-
PDF
- Split View
-
Views
-
Cite
Cite
Craig L. Nelson, Connie S. Karschimkus, George Dragicevic, David K. Packham, Andrew M. Wilson, David O'Neal, Gavin J. Becker, James D. Best, Alicia J. Jenkins, Systemic and vascular inflammation is elevated in early IgA and type 1 diabetic nephropathies and relates to vascular disease risk factors and renal function, Nephrology Dialysis Transplantation, Volume 20, Issue 11, November 2005, Pages 2420–2426, https://doi.org/10.1093/ndt/gfi067
Close - Share Icon Share
Abstract
Background. Inflammation is implicated in cardiovascular disease (CVD) and mortality in end-stage renal failure (ESRF). Its importance in early renal disease is yet to be defined.
Methods. Serum levels of systemic and vascular inflammatory markers in early IgA nephropathy (IgAN) and control subjects were measured and related to renal function and vascular risk factors. A parallel study in type 1 diabetes mellitus subjects with (T1DM Nx) and without nephropathy (T1DM No Nx) was performed.
Results. Fifty-one IgAN patients aged 46±2 years (mean±SEM), calculated creatinine clearance (CrCl) 88±5 ml/min, were compared with 51 matched control subjects. Forty-six T1DM Nx patients aged 40±2 years, CrCl 84±5 ml/min, and 73 T1DM No Nx patients aged 38±2 years were also compared. High sensitivity C-reactive protein (hsCRP) was elevated in IgAN, T1DM Nx and T1DM No Nx patients compared with controls [4.2±0.6 (P<0.001), 4.1±0.6 (P<0.001), 2.6±0.4 (P<0.05) vs 1.6±0.3 mg/l]. Levels in T1DM Nx patients were higher than in T1DM No Nx patients (P<0.05). Inflammation and vascular dysfunction as measured by pulse pressure (PP) were related. HsCRP correlated with PP in IgAN and T1DM Nx (r = 0.47, P = 0.001; r = 0.40, P<0.05). PP was the strongest independent predictor of hsCRP in IgAN (T = 2.45, P<0.001), while body mass index (T = 7.83, P<0.001) was the strongest predictor in T1DM Nx. Endothelial cell adhesion molecules were increased in T1DM Nx>IgAN>T1DM No Nx vs controls: soluble vascular adhesion molecule-1 (sVCAM-1) 760±30 (P<0.001)>663±34 (P = 0.001)>601±21 (P<0.05) vs 536±15 ng/ml; soluble intracellular adhesion molecule-1 (sICAM-1) 320±8 (P<0.001)>313±13 (P<0.001)>307±8 (P<0.001) vs 244±6 ng/ml. sVCAM-1 levels were higher in T1DM Nx than in T1DM No Nx, P<0.001. In IgAN and T1DM Nx, hsCRP correlated with sICAM-1 (r = 0.33, P = 0.017; r = 0.37; P = 0.017). sVCAM-1 was related to renal function in IgAN and T1DM Nx: serum cystatin C (r = 0.63, P<0.001: r = 0.425, P = 0.002), and urine protein:creatinine ratio in IgAN (r = 0.48; P = 0.001).
Conclusions. Systemic and vascular markers of inflammation are increased in early renal disease and relate to renal dysfunction and cardiovascular risk factors. Inflammation may be a common process in various renal diseases and may link and accelerate renal dysfunction and CVD.
Introduction
Cardiovascular disease (CVD) risk in dialysis patients is ∼30 times that of the general population, and remains 10- to 20-fold higher despite adjusting for age, gender and diabetes [1,2]. Atherosclerotic vascular disease most often becomes clinically evident in the setting of end-stage renal failure (ESRF), and most studies have focused on this phase of renal disease. In addition to traditional and uraemia-related risk factors [3], it has been demonstrated that there are associations between malnutrition, inflammation and atherosclerosis in ESRF. Inflammation has been associated with increased risk of cardiovascular death in haemodialysis-treated ESRF patients [4] and with carotid intimal-medial thickness in chronic renal failure patients not on dialysis [glomerular filtration rate (GFR) 7±1 ml/min] [5]. Other studies have linked inflammation and endothelial dysfunction in chronic renal failure (serum creatinine 0.53±0.32 mmol/l) [6]. Levels of soluble cell adhesion molecules (sCAMs), markers of endothelial dysfunction and inflammation, are risk factors for all-cause mortality in the pre-dialysis group (GFR 7±1 ml/min) [7]. Vascular damage begins many years before it becomes clinically apparent. A study utilizing pulse wave velocity as a surrogate vascular end-point has revealed increased large vessel stiffness in early renal disease subjects with serum creatinine ≤0.13 mmol/l [8]. Elucidation of inflammatory markers in early renal disease may guide innovative therapies to prevent or reverse vascular damage.
It is hypothesized that early renal disease patients, not symptomatically uraemic or undergoing dialysis, have an adverse CVD risk factor profile and that systemic and vascular inflammation is increased and relates to vascular risk and renal function. Many studies addressing CVD in the renal population have evaluated a mixture of diseases with varying aetiologies and treatments. Co-existing factors such as hyperglycaemia in diabetes may influence CVD risk factors independently of renal damage. To overcome potential confounding variables, we have studied two groups of patients with nephropathy: one with the histological diagnosis of IgA nephropathy (IgAN), the other with type 1 diabetic nephropathy (T1DM Nx). Comparator groups were healthy subjects and nephropathy-free type 1 diabetic subjects (T1DM No Nx). The latter group enabled the evaluation of the effects of diabetes per se on measured factors. Markers of inflammation were related to renal function and CVD risk factors.
We anticipated that early renal dysfunction would be associated with an adverse vascular risk profile, and that this relates to systemic and vascular inflammation and renal function.
Subjects and methods
Subjects with IgAN (n = 51) were recruited from hospital out-patient clinics and nephrologists’ private practices. All subjects had biopsy-proven IgAN as evidenced by mesangial proliferative glomerulonephritis with IgA deposition on immunohistochemical staining, reported by a pathologist trained in the interpretation of renal biopsy. Control subjects (n = 51) were matched with IgAN subjects for age, gender, body mass index (BMI) and smoking history, from a pool of >200 volunteers. Exclusion criteria were diabetes, clinically evident CVD and any acute illness or surgery in the previous 3 months. T1DM subjects were recruited from hospital out-patient clinics and nephrologists’ and endochrinologists’ private practices. T1DM subjects were classified as T1DM Nx if they had had at least two out of three positive timed microalbuminuria specimens with normal renal function or had proteinuria with impaired renal function. Exclusion criteria were clinically evident CVD and any acute illness or surgery in the previous 3 months. Studies were approved by the Human Research Ethics Committee of St. Vincent's Hospital, Melbourne Australia in accordance with National Health and Medical Research Council guidelines, and written, informed consent was obtained from each participant.
A lifestyle questionnaire was completed. BMI and waist to hip ratios were measured. Blood pressure and pulse pressure (PP) (systolic minus diastolic blood pressure) were measured and the mean of three measurements was used in data analysis. Subjects were venesected 60 ml after a 10 h overnight fast and blood was sent on ice for analysis by St Vincent's Hospital, Melbourne, pathology department for standard assays: serum electrolytes, urea, creatinine, lipid profile, glucose, HbA1C, iron studies (iron, percentage transferrin saturation and ferritin), full blood count and erythrocyte sedimentation rate (ESR). Creatinine clearance was calculated using the traditional Cockcroft–Gault formula. A mid-stream urine sample was analysed for cell count, phase contrast microscopy, and spot albumin and protein/creatinine. Research laboratory samples were centrifuged (3000 r.p.m., 10 min, 4°C) and serum and plasma aliquots stored at −80°C until analysis within 6 months of collection. High sensitive C-reactive protein (hsCRP) and cystatin C were measured via nephelometry with commercially available kits (Dade Behring-Marburg, Germany). Intra- and inter-assay coefficients of variation (CVs) were 2.8 and 4.6% for hsCRP, and 3.3 and 4.1% for cystatin C. Soluble vascular cell adhesion molecule-1 (sVCAM-1) and intracellular adhesion molecule-1 (sICAM-1) were measured using a commercially available quantitative sandwich enzyme immunoassay linked to horseradish peroxidase (R&D Systems, MN). Analysis was performed at 450 nm on the microplate reader Powerwave X (Biotek Instruments Inc., VT). Intra- and inter-assay CVs were: sVCAM-1 4.9 and 8.9%, and sICAM-1 4.6 and 6.1%.
Data were stored in Excel (Microsoft) and analysed using Minitab™ statistical software 13th edition, using unpaired Student t-tests assuming unequal variance, correlation and stepwise multivariate regression analysis where appropriate. Stepwise multivariate regression was performed on inflammatory markers including matching criteria, vascular risk factors, measures of vascular health and renal parameters. Parameters were removed from the regression equation if P>0.15. Data were tested for normality using Ryan Joyner analysis, and log10 transformed prior to regression analysis if not normally distributed. Results are reported as the mean±SEM. Statistical significance was taken at P<0.05.
Results
Clinical demographics of the 51 subjects with IgAN and 51 apparently healthy controls are shown in Table 1. The groups were matched for age, gender, BMI and smoking history. Mean calculated creatinine clearance in the IgAN group was 88 ml/min, consistent with early renal dysfunction, while all renal-related parameters were normal in the control group. Clinical demographics of T1DM subjects are also shown in Table 1. T1DM groups had a higher proportion of females than the IgAN group and controls, and T1DM Nx (n = 46) subjects had a greater BMI than those with T1DM No Nx (n = 73). Similar impairment of renal function and proteinuria were noted in both nephropathy groups.
Demographics, classical vascular risk factors and renal dependant parameters
| Study parameter . | Controls . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Matching criteria | ||||||||
| n (M:F) | 51 (38:13) | 51 (38:13) | 46 (18:28)†,‡ | 73 (32:41)†,‡ | ||||
| Age (years) | 42.7±1.7 | 45.7±2.0 | 40.1±1.9 | 38.1±1.7 | ||||
| BMI (kg/m2) | 26.8±0.5 | 28.3±0.7 | 30.0±1.2+ | 24.7±0.4 | ||||
| WHR | 0.87±0.01 | 0.89±0.02 | 0.90±0.01 | 0.85±0.01 | ||||
| Smoking (pack years) | 4.6±1.3 | 5.8±1.6 | 13.9±2.5 | 13.7±2.2 | ||||
| Haemodynamic parameters | ||||||||
| Diagnosed HT (n) | 8 | 24†,§ | 31†,§ | 14 | ||||
| ACEI (n) | 1 | 26†,§ | 25†,§ | 9 | ||||
| HR (b.p.m.) | 63±1 | 67±2 | 78±2* | 71±2 | ||||
| SBP (mmHg) | 124±2 | 134±2† | 141±2†,§ | 126±2 | ||||
| DBP (mmHg) | 72±2 | 78±1§ | 77±2 | 70±1 | ||||
| PP (mmHg) | 52±1 | 56±1* | 65±2†,**,+ | 56±1* | ||||
| Glycaemia and lipid profiles | ||||||||
| FBG (mmol/l) | 5.07±0.08 | 5.29±0.07 | 12.27±0.89†,‡ | 12.66±0.66†,‡ | ||||
| HbA1c (%) | 5.11±0.06 | 5.37±0.06 | 8.98±0.32†,‡ | 7.98±0.16†,‡ | ||||
| Total Chol (mmol/l) | 5.6±0.1 | 5.5±0.2 | 5.3±0.2 | 4.9±0.1* | ||||
| Trig (mmol/l) | 1.3±0.1 | 1.5±0.1 | 1.5±0.1 | 1.1±0.1 | ||||
| Calculated LDL-C (mmol/l) | 3.5±0.1 | 3.4±0.1 | 3.2±0.2 | 2.8±0.1* | ||||
| HDL-C (mmol/l) | 1.44±0.05 | 1.32±0.07 | 1.5±0.2 | 1.6±0.1 | ||||
| Statins (n) | 1 | 2 | 10† | 7† | ||||
| Renal parameters | ||||||||
| Serum Cr (mmol/l) | 0.09±0.00 | 0.12±0.01*,+ | 0.12±0.01*,+ | 0.08±0.00 | ||||
| Calculated Cr Cl (ml/min) | 102±3 | 88±5* | 84±5* | 94±7 | ||||
| Urinary Alb/Cr (mg/mmol) | 0.8±0.2 | 51.6±11.3†,§ | 66.4±20.9 | 0.9±0.1 | ||||
| Urinary Pr/Cr (mg/mmol) | 9.5±1.3 | 79.0±13.3†,§ | 91.7±36.9 | 8.8±1.0 | ||||
| Urine glomerular RCC (×106/l)‡‡ | <10 | 44:5:2* | <10 | <10 | ||||
| Cystatin C (mg/l) | 0.83±0.02 | 1.34±0.12† | 1.49±0.16†,§ | 0.75±0.01 | ||||
| Study parameter . | Controls . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Matching criteria | ||||||||
| n (M:F) | 51 (38:13) | 51 (38:13) | 46 (18:28)†,‡ | 73 (32:41)†,‡ | ||||
| Age (years) | 42.7±1.7 | 45.7±2.0 | 40.1±1.9 | 38.1±1.7 | ||||
| BMI (kg/m2) | 26.8±0.5 | 28.3±0.7 | 30.0±1.2+ | 24.7±0.4 | ||||
| WHR | 0.87±0.01 | 0.89±0.02 | 0.90±0.01 | 0.85±0.01 | ||||
| Smoking (pack years) | 4.6±1.3 | 5.8±1.6 | 13.9±2.5 | 13.7±2.2 | ||||
| Haemodynamic parameters | ||||||||
| Diagnosed HT (n) | 8 | 24†,§ | 31†,§ | 14 | ||||
| ACEI (n) | 1 | 26†,§ | 25†,§ | 9 | ||||
| HR (b.p.m.) | 63±1 | 67±2 | 78±2* | 71±2 | ||||
| SBP (mmHg) | 124±2 | 134±2† | 141±2†,§ | 126±2 | ||||
| DBP (mmHg) | 72±2 | 78±1§ | 77±2 | 70±1 | ||||
| PP (mmHg) | 52±1 | 56±1* | 65±2†,**,+ | 56±1* | ||||
| Glycaemia and lipid profiles | ||||||||
| FBG (mmol/l) | 5.07±0.08 | 5.29±0.07 | 12.27±0.89†,‡ | 12.66±0.66†,‡ | ||||
| HbA1c (%) | 5.11±0.06 | 5.37±0.06 | 8.98±0.32†,‡ | 7.98±0.16†,‡ | ||||
| Total Chol (mmol/l) | 5.6±0.1 | 5.5±0.2 | 5.3±0.2 | 4.9±0.1* | ||||
| Trig (mmol/l) | 1.3±0.1 | 1.5±0.1 | 1.5±0.1 | 1.1±0.1 | ||||
| Calculated LDL-C (mmol/l) | 3.5±0.1 | 3.4±0.1 | 3.2±0.2 | 2.8±0.1* | ||||
| HDL-C (mmol/l) | 1.44±0.05 | 1.32±0.07 | 1.5±0.2 | 1.6±0.1 | ||||
| Statins (n) | 1 | 2 | 10† | 7† | ||||
| Renal parameters | ||||||||
| Serum Cr (mmol/l) | 0.09±0.00 | 0.12±0.01*,+ | 0.12±0.01*,+ | 0.08±0.00 | ||||
| Calculated Cr Cl (ml/min) | 102±3 | 88±5* | 84±5* | 94±7 | ||||
| Urinary Alb/Cr (mg/mmol) | 0.8±0.2 | 51.6±11.3†,§ | 66.4±20.9 | 0.9±0.1 | ||||
| Urinary Pr/Cr (mg/mmol) | 9.5±1.3 | 79.0±13.3†,§ | 91.7±36.9 | 8.8±1.0 | ||||
| Urine glomerular RCC (×106/l)‡‡ | <10 | 44:5:2* | <10 | <10 | ||||
| Cystatin C (mg/l) | 0.83±0.02 | 1.34±0.12† | 1.49±0.16†,§ | 0.75±0.01 | ||||
*P<0.05 compared with controls; **P<0.05 compared with IgA nephropathy subjects; +P<0.05 compared with type 1 diabetes subjects without nephropathy; †P<0.001 compared with controls; ‡P<0.001 compared with IgA nephropathy subjects; §P<0.001 compared with type 1 diabetes subjects without nephropathy.
‡‡Mild <200; moderate 200–1000, severe >1000×106/l.
M = male; F = female; BMI = body mass index; WHR = waist:hip ratio; HT = hypertension; ACEI = angiotensin-converting enzyme inhibitor; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; FBG = fasting blood glucose; Chol = cholesterol; Trig = triglycerides, LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; statins = HMGCoA reductase inhibitors; Cr = creatinine, Cr Cl = creatinine clearance; Alb/Cr = albumin:creatinine ratio; Pr/Cr = protein:creatinine ratio; RCC = red cell count.
Demographics, classical vascular risk factors and renal dependant parameters
| Study parameter . | Controls . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Matching criteria | ||||||||
| n (M:F) | 51 (38:13) | 51 (38:13) | 46 (18:28)†,‡ | 73 (32:41)†,‡ | ||||
| Age (years) | 42.7±1.7 | 45.7±2.0 | 40.1±1.9 | 38.1±1.7 | ||||
| BMI (kg/m2) | 26.8±0.5 | 28.3±0.7 | 30.0±1.2+ | 24.7±0.4 | ||||
| WHR | 0.87±0.01 | 0.89±0.02 | 0.90±0.01 | 0.85±0.01 | ||||
| Smoking (pack years) | 4.6±1.3 | 5.8±1.6 | 13.9±2.5 | 13.7±2.2 | ||||
| Haemodynamic parameters | ||||||||
| Diagnosed HT (n) | 8 | 24†,§ | 31†,§ | 14 | ||||
| ACEI (n) | 1 | 26†,§ | 25†,§ | 9 | ||||
| HR (b.p.m.) | 63±1 | 67±2 | 78±2* | 71±2 | ||||
| SBP (mmHg) | 124±2 | 134±2† | 141±2†,§ | 126±2 | ||||
| DBP (mmHg) | 72±2 | 78±1§ | 77±2 | 70±1 | ||||
| PP (mmHg) | 52±1 | 56±1* | 65±2†,**,+ | 56±1* | ||||
| Glycaemia and lipid profiles | ||||||||
| FBG (mmol/l) | 5.07±0.08 | 5.29±0.07 | 12.27±0.89†,‡ | 12.66±0.66†,‡ | ||||
| HbA1c (%) | 5.11±0.06 | 5.37±0.06 | 8.98±0.32†,‡ | 7.98±0.16†,‡ | ||||
| Total Chol (mmol/l) | 5.6±0.1 | 5.5±0.2 | 5.3±0.2 | 4.9±0.1* | ||||
| Trig (mmol/l) | 1.3±0.1 | 1.5±0.1 | 1.5±0.1 | 1.1±0.1 | ||||
| Calculated LDL-C (mmol/l) | 3.5±0.1 | 3.4±0.1 | 3.2±0.2 | 2.8±0.1* | ||||
| HDL-C (mmol/l) | 1.44±0.05 | 1.32±0.07 | 1.5±0.2 | 1.6±0.1 | ||||
| Statins (n) | 1 | 2 | 10† | 7† | ||||
| Renal parameters | ||||||||
| Serum Cr (mmol/l) | 0.09±0.00 | 0.12±0.01*,+ | 0.12±0.01*,+ | 0.08±0.00 | ||||
| Calculated Cr Cl (ml/min) | 102±3 | 88±5* | 84±5* | 94±7 | ||||
| Urinary Alb/Cr (mg/mmol) | 0.8±0.2 | 51.6±11.3†,§ | 66.4±20.9 | 0.9±0.1 | ||||
| Urinary Pr/Cr (mg/mmol) | 9.5±1.3 | 79.0±13.3†,§ | 91.7±36.9 | 8.8±1.0 | ||||
| Urine glomerular RCC (×106/l)‡‡ | <10 | 44:5:2* | <10 | <10 | ||||
| Cystatin C (mg/l) | 0.83±0.02 | 1.34±0.12† | 1.49±0.16†,§ | 0.75±0.01 | ||||
| Study parameter . | Controls . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Matching criteria | ||||||||
| n (M:F) | 51 (38:13) | 51 (38:13) | 46 (18:28)†,‡ | 73 (32:41)†,‡ | ||||
| Age (years) | 42.7±1.7 | 45.7±2.0 | 40.1±1.9 | 38.1±1.7 | ||||
| BMI (kg/m2) | 26.8±0.5 | 28.3±0.7 | 30.0±1.2+ | 24.7±0.4 | ||||
| WHR | 0.87±0.01 | 0.89±0.02 | 0.90±0.01 | 0.85±0.01 | ||||
| Smoking (pack years) | 4.6±1.3 | 5.8±1.6 | 13.9±2.5 | 13.7±2.2 | ||||
| Haemodynamic parameters | ||||||||
| Diagnosed HT (n) | 8 | 24†,§ | 31†,§ | 14 | ||||
| ACEI (n) | 1 | 26†,§ | 25†,§ | 9 | ||||
| HR (b.p.m.) | 63±1 | 67±2 | 78±2* | 71±2 | ||||
| SBP (mmHg) | 124±2 | 134±2† | 141±2†,§ | 126±2 | ||||
| DBP (mmHg) | 72±2 | 78±1§ | 77±2 | 70±1 | ||||
| PP (mmHg) | 52±1 | 56±1* | 65±2†,**,+ | 56±1* | ||||
| Glycaemia and lipid profiles | ||||||||
| FBG (mmol/l) | 5.07±0.08 | 5.29±0.07 | 12.27±0.89†,‡ | 12.66±0.66†,‡ | ||||
| HbA1c (%) | 5.11±0.06 | 5.37±0.06 | 8.98±0.32†,‡ | 7.98±0.16†,‡ | ||||
| Total Chol (mmol/l) | 5.6±0.1 | 5.5±0.2 | 5.3±0.2 | 4.9±0.1* | ||||
| Trig (mmol/l) | 1.3±0.1 | 1.5±0.1 | 1.5±0.1 | 1.1±0.1 | ||||
| Calculated LDL-C (mmol/l) | 3.5±0.1 | 3.4±0.1 | 3.2±0.2 | 2.8±0.1* | ||||
| HDL-C (mmol/l) | 1.44±0.05 | 1.32±0.07 | 1.5±0.2 | 1.6±0.1 | ||||
| Statins (n) | 1 | 2 | 10† | 7† | ||||
| Renal parameters | ||||||||
| Serum Cr (mmol/l) | 0.09±0.00 | 0.12±0.01*,+ | 0.12±0.01*,+ | 0.08±0.00 | ||||
| Calculated Cr Cl (ml/min) | 102±3 | 88±5* | 84±5* | 94±7 | ||||
| Urinary Alb/Cr (mg/mmol) | 0.8±0.2 | 51.6±11.3†,§ | 66.4±20.9 | 0.9±0.1 | ||||
| Urinary Pr/Cr (mg/mmol) | 9.5±1.3 | 79.0±13.3†,§ | 91.7±36.9 | 8.8±1.0 | ||||
| Urine glomerular RCC (×106/l)‡‡ | <10 | 44:5:2* | <10 | <10 | ||||
| Cystatin C (mg/l) | 0.83±0.02 | 1.34±0.12† | 1.49±0.16†,§ | 0.75±0.01 | ||||
*P<0.05 compared with controls; **P<0.05 compared with IgA nephropathy subjects; +P<0.05 compared with type 1 diabetes subjects without nephropathy; †P<0.001 compared with controls; ‡P<0.001 compared with IgA nephropathy subjects; §P<0.001 compared with type 1 diabetes subjects without nephropathy.
‡‡Mild <200; moderate 200–1000, severe >1000×106/l.
M = male; F = female; BMI = body mass index; WHR = waist:hip ratio; HT = hypertension; ACEI = angiotensin-converting enzyme inhibitor; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; FBG = fasting blood glucose; Chol = cholesterol; Trig = triglycerides, LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; statins = HMGCoA reductase inhibitors; Cr = creatinine, Cr Cl = creatinine clearance; Alb/Cr = albumin:creatinine ratio; Pr/Cr = protein:creatinine ratio; RCC = red cell count.
Vascular risk factors
Twenty-four IgAN and 31 T1DM Nx subjects had previously diagnosed hypertension, differing from T1DM No Nx and control subjects. More IgAN and T1DM Nx subjects were taking angiotensin-converting enzyme (ACE) inhibitors, and haemodynamic parameters were worse in IgAN and T1DM Nx compared with controls and T1DM No Nx (Table 1).
Fasting blood glucose and HbA1c were elevated in diabetes. Levels in the other groups were within the non-diabetic range, but were significantly higher in the IgAN subjects than controls (Table 1).
Lipid profiles were similar between the groups except that the T1DM No Nx group had significantly lower total and low-density lipoprotein (LDL)-cholesterol than controls. There was greater use of lipid-lowering (HMG CoA reductase inhibitor) therapy in T1DM subjects (Table 1). Reported family history of vascular disease and self-reported physical activity did not differ significantly between the groups.
Systemic inflammatory markers
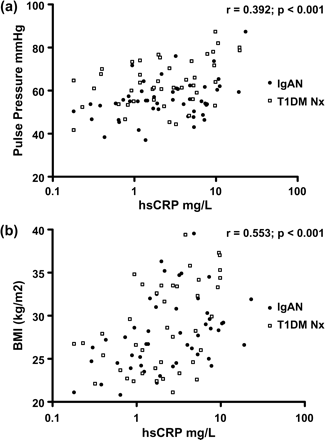
hsCRP, ESR and peripheral blood white cell count (WCC) were all significantly elevated in IgAN vs controls and T1DM Nx vs T1DM No Nx (Table 2). On univariate regression analysis, hsCRP in the IgAN group correlated with traditional vascular risk factors, in particular with PP, smoking packet years and BMI, but not with markers of renal function and renal disease activity. A similar result was found in the T1DM Nx group, with PP and BMI correlating with hsCRP levels (Table 3). On stepwise multivariate regression analysis excluding other systemic inflammatory markers, PP (T = 2.45, P<0.001) was the strongest independent correlate with hsCRP in the IgAN group. Multivariate analysis in the T1DM Nx group revealed BMI (T = 7.83, P<0.001) to be the strongest independent predictor. Combining nephropathy subgroups, BMI (T = 6.11, P<0.001) and PP (T = 2.26, P = 0.02) retained independent associations with hsCRP on multivariate regression analysis (Figure 1a and b).
Inflammatory markers
| Inflammatory marker . | Control . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Systemic | ||||||||
| WCC × 109/l | 5.4±0.2 | 6.3±0.2* | 7.8±0.4**,+ | 6.4±0.3* | ||||
| ESR (mm/h) | 7.2±0.8 | 13.4±0.8* | 21.2±3.3†,§ | 9.3±1.5 | ||||
| Ferritin (µg/l) | 144±46 | 169±25 | 150±45 | 168±39 | ||||
| hsCRP (mg/l) | 1.6±0.3 | 4.2±0.6†,+ | 4.1±0.6†,+ | 2.6±0.4* | ||||
| Vascular | ||||||||
| sVCAM-1 (ng/ml) | 536±15 | 663±34† | 760±30†,§ | 601±21* | ||||
| sICAM-1 (ng/ml) | 244±6 | 313±13† | 320±8† | 307±8† | ||||
| Inflammatory marker . | Control . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Systemic | ||||||||
| WCC × 109/l | 5.4±0.2 | 6.3±0.2* | 7.8±0.4**,+ | 6.4±0.3* | ||||
| ESR (mm/h) | 7.2±0.8 | 13.4±0.8* | 21.2±3.3†,§ | 9.3±1.5 | ||||
| Ferritin (µg/l) | 144±46 | 169±25 | 150±45 | 168±39 | ||||
| hsCRP (mg/l) | 1.6±0.3 | 4.2±0.6†,+ | 4.1±0.6†,+ | 2.6±0.4* | ||||
| Vascular | ||||||||
| sVCAM-1 (ng/ml) | 536±15 | 663±34† | 760±30†,§ | 601±21* | ||||
| sICAM-1 (ng/ml) | 244±6 | 313±13† | 320±8† | 307±8† | ||||
*P<0.05 compared with controls; **P<0.05 compared with IgA nephropathy subjects; +P<0.05 compared with type 1 diabetes subjects without nephropathy; †P<0.001 compared with controls; §P<0.001 compared with type 1 diabetes subjects without nephropathy.
IgAN = IgA nephropathy; T1DM Nx = type 1 diabetes mellitus with known nephropathy; T1DM No Nx = type 1 diabetes mellitus with no nephropathy; WCC = white cell count; ESR = erythrocyte sedimentation rate; hsCRP = high sensitivity C-reactive protein; sVCAM-1 = soluble vascular cell adhesion molecule-1; sICAM-1 = soluble intracellular adhesion molecule-1.
Inflammatory markers
| Inflammatory marker . | Control . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Systemic | ||||||||
| WCC × 109/l | 5.4±0.2 | 6.3±0.2* | 7.8±0.4**,+ | 6.4±0.3* | ||||
| ESR (mm/h) | 7.2±0.8 | 13.4±0.8* | 21.2±3.3†,§ | 9.3±1.5 | ||||
| Ferritin (µg/l) | 144±46 | 169±25 | 150±45 | 168±39 | ||||
| hsCRP (mg/l) | 1.6±0.3 | 4.2±0.6†,+ | 4.1±0.6†,+ | 2.6±0.4* | ||||
| Vascular | ||||||||
| sVCAM-1 (ng/ml) | 536±15 | 663±34† | 760±30†,§ | 601±21* | ||||
| sICAM-1 (ng/ml) | 244±6 | 313±13† | 320±8† | 307±8† | ||||
| Inflammatory marker . | Control . | IgAN . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Systemic | ||||||||
| WCC × 109/l | 5.4±0.2 | 6.3±0.2* | 7.8±0.4**,+ | 6.4±0.3* | ||||
| ESR (mm/h) | 7.2±0.8 | 13.4±0.8* | 21.2±3.3†,§ | 9.3±1.5 | ||||
| Ferritin (µg/l) | 144±46 | 169±25 | 150±45 | 168±39 | ||||
| hsCRP (mg/l) | 1.6±0.3 | 4.2±0.6†,+ | 4.1±0.6†,+ | 2.6±0.4* | ||||
| Vascular | ||||||||
| sVCAM-1 (ng/ml) | 536±15 | 663±34† | 760±30†,§ | 601±21* | ||||
| sICAM-1 (ng/ml) | 244±6 | 313±13† | 320±8† | 307±8† | ||||
*P<0.05 compared with controls; **P<0.05 compared with IgA nephropathy subjects; +P<0.05 compared with type 1 diabetes subjects without nephropathy; †P<0.001 compared with controls; §P<0.001 compared with type 1 diabetes subjects without nephropathy.
IgAN = IgA nephropathy; T1DM Nx = type 1 diabetes mellitus with known nephropathy; T1DM No Nx = type 1 diabetes mellitus with no nephropathy; WCC = white cell count; ESR = erythrocyte sedimentation rate; hsCRP = high sensitivity C-reactive protein; sVCAM-1 = soluble vascular cell adhesion molecule-1; sICAM-1 = soluble intracellular adhesion molecule-1.
Correlations of hsCRP and indices of vascular risk factors and renal function
| . | IgAN . | Controls . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Vascular risks | ||||||||
| SBP | 0.294* | 0.235 | 0.037 | 0.216 | ||||
| DBP | 0.059 | 0.061 | −0.324 | 0.303 | ||||
| PP | 0.467† | 0.339* | 0.403* | −0.022 | ||||
| T Chol | −0.232 | 0.226 | −0.224 | 0.068 | ||||
| LDL-C | −0.170 | 0.330* | −0.161 | 0.030 | ||||
| HDL-C | −0.206 | −0.223 | −0.143 | 0.022 | ||||
| Trig | −0.034 | 0.232 | −0.062 | 0.061 | ||||
| Smoking (pack years) | 0.431+ | 0.577† | 0.071 | 0.479* | ||||
| BMI | 0.311* | 0.324* | 0.767† | 0.221 | ||||
| FBG | 0.196 | 0.091 | −0.417 | −0.035 | ||||
| HbAIc | 0.144 | −0.151 | −0.022 | 0.016 | ||||
| Renal function | ||||||||
| Serum Cr | 0.066 | −0.081 | −0.071 | −0.208 | ||||
| Calculated Cr Cl | 0.072 | 0.003 | 0.181 | 0.090 | ||||
| Cystatin C | 0.216 | 0.288* | 0.152 | 0.034 | ||||
| Urine Pr/Cr | 0.053 | – | −0.159 | 0.218 | ||||
| Urine RCC | −0.180 | – | – | – | ||||
| . | IgAN . | Controls . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Vascular risks | ||||||||
| SBP | 0.294* | 0.235 | 0.037 | 0.216 | ||||
| DBP | 0.059 | 0.061 | −0.324 | 0.303 | ||||
| PP | 0.467† | 0.339* | 0.403* | −0.022 | ||||
| T Chol | −0.232 | 0.226 | −0.224 | 0.068 | ||||
| LDL-C | −0.170 | 0.330* | −0.161 | 0.030 | ||||
| HDL-C | −0.206 | −0.223 | −0.143 | 0.022 | ||||
| Trig | −0.034 | 0.232 | −0.062 | 0.061 | ||||
| Smoking (pack years) | 0.431+ | 0.577† | 0.071 | 0.479* | ||||
| BMI | 0.311* | 0.324* | 0.767† | 0.221 | ||||
| FBG | 0.196 | 0.091 | −0.417 | −0.035 | ||||
| HbAIc | 0.144 | −0.151 | −0.022 | 0.016 | ||||
| Renal function | ||||||||
| Serum Cr | 0.066 | −0.081 | −0.071 | −0.208 | ||||
| Calculated Cr Cl | 0.072 | 0.003 | 0.181 | 0.090 | ||||
| Cystatin C | 0.216 | 0.288* | 0.152 | 0.034 | ||||
| Urine Pr/Cr | 0.053 | – | −0.159 | 0.218 | ||||
| Urine RCC | −0.180 | – | – | – | ||||
*P<0.05; +P<0.005; †P<0.001. Significant values are in bold.
Correlations of hsCRP and indices of vascular risk factors and renal function
| . | IgAN . | Controls . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Vascular risks | ||||||||
| SBP | 0.294* | 0.235 | 0.037 | 0.216 | ||||
| DBP | 0.059 | 0.061 | −0.324 | 0.303 | ||||
| PP | 0.467† | 0.339* | 0.403* | −0.022 | ||||
| T Chol | −0.232 | 0.226 | −0.224 | 0.068 | ||||
| LDL-C | −0.170 | 0.330* | −0.161 | 0.030 | ||||
| HDL-C | −0.206 | −0.223 | −0.143 | 0.022 | ||||
| Trig | −0.034 | 0.232 | −0.062 | 0.061 | ||||
| Smoking (pack years) | 0.431+ | 0.577† | 0.071 | 0.479* | ||||
| BMI | 0.311* | 0.324* | 0.767† | 0.221 | ||||
| FBG | 0.196 | 0.091 | −0.417 | −0.035 | ||||
| HbAIc | 0.144 | −0.151 | −0.022 | 0.016 | ||||
| Renal function | ||||||||
| Serum Cr | 0.066 | −0.081 | −0.071 | −0.208 | ||||
| Calculated Cr Cl | 0.072 | 0.003 | 0.181 | 0.090 | ||||
| Cystatin C | 0.216 | 0.288* | 0.152 | 0.034 | ||||
| Urine Pr/Cr | 0.053 | – | −0.159 | 0.218 | ||||
| Urine RCC | −0.180 | – | – | – | ||||
| . | IgAN . | Controls . | T1DM Nx . | T1DM No Nx . | ||||
|---|---|---|---|---|---|---|---|---|
| Vascular risks | ||||||||
| SBP | 0.294* | 0.235 | 0.037 | 0.216 | ||||
| DBP | 0.059 | 0.061 | −0.324 | 0.303 | ||||
| PP | 0.467† | 0.339* | 0.403* | −0.022 | ||||
| T Chol | −0.232 | 0.226 | −0.224 | 0.068 | ||||
| LDL-C | −0.170 | 0.330* | −0.161 | 0.030 | ||||
| HDL-C | −0.206 | −0.223 | −0.143 | 0.022 | ||||
| Trig | −0.034 | 0.232 | −0.062 | 0.061 | ||||
| Smoking (pack years) | 0.431+ | 0.577† | 0.071 | 0.479* | ||||
| BMI | 0.311* | 0.324* | 0.767† | 0.221 | ||||
| FBG | 0.196 | 0.091 | −0.417 | −0.035 | ||||
| HbAIc | 0.144 | −0.151 | −0.022 | 0.016 | ||||
| Renal function | ||||||||
| Serum Cr | 0.066 | −0.081 | −0.071 | −0.208 | ||||
| Calculated Cr Cl | 0.072 | 0.003 | 0.181 | 0.090 | ||||
| Cystatin C | 0.216 | 0.288* | 0.152 | 0.034 | ||||
| Urine Pr/Cr | 0.053 | – | −0.159 | 0.218 | ||||
| Urine RCC | −0.180 | – | – | – | ||||
*P<0.05; +P<0.005; †P<0.001. Significant values are in bold.
Pulse pressure (a) and body mass index (b) vs high sensitivity C-reactive protein (hsCRP) in IgA nephropathy and type 1 diabetes mellitus subjects with nephropathy.
In control subjects, hsCRP also correlated with classical vascular risk factors (Table 3). Stepwise multivariate regression analysis revealed BMI to be an independent predictor (T = 2.5; P = 0.017) as well as smoking packet years (T = 2.72; P = 0.011), while an inverse independent association was found with high-density lipoprotein (HDL; T = −2.08, P = 0.045) in the control subjects.
Vascular inflammatory markers
Levels of sVCAM-1 and sICAM-1 were significantly elevated in IgAN and T1DM subjects vs control subjects (Table 2).
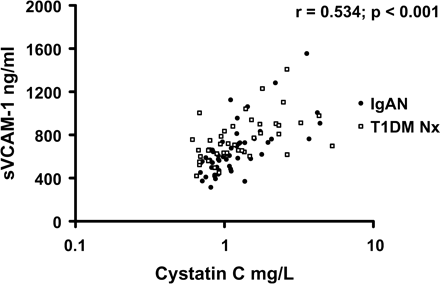
There was an association between sVCAM-1 and PP and an inverse association with HDL-cholesterol levels in T1DM Nx subjects (Table 4). In IgAN and T1DM Nx, sVCAM-1 levels correlated positively with measures of renal dysfunction (Table 4). Cystatin C was the only independent predictor of sVCAM-1 on stepwise multivariate regression analysis in IgAN and T1DM Nx (T = 6.12, P<0.001; T = 2.73, P = 0.009). This relationship remained strong when combining nephropathy groups (Figure 2). sVCAM-1 levels also correlated with cystatin C levels in control subjects (Table 4). There was no statistically significant correlation of renal function with VCAM-1 in T1DM No Nx.
Correlations of vascular indices of inflammation and vascular health, vascular risk factors and renal function
| . | sVCAM-1 . | . | . | sICAM-1 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | IgAN . | Controls . | TIDM Nx . | IgAN . | Controls . | TIDM Nx . | ||||||
| Vascular risks | ||||||||||||
| SBP | 0.110 | −0.087 | 0.171 | 0.084 | −0.189 | −0.142 | ||||||
| DBP | 0.122 | 0.094 | −0.101 | 0.032 | −0.140 | −0.268 | ||||||
| PP | 0.053 | −0.295 | 0.331* | 0.131 | −0.135 | 0.077 | ||||||
| T Chol | −0.233 | −0.079 | −0.217 | −0.180 | −0.230 | −0.196 | ||||||
| LDL-C | −0.201 | −0.307 | −0.157 | −0.138 | −0.115 | −0.165 | ||||||
| HDL-C | −0.079 | 0.134 | −0.319* | −0.323* | −0.256 | −0.102 | ||||||
| Trig | −0.098 | −0.164 | 0.189 | 0.180 | −0.033 | 0.004 | ||||||
| Smoking (pack years) | 0.268 | −0.075 | 0.165 | 0.528† | −0.160 | −0.055 | ||||||
| BMI | −0.262 | −0.240 | 0.102 | 0.130 | −0.155 | 0.227 | ||||||
| FBG | −0.005 | −0.216 | −0.122 | 0.286* | 0.000 | −0.092 | ||||||
| HbAIc | 0.360* | 0.002 | 0.187 | −0.038 | −0.184 | 0.062 | ||||||
| Renal function | ||||||||||||
| Serum Cr | 0.464† | 0.225 | 0.416* | −0.034 | 0.040 | 0.166 | ||||||
| Calc Cr Cl | −0.562† | −0.278* | −0.134 | 0.117 | −0.028 | −0.076 | ||||||
| Cystatin C | 0.632† | 0.322* | 0.425+ | −0.007 | 0.291* | 0.054 | ||||||
| Urine Pr/Cr | 0.480† | – | 0.185 | −0.066 | – | −0.157 | ||||||
| Urine RCC | −0.071 | – | – | −0.284 | – | – | ||||||
| . | sVCAM-1 . | . | . | sICAM-1 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | IgAN . | Controls . | TIDM Nx . | IgAN . | Controls . | TIDM Nx . | ||||||
| Vascular risks | ||||||||||||
| SBP | 0.110 | −0.087 | 0.171 | 0.084 | −0.189 | −0.142 | ||||||
| DBP | 0.122 | 0.094 | −0.101 | 0.032 | −0.140 | −0.268 | ||||||
| PP | 0.053 | −0.295 | 0.331* | 0.131 | −0.135 | 0.077 | ||||||
| T Chol | −0.233 | −0.079 | −0.217 | −0.180 | −0.230 | −0.196 | ||||||
| LDL-C | −0.201 | −0.307 | −0.157 | −0.138 | −0.115 | −0.165 | ||||||
| HDL-C | −0.079 | 0.134 | −0.319* | −0.323* | −0.256 | −0.102 | ||||||
| Trig | −0.098 | −0.164 | 0.189 | 0.180 | −0.033 | 0.004 | ||||||
| Smoking (pack years) | 0.268 | −0.075 | 0.165 | 0.528† | −0.160 | −0.055 | ||||||
| BMI | −0.262 | −0.240 | 0.102 | 0.130 | −0.155 | 0.227 | ||||||
| FBG | −0.005 | −0.216 | −0.122 | 0.286* | 0.000 | −0.092 | ||||||
| HbAIc | 0.360* | 0.002 | 0.187 | −0.038 | −0.184 | 0.062 | ||||||
| Renal function | ||||||||||||
| Serum Cr | 0.464† | 0.225 | 0.416* | −0.034 | 0.040 | 0.166 | ||||||
| Calc Cr Cl | −0.562† | −0.278* | −0.134 | 0.117 | −0.028 | −0.076 | ||||||
| Cystatin C | 0.632† | 0.322* | 0.425+ | −0.007 | 0.291* | 0.054 | ||||||
| Urine Pr/Cr | 0.480† | – | 0.185 | −0.066 | – | −0.157 | ||||||
| Urine RCC | −0.071 | – | – | −0.284 | – | – | ||||||
*P<0.05; +P<0.005; †P<0.001. Significant values are in bold.
Correlations of vascular indices of inflammation and vascular health, vascular risk factors and renal function
| . | sVCAM-1 . | . | . | sICAM-1 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | IgAN . | Controls . | TIDM Nx . | IgAN . | Controls . | TIDM Nx . | ||||||
| Vascular risks | ||||||||||||
| SBP | 0.110 | −0.087 | 0.171 | 0.084 | −0.189 | −0.142 | ||||||
| DBP | 0.122 | 0.094 | −0.101 | 0.032 | −0.140 | −0.268 | ||||||
| PP | 0.053 | −0.295 | 0.331* | 0.131 | −0.135 | 0.077 | ||||||
| T Chol | −0.233 | −0.079 | −0.217 | −0.180 | −0.230 | −0.196 | ||||||
| LDL-C | −0.201 | −0.307 | −0.157 | −0.138 | −0.115 | −0.165 | ||||||
| HDL-C | −0.079 | 0.134 | −0.319* | −0.323* | −0.256 | −0.102 | ||||||
| Trig | −0.098 | −0.164 | 0.189 | 0.180 | −0.033 | 0.004 | ||||||
| Smoking (pack years) | 0.268 | −0.075 | 0.165 | 0.528† | −0.160 | −0.055 | ||||||
| BMI | −0.262 | −0.240 | 0.102 | 0.130 | −0.155 | 0.227 | ||||||
| FBG | −0.005 | −0.216 | −0.122 | 0.286* | 0.000 | −0.092 | ||||||
| HbAIc | 0.360* | 0.002 | 0.187 | −0.038 | −0.184 | 0.062 | ||||||
| Renal function | ||||||||||||
| Serum Cr | 0.464† | 0.225 | 0.416* | −0.034 | 0.040 | 0.166 | ||||||
| Calc Cr Cl | −0.562† | −0.278* | −0.134 | 0.117 | −0.028 | −0.076 | ||||||
| Cystatin C | 0.632† | 0.322* | 0.425+ | −0.007 | 0.291* | 0.054 | ||||||
| Urine Pr/Cr | 0.480† | – | 0.185 | −0.066 | – | −0.157 | ||||||
| Urine RCC | −0.071 | – | – | −0.284 | – | – | ||||||
| . | sVCAM-1 . | . | . | sICAM-1 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | IgAN . | Controls . | TIDM Nx . | IgAN . | Controls . | TIDM Nx . | ||||||
| Vascular risks | ||||||||||||
| SBP | 0.110 | −0.087 | 0.171 | 0.084 | −0.189 | −0.142 | ||||||
| DBP | 0.122 | 0.094 | −0.101 | 0.032 | −0.140 | −0.268 | ||||||
| PP | 0.053 | −0.295 | 0.331* | 0.131 | −0.135 | 0.077 | ||||||
| T Chol | −0.233 | −0.079 | −0.217 | −0.180 | −0.230 | −0.196 | ||||||
| LDL-C | −0.201 | −0.307 | −0.157 | −0.138 | −0.115 | −0.165 | ||||||
| HDL-C | −0.079 | 0.134 | −0.319* | −0.323* | −0.256 | −0.102 | ||||||
| Trig | −0.098 | −0.164 | 0.189 | 0.180 | −0.033 | 0.004 | ||||||
| Smoking (pack years) | 0.268 | −0.075 | 0.165 | 0.528† | −0.160 | −0.055 | ||||||
| BMI | −0.262 | −0.240 | 0.102 | 0.130 | −0.155 | 0.227 | ||||||
| FBG | −0.005 | −0.216 | −0.122 | 0.286* | 0.000 | −0.092 | ||||||
| HbAIc | 0.360* | 0.002 | 0.187 | −0.038 | −0.184 | 0.062 | ||||||
| Renal function | ||||||||||||
| Serum Cr | 0.464† | 0.225 | 0.416* | −0.034 | 0.040 | 0.166 | ||||||
| Calc Cr Cl | −0.562† | −0.278* | −0.134 | 0.117 | −0.028 | −0.076 | ||||||
| Cystatin C | 0.632† | 0.322* | 0.425+ | −0.007 | 0.291* | 0.054 | ||||||
| Urine Pr/Cr | 0.480† | – | 0.185 | −0.066 | – | −0.157 | ||||||
| Urine RCC | −0.071 | – | – | −0.284 | – | – | ||||||
*P<0.05; +P<0.005; †P<0.001. Significant values are in bold.
Soluble vascular cell adhesion molecule-1 (sVCAM-1) vs cystatin C in IgA nephropathy and type 1 diabetes mellitus subjects with nephropathy.
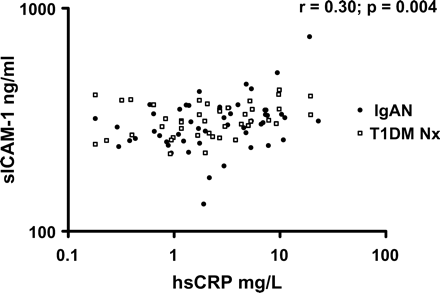
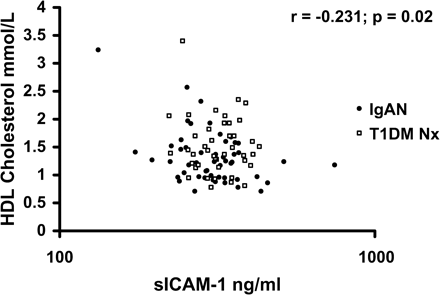
In IgAN subjects, significant correlations were noted between sICAM-1 and hsCRP (r = 0.33, P = 0.017) and smoking pack years (Table 4). An inverse association was found between sICAM-1 and HDL-cholesterol. There was also a significant correlation with sICAM-1 and CRP in the T1DM Nx group (r = 0.37, P = 0.017), and combining nephropathy groups revealed that the relationship between sICAM-1 and CRP was stronger (Figure 3). The relationship with hsCRP and HDL-cholesterol remained significant when combining the two nephropathy groups (Figure 4). There was no association with sICAM-1 and haemodynamic or renal disease parameters in either nephropathy group. On multivariate regression analysis, the only statistically significant independent predictor of sICAM-1 in IgAN was smoking packet years (T = 2.06, P = 0.046).
Soluble intracellular adhesion molecule-1 (sICAM-1) vs high sensitivity C-reactive protein (hsCRP) in IgA nephropathy and type 1 diabetes mellitus subjects with nephropathy.
High-density lipoprotein (HDL) cholesterol vs soluble intracellular adhesion molecule-1 (sICAM-1) in IgA nephropathy and type 1 diabetes mellitus subjects with nephropathy.
Discussion
CVD is well known to be accelerated in renal disease and to occur earlier than in the general population [2]. The pathological arterial changes associated with renal disease are associated with increased vascular stiffness, which is reflected by widened PP. Earlier detection of vascular disease in this patient population is a clinical priority so as to direct more intensive preventive treatments.
Measures of inflammation previously have been associated with increased cardiovascular mortality in the general population and in subjects with late or ESRF. We have demonstrated elevation of systemic inflammatory markers and sCAMs in very early IgAN vs controls and early T1DM Nx vs T1DM No Nx. The associations of the systemic and vascular inflammatory markers in the two nephropathy groups were similar.
The inflammatory marker hsCRP has been implicated in the accelerated atherosclerosis of ESRF. hsCRP is a strong independent predictor of overall death and cardiovascular death in haemodialysis patients [4]. Other studies have found inverse relationships between renal function and levels of the inflammatory markers CRP and interleukin-6 in the pre-dialysis population [9]. Inflammation may be elevated from very early stages of renal decline as evidenced by large studies in the general community [10]. In keeping with this, we have also found elevations of hsCRP early in two common types of renal disease, IgAN compared with general community controls and T1DM with nephropathy compared with those without nephropathy. Our results also agree with a study showing elevation of hsCRP in IgAN as well as non-immune renal disease that could not be explained by markers of IgAN activity [11]. It was hypothesized that this elevation of hsCRP may relate to vascular disease rather than the IgAN. Our findings of the higher hsCRP in IgAN and T1DM Nx correlating with markers of vascular dysfunction (PP) and vascular risk factors rather than renal dysfunction are supportive.
CRP is associated with adiposity in the general community. This association remains true in IgAN and T1DM Nx, with BMI being the strongest independent predictor on multivariate regression analysis of CRP levels in T1DM alone and when both nephropathy groups were pooled (Figure 1b). HsCRP had the strongest independent association on multivariate regression analysis with PP in IgAN, was a significant correlate in the T1DM Nx group and remained independent of BMI on multivariate regression analysis when these groups were pooled (Figure 1a). This suggests a relationship between CRP and vascular dysfunction in early renal disease. This relationship between CRP and PP has also been demonstrated in larger population studies in apparently healthy individuals [12]. The elevated BMI in our T1DM Nx group compared with the T1DM No Nx group also raises interesting questions regarding the role of inflammation and insulin resistance in the propensity of T1DM to develop microvascular as well as macrovascular complications.
Cell culture and animal studies have shown that CRP and lipids may contribute to endothelial dysfunction via induction of CAMs [13,14]. VCAM-1 and ICAM-1 expression may increase arterial intimal accumulation of T lymphocytes, contributing to atherosclerosis [15]. Circulating forms of these adhesion molecules have been associated with clinical vascular events and all-cause mortality in the renal population [7]. We have shown in early renal disease, significant increases of sVCAM-1 and sICAM-1 in IgAN and T1DM Nx and T1DM No Nx, with greater levels of sVCAM-1 in T1DM Nx compared with T1DM No Nx. We have shown a strong relationship between hsCRP and sICAM-1 in IgAN and T1DM Nx which was more significant when both nephropathy groups were pooled (Figure 3). A similar association has been reported previously in near ESRF [7]. This previous study revealed associations between inflammation, malnutrition and CVD, as well as all-cause mortality. Our study in early renal disease demonstrates an association between increased BMI, inflammation and PP. Although inflammation is present in early renal disease, the stimulus for its generation may be different from that in ESRF, but the vascular consequences may be similar.
Increases in CAMs have been associated with several forms of glomerulonephritis, and these are believed to promote leukocyte activation, antigen presentation, free radical generation and cytokine production, contributing to structural and functional glomerular and interstitial injury [15]. A histopathological study in IgAN found an association between VCAM-1 tissue expression and disease activity in biopsy sections as well as an association between VCAM-1 histochemical staining and sVCAM-1, though in this study there was no direct correlation with calculated creatinine clearance [16]. The correlation of soluble forms of CAMs with the severity of glomerulonephritis or with disease progression is not known. In our study, sVCAM-1 correlated with measures of worsening renal function in IgAN including cystatin C, calculated creatinine clearance and urine protein:creatinine ratio. A similar association was found with T1DM Nx subjects with serum creatinine and cystatin C. There was no association with calculated creatinine clearance in the T1DM Nx group, which may relate to inaccuracies in this measure of renal function with increasing BMI. The relationship between sVCAM-1 and cystatin C remained strong when nephropathy groups were combined (Figure 2). This result is consistent with studies in near ESRF (average GFR 7 ml/min) [7]. This result could be interpreted as indicating a renal clearance mechanism for sVCAM-1 and may also support histopathological studies suggesting a relationship between disease activity and VCAM-1 [16]. In our study, sICAM-1 was not associated with renal function or disease activity in IgAN or T1DM, consistent with previous histopathological and serological studies in IgAN [16]. Further studies are required addressing the effects of VCAM-1 blockade on renal function and renal disease progression as well as the incidence of vascular disease.
We found an inverse association between HDL-cholesterol and sICAM-1 in IgAN, which remains significant when T1DM Nx subjects are added to the analysis (Figure 4), and an inverse association between HDL-cholesterol and sVCAM-1 in T1DM Nx (Table 4). This suggests a potential anti-inflammatory role for HDL in early renal disease. It has been shown in vitro with endothelial cell culture systems that HDL suppresses CAM expression [17]. We believe this to be the first report of a similar association in vivo in subjects with renal disease. Such a link would suggest that increasing HDL with pharmacotherapy or lifestyle modification prior to the onset of ESRF may be beneficial.
In summary, systemic inflammatory markers and circulating CAMs are elevated in the early stages of diabetic and non-diabetic renal disease, before the onset of pre-dialysis and dialysis-dependent renal failure. Elevated levels of CRP from vascular risks including adiposity and smoking may potentiate vascular damage as reflected by increased PP. CRP may potentiate the atherogenicity of lipids and may induce expression of CAMs, such as sICAM-1. Renal dysfunction itself may be important in promoting elevations of sVCAM-1. These responses could be involved in the acceleration of atherosclerosis and the progression of renal disease. Intervention trials are warranted in early renal disease to assess whether lowering these inflammatory markers translates into improved vascular and renal outcomes.
We thank Kevin G. Rowley, Eve Anwar, Andzej Januszewski and Jasmine Chung for assistance with patient characterization. The following doctors are also thanked for their contribution of patients to this study: Anastasia Chrysostomou, Paul Champion DeCrespigny, Petrova Lee, Alex Harper and Richard Gilbert. This work was supported by grants from: University of Melbourne and St Vincent's Hospital, Melbourne, Australia; National Health and Medical Research Council, Australia; Pfizer Cardiovascular Lipid Research Grants Scheme; Juvenile Diabetes Research Foundation, International. American Diabetes Association Lions Sight First Program, and the National Heart Foundation (Australia).
Conflict of interest statement. None declared.
References
Foley RN, Parfrey PS, Sarnack MJ. Clinical epidemiology of cardiovascular disease in chronic renal failure.
Iseki K, Fukiyama K. Long term prognosis and incidence of acute myocardial infarction in patients on chronic haemodialysis.
London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure.
Zimmerman J, Herrlinger S, Pruy A et al. Inflammation enhances cardiovascular risk and mortality in haemodialysis patients.
Stenvinkel P, Heimburger O, Paultre F et al. Strong association between malnutrition, inflammation and atherosclerosis in chronic renal failure.
Mezzano D, Pais EO, Aranda E et al. Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uraemia.
Stenvinkel P, Lindholm B, Heimburger M et al. Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: association with malnutrition, inflammation and cardiovascular disease.
Mourad J, Pannier B, Blacher J et al. Creatinine clearance, pulse wave velocity, carotid compliance and essential hypertension.
Panichi V, Migliori M, De Pietro S et al. C-reactive protein as a marker of chronic inflammation in uraemic patients.
Stuveling EM, Hillege HL, Bakker SJ et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population.
Janssen U, Bahlmann F, Kohl J et al. Activation of the acute phase response and complement C3 in patients with IgA nephropathy.
Amar J, Ruidavets JB, Sollier CB et al. Relationship between C reactive protein and pulse pressure is not mediated by atherosclerosis or aortic stiffness.
Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells.
Hattori M, Nicolic-Paterson DJ, Miyazaki K et al. Mechanisms of glomerular macrophage infiltration in lipid-induced renal injury.
Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease.
Mrowka C, Heintz B, Sieberth HG. VCAM-1, ICAM-1, and E-selectin in IgA nephropathy and Schonlein–Henoch syndrome: differences between tissue expression and serum concentration.
Author notes
1The University of Melbourne, Department of Medicine, St Vincent's Hospital and 2Department of Nephrology, The Royal Melbourne Hospital, Victoria, Australia
- body mass index procedure
- kidney diseases
- cardiovascular diseases
- diabetic nephropathy
- inflammatory markers
- heart disease risk factors
- inflammation
- cell adhesion molecules
- renal function
- endothelial cells
- vascular diseases
- kidney failure, chronic
- diabetes mellitus, type 1
- creatinine
- kidney failure
- adhesions
- iga glomerulonephritis
- immunoglobulin a
- c-reactive protein
- mortality
- urine protein test
- creatinine clearance
- cystatin c measurement
- pulse pressure
- parallel study
- molecule





Comments