-
PDF
- Split View
-
Views
-
Cite
Cite
Richard E. Gilbert, Aysel Akdeniz, Terri J. Allen, George Jerums, Urinary transforming growth factor‐β in patients with diabetic nephropathy: implications for the pathogenesis of tubulointerstitial pathology, Nephrology Dialysis Transplantation, Volume 16, Issue 12, December 2001, Pages 2442–2443, https://doi.org/10.1093/ndt/16.12.2442
Close - Share Icon Share
Sir,
Proteinuria is a major risk factor for the progression of diabetic nephropathy [1] and directly contributes to its pathogenesis by inducing tubulointerstitial pathology [2], itself a major correlate of declining renal function [3]. While the pathogenetic effects of proteinuria may represent a non‐specific response to protein overload [2] other mechanisms such as the enhanced ultrafiltration of growth factors may also contribute [4]. A candidate growth factor for such a role is transforming growth factor‐β (TGF‐β), a predominantly profibrotic factor that has been consistently implicated as playing a pivotal role in the pathogenesis of the glomerulosclerosis and tubulointerstitial fibrosis which characterize diabetic nephropathy [5].
Recent studies in experimental renal disease have shown that proteinuria is accompanied by increased ultrafiltration of high molecular weight growth factors into tubular fluid where they act on specific apical receptors, inducing matrix synthesis in both tubular epithelial cells and interstitial myofibroblasts [4]. We therefore examined the relationship between renal disease and urinary TGF‐β in patients with type 1 diabetes and nephropathy in 14 patients (7 with microalbuminuria and 7 with macroalbuminuria) prior to ACE inhibitor therapy being initiated. These patients were compared with eight subjects who remained persistently normoalbuminuric after at least 15 years of type 1 diabetes (nephropathy resistant) and eight healthy volunteers.
A 24 h urine collection was performed in all study subjects. In addition, in patients with diabetes, blood was drawn for the measurement of haemoglobin A1c (HbA1c) and blood pressure was measured after 5 min of quiet recumbency. A 2.0 ml aliquot from each urine collection was stored at −80 °C. Prior to assay, specimens were thawed, placed in a filter unit (Centricon‐10 filter, Amicon, Bedford, MA, USA) and concentrated 40‐fold, by centrifugation for 60 min at 6500 rpm, as previously described [6]. Urinary TGF‐β1 was assayed by solid‐phase ELISA (Quantikine, R & D Systems, Abingdon, UK) according to the manufacturer's instructions. The intra‐assay and inter‐assay coefficients of variation were 7.5% and 12.2%, respectively.
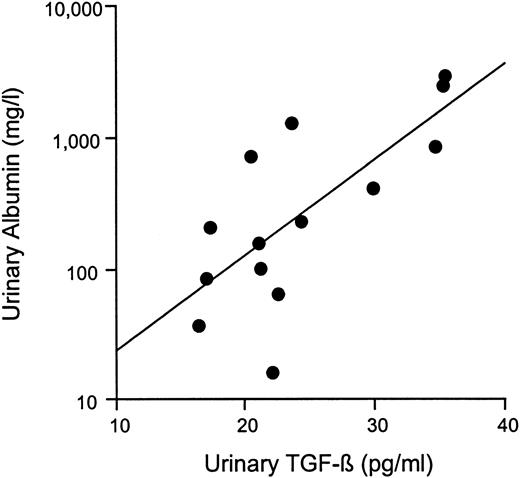
Patients with diabetic nephropathy had higher systolic and diastolic blood pressures than either persistently normoalbuminuric diabetic or non‐diabetic subjects. However, there was no difference in HbA1c or diabetes duration between the diabetic groups. Patients with diabetic nephropathy had higher urinary TGF‐β1 concentration when compared with both persistently normoalbuminuric diabetic patients and control subjects (Table 1). In addition, urinary TGF‐β correlated closely with albuminuria in patients with nephropathy (r=0.77, P=0.0013; Figure 1). No difference in urinary TGF‐β was noted between control and persistently normoalbuminuric diabetic subjects.
The magnitude of proteinuria, the extent of tubulointerstitial disease and progression of renal dysfunction in diabetic nephropathy are inter‐related [3]. As in experimental animals [4], the present study suggests that growth factor ultrafiltration is also a component of diabetic nephropathy in humans. Indeed, the presence of elevated urinary TGF‐β1 in patients with diabetic nephropathy, but not in those who were persistently normoalbuminuric, suggests that nephropathy rather than hyperglycaemia is the major determinant of urinary TGF‐β1 excretion.
As previously reported [4], there are several mechanisms whereby increased urinary TGF‐β1 may lead to progressive renal disease. First, TGF‐β in tubular fluid may act directly on tubular epithelial cells to induce the expression of extracellular matrix proteins by receptor‐mediated mechanisms. Second, TGF‐β mediated activation of tubular epithelial cells induces these cells to elaborate platelet‐derived growth factor, another prosclerotic growth factor which leads to matrix production by neighbouring interstitial fibroblasts. Third, TGF‐β may induce C–C chemokine expression leading to macrophage infiltration and acceleration of tissue injury.
In summary, the findings of the present study suggest that urinary TGF‐β1 may be an important contributor to the relationship between proteinuria and the progression of diabetic nephropathy.
Correlation between urinary albumin and transforming growth factor‐β in patients with type 1 diabetes and nephropathy. r=0.77; P=0.0013.
Characteristics of study patients
| Non‐diabetic | Diabetic | |||
| Non‐nephropathic | Nephropathic | |||
| n | 8 | 8 | 14 | |
| Age (years) | 33±4 | 40±6 | 32±5 | |
| Sex (male:female) | 4:4 | 3:5 | 9:5 | |
| Diabetes duration (years) | – | 18±5 | 21±4 | |
| SBP (mmHg) | 128±5 | 130±6 | 140±5* | |
| DBP (mmHg) | 76±1 | 73±2 | 80±2* | |
| Haemoglobin A1c (%) | – | 7.8±0.6 | 8.4±0.6 | |
| Urinary albumin (mg/l)‡ | 4.5×/÷1.3 | 6.8×/÷1.1 | 73.6×/÷1.4† | |
| TGF‐β (ng/l) | 18.8±1.8 | 15.8±1.6 | 26.1±2.4† | |
| Non‐diabetic | Diabetic | |||
| Non‐nephropathic | Nephropathic | |||
| n | 8 | 8 | 14 | |
| Age (years) | 33±4 | 40±6 | 32±5 | |
| Sex (male:female) | 4:4 | 3:5 | 9:5 | |
| Diabetes duration (years) | – | 18±5 | 21±4 | |
| SBP (mmHg) | 128±5 | 130±6 | 140±5* | |
| DBP (mmHg) | 76±1 | 73±2 | 80±2* | |
| Haemoglobin A1c (%) | – | 7.8±0.6 | 8.4±0.6 | |
| Urinary albumin (mg/l)‡ | 4.5×/÷1.3 | 6.8×/÷1.1 | 73.6×/÷1.4† | |
| TGF‐β (ng/l) | 18.8±1.8 | 15.8±1.6 | 26.1±2.4† | |
TGF‐β, transforming growth factor‐β; *P<0.05 vs non‐nephropathic diabetic patients and control subjects;†P<0.01 vs non‐nephropathic diabetic patients and control subjects; ‡data expressed as geometric mean×/÷ tolerance factor.
Characteristics of study patients
| Non‐diabetic | Diabetic | |||
| Non‐nephropathic | Nephropathic | |||
| n | 8 | 8 | 14 | |
| Age (years) | 33±4 | 40±6 | 32±5 | |
| Sex (male:female) | 4:4 | 3:5 | 9:5 | |
| Diabetes duration (years) | – | 18±5 | 21±4 | |
| SBP (mmHg) | 128±5 | 130±6 | 140±5* | |
| DBP (mmHg) | 76±1 | 73±2 | 80±2* | |
| Haemoglobin A1c (%) | – | 7.8±0.6 | 8.4±0.6 | |
| Urinary albumin (mg/l)‡ | 4.5×/÷1.3 | 6.8×/÷1.1 | 73.6×/÷1.4† | |
| TGF‐β (ng/l) | 18.8±1.8 | 15.8±1.6 | 26.1±2.4† | |
| Non‐diabetic | Diabetic | |||
| Non‐nephropathic | Nephropathic | |||
| n | 8 | 8 | 14 | |
| Age (years) | 33±4 | 40±6 | 32±5 | |
| Sex (male:female) | 4:4 | 3:5 | 9:5 | |
| Diabetes duration (years) | – | 18±5 | 21±4 | |
| SBP (mmHg) | 128±5 | 130±6 | 140±5* | |
| DBP (mmHg) | 76±1 | 73±2 | 80±2* | |
| Haemoglobin A1c (%) | – | 7.8±0.6 | 8.4±0.6 | |
| Urinary albumin (mg/l)‡ | 4.5×/÷1.3 | 6.8×/÷1.1 | 73.6×/÷1.4† | |
| TGF‐β (ng/l) | 18.8±1.8 | 15.8±1.6 | 26.1±2.4† | |
TGF‐β, transforming growth factor‐β; *P<0.05 vs non‐nephropathic diabetic patients and control subjects;†P<0.01 vs non‐nephropathic diabetic patients and control subjects; ‡data expressed as geometric mean×/÷ tolerance factor.
Richard Gilbert is a Recipient of a career development award from the Juvenile Diabetes Research Foundation.
References
Jerums G, Panagiotopoulos S, Tsalamandris C, Allen TJ, Gilbert RE, Comper WD. Why is proteinuria such an important risk factor for progression in clinical trials?
Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies.
Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury.
Wang SN, Hirschberg R. Growth factor ultrafiltration in experimental diabetic nephropathy contributes to interstitial fibrosis.
Border WA, Yamamoto T, Noble NA. Transforming growth factor‐β in diabetic nephropathy.





Comments