waste from agriculture, namely agro-waste, fits the objectives of a circular economy. Given that the volume of non-edible plant waste from crops, mainly cereals, starchy roots, fruits, and other vegetables, is ~250 million tons per year (value calculated from data provided by FAO, 2013), they represent an obvious potential source of agro-waste. Such waste is rich in many useful substances such as lipids, polysaccharides, and aromatic compounds, which could be used for the fabrication of polymeric materials. Nevertheless, agro-waste needs to be chemically treated in order to extract or isolate specific macromolecules, such as cellulose, lignin, and suberin, or monomers, such as vegetable oils, tannins, and terpenes, from which bioplastics are produced (Gandini, 2008; Gandini, 2011; Gandini and Lacerda, 2015; Gandini et al., 2016). An exhaustive review about polymers from renewable resources can be found elsewhere (Belgacem and Gandini, 2008).
Cellulose, the major component of plant cell walls (Cosgrove, 2005), is the most common natural polymer on Earth, with an estimated annual biomass production between 1011 and 1012 tons (Klemm et al., 2005). Cellulose is mainly used in the fabrication of paper, cardboard, cellophane, and rayon. Lignin, another plant biopolymer, is a complex mixture of aromatic alcohols, namely coniferyl, sinapyl, and p-coumaryl alcohols (Grabber, 2005). With an estimated biomass production of 107 tons/year, lignin is the most abundant natural aromatic polymer (Thielemans et al., 2002). This biopolymer is used in many applications such as a base-exchange material for water softening, an emulsifying agent, a component of adhesives and phenolic resins, and as a precursor for the synthesis of dyes and plastics. (Bhat et al., 2015). Another well-studied natural polymer is suberin, a barrier polymer largely found in the periderm of plants (Schreiber, 2010). It is is an aromatic-aliphatic polyester (Bernards, 2002), which is particularly abundant in the outer bark of cork oak (Quercus suber L.; Graça, 2015). Aliphatic suberin monomers can be used to fabricate inks, paints, coatings, drying oils, plasticizers, wetting agents, and as precursors of polyurethanes, polyesters, and polyamides (Gandini et al., 2006).
Cutin, on the other hand, is an interesting plant biopolymer that has gained less attention than the above-mentioned polymers. Its main features, such as its molecular structure, chemical composition, biodegradability, and mechanical, thermal, and hydrodynamic properties, are summarized in the following sections. Moreover, an evaluation of the potential use of cutin as a raw material for industrial applications is also carried out. Cutin is compared with common plastics, its annual biomass is estimated, and its main sources are reported. Finally, methods for the isolation and depolymerization of cutin as well as the fabrication of materials from cutin monomers are also reviewed.
Cutin as a source for packaging materials
The plant cuticle and cutin
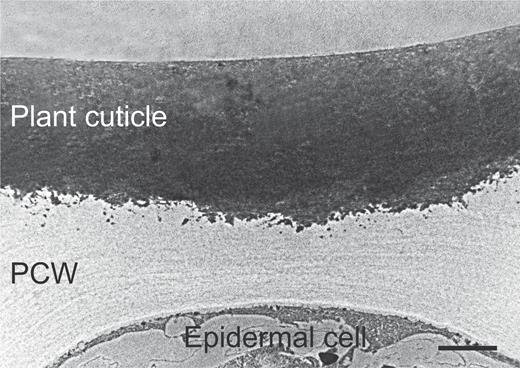
The plant cuticle is the outermost membrane that covers and protects the epidermis of non-lignified aerial parts of plants such as leaves, fruits, stems, and flowers (Heredia, 2003). The thickness of the cuticle usually ranges between 0.02 and 200 μm (Nawrath, 2006). Fig. 1 shows a TEM image of an epidermal cross-section of a tomato fruit (Segado et al., 2016). The plant cuticle is a composite material that consists of polysaccharides from the cell wall, the biopolyester cutin acting as a polymer matrix, waxes that can be located in the outer part, namely epicuticular waxes, or distributed inside, namely intracuticular waxes, the cuticle, and phenolic compounds such as cinnamic acids and flavonoids (España et al., 2014; Heredia-Guerrero et al., 2014). An alternative macromolecule to cutin, named cutan, can be found in certain species. Cutan is a refractive polymer of polyether nature (Villena et al., 1999). The chemical composition and distribution of the cuticle components can be different among plants, organs, and developmental stages (Fernández et al., 2016).
High magnification TEM picture of the epidermis of a ripe tomato fruit epicarp. Image from Segado et al. (2016). PCW, plant cell wall. Scale bar, 2 μm.
The main role attributed to plant cuticles is to avoid water loss from internal tissues (Riederer and Schreiber, 2001). However, it is also involved in other important physiological roles such as acting as a gas barrier and thermal regulator, for defense against pests and pathogens, reduction of nutrient leaching, and protection from mechanical injuries and UV damage (Heredia, 2003; Martin and Rose, 2014). With all these features, the plant cuticle can be considered as an ideal food packaging material. In fact, there is increasing interest in the production of plant cuticle-like materials with similar properties to standard plastics for food packaging (Zhang and Uyama, 2016; Heredia-Guerrero et al., 2017).
Cutin is the polymeric network support of the plant cuticle. It represents 40–80% of the cuticle’s dry weight and consists of esterified bi- and trifunctional fatty acids (Heredia, 2003; Domínguez et al., 2015). This molecular architecture results in a branched, amorphous, and flexible 3-dimensional network that fosters interactions with waxes, phenolic compounds, and polysaccharides (Luque et al., 1995; Pollard et al., 2008; Chatterjee et al., 2016). The following subsections will describe the chemical composition and physical properties of cutin.
Chemical composition and depolymerization of cutin
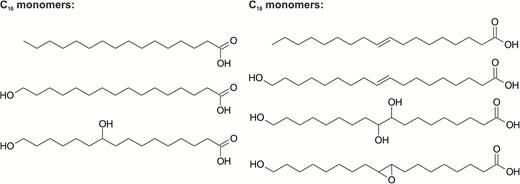
Cutin is mainly composed of C16, C18, or a mixture of both fatty acids (Domínguez et al., 2011b). They are usually functionalized in the ω position with hydroxyl groups and in the mid-chain with oxygen-containing functional groups, namely epoxy, oxo, hydroxy or vicinal diol, or double bonds (Pollard et al., 2008). Fig. 2 shows the chemical structure of the main cutin monomers. C16 cutins are rich in 9(10),16-dihydroxyhexadecanoic and 16-hydroxyhexadecanoic acids, whereas 16-hydroxy-10-oxo-hexadecanoic and 16-oxo-9 or 10-hydroxyhexadecanoic acids are uncommon. On the other hand, C18 cutins consist of 18-hydroxy-9,10-epoxyoctadecanoic and 9,10,18-trihydroxyoctadecanoic acids together with their mono-unsaturated homologs. Glycerol and phenolic compounds are also found in the cutin of some species (Graça et al., 2002; Pollard et al., 2008).
Chemical structure of the most common fatty acids found in cutin. Image adapted from Domínguez et al. (2011b).
Table 1 shows general cutin composition (Pollard et al., 2008) together with the specific chemical description of the tomato (Solanum lycopersicum), pepper (Capsicum annuum), lime (Citrus aurantifolia), cherry (Prunus avium), and apple (Malus pumila) fruit cutins (Parsons et al., 2013; Philippe et al., 2016), five agronomically important crops.
Main composition of cutin from various species and specific composition of tomato, pepper, and fruit cutins
| General composition for cutin (Pollard et al., 2008) . | . | Tomato fruit cutin (Philippe et al., 2016) . | Pepper fruit cutin (Parsons et al., 2013) . | Lime fruit cutin (Ray et al., 1995) . | Cherry fruit cutin (Belge et al., 2014) . | Apple fruit cutin (Eglinton and Hunneman, 1968) . | ||
|---|---|---|---|---|---|---|---|---|
| Monomer type . | Common monomers . | Abundance (%) . | Monomer . | Abundance (%) . | ||||
| Unsubstituted fatty acids | C16:0, C18:0, C18:1, C18:2 | 1–25 | Hexadecanoic acid | 0.2 | 3.7a | 3.7 | 0.3 | |
| Octadecanoic acid | 0.2 | 1.0 | 0.1 | |||||
| 9-octadecenoic acid | 11.5 | 0.6b | ||||||
| 9,12-octadecadienoic acid | 3.3 | |||||||
| Eicosanoic acid | 0.1 | |||||||
| Docosanoic acid | 1.9 | 0.1 | ||||||
| ω-hydroxy fatty acids | C16:0, C18:1, C18:2 | 1–32 | 16-hydroxyhexadecanoic acid | 2.9 | 2.7 | 2.8 | 5.0 | 8.0 |
| 16-hydroxy-9-oxo-hexadecanoic acid | 4.8 | |||||||
| 16-hydroxy-10-oxo-hexadecanoic acid | 51.0 | |||||||
| 18-hydroxyoctadecanoic acid | 0.1 | 1.9 | ||||||
| 18-hydroxyoctadeca-9-enoic acid | 1.7 | 14.2 | 5.0 | |||||
| 18-hydroxyoctadeca-9,12-dienoic acid | 0.8 | 16.0 | 13.0 | |||||
| 24-hydroxytetracosanoic acid | 0.2 | |||||||
| Other hydroxy fatty acids | 10-hydroxyhexadecanoic | 1.0 | ||||||
| 2-hydroxyhexadecanoic | 0.7 | |||||||
| α,ω-dicarboxylic acids | C16:0, C18:0, C18:1, C18:2 | 0–5 | 1,16-hexadecanedioic acid | 0.5 | 1.0 | 7.7 | 0.2 | |
| 9-hydroxy-1,16-hexadecanedioic acid | 2.7 | |||||||
| 1,18-octadecenedioic acid | 18.1 | 0.3 | ||||||
| 1,18-octadeca-9,10-dihydroxydioic acid | 0.1 | |||||||
| 1,18-octadecadienedioic acid | 1.2 | 0.1 | ||||||
| Epoxy-fatty acids | C18:0 (9,10-epoxy) C18:1 (9,10-epoxy) | 0–34 | 9,10-epoxy-18-hydroxyoctadecenoic acid | 0.2 | 9.3 | |||
| 9,10-epoxy-18-hydroxyoctadecanoic acid | 0.1 | 1.3 | ||||||
| Polyhydroxy-fatty acids | C16:0 (10,16-dihydroxy), C18:0 (9,10,18-trihydroxy) | 16–92 | 9(10),16-dihydroxyhexadecanoic acid | 81.6 | 63.8 | 30.8 | 24.0 | |
| 9(10),18-dihydroxyoctadecanoic acid | 1.4 | 1.3 | ||||||
| 9,10,18-trihydroxyoctadecenoic acid | 0.2 | 6.5 | 3.0 | |||||
| 9,10,18-trihydroxyoctadecanoic acid | 0.4 | 24.0 | ||||||
| Fatty alcohols | C16:0, C18:1 | 0–13 | ||||||
| Glycerol | 1–14 | Glycerol | 0.6 | |||||
| Phenolic compounds | 0–1 | p- and m-coumaric acids | 2.8 | 2.3 | ||||
| Unidentified compounds | 5.8 | 9.9 | 3.4 | 10.2 | 21.7 | |||
| General composition for cutin (Pollard et al., 2008) . | . | Tomato fruit cutin (Philippe et al., 2016) . | Pepper fruit cutin (Parsons et al., 2013) . | Lime fruit cutin (Ray et al., 1995) . | Cherry fruit cutin (Belge et al., 2014) . | Apple fruit cutin (Eglinton and Hunneman, 1968) . | ||
|---|---|---|---|---|---|---|---|---|
| Monomer type . | Common monomers . | Abundance (%) . | Monomer . | Abundance (%) . | ||||
| Unsubstituted fatty acids | C16:0, C18:0, C18:1, C18:2 | 1–25 | Hexadecanoic acid | 0.2 | 3.7a | 3.7 | 0.3 | |
| Octadecanoic acid | 0.2 | 1.0 | 0.1 | |||||
| 9-octadecenoic acid | 11.5 | 0.6b | ||||||
| 9,12-octadecadienoic acid | 3.3 | |||||||
| Eicosanoic acid | 0.1 | |||||||
| Docosanoic acid | 1.9 | 0.1 | ||||||
| ω-hydroxy fatty acids | C16:0, C18:1, C18:2 | 1–32 | 16-hydroxyhexadecanoic acid | 2.9 | 2.7 | 2.8 | 5.0 | 8.0 |
| 16-hydroxy-9-oxo-hexadecanoic acid | 4.8 | |||||||
| 16-hydroxy-10-oxo-hexadecanoic acid | 51.0 | |||||||
| 18-hydroxyoctadecanoic acid | 0.1 | 1.9 | ||||||
| 18-hydroxyoctadeca-9-enoic acid | 1.7 | 14.2 | 5.0 | |||||
| 18-hydroxyoctadeca-9,12-dienoic acid | 0.8 | 16.0 | 13.0 | |||||
| 24-hydroxytetracosanoic acid | 0.2 | |||||||
| Other hydroxy fatty acids | 10-hydroxyhexadecanoic | 1.0 | ||||||
| 2-hydroxyhexadecanoic | 0.7 | |||||||
| α,ω-dicarboxylic acids | C16:0, C18:0, C18:1, C18:2 | 0–5 | 1,16-hexadecanedioic acid | 0.5 | 1.0 | 7.7 | 0.2 | |
| 9-hydroxy-1,16-hexadecanedioic acid | 2.7 | |||||||
| 1,18-octadecenedioic acid | 18.1 | 0.3 | ||||||
| 1,18-octadeca-9,10-dihydroxydioic acid | 0.1 | |||||||
| 1,18-octadecadienedioic acid | 1.2 | 0.1 | ||||||
| Epoxy-fatty acids | C18:0 (9,10-epoxy) C18:1 (9,10-epoxy) | 0–34 | 9,10-epoxy-18-hydroxyoctadecenoic acid | 0.2 | 9.3 | |||
| 9,10-epoxy-18-hydroxyoctadecanoic acid | 0.1 | 1.3 | ||||||
| Polyhydroxy-fatty acids | C16:0 (10,16-dihydroxy), C18:0 (9,10,18-trihydroxy) | 16–92 | 9(10),16-dihydroxyhexadecanoic acid | 81.6 | 63.8 | 30.8 | 24.0 | |
| 9(10),18-dihydroxyoctadecanoic acid | 1.4 | 1.3 | ||||||
| 9,10,18-trihydroxyoctadecenoic acid | 0.2 | 6.5 | 3.0 | |||||
| 9,10,18-trihydroxyoctadecanoic acid | 0.4 | 24.0 | ||||||
| Fatty alcohols | C16:0, C18:1 | 0–13 | ||||||
| Glycerol | 1–14 | Glycerol | 0.6 | |||||
| Phenolic compounds | 0–1 | p- and m-coumaric acids | 2.8 | 2.3 | ||||
| Unidentified compounds | 5.8 | 9.9 | 3.4 | 10.2 | 21.7 | |||
aOctadecanoic acid is included in this percentage; b9,12-octadecadienoic acid is included in this percentage.
Main composition of cutin from various species and specific composition of tomato, pepper, and fruit cutins
| General composition for cutin (Pollard et al., 2008) . | . | Tomato fruit cutin (Philippe et al., 2016) . | Pepper fruit cutin (Parsons et al., 2013) . | Lime fruit cutin (Ray et al., 1995) . | Cherry fruit cutin (Belge et al., 2014) . | Apple fruit cutin (Eglinton and Hunneman, 1968) . | ||
|---|---|---|---|---|---|---|---|---|
| Monomer type . | Common monomers . | Abundance (%) . | Monomer . | Abundance (%) . | ||||
| Unsubstituted fatty acids | C16:0, C18:0, C18:1, C18:2 | 1–25 | Hexadecanoic acid | 0.2 | 3.7a | 3.7 | 0.3 | |
| Octadecanoic acid | 0.2 | 1.0 | 0.1 | |||||
| 9-octadecenoic acid | 11.5 | 0.6b | ||||||
| 9,12-octadecadienoic acid | 3.3 | |||||||
| Eicosanoic acid | 0.1 | |||||||
| Docosanoic acid | 1.9 | 0.1 | ||||||
| ω-hydroxy fatty acids | C16:0, C18:1, C18:2 | 1–32 | 16-hydroxyhexadecanoic acid | 2.9 | 2.7 | 2.8 | 5.0 | 8.0 |
| 16-hydroxy-9-oxo-hexadecanoic acid | 4.8 | |||||||
| 16-hydroxy-10-oxo-hexadecanoic acid | 51.0 | |||||||
| 18-hydroxyoctadecanoic acid | 0.1 | 1.9 | ||||||
| 18-hydroxyoctadeca-9-enoic acid | 1.7 | 14.2 | 5.0 | |||||
| 18-hydroxyoctadeca-9,12-dienoic acid | 0.8 | 16.0 | 13.0 | |||||
| 24-hydroxytetracosanoic acid | 0.2 | |||||||
| Other hydroxy fatty acids | 10-hydroxyhexadecanoic | 1.0 | ||||||
| 2-hydroxyhexadecanoic | 0.7 | |||||||
| α,ω-dicarboxylic acids | C16:0, C18:0, C18:1, C18:2 | 0–5 | 1,16-hexadecanedioic acid | 0.5 | 1.0 | 7.7 | 0.2 | |
| 9-hydroxy-1,16-hexadecanedioic acid | 2.7 | |||||||
| 1,18-octadecenedioic acid | 18.1 | 0.3 | ||||||
| 1,18-octadeca-9,10-dihydroxydioic acid | 0.1 | |||||||
| 1,18-octadecadienedioic acid | 1.2 | 0.1 | ||||||
| Epoxy-fatty acids | C18:0 (9,10-epoxy) C18:1 (9,10-epoxy) | 0–34 | 9,10-epoxy-18-hydroxyoctadecenoic acid | 0.2 | 9.3 | |||
| 9,10-epoxy-18-hydroxyoctadecanoic acid | 0.1 | 1.3 | ||||||
| Polyhydroxy-fatty acids | C16:0 (10,16-dihydroxy), C18:0 (9,10,18-trihydroxy) | 16–92 | 9(10),16-dihydroxyhexadecanoic acid | 81.6 | 63.8 | 30.8 | 24.0 | |
| 9(10),18-dihydroxyoctadecanoic acid | 1.4 | 1.3 | ||||||
| 9,10,18-trihydroxyoctadecenoic acid | 0.2 | 6.5 | 3.0 | |||||
| 9,10,18-trihydroxyoctadecanoic acid | 0.4 | 24.0 | ||||||
| Fatty alcohols | C16:0, C18:1 | 0–13 | ||||||
| Glycerol | 1–14 | Glycerol | 0.6 | |||||
| Phenolic compounds | 0–1 | p- and m-coumaric acids | 2.8 | 2.3 | ||||
| Unidentified compounds | 5.8 | 9.9 | 3.4 | 10.2 | 21.7 | |||
| General composition for cutin (Pollard et al., 2008) . | . | Tomato fruit cutin (Philippe et al., 2016) . | Pepper fruit cutin (Parsons et al., 2013) . | Lime fruit cutin (Ray et al., 1995) . | Cherry fruit cutin (Belge et al., 2014) . | Apple fruit cutin (Eglinton and Hunneman, 1968) . | ||
|---|---|---|---|---|---|---|---|---|
| Monomer type . | Common monomers . | Abundance (%) . | Monomer . | Abundance (%) . | ||||
| Unsubstituted fatty acids | C16:0, C18:0, C18:1, C18:2 | 1–25 | Hexadecanoic acid | 0.2 | 3.7a | 3.7 | 0.3 | |
| Octadecanoic acid | 0.2 | 1.0 | 0.1 | |||||
| 9-octadecenoic acid | 11.5 | 0.6b | ||||||
| 9,12-octadecadienoic acid | 3.3 | |||||||
| Eicosanoic acid | 0.1 | |||||||
| Docosanoic acid | 1.9 | 0.1 | ||||||
| ω-hydroxy fatty acids | C16:0, C18:1, C18:2 | 1–32 | 16-hydroxyhexadecanoic acid | 2.9 | 2.7 | 2.8 | 5.0 | 8.0 |
| 16-hydroxy-9-oxo-hexadecanoic acid | 4.8 | |||||||
| 16-hydroxy-10-oxo-hexadecanoic acid | 51.0 | |||||||
| 18-hydroxyoctadecanoic acid | 0.1 | 1.9 | ||||||
| 18-hydroxyoctadeca-9-enoic acid | 1.7 | 14.2 | 5.0 | |||||
| 18-hydroxyoctadeca-9,12-dienoic acid | 0.8 | 16.0 | 13.0 | |||||
| 24-hydroxytetracosanoic acid | 0.2 | |||||||
| Other hydroxy fatty acids | 10-hydroxyhexadecanoic | 1.0 | ||||||
| 2-hydroxyhexadecanoic | 0.7 | |||||||
| α,ω-dicarboxylic acids | C16:0, C18:0, C18:1, C18:2 | 0–5 | 1,16-hexadecanedioic acid | 0.5 | 1.0 | 7.7 | 0.2 | |
| 9-hydroxy-1,16-hexadecanedioic acid | 2.7 | |||||||
| 1,18-octadecenedioic acid | 18.1 | 0.3 | ||||||
| 1,18-octadeca-9,10-dihydroxydioic acid | 0.1 | |||||||
| 1,18-octadecadienedioic acid | 1.2 | 0.1 | ||||||
| Epoxy-fatty acids | C18:0 (9,10-epoxy) C18:1 (9,10-epoxy) | 0–34 | 9,10-epoxy-18-hydroxyoctadecenoic acid | 0.2 | 9.3 | |||
| 9,10-epoxy-18-hydroxyoctadecanoic acid | 0.1 | 1.3 | ||||||
| Polyhydroxy-fatty acids | C16:0 (10,16-dihydroxy), C18:0 (9,10,18-trihydroxy) | 16–92 | 9(10),16-dihydroxyhexadecanoic acid | 81.6 | 63.8 | 30.8 | 24.0 | |
| 9(10),18-dihydroxyoctadecanoic acid | 1.4 | 1.3 | ||||||
| 9,10,18-trihydroxyoctadecenoic acid | 0.2 | 6.5 | 3.0 | |||||
| 9,10,18-trihydroxyoctadecanoic acid | 0.4 | 24.0 | ||||||
| Fatty alcohols | C16:0, C18:1 | 0–13 | ||||||
| Glycerol | 1–14 | Glycerol | 0.6 | |||||
| Phenolic compounds | 0–1 | p- and m-coumaric acids | 2.8 | 2.3 | ||||
| Unidentified compounds | 5.8 | 9.9 | 3.4 | 10.2 | 21.7 | |||
aOctadecanoic acid is included in this percentage; b9,12-octadecadienoic acid is included in this percentage.
Cutin depolymerization is usually carried out after the cuticle has been isolated. Enzymes such as pectinases, cellulases, and hemicellulases are usually employed for cuticle isolation, although harsh chemical extractions with ammonium oxalate/oxalic acid or ZnCl2/HCl are also possible (Huelin and Gallop, 1951; Orgell, 1955; Holloway and Baker, 1968; Pacchiano et al., 1993). Isolated plant cuticles are dewaxed by immersion in organic solvents and polysaccharides are removed by acid hydrolysis, usually using HCl. Cutin can then be depolymerized by cleaving the ester bonds using alkaline hydrolysis, with NaOH or KOH in water, transesterification with methanol containing BF3 or NaOCH3, reductive cleavage by exhaustive treatment with LiAlH4 in tetrahydrofuran, or with trimethylsilyl iodide in organic solvents (Kolattukudy, 2001). These methodologies are not adequate for large-scale cutin extraction due to the number of steps involved, some of which are time consuming, and the cost of solvents and chemicals. Alternative approaches that involve cheaper chemical reagents and/or avoid cuticle isolation have therefore been investigated. In line with this, cutin monomers have been directly obtained from tomato peel waste via alkaline hydrolysis (Cigognini et al., 2015; Benítez et al., 2016), using either sodium carboxylate or sodium hydroxide/hydrogen peroxide (Cifarelli et al., 2016). Similarly, cutin monomers from the epidermis of leaves and stems of Malabar Spinach (Basella alba) were obtained by sequential hydrolysis in KOH and H2SO4 (Phu et al., 2017).
Cutin versus common plastics: a question of physical properties and biodegradability
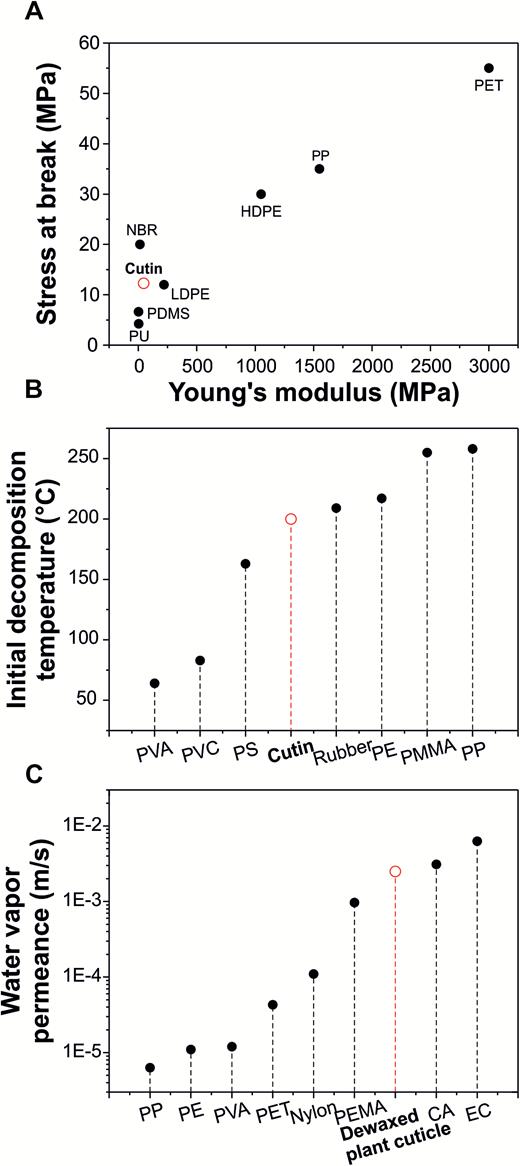
The mechanical properties of isolated tomato fruit cutin have been exhaustively characterized (López-Casado et al., 2007; Domínguez et al., 2011a). These properties change during organ growth and development as well as with humidity and temperature. For instance, Young’s modulus, stress at break, and elongation at break for the cutin of red ripe tomatoes at 23°C and 40% relative humidity are 45.0 MPa, 12.3 MPa, and 27%, respectively (López-Casado et al., 2007). These values are far from the standards for petroleum-based plastics with Young’s modulus in the order of GPa and elongations at break of several hundred percent. Fig. 3A compares the values of stress at break versus Young’s modulus of cutin with common plastics materials such as polyurethane (PU), polydimethylsiloxane (PDMS), low- and high-density polyethylene (LDPE and HDPE, respectively), acrylonitrile-butadiene rubber (NBR), polypropylene (PP), and poly(ethylene terephthalate) (PET) (Mark, 2007). Cutin is found in the low value range between NBR and LDPE. However, these polymers have elongations at break that are much higher, 350–800% for NBR and 200–900% for LDPE (Mark, 2007). The elongation at break of cutin is comparable with or even higher than that of rigid polymers such as polystyrene (PS) at 1–4%, styrene-acrylonitrile (SAN) at 2–5%), nylon 66 at 20%, poly(methyl methacrylate) (PMMA) at 3–5%, melamine-formaldehyde resin (MF) at 0.5–15%, or epoxy resin at 1–2% (Mark, 2007).
Stress at break, initial deomposition temperature, and water vapor permanence of cutin and various common plastics A) Comparison of stress at break versus Young’s modulus of ripe tomato fruit cutin with different man-made polymeric materials. Data from López-Casado et al. (2007) and Mark (2007). B) Initial decomposition temperature of watermelon cutin compared with conventional plastics. Data from Chaudhari and Singhal (2015) and Mark (2007). C) Water vapor permeance values of dewaxed plant cuticles of Ficus elastica leaves and some industrial plastics and polymers. Data from Schreiber and Schonherr (2009).
Similarly, the thermal properties of tomato fruit cutin have also been investigated by differential scanning calorimetry (Matas et al., 2004). No melting point has been detected for cutin, most probably due to its cross-linked nature, indicating that it is a thermoset polymer. On the other hand, its glass transition temperature, namely the temperature at which the segment motion of macromolecules becomes thermally activated, has been detected at ~22°C. Thermal degradation is also of interest for the characterization of polymers and is usually measured by thermogravimetric analysis. Fig. 3B shows the comparison between the onset of thermal decomposition of watermelon cutin with various conventional plastics such as poly(vinyl alcohol) (PVA), poly(vinyl chloride) (PVC), PS, rubber, polyethylene (PE), PMMA, and PP. Interestingly, cutin showed a relative high initial temperature of degradation of 200°C similar to rubber and PE and higher than PVA, PVC, and PS (Chaudhari and Singhal, 2015).
In terms of hydrophobicity and hydrodynamic properties, the comparison of cutin with other polymers is more difficult because data for isolated cutin is very scarce or even non-existent. For example, there is no data about the water contact angle and surface energy of cutin. Nevertheless, some degree of hydrophobicity can be expected due to its chemical composition. In this sense, water adsorption of tomato fruit cutin is very low at 2.5 wt.%, indicating that the biopolymer shows a low interaction with water molecules (Heredia-Guerrero et al., 2009). The water vapor permeance (Pwv) of dewaxed plant cuticles has been compared with some petroleum-based plastics and cellulose derivatives such as PP, PE, PVA, PET, nylon, poly(ethyl methacrylate) (PEMA), cellulose acetate (CA) and ethyl cellulose (EC) (Schreiber and Schonherr, 2009), as illustrated in Fig. 3C. Results show that the dewaxed cuticle of Ficus elastica leaves has a relatively low water permeance, between conventional plastics and cellulose derivatives.
Another important aspect for polymeric materials is biodegradability. Cutin can be fully hydrolyzed by soil microorganisms in a period of 3–8 months depending on the season, the characteristics of the soil, and the microbiota (De Vries et al., 1967). Such degradation, namely cleavage of ester groups, is mainly catalyzed by cutinases secreted by microorganisms and some fungi (Kolattukudy, 2001). On the other hand, plastics such as LDPE, PS, polyvinyl chloride, and urea formaldehyde resin buried over thirty years showed insignificant degradation (Otake et al., 1995).
Is cutin abundant enough to be used as a raw material at an industrial scale?
Estimation of the volume of cutin biomass and main agronomic sources
The amount of plant cuticle material in both natural and agricultural plant communities has been reported to range between 180 and 1500 kg/ha (Heredia, 2003). Considering that 40–80 wt.% is cutin and that the total area of land for agricultural uses and forests is ~8.9 ⋅ 109 ha (FAO, 2017), the volume of total cutin biomass could reach 109–1010 tons. Despite this huge amount, not all this cutin is easily accessible due to technical reasons, such as accessing forests and jungles, and/or due to ethical reasons, such as making use of edible plants, sustainability, and deforestation. In this sense, a better approach for the industrial exploitation of this biopolyester could be the use of cutin-rich waste from species of high agronomic importance such as tomatoes. In fact, global tomato production reached 171 million tons in 2014, with leading producers being China at 53 million tons and Europe at 23 million tons, where Spain and Italy were the main tomato producers, followed by India, USA, and Turkey (FAOSTAT, 2017). The global tomato processing market reached a volume of 36 million tons in the same year. Tomato fruits are mainly processed for the preparation of peeled tomatoes, purées, juices, concentrates, and sauces (European Comission, 2015). Peeling is performed before the manufacture of these products, resulting in a final residue of tomato peel rich in cutin (Rock et al., 2012). Considering the average amount of cutin, expressed as weight/area, and the area and weight of a typical tomato fruit, the volume of cutin from this agro-waste could reach 105–106 tons/year, which is comparable to lignin stock but considerably lower than annual cellulose biomass production. Nowadays, this inexpensive by-product, at ∼0.04€/kg (as reported in Cifarelli et al., 2016), is wasted or, to a lesser extent, used for animal feed as tomato pomace.
Plant breeding strategies to increase the amount of cutin in plants
Natural variability present within a given species or among a cultivated crop and its related wild species can be used in plant breeding to modify cuticle deposition, mainly to improve the quality of fruits, but also to increase the amount cuticle components and, hence, the productivity of plants as raw materials. In this sense, significant differences have been observed for the amount of cuticle and waxes among different tomato and pepper genotypes (Domínguez et al., 2008; Domínguez et al., 2009; Parsons et al., 2012; Yeats et al., 2012; Parsons et al., 2013) and for epicuticular waxes in apples (Belding et al., 1998). These differences can be used to uncover some of the genes involved in cuticle deposition.
The ability of plants to interact with the environment implies that they perceive environmental changes and modulate their growth accordingly. Cuticle deposition is strongly affected by the environment. Drought, radiation, relative humidity, and temperature, to name a few, have been shown to affect the amount of cuticle deposited, its thickness, or the relative amount of its components (Hull et al., 1975). However, it is very difficult to isolate the effect of one environmental trait from the rest of them. Arabidopsis thaliana plants subjected to water deficit or irrigated with saline water showed an increase in the amount of cutin and waxes (Kosma et al., 2009). Similarly, the radiation and temperature differences between spring and winter in Mediterranean conditions promoted changes in the amount of cuticle deposited (Domínguez et al., 2012). Hormones play an important role in mediating plant adaptation to adverse environmental conditions (Wolters and Jurgens, 2009). Yet, their regulation of cuticle synthesis is still poorly understood. Gibberellins have been shown to promote cuticle deposition during early stages of development in various species such as rice, pea, and tomato (Bowen and Walton, 1988; Hoffmann-Benning and Kende, 1994; Knoche and Peschel, 2007). Cytokinins have also been postulated to play a role in cutin deposition (Wu et al., 2015). Abscisic acid (ABA), a hormone related to abiotic stresses, has been reported to induce wax accumulation but not cuticle deposition in A. thaliana (Kosma et al., 2009). More recently, the use of ABA biosynthesis impaired mutants in tomato has shown a reduction of cutin in the leaves of some of these mutants, whereas an opposite effect was observed in fruits (Martin et al., 2017).
Materials inspired by plant cuticles
The protective properties of the plant cuticle against the environment, namely water loss and gas exchange, humidity, temperature oscillations, UV radiation, and pathogen attack, have always attracted the attention of researchers to create a synthetic replica for a variety of applications such as packaging materials, membranes, and UV filters. Additionally, a manufactured material inspired by the plant cuticle would provide key characteristics, such as innocuousness and biodegradability, to become an attractive alternative to petroleum-based plastics and to alleviate the environmental implications connected with their production, use, and disposal. As mentioned above, abundancy and ubiquity of this tissue in nature add potential economic viability to any industrial process that values the plant cuticle. Furthermore, the fact that this biomaterial is mostly a by-product from other production activities, such as crop and fruit processing, is a very important and valuable aspect, particularly, in underdeveloped countries, since no resources are retracted from human and/or animal feeding.
As it has already been mentioned, direct processing of cuticles is not straightforward because of its insolubility and infusibility and such drawbacks have seriously hampered advances in this field. Hence, efforts have been redirected to mimic the plant cuticle through a combination of cellulosic and long- or mid-chain polyester phases. For instance, bimodal hydrophilic-hydrophobic, UV-blocking transparent films using ricinoleic acid, which is the main component of castor oil, polyglycerol, and cellulose have been obtained, with potential uses as packaging and displaying materials (Zhang and Uyama, 2016). In a similar approach, a naturally occurring polyhydroxy fatty acid, aleuritic (9,10,16-trihydroxyhexadecanoic) acid, fibrous cellulose and carnauba wax have been combined (Heredia-Guerrero et al., 2017). Aleuritic acid is a renewable trihydroxy C16 carboxylic acid obtained from a natural lac resin. The molecular functionalization of aleuritic acid is very similar to that of cutin monomers and, therefore, the nominal similarity of this composite with the plant cuticle is evident. Polymerization was conducted through the well-implemented industrial procedure of hot pressing the cellulose support, previously impregnated with a mixture of the acid and the wax. The process uses no catalysts and the obtained films show slightly improved mechanical properties with respect to cellulose and other common polysaccharide materials like cellulose acetate and ethyl and methyl cellulose. Meanwhile, water absorption and permeability values are reduced and become similar to commercial cellulose esters. Also, edible films from inexpensive and renewable resources such as tomato cutin and citrus pectin were blended via a low temperature casting method (Manrich et al., 2017). The composite is intended for use as a short-term packaging material; it resembles and even improves upon the properties of tomato peels in terms of water resistance, hydrophobicity, and thermal and mechanical behavior.
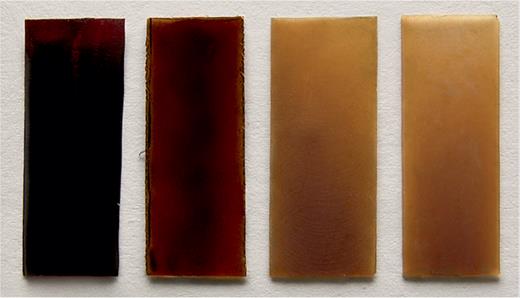
Attempts to mimic exclusively the cutin fraction of the plant cuticle are few in number. De Vries reported one of the earliest attempts in 1970, using acids extracted from ‘Golden Delicious’ apple skins and a direct polymerization procedure at 100°C (De Vries, 1970). Final products took several days to be formed and were insoluble in common organic solvents. Unfortunately, the physical and chemical characterization in this report is scarce and no further results were provided. Much later, the viability of the synthesis of a cutin mimetic polymer by a low temperature polycondensation using DBSA (4-dodecylbenzenesulfonic acid) as surfactant catalyst was demonstrated (Benı́tez et al., 2004). The product, obtained from apple cutin monomers, was insoluble in common organic solvents and Fourier transform infrared spectroscopy and NMR data confirmed the formation of the polyester. This methodology was extended to the polycondensation of a reference fatty polyhydroxyacid, aleuritic acid (Heredia-Guerrero et al., 2009). The rubbery product was characterized as an amorphous, insoluble, and hydrophobic polyhydroxyester, chemically and physically very similar to the one obtained from the tomato cutin monomeric mixture. The polycondensation of monomers extracted from tomato cutin monomers was addressed a few years later (Benítez et al., 2015). In this case, non-catalyzed self-esterification in a molten state route in air was selected. The study was extended to other similar fatty acids, namely hydroxyacids, with variable degrees of hydroxylation to those present in plant cutin. In general, amorphous, insoluble, and infusible solids were obtained, as shown in Fig. 4. Their structure was mainly directed by the preferential esterification, when available, of the primary hydroxyl group, as observed in natural cutin. The novelty of this study was the use of non-metallic or aromatic catalysts, which upholds the non-toxic and environment friendly vision of these polymeric materials. More strictly, isolated 10,16-dihydroxyhexadecanoic acid from tomato fruit cutin has been used to conduct the esterification reaction. First, lipases were essayed as catalysts at low temperature and in organic media (Gómez-Patiño et al., 2013). In these conditions, linear oligomers were obtained and proposed as building units to design high molecular weight functionalizable polyesters. In a second stage, an ionic liquid, namely choline chloride.2ZnCl2, was employed as a catalyst (Gómez-Patiño et al., 2015). In these conditions, some esterification of the secondary hydroxyl was observed and materials from partially crystalline solids to viscous insoluble materials were synthesized. The ionic liquid could be recovered and reused, which makes the process economically interesting and preserves the innocuousness of the polyester.
Photograph of different free-standing films from cutin monomers and similar hydroxylated fatty acids. Image from Benítez et al. (2015).
Alternatively, the usage of tomato cutin as a lacquer to coat the internal part of food metal packaging has been explored. This would be a good replacement of bisphenol A diglycidyl ether (BADGE) resins, which have a potential impact on human health. Though preliminary results have been promising (Montanari et al., 2014), to date, no further information has been made available. In any case, this is a most suitable application because it combines the need for an alternative to the current BADGE resins, a highly demanding global market, accessibility to the raw material, affordability and the environmental care of the process and the inherent good physical and chemical qualities of a plant cutin mimetic polymeric coating.
Development of cutin-based materials is not restricted to the ex-situ mimicry of the plant biopolyester and the valorization of fruit peel residues. Recently, a patent has been filed to engineer 2-dimensional arrays of cutin-like materials (CLM) for the next generation of functional coatings with potential applications in aircraft infrastructure, flexible electronics, bio-controlled instrumentation, and biological tissue protection using the epidermis of several cacti species, specifically Opuntia ficus-indica, Agave americana, Cereus peruvianus, and Rhipsalis (Alcantar et al., 2016).
Concluding remarks
Cutin is an abundant biopolyester with a complex chemical composition in comparison with other traditional biopolymers such as cellulose or lignin. The wide variety of cutin monomers could be a problem for the production of bioplastics. However, focusing on specific agro-waste rich in determined fatty acids can increase the reproducibility of the chemical profile of monomers. This is the case for cutin obtained from tomato peels whose main component is by far 9(10),16-dihydroxyhexadecanoic acid. In this sense, low-cost and scalable technologies based on aqueous hydrolysis by NaOH have been developed to obtain cutin monomers, though more sustainable processes, such as steam explosion, should be investigated. Furthermore, plant breeding strategies could be used to increase the amount of cutin and control monomer composition.
Most reviewed applications for cutin-based materials are related to packaging. However, the potential use of cutin monomers in other applications has been proprosed by Arrieta-Baez et al. (2011), such as for the fabrication of biomedical devices, fragrances, lubricants, and adhesives. In our opinion, these possibilities are very interesting and should be investigated in order to explore new applications of such monomers.
A lot of information is still missing in order to properly compare cutin with conventional plastics. For instance, the hydrophobicity of cutin should be characterized. On the other hand, cutin shows full biodegradability in short times, relatively high thermal resistance, and low values of water adsorption and water vapor permeance. In any case, physical properties of cutin-based materials can be improved by the addition of different monomers, cross-linkers or fillers. The use of polysaccharides and phenolic compounds to increase mechanical properties or waxes to improve hydrophobicity could also be interesting topics to explore. Finally, the study of other geometries different to films, such as coatings or fibers, is promising.
Abbreviations:
- LDPE
low-density polyethylene
- NBR
acrylonitrile-butadiene rubber
- PE
polyethylene
- PET
poly(ethylene terephthalate
- PMMA
poly(methyl methacrylate
- PP
polypropylene
- PS
polystyrene
- PVA
poly(vinyl alcohol)
- PVC
poly(vinyl chloride).
References
Author notes
Editor: Christine Raines, University of Essex, UK




Comments