-
PDF
- Split View
-
Views
-
Cite
Cite
C. M. BRAUNS, J. M. HERGT, J. D. WOODHEAD, R. MAAS, Os Isotopes and the Origin of the Tasmanian Dolerites, Journal of Petrology, Volume 41, Issue 7, July 2000, Pages 905–918, https://doi.org/10.1093/petrology/41.7.905
Close - Share Icon Share
Abstract
New Os isotope data obtained for oxides separated from samples of the Jurassic dolerites of Tasmania (Australia) are used to constrain the petrogenesis of the Ferrar continental flood basalt province. It is proposed here that the unradiogenic initial Os ratio (187Os/188Os = 0·145 ± 0·049 2σ) of these rocks is inconsistent with petrogenetic models involving the assimilation of continental crust by mantle-derived magmas. Instead, the results are more readily explained by models in which the introduction of upper-crustal materials into the mantle contaminates the source of these magmas before melting. The increasing body of Os isotope data for mantle-derived rocks also provides new opportunities to assess whether any distinction between the roles of asthenospheric vs lithospheric mantle in the genesis of continental flood basalts might be possible. It is concluded that, in the absence of an Os-rich phase with low Re/Os, the uncertainties in calculated initial ratios preclude the distinction between mantle sources in this particular case.
INTRODUCTION
Continental flood basalts (CFBs; including both lava flows and high-level intrusions) are the expressions of dramatic partial melting episodes within the Earth’s mantle. Increasingly precise geochronological constraints have highlighted the short-lived nature of igneous activity in some provinces (e.g. <1 my for the bulk of magmatism in the Deccan; Officer & Drake, 1985; Courtillot et al., 1986), which is remarkable as the volumes of magma generated are thought to be in excess of 1 × 106 km3 (e.g. the Deccan case noted above). In general, CFBs are not primary magmas and have compositions that differ from typical mid-ocean ridge and ocean island basalts (MORB and OIB, respectively). In short, many CFBs formed from enormous volumes of basaltic magma that appear to have been generated over a restricted timespan, and with compositions unlike those of their oceanic counterparts.
For more than half a century CFBs have presented the geological community with cause for much enthusiastic scientific debate. In referring to the likely compositions of flood basalt parental magmas (picritic vs basaltic), Cox (1980) made the perceptive observation: ‘In the rather well-matched and evenly-balanced debate which has taken place so far it is tempting to discern that the views of various authors are coloured by the flood basalt provinces they have been most concerned with or influenced by.’ This comment could equally well apply to most issues surrounding CFB magmatism.
BACKGROUND
Continental flood basalts are now known to vary significantly in composition, both within and between magmatic provinces. For example, Cox et al. (1967) were the first to note the north–south variation in the Jurassic Karoo CFB magmatism of southern Africa in which Ti contents are distinctly higher in the northern sub-province. This observation was extended into studies of the Early Cretaceous Paraná (South America), and Jurassic Antarctic (Queen Maud Land) provinces by other workers (e.g. Ford & Kistler, 1980; Mantovani et al., 1985) where similar ‘high-Ti’ and ‘low-Ti’ provinces were recognized. Apart from their major element compositions, low- and high-Ti CFBs also tend to display constrasting incompatible trace element and isotopic compositions. In the case of low-Ti rocks, this can include unusual features, more reminiscent of continental crust than mantle-derived magmas (e.g. elevated initial 87Sr/86Sr ratios; Heier et al., 1965).
This clear ‘continental crustal’ influence, particularly encountered in the low-Ti CFBs, has sparked considerable debate. As these magmas clearly migrated through continental lithosphere, strong arguments have been forwarded in favour of crustal assimilation processes [e.g. bulk assimilation, AFC (assmilation–fractional crystallization), selective contamination; Faure et al., 1972; Allègre et al., 1982]. Although a number of clear examples of crustal assimilation have convincingly been demonstrated (e.g. Hoefs et al., 1980), it is also apparent that ‘At least some part of the evolved isotopic compositions seen in flood basalts must be attributed to their mantle source’ (Carlson, 1991). Continuing this theme, other workers have argued that crustal material might somehow pollute the mantle source region before magma generation (e.g. Hergt et al., 1989a, 1989b).
The sub-continental lithospheric mantle
The isotopic features of many CFBs are consistent with their derivation from a mantle source region with a long history of enrichment in incompatible elements. This is particularly true for the low-Ti CFBs and has led many workers to propose that the origin of these magmas lies within the sub-continental lithospheric mantle (SCLM) (e.g. Cox et al., 1984). Such hypotheses are also consistent with the depleted major element compositions of these particular CFB magmas (e.g. generally low Ti compared with MORB at the same MgO content). Whether the ‘re-enrichment’ of incompatible trace elements relates to the subduction of sedimentary material or fluids, or introduction of small-degree melts into the lithospheric mantle, this reservoir is viewed as a place in which long-term storage of such trace element enriched components might be achieved.
The asthenospheric mantle (e.g. upwelling mantle plumes)
The development of models in which the heads of ‘starting plumes’ were proposed to supply both the heat and mantle source of voluminous magmatism (e.g. Richards et al., 1989; White & McKenzie, 1989) provided an explanation for both the size and rapid outpouring of CFB magmatism. Proponents of these models argue that the lithospheric mantle is too cold and infertile to supply the large volumes of magma observed (e.g. McKenzie & Bickle, 1988; Arndt & Christensen, 1992). Furthermore, the correlation between flood basalt magmatism and the location of hotspot tracks provides convincing support for this hypothesis (Richards et al., 1989).
Although models for CFB genesis involving upwelling mantle plumes provide an explanation for the generation of large volumes of magma over short timespans (particularly if rifting accompanies the arrival of the plume head; White & McKenzie, 1989) this is at odds with the observation that the geochemical characteristics of many CFBs differ from those of plume-derived magmas located within the oceanic environment.
Hybrid solutions
In an effort to reconcile competing hypotheses, Ellam & Cox (1991) proposed that the chemistry of the Nuanetsi picrites (Karoo) might be explained if the bulk of the magma was derived from the plume, with the incorporation of incompatible element enriched (e.g. lamproitic) melts derived from the lithospheric mantle. In other words, the major elements might broadly reflect one source region (plume) whereas the trace element and isotope characteristics are dominated by a component extracted from another (the lithospheric mantle). A study of mafic potassic rocks from the Paraná province appears to provide some evidence in support of this. Gibson et al. (1996) noted a correlation between the distribution of low- and high-Ti mafic potassic rocks with low- and high-Ti CFBs in the Paraná province. Although not constraining the source of the major element component, these workers demonstrated the plausibility of extracting incompatible trace element and isotope ‘enriched’ melts from the SCLM with compositions linked to those observed in the CFBs of this province.
An alternative approach has been to suggest that delaminated lithospheric mantle might become recycled into some mantle plumes (e.g. McKenzie & O’Nions, 1983; Castillo, 1988; Hart, 1988). Such models ‘recouple’ the source regions of the major and trace elements in CFB, but difficulties in explaining the lack of intra-oceanic magmas with continental crustal signatures as strongly developed as those in CFBs remain. In short, why might delaminated SCLM return in the form of a plume, yet impinge only on continental crust, never in the oceanic environment? In support of delamination sources for mantle plumes, it has been suggested that the enriched characteristics of Dupal-type OIB do provide examples of upwelling material of unusual composition (e.g. Menzies & Kyle, 1990). These same ‘Dupal’ features, however, have been attributed by others as evidence for contamination of the plume (e.g. entrainment of lithospheric mantle) during its residence beneath the continents before separation of Africa and South America (Hawkesworth et al., 1986).
Gallagher & Hawkesworth (1992) proposed that rehydrated lithospheric mantle could well generate large melt volumes, even in the absence of a plume. It was suggested that such a model could explain the CFBs with well-developed lithospheric signatures rather than clear ‘plume’ features, particularly in cases for which apparently no plume had existed and there was a possibility that magmatism had been triggered by extension (e.g. the Gondwana Ferrar province: Cox, 1978; Brewer et al., 1992).
A POTENTIAL ROLE FOR Re–Os ISOTOPE GEOCHEMISTRY?
Most workers now agree that, despite the evidence for the assimilation of continental crust by some CFB magmas, in a number of other cases the ‘enriched’ incompatible trace element and isotope characteristics have been inherited from their mantle source regions. However, as noted by Carlson (1991), ‘the issue of a shallow [mantle lithosphere] or deep [mantle plume] origin for the enriched isotopic composition seen in these basalts is not likely to be answered by the current base of Sr, Nd and Pb isotopic and trace element data’. In other words, in any study of CFB petrogenesis there are two main issues that need to be addressed. First, if the magmas show ‘enriched’ signatures, were these inherited from the mantle source region, or adopted by magmas at a later stage as they interacted with and assimilated continental crust? Second, how do we determine whether the mantle source region of the magmas lies primarily within the shallow sub-continental lithosphere, or within asthenosphere-derived upwelling mantle plumes?
Recently, technical advances in Re–Os isotope geochemistry [e.g. negative thermal ionization mass spectrometry (N-TIMS), solvent extraction chemistry] have allowed materials other than Os-rich minerals and peridotites to be studied (e.g. Roy-Barman, 1993; Birck et al., 1997). Few workers studying flood basalt provinces have employed this technique to date, and yet, as noted by McDonough (1991), the variations between the Os isotope compositions of the convecting asthenosphere versus that of depleted lithospheric mantle might provide the ultimate test for tracking the sources of CFB magmatism.
The current database
Sufficient Os isotope data now exist to delineate broad compositional ranges for key mantle reservoirs. The histograms featured in Fig. 1a–c summarize γOs data currently available for the upper oceanic mantle, mantle plumes and SCLM. Also included are ranges in initial γOs values for several CFB provinces (Fig. 1d) and a representation of the possible γOs values for the SCLM of SE Australia around the time of Tasmanian Dolerite emplacement (∼175 Ma, Fig. 1e). In assembling Fig. 1, ∼400 results were employed from 25 separate studies (references are provided in the caption to Fig. 1).
Histograms based on reported Os isotope data (γOs) for a range of mantle domains. (a) Upper oceanic mantle (including MORB and abyssal peridotites). (b) Mantle plumes (including EMI, EMII, HIMU endmember examples and OIB of intermediate chemical affinities). (c) Sub-continental lithospheric mantle (including kimberlite-hosted xenoliths and lamproites as measures of the mantle lithosphere beneath ancient cratonic areas, and basalt-hosted peridotites as samples from non-cratonized regions). (d) Examples of CFBs, age corrected to initial γOs values. (e) An estimate of the SCLM in SE Australia at the time of Ferrar province magmatism (∼175 Ma) calculated by age correcting published peridotite xenolith data to an age of 175 Ma. References used to construct these histograms are either referred to elsewhere in the text or are listed here in chronological order and include: Walker et al. (1989), Martin (1991), Reisberg et al. (1991, 1993), Pegram & Allègre (1992), Hauri & Hart (1993), Carlson & Irving (1994), Martin et al. (1994), Roy-Barman & Allègre (1994, 1995), Marcantonio et al. (1995), Pearson et al. (1995a, 1995b), Reisberg & Lorand (1995), Shirey & Walker (1995), Snow & Reisberg (1995), Bennett et al. (1996), Brandon et al. (1996), Carlson et al. (1996), Hart & Ravizza (1996), Hauri (1996), Hauri et al. (1996), McBride et al. (1996), Roy-Barman et al. (1996), Handler et al. (1997), Chesley & Ruiz (1998). γOs values, where γOs = {[(187Os/188Ossample)/(187Os/188Osmantle)] − 1} × 100, were calculated relative to a chondritic upper-mantle value of 0·1275 following the technique of Handler et al. (1997). Os isotope ratios quoted as 187Os/186Os were converted to equivalent 187Os/188Os values first, by dividing through by 8·340 [after Walker et al. (1991)].
On the basis of Fig. 1, Re–Os geochemistry would appear to be a powerful technique for addressing the ultimate mantle source region of CFBs. The SCLM (as sampled in kimberlite-hosted xenoliths and lamproite melts in cratonic regions, as well as peridotites in non-cratonic areas) has distinctly low, predominantly negative, γOs values. In contrast, ocean-island (plume) magmas generally have values greater than zero. Certainly there is a degree of overlap between these groups as might be expected if, for example, areas of lithospheric mantle attained radiogenic γOs values resulting from the infiltration of melts with high Re/Os; however, the general subdivision is clear.
Limitations in applying the Os technique to CFB studies
The potential utility of the Re–Os system in the study of CFBs is twofold. First, where unusually ‘enriched’ trace element and isotope characteristics are observed, the isotopic composition of Os should help distinguish between the sources of such enrichment; that is, enriched mantle (low Re/Os, hence unradiogenic) vs assimilated continental crust (high Re/Os, therefore radiogenic). Second, given the broad distinctions between plume and SCLM Os isotope compositions (Fig. 1), it may be possible to argue that in cases where CFBs have unusually unradiogenic Os isotope compositions, at least a significant proportion of the melting must take place in the SCLM rather than a plume (e.g. Ellam et al., 1992).
The advances in chemical separation and sample handling techniques now mean that blank contributions can be kept sufficiently low, and sample signals sufficiently intense, to permit the analysis of rocks with low Os contents (e.g. ≤∼10 ppt) and high Re/Os (e.g. Molzahn et al., 1996; Allègre et al., 1999). Nevertheless, both applications of Re–Os geochemistry to the study of CFBs outlined above require the calculation of initial ratios, and these often necessitate large extrapolations when age correcting the data, which generally have high 187Re/188Os and 187Os/188Os ratios and may also be old (e.g. Mesozoic flood basalts of Gondwana). This requirement becomes important because no reliable means yet exists for combining and propagating true uncertainties in spike calibrations, weighing errors and in-run precision in mass spectrometry runs, together with uncertainties introduced in identifying the isotopic composition and concentration of the blank, and subtracting this contribution. As noted by Allègre et al. (1999), the best means of achieving confidence in the quality of Re–Os data is to establish that the results can be closely replicated. In the case of a closed magmatic system, the quality of the isochron generated provides an additional means of assessing the data (i.e. whether or not it reproduces the known age of the rocks). Additional problems arise with the latter, however, in relation to the proper estimation of uncertainties, and adopting an approach to isochron fitting that can at least be replicated by other workers for the purposes of comparison.
In the case where an isochron can be established, the initial ratio estimated has the potential to be well constrained. As an example of this we will examine an isochron recently reported for the Deccan Traps volcanic province of India. Allègre et al. (1999) employed the Re–Os system to estimate the age and duration of magmatism in the Deccan Traps. Their calculated initial ratio of 0·12843 yields a gamma value of 1·1 at 65·6 Ma, which, according to their reported 2σ uncertainties, provides a range in γOs of 0·7–1·4. From Fig. 1, this range is consistent with either lithospheric or asthenospheric mantle sources (including MORB and OIB) and would appear to preclude any crustal contamination by radiogenic Os, or the necessity for involving melts with unradiogenic Os extracted from the SCLM. The tight constraints upon the range of γOs values at the time of eruption derive from the fact that each sample contributes to the isochron from which the initial ratio is determined. We caution that the calculated age, initial ratio and, in particular, the uncertainties depend critically on the software employed to generate the results. For example, Allègre et al. (1999) followed the method of Minster et al. (1979) to perform their best-fit calculations. In processing the same results using Isoplot/Ex v. 2.00 (Ludwig, 1999) we calculate an age of 65·64 ± 0·85 and an initial Os isotope ratio of 0·127 ± 0·012 (both uncertainty estimates quoted at the 2σ level). Although the ages are the same in each case, the initial ratios are slightly different and the uncertainties show significant discrepancies (the range in γOs values is extended to −9·5 to +9·4 using Isoplot).
Notwithstanding these issues, the key point is that most workers would not consider the construction of an isochron necessary when their goal is to employ initial ratios as isotopic tracers. In many cases the age of CFB magmatism is known by independent means and, as is common practice with other isotope systems (e.g. Rb–Sr, Sm–Nd) individual samples are then age corrected using this information. To examine the potential danger in this approach when employing the Re–Os system for CFBs, let us again consider the Deccan data. This time, using the known age of 65·6 Ma we age correct each sample independently from its measured 187Re/188Os and 187Os/188Os ratios. In this case, the range in γOs of 0·7–1·4 (or −9·5 to 9·4) expands to become −18 to 24. Clearly, the errors on these calculations are likely to be very large (although these are difficult if not impossible to propagate with any confidence); however, this example highlights the problem many workers must face when dealing with aged high Re/Os rocks, particularly in the absence of an Re–Os isochron (e.g. in cases where magma mixing or crustal assimilation has taken place). As noted by one of the reviewers of this paper, ‘what people are trying to do with Re–Os in evolved basalts is effectively like trying to get common Pb constraints out of zircons’.
Yet the evolved CFBs are precisely those rocks that might be most informative in constraining the source of enrichment (i.e. SCLM vs assimilation of continental crust; Lassiter & DePaolo, 1997). In contrast to rare picrites (with levels of the order of 102−103 ppt Os; Ellam et al., 1992), these more common CFBs will have low Os contents (generally <10 ppt; Molzahn et al., 1996; Allègre et al., 1999) and therefore will be more sensitive to the assimilation of continental crust. Thus, the absence of radiogenic Os isotope ratios in rocks with ‘enriched’ incompatible trace element characteristics would support an SCLM source for these features.
We argue that there is merit in employing Re–Os in the study of evolved basalts, and that the best means of minimizing uncertainties in initial ratios is to generate an isochron, where possible. The preferred approach, and one adopted here, is also to consider possible solutions to the other problems faced in applying the Re–Os isotope system to evolved CFB magmas. A first step is to locate rock samples related to evolved CFBs, but in which Os is likely to be most abundant; mafic cumulates, for example, may well serve this purpose. Second, where possible, one should identify a mineral phase present in these rocks that has sequestered the Os (ideally in preference to Re) and perform Re–Os isotope determinations on mineral separates. It has been well known for some time that Os is preferentially enriched in sulphides and oxides [e.g. Shirey & Walker (1998) and references therein]. Third, one should analyse a range of chemically different samples in the attempt to generate a well-constrained isochron (and hence, as noted previously, generate the best possible constraints on the initial Os isotope ratio).
A final issue is that of selecting the most appropriate CFBs to address the particular question being asked. The Ferrar magmatic province represents the most extreme endmember of the low-Ti CFBs in preserving trace element and Sr, Nd, Pb isotope compositions closest to upper continental crust, as well as the greatest level of major element depletion. To explain the characteristics of the Ferrar rocks, some workers have proposed that asthenospheric magmas must have undergone extensive assimilation of continental crust (e.g. Faure et al., 1974) whereas others have argued that the mantle source resided, perhaps exclusively, within a very depleted, possibly lithospheric mantle to which upper-crustal material had been added before partial melting (e.g. Hergt et al., 1989a, 1989b, 1991). Here then is a CFB province in which the rocks are characterized by both strong continental crustal signatures and extreme source depletion. The application of Re–Os isotope geochemistry to the Ferrar rocks provides the opportunity to test existing models proposed to explain both of these features.
THE FERRAR MAGMATIC PROVINCE, ANTARCTICA–AUSTRALIA
The Ferrar CFB province extends from southern Australia, through the Transantarctic Mountains and into Queen Maud Land, a distance of >4000 km. The similarity in the compositions generated over this vast area is remarkable, and although this includes lavas erupted as basalts at the Earth’s surface, the province is dominated by magmas emplaced as dykes and sills at shallow depths (e.g. Mensing et al., 1984; Hergt et al., 1989a, 1989b, 1991; Brewer et al., 1992).
Across this vast province, the Ferrar CFBs share important chemical features, which have been used to constrain their magmagenesis. These include:
high Si and low Fe, Ti, Na and P at a given mg-number. These features are well documented in magmas with mg-number ranging from 58 to 62 (e.g. Hergt et al., 1989a), but are also present in rare, more primitive samples from Antarctica (e.g. reported mg-number of 72; Molzahn et al., 1996).
Incompatible trace element compositions show remarkable similarities with upper continental crust, but translated to lower, more ‘basaltic’, concentrations (see Fig. 4, below).
The isotopic compositions of incompatible trace elements Sr, Nd and Pb are more typical of continental crust than mantle (e.g. Heier et al., 1965; Hergt et al., 1989a).
Isotopic compositions of the compatible major element oxygen vary significantly and display weak correlations with other parameters. At mantle-like oxygen isotope compositions, other incompatible trace element and isotope characteristics still resemble upper continental crustal rocks (e.g. initial 87Sr/86Sr ∼ 0·710 at δ18O ∼ +6‰: Hergt et al., 1989b).
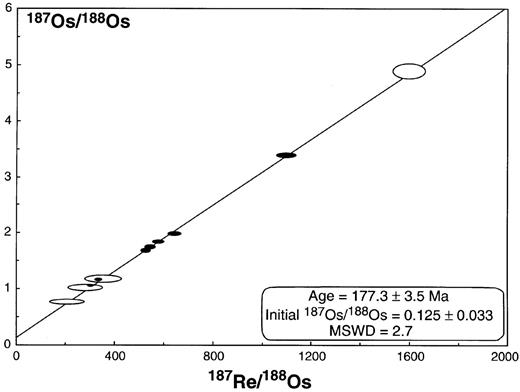
An illustration of the Model 3 isochron (i.e. the uncertainties on the line of best-fit exceed those anticipated from analytical uncertainties alone) calculated for the combined Tasmanian (filled symbols) and Antarctic (open symbols) datasets using Isoplot/Ex v. 2.00 (Ludwig, 1999). Three points were excluded from the Antarctic dataset published by Molzahn et al. (1996), restricting this plot to 11 data. Numerical uncertainties are given at the 2σ level; however, the sizes of the error ellipses for all data points have been more than doubled to ensure reproduction when this figure was reduced in size for publication. In doing so, the relative sizes of the uncertainties have been approximated.
The samples
Four separate dolerite intrusions were sampled from different areas throughout Tasmania (Fig. 2): Mt Wellington (S), Red Hill (S), Ben Lomond (NE) and Mt Sedgwick (NW). Although demonstrably from the same period of magmatism, any intra-crustal evolution of these different sills should be unrelated. This is particularly true in the case of the Mt Sedwick sample, as the basement in NW Tasmania contrasts with that in the east, the two being entirely different terranes separated by the Tamar Fracture System (Williams, 1978). Thus crustal-level assimilation processes should not only be reflected in radiogenic initial ratios of these samples, but would also be expected to produce a scatter on any ‘isochron’ diagram in response to variations in the isotopic compositions of the crustal component.
Sketch map of Tasmania indicating the extent of exposed dolerite (shaded) and locations of widely separated sampling sites chosen for this study. The Hobart sample was taken from the Mt Wellington sheet. It should be noted that the shaded region includes numerous separate intrusions that are distributed across a major basement divide in Tasmania. The southern extension of the Tamar Fracture System is unclear; however, it extends from ∼147° on the northern coast, through to a location close to Hobart in the SE [modified from Hergt et al. (1989a)].
A total of seven samples from these localities were chosen for the Re–Os study. These were selected on the basis of previous work (Hergt, 1987), and we focused on known cumulate units (cpx + plag ± opx ± pig) within differentiated intrusions in an effort to optimize the level of Os in the samples. Major and trace element data for six of these samples are provided in Table 1 and the mantle-normalized trace element characteristics are illustrated in Fig. 4 (below). Some of the samples exhibit lower incompatible element abundances than the proposed parent magma (indicated by the average chilled margin composition shown), which is consistent with their mafic cumulate origin (i.e. the preferential exclusion of incompatible trace elements during the earliest stages of crystallization), but the overall patterns retain typical ‘Ferrar’ upper-crustal signatures.
Major and trace element data for six of the samples described in this study
| Mt | Red Hill | Red Hill | Red Hill | Ben | Ben | |
| Wellington 1 | 3 | 4 | 5 | Lomond 6 | Lomond 7 | |
| Latitude: | −42°53·5’ | −43°04·4’ | −43°03·6’ | −43°03·6’ | −41°31·0’ | −41°31·0’ |
| Longitude: | 147°14·2’ | 147°14·1’ | 147°13·1’ | 147°12·9’ | 147°39·5’ | 147°39·5’ |
| SiO2 | 54·02 | 54·75 | 55·11 | 55·67 | 53·29 | 52·89 |
| TiO2 | 0·44 | 0·66 | 0·69 | 0·81 | 0·44 | 0·38 |
| Al2O3 | 14·59 | 16·02 | 14·90 | 15·34 | 13·66 | 14·65 |
| Fe2O3* | 8·92 | 10·16 | 10·56 | 10·69 | 9·15 | 8·19 |
| MnO | 0·13 | 0·13 | 0·18 | 0·17 | 0·19 | 0·17 |
| MgO | 8·40 | 4·49 | 5·48 | 3·84 | 9·05 | 9·26 |
| CaO | 11·64 | 10·55 | 10·31 | 9·27 | 11·89 | 12·43 |
| Na2O | 0·91 | 1·33 | 1·71 | 1·85 | 1·21 | 1·23 |
| K2O | 0·54 | 0·91 | 0·87 | 1·12 | 0·55 | 0·47 |
| SO3 | 0·03 | 0·00 | 0·14 | 0·10 | 0·04 | 0·04 |
| P2O5 | 0·02 | 0·06 | 0·10 | 0·11 | 0·06 | 0·05 |
| LOI | 0·08 | 0·65 | 0·22 | 0·48 | 0·66 | 0·60 |
| Total | 99·72 | 99·71 | 100·27 | 99·45 | 100·19 | 100·36 |
| Cs | 1·3 | 1·2 | 1·6 | 2·7 | 0·7 | 0·8 |
| Ba | 184 | 279 | 256 | 258 | 170 | 104 |
| Rb | 17 | 26 | 24 | 34 | 12 | 12 |
| Sr | 116 | 145 | 137 | 135 | 106 | 107 |
| Th | 2·3 | 4·1 | 3·3 | 4·1 | 1·9 | 2·2 |
| U | 0·67 | 1·07 | 0·96 | 1·18 | 0·57 | 0·54 |
| Pb | 8·6 | 18·4 | 15·7 | 20·4 | 8·1 | 7·7 |
| Zr | 67 | 102 | 95 | 120 | 60 | 55 |
| Nb | 4·0 | 6·1 | 6·0 | 7·8 | 3·1 | 3·4 |
| Y | 18 | 25 | 25 | 29 | 18 | 14 |
| V | 240 | 250 | 241 | 214 | 250 | 217 |
| Cr | 265 | 18 | 28 | 10 | 108 | 212 |
| Co | 42 | 44 | 49 | 42 | 54 | 46 |
| Ni | 76 | 18 | 85 | 62 | 130 | 136 |
| Cu | 32 | 46 | 73 | 79 | 46 | 41 |
| Zn | 39 | 54 | 78 | 82 | 59 | 55 |
| Ga | 0 | 0 | 8 | 10 | 9 | 9 |
| Sc | 42·2 | 41·6 | 45·2 | 41·4 | 44·5 | 47·4 |
| La | 8·9 | 14·0 | 12·9 | 16·0 | 7·5 | 7·2 |
| Ce | 19·0 | 30·3 | 28·7 | 34·8 | 16·1 | 15·4 |
| Pr | 2·5 | 3·9 | 3·6 | 4·5 | 2·1 | 2·0 |
| Nd | 9·9 | 15·1 | 14·2 | 17·1 | 8·5 | 8·3 |
| Sm | 2·2 | 3·4 | 3·4 | 4·1 | 1·9 | 2·0 |
| Eu | 0·66 | 0·98 | 0·92 | 1·14 | 0·61 | 0·61 |
| Gd | 2·6 | 4·0 | 3·8 | 4·5 | 2·3 | 2·3 |
| Tb | 0·45 | 0·65 | 0·66 | 0·78 | 0·40 | 0·38 |
| Dy | 2·8 | 4·1 | 4·1 | 4·8 | 2·5 | 2·4 |
| Ho | 0·61 | 0·90 | 0·91 | 1·02 | 0·56 | 0·54 |
| Er | 1·7 | 2·5 | 2·5 | 2·9 | 1·5 | 1·5 |
| Tm | 0·28 | 0·41 | 0·38 | 0·43 | 0·24 | 0·24 |
| Yb | 1·8 | 2·7 | 2·6 | 3·0 | 1·6 | 1·6 |
| Lu | 0·28 | 0·42 | 0·41 | 0·48 | 0·26 | 0·24 |
| Hf | 1·9 | 2·8 | 2·8 | 3·5 | 1·6 | 1·6 |
| Ta | 0·41 | 0·52 | 0·48 | 0·61 | 0·32 | 0·38 |
| Mt | Red Hill | Red Hill | Red Hill | Ben | Ben | |
| Wellington 1 | 3 | 4 | 5 | Lomond 6 | Lomond 7 | |
| Latitude: | −42°53·5’ | −43°04·4’ | −43°03·6’ | −43°03·6’ | −41°31·0’ | −41°31·0’ |
| Longitude: | 147°14·2’ | 147°14·1’ | 147°13·1’ | 147°12·9’ | 147°39·5’ | 147°39·5’ |
| SiO2 | 54·02 | 54·75 | 55·11 | 55·67 | 53·29 | 52·89 |
| TiO2 | 0·44 | 0·66 | 0·69 | 0·81 | 0·44 | 0·38 |
| Al2O3 | 14·59 | 16·02 | 14·90 | 15·34 | 13·66 | 14·65 |
| Fe2O3* | 8·92 | 10·16 | 10·56 | 10·69 | 9·15 | 8·19 |
| MnO | 0·13 | 0·13 | 0·18 | 0·17 | 0·19 | 0·17 |
| MgO | 8·40 | 4·49 | 5·48 | 3·84 | 9·05 | 9·26 |
| CaO | 11·64 | 10·55 | 10·31 | 9·27 | 11·89 | 12·43 |
| Na2O | 0·91 | 1·33 | 1·71 | 1·85 | 1·21 | 1·23 |
| K2O | 0·54 | 0·91 | 0·87 | 1·12 | 0·55 | 0·47 |
| SO3 | 0·03 | 0·00 | 0·14 | 0·10 | 0·04 | 0·04 |
| P2O5 | 0·02 | 0·06 | 0·10 | 0·11 | 0·06 | 0·05 |
| LOI | 0·08 | 0·65 | 0·22 | 0·48 | 0·66 | 0·60 |
| Total | 99·72 | 99·71 | 100·27 | 99·45 | 100·19 | 100·36 |
| Cs | 1·3 | 1·2 | 1·6 | 2·7 | 0·7 | 0·8 |
| Ba | 184 | 279 | 256 | 258 | 170 | 104 |
| Rb | 17 | 26 | 24 | 34 | 12 | 12 |
| Sr | 116 | 145 | 137 | 135 | 106 | 107 |
| Th | 2·3 | 4·1 | 3·3 | 4·1 | 1·9 | 2·2 |
| U | 0·67 | 1·07 | 0·96 | 1·18 | 0·57 | 0·54 |
| Pb | 8·6 | 18·4 | 15·7 | 20·4 | 8·1 | 7·7 |
| Zr | 67 | 102 | 95 | 120 | 60 | 55 |
| Nb | 4·0 | 6·1 | 6·0 | 7·8 | 3·1 | 3·4 |
| Y | 18 | 25 | 25 | 29 | 18 | 14 |
| V | 240 | 250 | 241 | 214 | 250 | 217 |
| Cr | 265 | 18 | 28 | 10 | 108 | 212 |
| Co | 42 | 44 | 49 | 42 | 54 | 46 |
| Ni | 76 | 18 | 85 | 62 | 130 | 136 |
| Cu | 32 | 46 | 73 | 79 | 46 | 41 |
| Zn | 39 | 54 | 78 | 82 | 59 | 55 |
| Ga | 0 | 0 | 8 | 10 | 9 | 9 |
| Sc | 42·2 | 41·6 | 45·2 | 41·4 | 44·5 | 47·4 |
| La | 8·9 | 14·0 | 12·9 | 16·0 | 7·5 | 7·2 |
| Ce | 19·0 | 30·3 | 28·7 | 34·8 | 16·1 | 15·4 |
| Pr | 2·5 | 3·9 | 3·6 | 4·5 | 2·1 | 2·0 |
| Nd | 9·9 | 15·1 | 14·2 | 17·1 | 8·5 | 8·3 |
| Sm | 2·2 | 3·4 | 3·4 | 4·1 | 1·9 | 2·0 |
| Eu | 0·66 | 0·98 | 0·92 | 1·14 | 0·61 | 0·61 |
| Gd | 2·6 | 4·0 | 3·8 | 4·5 | 2·3 | 2·3 |
| Tb | 0·45 | 0·65 | 0·66 | 0·78 | 0·40 | 0·38 |
| Dy | 2·8 | 4·1 | 4·1 | 4·8 | 2·5 | 2·4 |
| Ho | 0·61 | 0·90 | 0·91 | 1·02 | 0·56 | 0·54 |
| Er | 1·7 | 2·5 | 2·5 | 2·9 | 1·5 | 1·5 |
| Tm | 0·28 | 0·41 | 0·38 | 0·43 | 0·24 | 0·24 |
| Yb | 1·8 | 2·7 | 2·6 | 3·0 | 1·6 | 1·6 |
| Lu | 0·28 | 0·42 | 0·41 | 0·48 | 0·26 | 0·24 |
| Hf | 1·9 | 2·8 | 2·8 | 3·5 | 1·6 | 1·6 |
| Ta | 0·41 | 0·52 | 0·48 | 0·61 | 0·32 | 0·38 |
The trace elements Cs, Th, U, Pb, Nb, Sc, REE, Hf and Ta were obtained via ICPMS analysis of solutions employing an indium internal standard for drift correction. Major element oxides (expressed in wt %) and remaining trace element data were obtained using standard XRF techniques. Total Fe is included as Fe2O3. LOI, loss on ignition.
Major and trace element data for six of the samples described in this study
| Mt | Red Hill | Red Hill | Red Hill | Ben | Ben | |
| Wellington 1 | 3 | 4 | 5 | Lomond 6 | Lomond 7 | |
| Latitude: | −42°53·5’ | −43°04·4’ | −43°03·6’ | −43°03·6’ | −41°31·0’ | −41°31·0’ |
| Longitude: | 147°14·2’ | 147°14·1’ | 147°13·1’ | 147°12·9’ | 147°39·5’ | 147°39·5’ |
| SiO2 | 54·02 | 54·75 | 55·11 | 55·67 | 53·29 | 52·89 |
| TiO2 | 0·44 | 0·66 | 0·69 | 0·81 | 0·44 | 0·38 |
| Al2O3 | 14·59 | 16·02 | 14·90 | 15·34 | 13·66 | 14·65 |
| Fe2O3* | 8·92 | 10·16 | 10·56 | 10·69 | 9·15 | 8·19 |
| MnO | 0·13 | 0·13 | 0·18 | 0·17 | 0·19 | 0·17 |
| MgO | 8·40 | 4·49 | 5·48 | 3·84 | 9·05 | 9·26 |
| CaO | 11·64 | 10·55 | 10·31 | 9·27 | 11·89 | 12·43 |
| Na2O | 0·91 | 1·33 | 1·71 | 1·85 | 1·21 | 1·23 |
| K2O | 0·54 | 0·91 | 0·87 | 1·12 | 0·55 | 0·47 |
| SO3 | 0·03 | 0·00 | 0·14 | 0·10 | 0·04 | 0·04 |
| P2O5 | 0·02 | 0·06 | 0·10 | 0·11 | 0·06 | 0·05 |
| LOI | 0·08 | 0·65 | 0·22 | 0·48 | 0·66 | 0·60 |
| Total | 99·72 | 99·71 | 100·27 | 99·45 | 100·19 | 100·36 |
| Cs | 1·3 | 1·2 | 1·6 | 2·7 | 0·7 | 0·8 |
| Ba | 184 | 279 | 256 | 258 | 170 | 104 |
| Rb | 17 | 26 | 24 | 34 | 12 | 12 |
| Sr | 116 | 145 | 137 | 135 | 106 | 107 |
| Th | 2·3 | 4·1 | 3·3 | 4·1 | 1·9 | 2·2 |
| U | 0·67 | 1·07 | 0·96 | 1·18 | 0·57 | 0·54 |
| Pb | 8·6 | 18·4 | 15·7 | 20·4 | 8·1 | 7·7 |
| Zr | 67 | 102 | 95 | 120 | 60 | 55 |
| Nb | 4·0 | 6·1 | 6·0 | 7·8 | 3·1 | 3·4 |
| Y | 18 | 25 | 25 | 29 | 18 | 14 |
| V | 240 | 250 | 241 | 214 | 250 | 217 |
| Cr | 265 | 18 | 28 | 10 | 108 | 212 |
| Co | 42 | 44 | 49 | 42 | 54 | 46 |
| Ni | 76 | 18 | 85 | 62 | 130 | 136 |
| Cu | 32 | 46 | 73 | 79 | 46 | 41 |
| Zn | 39 | 54 | 78 | 82 | 59 | 55 |
| Ga | 0 | 0 | 8 | 10 | 9 | 9 |
| Sc | 42·2 | 41·6 | 45·2 | 41·4 | 44·5 | 47·4 |
| La | 8·9 | 14·0 | 12·9 | 16·0 | 7·5 | 7·2 |
| Ce | 19·0 | 30·3 | 28·7 | 34·8 | 16·1 | 15·4 |
| Pr | 2·5 | 3·9 | 3·6 | 4·5 | 2·1 | 2·0 |
| Nd | 9·9 | 15·1 | 14·2 | 17·1 | 8·5 | 8·3 |
| Sm | 2·2 | 3·4 | 3·4 | 4·1 | 1·9 | 2·0 |
| Eu | 0·66 | 0·98 | 0·92 | 1·14 | 0·61 | 0·61 |
| Gd | 2·6 | 4·0 | 3·8 | 4·5 | 2·3 | 2·3 |
| Tb | 0·45 | 0·65 | 0·66 | 0·78 | 0·40 | 0·38 |
| Dy | 2·8 | 4·1 | 4·1 | 4·8 | 2·5 | 2·4 |
| Ho | 0·61 | 0·90 | 0·91 | 1·02 | 0·56 | 0·54 |
| Er | 1·7 | 2·5 | 2·5 | 2·9 | 1·5 | 1·5 |
| Tm | 0·28 | 0·41 | 0·38 | 0·43 | 0·24 | 0·24 |
| Yb | 1·8 | 2·7 | 2·6 | 3·0 | 1·6 | 1·6 |
| Lu | 0·28 | 0·42 | 0·41 | 0·48 | 0·26 | 0·24 |
| Hf | 1·9 | 2·8 | 2·8 | 3·5 | 1·6 | 1·6 |
| Ta | 0·41 | 0·52 | 0·48 | 0·61 | 0·32 | 0·38 |
| Mt | Red Hill | Red Hill | Red Hill | Ben | Ben | |
| Wellington 1 | 3 | 4 | 5 | Lomond 6 | Lomond 7 | |
| Latitude: | −42°53·5’ | −43°04·4’ | −43°03·6’ | −43°03·6’ | −41°31·0’ | −41°31·0’ |
| Longitude: | 147°14·2’ | 147°14·1’ | 147°13·1’ | 147°12·9’ | 147°39·5’ | 147°39·5’ |
| SiO2 | 54·02 | 54·75 | 55·11 | 55·67 | 53·29 | 52·89 |
| TiO2 | 0·44 | 0·66 | 0·69 | 0·81 | 0·44 | 0·38 |
| Al2O3 | 14·59 | 16·02 | 14·90 | 15·34 | 13·66 | 14·65 |
| Fe2O3* | 8·92 | 10·16 | 10·56 | 10·69 | 9·15 | 8·19 |
| MnO | 0·13 | 0·13 | 0·18 | 0·17 | 0·19 | 0·17 |
| MgO | 8·40 | 4·49 | 5·48 | 3·84 | 9·05 | 9·26 |
| CaO | 11·64 | 10·55 | 10·31 | 9·27 | 11·89 | 12·43 |
| Na2O | 0·91 | 1·33 | 1·71 | 1·85 | 1·21 | 1·23 |
| K2O | 0·54 | 0·91 | 0·87 | 1·12 | 0·55 | 0·47 |
| SO3 | 0·03 | 0·00 | 0·14 | 0·10 | 0·04 | 0·04 |
| P2O5 | 0·02 | 0·06 | 0·10 | 0·11 | 0·06 | 0·05 |
| LOI | 0·08 | 0·65 | 0·22 | 0·48 | 0·66 | 0·60 |
| Total | 99·72 | 99·71 | 100·27 | 99·45 | 100·19 | 100·36 |
| Cs | 1·3 | 1·2 | 1·6 | 2·7 | 0·7 | 0·8 |
| Ba | 184 | 279 | 256 | 258 | 170 | 104 |
| Rb | 17 | 26 | 24 | 34 | 12 | 12 |
| Sr | 116 | 145 | 137 | 135 | 106 | 107 |
| Th | 2·3 | 4·1 | 3·3 | 4·1 | 1·9 | 2·2 |
| U | 0·67 | 1·07 | 0·96 | 1·18 | 0·57 | 0·54 |
| Pb | 8·6 | 18·4 | 15·7 | 20·4 | 8·1 | 7·7 |
| Zr | 67 | 102 | 95 | 120 | 60 | 55 |
| Nb | 4·0 | 6·1 | 6·0 | 7·8 | 3·1 | 3·4 |
| Y | 18 | 25 | 25 | 29 | 18 | 14 |
| V | 240 | 250 | 241 | 214 | 250 | 217 |
| Cr | 265 | 18 | 28 | 10 | 108 | 212 |
| Co | 42 | 44 | 49 | 42 | 54 | 46 |
| Ni | 76 | 18 | 85 | 62 | 130 | 136 |
| Cu | 32 | 46 | 73 | 79 | 46 | 41 |
| Zn | 39 | 54 | 78 | 82 | 59 | 55 |
| Ga | 0 | 0 | 8 | 10 | 9 | 9 |
| Sc | 42·2 | 41·6 | 45·2 | 41·4 | 44·5 | 47·4 |
| La | 8·9 | 14·0 | 12·9 | 16·0 | 7·5 | 7·2 |
| Ce | 19·0 | 30·3 | 28·7 | 34·8 | 16·1 | 15·4 |
| Pr | 2·5 | 3·9 | 3·6 | 4·5 | 2·1 | 2·0 |
| Nd | 9·9 | 15·1 | 14·2 | 17·1 | 8·5 | 8·3 |
| Sm | 2·2 | 3·4 | 3·4 | 4·1 | 1·9 | 2·0 |
| Eu | 0·66 | 0·98 | 0·92 | 1·14 | 0·61 | 0·61 |
| Gd | 2·6 | 4·0 | 3·8 | 4·5 | 2·3 | 2·3 |
| Tb | 0·45 | 0·65 | 0·66 | 0·78 | 0·40 | 0·38 |
| Dy | 2·8 | 4·1 | 4·1 | 4·8 | 2·5 | 2·4 |
| Ho | 0·61 | 0·90 | 0·91 | 1·02 | 0·56 | 0·54 |
| Er | 1·7 | 2·5 | 2·5 | 2·9 | 1·5 | 1·5 |
| Tm | 0·28 | 0·41 | 0·38 | 0·43 | 0·24 | 0·24 |
| Yb | 1·8 | 2·7 | 2·6 | 3·0 | 1·6 | 1·6 |
| Lu | 0·28 | 0·42 | 0·41 | 0·48 | 0·26 | 0·24 |
| Hf | 1·9 | 2·8 | 2·8 | 3·5 | 1·6 | 1·6 |
| Ta | 0·41 | 0·52 | 0·48 | 0·61 | 0·32 | 0·38 |
The trace elements Cs, Th, U, Pb, Nb, Sc, REE, Hf and Ta were obtained via ICPMS analysis of solutions employing an indium internal standard for drift correction. Major element oxides (expressed in wt %) and remaining trace element data were obtained using standard XRF techniques. Total Fe is included as Fe2O3. LOI, loss on ignition.
Normal mid-ocean ridge basalt (N-MORB) normalized incompatible trace element diagrams illustrating: (a) our preferred model for the petrogenesis of the Ferrar province CFBs and (b) the liquid (chilled margin) and cumulate compositions for five of the rock samples examined in this study. The top panel includes the compositions of the mantle (depleted MORB source) and continental crustal (average shale) components employed in the model. The contaminated source results from the addition of 3% of the average shale into the depleted mantle source. The cross-hatched area brackets a range of possible magmas derived from the contaminated source via 8–15% partial melting (making the simplifying assumption that all incompatible elements will completely partition into the magma). As indicated by the key in the lower panel, the pattern within this cross-hatched region is the Tasmanian Dolerite average chilled margin composition [reported by Hergt et al. (1989a)].
Analytical methods
It has been known for some time that oxides and sulphides sequester Os (and Re; Shirey & Walker, 1998) and therefore, to limit uncertainties in individual results (e.g. reduce the volumes of reagents and hence the blank contributions), we preconcentrated the oxide phases from most samples rather than processing bulk-rock material (sulphide grains were rare or absent in the rocks studied). The opaque oxides in the Tasmanian Dolerites vary in composition, and most have exsolved to generate separate magnetite and ilmenite domains during the slow cooling and crystallization process. Extraction of these mixed oxide phases from the rock samples is very labour intensive, but five samples provided sufficient material for X-ray fluorescence (XRF) spectrometric analysis to be conducted (electron microprobe analyses would sample these grains at a scale too small to give reliable estimates of their bulk composition). The data are presented in Table 2 and indicate significant variations in Ti and Fe, with highest Ti oxides occurring in the more Mg-rich rocks.
Estimated major element compositions of separated oxides from five of the Tasmanian Dolerite samples discussed in this study
| Red Hill 3 | Red Hill 4 | Red Hill 5 | Ben | Ben | |
| Lomond 6 | Lomond 7 | ||||
| TiO2 | 11·62 | 15·03 | 13·68 | 25·85 | 27·68 |
| Fe2O3 | 85·13 | 81·50 | 85·59 | 68·61 | 64·84 |
| MnO | 0·53 | 0·54 | 0·37 | 1·12 | 1·12 |
| MgO | 2·57 | 2·27 | 0·12 | 4·22 | 6·03 |
| SO3 | 0·16 | 0·66 | 0·24 | 0·20 | 0·33 |
| Red Hill 3 | Red Hill 4 | Red Hill 5 | Ben | Ben | |
| Lomond 6 | Lomond 7 | ||||
| TiO2 | 11·62 | 15·03 | 13·68 | 25·85 | 27·68 |
| Fe2O3 | 85·13 | 81·50 | 85·59 | 68·61 | 64·84 |
| MnO | 0·53 | 0·54 | 0·37 | 1·12 | 1·12 |
| MgO | 2·57 | 2·27 | 0·12 | 4·22 | 6·03 |
| SO3 | 0·16 | 0·66 | 0·24 | 0·20 | 0·33 |
Analyses were performed on fused discs via XRF after doping with ≥60% silica. The results represent compositions recalculated to a total of 100% after removing this silica, in addition to trace levels of elements associated with slight feldspar contamination (Al, Ca, K).
Estimated major element compositions of separated oxides from five of the Tasmanian Dolerite samples discussed in this study
| Red Hill 3 | Red Hill 4 | Red Hill 5 | Ben | Ben | |
| Lomond 6 | Lomond 7 | ||||
| TiO2 | 11·62 | 15·03 | 13·68 | 25·85 | 27·68 |
| Fe2O3 | 85·13 | 81·50 | 85·59 | 68·61 | 64·84 |
| MnO | 0·53 | 0·54 | 0·37 | 1·12 | 1·12 |
| MgO | 2·57 | 2·27 | 0·12 | 4·22 | 6·03 |
| SO3 | 0·16 | 0·66 | 0·24 | 0·20 | 0·33 |
| Red Hill 3 | Red Hill 4 | Red Hill 5 | Ben | Ben | |
| Lomond 6 | Lomond 7 | ||||
| TiO2 | 11·62 | 15·03 | 13·68 | 25·85 | 27·68 |
| Fe2O3 | 85·13 | 81·50 | 85·59 | 68·61 | 64·84 |
| MnO | 0·53 | 0·54 | 0·37 | 1·12 | 1·12 |
| MgO | 2·57 | 2·27 | 0·12 | 4·22 | 6·03 |
| SO3 | 0·16 | 0·66 | 0·24 | 0·20 | 0·33 |
Analyses were performed on fused discs via XRF after doping with ≥60% silica. The results represent compositions recalculated to a total of 100% after removing this silica, in addition to trace levels of elements associated with slight feldspar contamination (Al, Ca, K).
To achieve mineral separation, the very fine grained magnetite-rich particles were extracted with magnetic foil, which was wrapped around the outside of thin-walled, disposable plastic beakers. For this procedure finely ground sample powder was made into a suspension with 18 MΩ water. Because of the weak magnetic attraction, mainly clean magnetite-rich grains were collected on the walls of the beaker, with quartz and feldspar grains remaining in suspension. The same procedure was repeated on the preconcentrated magnetic separates to achieve a final purity of ∼97%.
To determine the Re and Os contents and 187Re/188Os for each sample, a mixed spike was employed (185Re/190Os ∼ 300). The homogenization between spike and sample was achieved by applying the Carius tube technique described by Shirey & Walker (1995). Samples were leached (none of the samples were completely dissolved), with inverse aqua regia (2:1 of conc. HNO3–conc. HCl) in sealed Pyrex Carius tubes. Each Carius tube was cooled in a mixture of ethanol and dry ice, then loaded with sample powder, spike solution and 6 ml inverse aqua regia before being sealed and placed in an oven at 240°C for 3 days.
In all but one sample (Mt Sedwick 2), 1·5 ± 0·1 g of ‘magnetite’ were used per analysis. In the case of the Mt Sedwick sample, the size of the rock available for study restricted the oxide separate to <1 g. Controlling the volume of sample was considered an important step in limiting the variations in Re and Os extracted during chemical processing (important both in the consideration of blank contributions and in the reproducibility of uncertainties during mass spectrometry). To monitor the recovery of Re and Os we used the same mass of ‘magnetite’ (with known Re and Os contents) separated from one sample (Ben Lomond 6) and performed the same chemical separation procedure with the exception that the spike solution was added at the final stage before loading onto the filament. Importantly, no variations in isotope ratios were observed during these runs, suggesting there were no equilibration problems between spike and sample. Yields calculated for Re and Os in this study are at least 70% and 66%, respectively.
After the Carius tube digestion, bromine was added to the inverse aqua regia and Os (as OsO4) was extracted from the aqueous phase. The OsO4 was subsequently reduced by mixing the bromine with hydrobromic acid as described by Birck et al. (1997). Final purification was accomplished for osmium using the micro distillation technique described by Roy-Barman (1993) and Birck et al. (1997). Rhenium was recovered by anion exchange using Dowex AG 1X8 with an HNO3 chemistry.
Total procedural blanks average 0·7 ± 0·5 pg for Os and 15 ± 5 pg for Re. The isotopic composition of Os in the blank is very close to the natural composition of Os and has a 187Os/188Os ratio of 0·108. All data were blank corrected on the basis of these measurements in combination with the determined yield for an Os blank of 1 pg Os and 20 pg Re. The blank corrections were minor (<1·5%) for both Os and Re data in this study.
The isotopic compositions of Re and Os were measured at La Trobe University using N-TIMS (Finnigan MAT 262). The mass spectrometric procedures used have been discussed by Walker et al. (1994) and Morgan et al. (1995). All Re analyses were performed using Faraday detectors, whereas the osmium isotope ratios were determined using an ion counter in peak-jumping mode. The 2σ in-run analytical uncertainties for Re and Os are <0·1‰ and <0·2‰, respectively.
The external reproducibility for repeated analyses of comparable amounts of Re (5 ng Re per measurement) and Os (100 pg Os per measurement) standards, analysed with Faraday detectors and ion counter, respectively, is approximately ±0·05% and ±0·2%.
In the absence of a suitable method for combining and propagating these errors (including uncertainties in spike calibrations), our best estimates for the maximum overall 2σ uncertainties are ±1·3% for 187Re/188Os, ±0·32% for 187Os/188Os, ±0·2% for the Re concentrations and ±0·5% for the Os concentrations. These are listed in Table 3 and used in the isochron fitting.
Re–Os results
The Os isotope data for the Ferrar tholeiites obtained in this study are presented in Table 3 and illustrated (using the filled symbols) in Fig. 3. Present-day 187Re/188Os ratios vary between 286 and 1091 in the samples we have examined. The isochron calculated for these new data employed Isoplot/Ex v. 2.00 (Ludwig, 1999) and yields an age of 175·1 ± 5·4 Ma (2σ), almost identical to the K–Ar age of 174 Ma [Schmidt & McDougall (1977), recalculated using the revised decay constants of Steiger & Jager (1977)]. The initial 187Os/188Os is within the range 0·145 ± 0·049 (2σ), corresponding to an initial γOs value of 15 ± 38 at 175 Ma.
Re–Os isotope ratios and concentration data for separated oxides from Tasmanian Dolerite samples
| Sample | 187Re/188Os | ±2σ | 187Os/188Os | ±2σ | ppb Re | ±2σ | ppt Os | ±2σ |
| Mt Wellington 1 | 629 | 8 | 1·948 | 0·006 | 0·863 | 0·002 | 8·2 | 0·04 |
| Mt Sedgwick 2 | 322 | 4 | 1·100 | 0·003 | 16·396 | 0·033 | 278·6 | 1·39 |
| Red Hill 3 | 1091 | 14 | 3·352 | 0·011 | 11·117 | 0·022 | 70·2 | 0·35 |
| Red Hill 4 | 516 | 7 | 1·634 | 0·005 | 14·458 | 0·029 | 163·1 | 0·82 |
| Red Hill 5 | 533 | 7 | 1·699 | 0·005 | 10·795 | 0·022 | 118·5 | 0·59 |
| Ben Lomond 6 | 564 | 7 | 1·794 | 0·006 | 9·669 | 0·019 | 101·4 | 0·51 |
| Ben Lomond 7 | 286 | 4 | 0·983 | 0·003 | 16·573 | 0·033 | 313·4 | 1·57 |
| Sample | 187Re/188Os | ±2σ | 187Os/188Os | ±2σ | ppb Re | ±2σ | ppt Os | ±2σ |
| Mt Wellington 1 | 629 | 8 | 1·948 | 0·006 | 0·863 | 0·002 | 8·2 | 0·04 |
| Mt Sedgwick 2 | 322 | 4 | 1·100 | 0·003 | 16·396 | 0·033 | 278·6 | 1·39 |
| Red Hill 3 | 1091 | 14 | 3·352 | 0·011 | 11·117 | 0·022 | 70·2 | 0·35 |
| Red Hill 4 | 516 | 7 | 1·634 | 0·005 | 14·458 | 0·029 | 163·1 | 0·82 |
| Red Hill 5 | 533 | 7 | 1·699 | 0·005 | 10·795 | 0·022 | 118·5 | 0·59 |
| Ben Lomond 6 | 564 | 7 | 1·794 | 0·006 | 9·669 | 0·019 | 101·4 | 0·51 |
| Ben Lomond 7 | 286 | 4 | 0·983 | 0·003 | 16·573 | 0·033 | 313·4 | 1·57 |
Uncertainties represent maximum values estimated from a combination of sources for error (see Analytical Methods for discussion).
Re–Os isotope ratios and concentration data for separated oxides from Tasmanian Dolerite samples
| Sample | 187Re/188Os | ±2σ | 187Os/188Os | ±2σ | ppb Re | ±2σ | ppt Os | ±2σ |
| Mt Wellington 1 | 629 | 8 | 1·948 | 0·006 | 0·863 | 0·002 | 8·2 | 0·04 |
| Mt Sedgwick 2 | 322 | 4 | 1·100 | 0·003 | 16·396 | 0·033 | 278·6 | 1·39 |
| Red Hill 3 | 1091 | 14 | 3·352 | 0·011 | 11·117 | 0·022 | 70·2 | 0·35 |
| Red Hill 4 | 516 | 7 | 1·634 | 0·005 | 14·458 | 0·029 | 163·1 | 0·82 |
| Red Hill 5 | 533 | 7 | 1·699 | 0·005 | 10·795 | 0·022 | 118·5 | 0·59 |
| Ben Lomond 6 | 564 | 7 | 1·794 | 0·006 | 9·669 | 0·019 | 101·4 | 0·51 |
| Ben Lomond 7 | 286 | 4 | 0·983 | 0·003 | 16·573 | 0·033 | 313·4 | 1·57 |
| Sample | 187Re/188Os | ±2σ | 187Os/188Os | ±2σ | ppb Re | ±2σ | ppt Os | ±2σ |
| Mt Wellington 1 | 629 | 8 | 1·948 | 0·006 | 0·863 | 0·002 | 8·2 | 0·04 |
| Mt Sedgwick 2 | 322 | 4 | 1·100 | 0·003 | 16·396 | 0·033 | 278·6 | 1·39 |
| Red Hill 3 | 1091 | 14 | 3·352 | 0·011 | 11·117 | 0·022 | 70·2 | 0·35 |
| Red Hill 4 | 516 | 7 | 1·634 | 0·005 | 14·458 | 0·029 | 163·1 | 0·82 |
| Red Hill 5 | 533 | 7 | 1·699 | 0·005 | 10·795 | 0·022 | 118·5 | 0·59 |
| Ben Lomond 6 | 564 | 7 | 1·794 | 0·006 | 9·669 | 0·019 | 101·4 | 0·51 |
| Ben Lomond 7 | 286 | 4 | 0·983 | 0·003 | 16·573 | 0·033 | 313·4 | 1·57 |
Uncertainties represent maximum values estimated from a combination of sources for error (see Analytical Methods for discussion).
DISCUSSION
As we have not measured either the bulk-rock Re and Os contents, nor the modal proportion of oxides in the samples (the aim being to reduce the mineral separation yields in favour of higher purity oxide separates) it is impossible to assess whether or not another phase may occur in these rocks containing either Re or Os. A study by Molzahn et al. (1996) examined the Re–Os isotope compositions of whole-rock Ferrar province samples from the Transantarctic Mountains. Three of their bulk-rock samples (MUR-04, MJY-02 and RIH-02) have 187Re/188Os ratios very similar to those of three of our magnetite-rich separates (Ben Lomond 7, Mt Sedwick 2 and Ben Lomond 6, respectively). The rock samples have Re and Os contents between ∼330–480 ppt and 4·4–7·9 ppt, respectively, whereas the oxide separates contain ∼9670–16 570 ppt Re and 100–313 ppt Os. If we make two assumptions—first, that magma differentiation processes (e.g. the minerals formed) were similar in the two provinces, and, second, that Fe–Ti oxides are the only phases in these rocks to sequester Re and Os—the modal proportions of ‘magnetite’ estimated for the three sample ‘pairs’ are 2–4%. These values appear reasonable (i.e. <5%) and in this way broadly support the assumptions made. The fact that Re/Os correlates well with the bulk-rock mg-number (Molzahn et al., 1996), and that we have observed a correlation between the TiO2 content of the oxides and MgO contents of the bulk-rock samples, also means that there is no requirement for the presence of another phase in these rocks containing either Re or Os.
Importantly, the age estimate based on the new Tasmanian Re–Os data is remarkably close to those already published for rocks from this part of the Ferrar province. We use this to argue that both the age and the initial ratio represent the results of an isotopically equilibrated system at the time of emplacement. As noted above, there are distinct variations in the basement geology between the locations of some of these samples and the lack of significant scatter is therefore interpreted to indicate that there has been no local crustal-level contamination to modify the Os isotopic compositions of these magmas.
In the study on Antarctic samples reported by Molzahn et al. (1996), the scatter in their data in an isochron plot (187Os/188Os vs 187Re/188Os) results in large uncertainties in the calculated initial ratio for these magmas. To minimize the Re blank, those workers processed separate aliquots of sample for Re and Os determinations, and it is possible that this contributed to the scatter in their results. It should be noted here that although Molzahn et al. (1996) quoted an initial 187Os/188Os ratio of 0·194 ± 0·023 based on their isochron calculation, again, we have been unable to reproduce this result using Isoplot/Ex v. 2.00 (Ludwig, 1999). For the purposes of comparison with our new dataset, we derive an estimated initial ratio of 0·13 ± 0·18 (2σ) for their data, which is significantly lower and with almost an order of magnitude greater uncertainty. Although uncertainties for the data reported by Molzahn et al. (1996) are unclear, these have been estimated from their discussion (i.e. using an absolute uncertainty of ±25 for each 187Re/188Os ratio and the in-run precisions highlighted in their table for 187Os/188Os).
As noted above, we believe some of the scatter in the data of Molzahn et al. (1996) might be attributed to aspects of the chemical procedures used to control their Re blanks. Indeed, examination of their isochron indicates three points that lie significantly off their line of best fit. If their dataset is combined with that of this study, the same three points are clearly removed from our isochron (not shown), whereas four of their results plot along it (Fig. 3; open symbols). Despite the great distances between the sample localities (Tasmania vs Antarctica), even at the time of emplacement when the two were adjacent, these areas have been inferred to belong to the same, remarkably homogeneous, Ferrar igneous province (e.g. Compston et al., 1968). We have combined our new data with what we believe to be the most reliable of the Molzahn et al. (1996) results, and calculated a further isochron. The results of this calculation (177·3 ± 3·5 Ma; 187Os/188Osi 0·125 ± 0·033 2σ) are illustrated in Fig. 3, and are entirely consistent with the results already discussed for our new Tasmanian data alone.
The current most precise estimates for the age of the Ferrar magmatism are 183·6 ± 2·1 Ma based on 206Pb/238U zircon and baddeleyite data from two sills in the Transantarctic Mountains (Encarnación et al., 1996), and 180·4 ± 1·5 Ma based on an 40Ar/39Ar age of Heimann et al. (1994) recalculated using revised parameters for the Ar standard employed (see Encarnación et al., 1996). The age of the combined dataset, illustrated in Fig. 3, lies within the range of the Ar age estimate, but is slightly low compared with the 206Pb/238U age.
As a consequence of combining these two datasets, our best estimate for the initial 187Os/188Os ratio for the Ferrar CFB magmas corresponds to a γOs of −1 ± 26 (Fig. 1). This is a significant range and spans much of the variation in mantle components illustrated in Fig. 1, including our best estimate of the likely Os isotope composition of the SCLM beneath SE Australia around the time of igneous activity (Fig. 1e). Thus no attempt can be made here to independently assess the location of the depleted mantle source region (plume vs lithosphere) beyond that proposed in previous studies (e.g. Hergt et al., 1991). Despite this range in initial γOs, however, we can examine the possible role of crustal assimilation in the origin of these magmas.
We have modelled two cases, both of which employ a crustal component with 64 ppt Os and a γOs of 740 (the median values obtained from >60 continental sediments in our database), and a mantle component with a γOs of −2 (taken from the SCLM available in SE Australia at 175 Ma, Fig. 1e). In the case of crustal-level assimilation the basalt with this gamma value is assigned an Os concentration of 100 ppt, whereas in the case of mantle contamination, the source region with this gamma value is estimated to contain 2200 ppt Os. After 10% assimilation (considered to be the minimum amount required, e.g. Hergt et al., 1989a) and using these parameters, the γOs of the contaminated magma would be +48, well beyond even the large range determined for the Ferrar magmas. Certainly the sediment Os values are somewhat arbitrarily chosen; however, it might also be argued that in the assimilation case, the γOs of the basalt endmember is likely to be higher (e.g. >1 rather than −2) so that at least some of these effects should compensate for each other. In contrast, the addition of 3% of the same crust into the mantle source only serves to shift the γOs of the mantle to −1, well within (in fact at the mean value of) the Ferrar initial γOs range.
The top panel in Fig. 4 illustrates the model believed to explain the petrogenesis of the dolerite magmas [based on Hergt et al. (1989a, 1989b, 1991)]. A depleted mantle source (with incompatible trace element abundances significantly less than typical MORB source), therefore perhaps lithospheric, is contaminated by the addition of a small amount of upper-crustal sedimentary material (≤3%). As a result, this polluted source region has trace element (and Sr, Nd and Pb isotope) characteristics dominated by the sedimentary component, but major elements (including oxygen and Os) controlled by the original mantle peridotite. During partial melting, primary magmas preserve evidence for the major element depletion of their source (low Ti, Fe, Na and P; high Si) and mantle-like oxygen and Os isotope values, while at the same time inheriting the unusual incompatible trace element and Sr, Nd and Pb isotope compositions imposed by the subducted contaminant. In this model, the choice of the average shale composition [from Taylor & McLennan (1985)] is somewhat arbitrary, but relates to the close resemblance in incompatible trace element characteristics of the dolerite magmas compared with upper-crustal rocks (Fig. 4). Neither is there any means of constraining the composition of the proposed depleted mantle except that it must have low values for at least some incompatible elements (e.g. Ti) before sediment addition. As there is no a priori reason to assume the mantle source had unusual trace element features before the addition of sediment, the composition used in Fig. 4 is simply an N-MORB (normal MORB) average (Sun & McDonough, 1989) divided by 20 (i.e. a smooth trace element pattern, with an incompatible trace element budget approximately one-third that of N-MORB source).
CONCLUSIONS
Despite decades of controversy, our understanding of CFB magmatism has grown, perhaps exponentially, in the last 10–15 years. A general consensus is emerging, that there are different sources of CFBs and that at least some owe their unusual incompatible trace element and isotopic compositions to their mantle source region, possibly deriving a significant melt contribution from sub-continental lithosphere. Although there are several means of distinguishing the effects of crustal assimilation from enriched mantle in basalt genesis (O and Os isotope compositions perhaps being among the least ambiguous) it remains very difficult to distinguish between lithospheric and asthenospheric mantle sources in CFBs.
This remains true even in the case of the Ferrar CFB province, which has the most clearly developed ‘upper continental crustal’ incompatible trace element and isotope characteristics, although retaining the most depleted major element compositions (e.g. Hergt et al., 1991). New Os isotope data are consistent with existing results (i.e. based on δ18O coupled with Sr, Nd and Pb isotope data and incompatible trace element compositions) and support an origin for the ‘crustal’ component from the SCLM (Hergt et al., 1989a, 1989b, 1991). Efforts made in this study to reduce the uncertainties in initial γOs estimates included careful sample selection, preconcentration of Os-bearing oxides, and the generation of an isochron. Despite these measures, the range in initial γOs values precludes any assessment of the role of the SCLM as the source of the major element component of the magmas.
It would appear we are tantalizingly close to resolving key issues relating to the ultimate mantle source of these magmas, yet present uncertainties in calculating initial Os isotope ratios, even in cases where apparently well-constrained isochrons can be generated, are likely to make this a discovery for the future.
*Present address: Institut für Geowissenschaften und Lithosphärenforschungen, Universität Giessen, Senckenbergstrasse 3, 35390 Giessen, Germany.
†Corresponding author. Telephone: +61-3-8344-6522. Fax: +61-3-8344-7761. e-mail: j.hergt@earthsci.unimelb.edu.au
Keith Cox played a key role in bringing the scientific community to its current level of understanding of CFB petrogenesis. His contributions extend beyond benchmark papers relating to the petrology and geochemistry of magmas, to the geodynamic and erosional implications of CFB activity. J.M.H. and J.D.W. feel privileged to have known and discussed these issues with him (ever accompanied by his pipe, which always refused to stay alight!). We dedicate this paper, and our future pursuit of understanding CFBs, to his memory. We would like to thank Rob Ellam, Chris Hawkesworth and Marjorie Wilson for their thorough and helpful reviews. This work was funded by the Australian Research Council through both the International Research Exchange and Small Grants schemes.
REFERENCES
Allègre, C. J., Dupre, B., Richard, P., Rousseau, D. & Brooks, C. (
Allègre, C. J., Birck, J. L., Capmas, F. & Courtillot, V. (
Arndt, N. T. & Christensen, U. R. (
Bennett, V. C., Esat, T. M. & Norman, M. D. (
Birck, J.-L., Roy-Barman, M. & Copmas, F. (
Brandon, A. D., Creaser, R. A., Shirey, S. B. & Carlson, R. W. (
Brewer, T. S., Hergt, J. M., Hawkesworth, C. J., Rex, D. & Storey, B. C. (
Carlson, R. W. (
Carlson, R. W. & Irving, A. J. (
Carlson, R. W., Esperanca, S. & Svisero, D. P. (
Castillo, P. R. (
Chesley, J. T. & Ruiz, J. (
Compston, W., McDougall, I. & Heier, K. S. (
Courtillot, V., Besse, J., Vandamme, D., Montigny, R., Jaeger, J. J. & Cappetta, H. (
Cox, K. G., MacDonald, R. & Hornung, G. (
Cox, K. G., Duncan, A. R., Bristow, J. W., Taylor, S. R. & Erlank, A. J. (
Ellam, R. M. & Cox, K. G. (
Ellam, R. M., Carlson, R. W. & Shirey, S. B. (
Encarnación, J., Fleming, T. H., Elliot, D. H. & Eales, H. V. (
Faure, G., Hill, R. L., Jones, L. M. & Elliot, D. H. (
Faure, G., Bowman, J. R., Elliot, D. H. & Jones, L. M. (
Ford, A. B. & Kistler, R. W. (
Gallagher, K. & Hawkesworth, C. J. (
Gibson, S. A., Thompson, R. N., Dickin, A. P. & Leonardos, O. H. (
Handler, M. R., Bennett, V. C. & Esat, T. M. (
Hart, S. R. (
Hart, S. R. & Ravizza, G. E. (
Hauri, E. H. & Hart, S. R. (
Hauri, E. H., Lassiter, J. C. & DePaolo, D. J. (
Hawkesworth, C. J., Mantovani, M. S. M., Taylor, P. N. & Palacz, Z. (
Heier, K. S., Compston, W. & McDougall, I. (
Heimann, A., Fleming, T. H., Elliot, D. H. & Foland, K. A. (
Hergt, J. M. (
Hergt, J. M., Chappell, B. W., McCulloch, M. T., McDougall, I. & Chivas, A. R. (
Hergt, J. M., Chappell, B. W., Faure, G. & Mensing, T. M. (
Hergt, J. M., Peate, D. W. & Hawkesworth, C. J. (
Hoefs, J., Faure, G. & Elliot, D. H. (
Lassiter, J. C. & DePaolo, D. J. (
Ludwig, K. R. (
Mantovani, M. S. M., Marques, L. S., De Sousa, M. A., Civetta, L., Atalla, L. & Innocenti, F. (
Marcantonio, F., Zindler, A., Elliott, T. & Staudigel, H. (
Martin, C. E. (
Martin, C. E., Carlson, R. W., Shirey, S. B., Frey, F. A. & Chen, C.-Y. (
McBride, J. S., Lambert, D. D., Greig, A. & Nicholls, I. A. (
McDonough, W. F. (
McKenzie, D. P. & Bickle, M. J. (
McKenzie, D. P. & O’Nions, R. K. (
Mensing, T. M., Faure, G., Jones, L. M., Bowman, J. R. & Hoefs, J. (
Menzies, M. A. & Kyle, P. R. (
Minster, J. F., Ricard, L. P. & Allègre, C. J. (
Molzahn, M., Reisberg, L. & Wörner, G. (
Morgan, J. W., Horan, M. F., Walker, R. J. & Grossmann, J. N. (
Officer, C. B. & Drake, C. L. (
Pearson, D. G., Carlson, R. W., Shirey, S. B., Boyd, F. R. & Nixon, P. H. (
Pearson, D. G., Shirey, S. B., Carlson, R. W., Boyd, F. R., Pokhilenko, N. P. & Shimizu, N. (
Pegram, W. J. & Allègre, C. J. (
Reisberg, L. & Lorand, J. P. (
Reisberg, L. C., Allègre, C. J. & Luck, J. M. (
Reisberg, L., Zindler, A., Marcantonio, F., White, W. Wyman, D. & Weaver, B. (
Richards, M. A., Duncan, R. A. & Courtillot, V. E. (
Roy-Barman, M. (
Roy-Barman, M. & Allègre, C. J. (
Roy-Barman, M. & Allègre, C. J. (
Roy-Barman, M., Luck, J. M. & Allègre, C. J. (
Schmidt, P. W. & McDougall, I. (
Shirey, S. B. & Walker, R. J. (
Shirey, S. B. & Walker, R. J. (
Snow, J. & Reisberg, L. (
Steiger, R. H. & Jager, E. (
Sun, S.-S. & McDonough, W. F. (
Taylor, S. R. & McLennan, S. M. (
Walker, R. J., Carlson, R. W., Shirey, S. B. & Boyd, F. R. (
Walker, R. J., Morgan, J. W., Naldrett, A. J., Li, C. & Fassett, J. D. (
Walker, R. J., Morgan, J. W., Horan, M. F., Czamanske, G. F., Krogstad, E. J., Fedorenko, V. & Kunilov, V. E. (
White, R. & McKenzie, D. (


![Histograms based on reported Os isotope data (γOs) for a range of mantle domains. (a) Upper oceanic mantle (including MORB and abyssal peridotites). (b) Mantle plumes (including EMI, EMII, HIMU endmember examples and OIB of intermediate chemical affinities). (c) Sub-continental lithospheric mantle (including kimberlite-hosted xenoliths and lamproites as measures of the mantle lithosphere beneath ancient cratonic areas, and basalt-hosted peridotites as samples from non-cratonized regions). (d) Examples of CFBs, age corrected to initial γOs values. (e) An estimate of the SCLM in SE Australia at the time of Ferrar province magmatism (∼175 Ma) calculated by age correcting published peridotite xenolith data to an age of 175 Ma. References used to construct these histograms are either referred to elsewhere in the text or are listed here in chronological order and include: Walker et al. (1989), Martin (1991), Reisberg et al. (1991, 1993), Pegram & Allègre (1992), Hauri & Hart (1993), Carlson & Irving (1994), Martin et al. (1994), Roy-Barman & Allègre (1994, 1995), Marcantonio et al. (1995), Pearson et al. (1995a, 1995b), Reisberg & Lorand (1995), Shirey & Walker (1995), Snow & Reisberg (1995), Bennett et al. (1996), Brandon et al. (1996), Carlson et al. (1996), Hart & Ravizza (1996), Hauri (1996), Hauri et al. (1996), McBride et al. (1996), Roy-Barman et al. (1996), Handler et al. (1997), Chesley & Ruiz (1998). γOs values, where γOs = {[(187Os/188Ossample)/(187Os/188Osmantle)] − 1} × 100, were calculated relative to a chondritic upper-mantle value of 0·1275 following the technique of Handler et al. (1997). Os isotope ratios quoted as 187Os/186Os were converted to equivalent 187Os/188Os values first, by dividing through by 8·340 [after Walker et al. (1991)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/41/7/10.1093_petrology_41.7.905/2/m_egd061.f1.jpeg?Expires=1716642055&Signature=TIGWCdkAPJMt5nIhkX03ajEmiz3PxPZACJxPZSv2p0BoLMYFhS0bUaTc3COj1A1omd4G8W3jZ9E-EsHpBbFrxbnvv3LcWEYM0FcS8PqdTuY2gf9AioxKB~ZzNoYKWQyIQEBOu02rerdzUPYmx4Bg~p18cfXginqPVyfeOUsScd9bT2LfS6iSm~q1djHHg0OTTXgr-4AprLPIkz3Bn0IWSJe2sMkoNN~bpdDbmShtkJ4VMfS~GJFYCHwetSFXLgyIzLrBpQwqTtvc8EWgVwEfye2NZqdOt3qc-AYCJM9vWUmPCfM00zh-6KDs9hu2HxEsBzcid7lagtbkkFeWpSM-Cg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Sketch map of Tasmania indicating the extent of exposed dolerite (shaded) and locations of widely separated sampling sites chosen for this study. The Hobart sample was taken from the Mt Wellington sheet. It should be noted that the shaded region includes numerous separate intrusions that are distributed across a major basement divide in Tasmania. The southern extension of the Tamar Fracture System is unclear; however, it extends from ∼147° on the northern coast, through to a location close to Hobart in the SE [modified from Hergt et al. (1989a)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/41/7/10.1093_petrology_41.7.905/2/m_egd061.f2.jpeg?Expires=1716642055&Signature=4Lgn3U~2lN-sknbziBy79CF5-6ykNv6s1MvysSoGCSyPGzB0smuU9AJ70HWTV7uUvXSBRskGm1lP8KgjjoAMi3Hx1KLM7uIgcrPdfShKl2j0K4ceGVbcTtJShOuXYK50EdiQInEKD2NisNp0bQTSB5Lk2xfSy2hmKBBhGfwaVx~yWuspr9FJnG7HzeWvy2IpgGcCazdeH4EgzYDCUC~z42Dbvo~04BwS7s2qspa4-8HsOyW~ijSoH3qcQL1xNlK6xxO8Ybdwzx9Yh2Lna4OrgK-yLl5Z3SS7AdWGpKCC88qx~kmVBJFD~IgfodpiYGqqhLFN9wrgSfnCikZNu6qI7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Normal mid-ocean ridge basalt (N-MORB) normalized incompatible trace element diagrams illustrating: (a) our preferred model for the petrogenesis of the Ferrar province CFBs and (b) the liquid (chilled margin) and cumulate compositions for five of the rock samples examined in this study. The top panel includes the compositions of the mantle (depleted MORB source) and continental crustal (average shale) components employed in the model. The contaminated source results from the addition of 3% of the average shale into the depleted mantle source. The cross-hatched area brackets a range of possible magmas derived from the contaminated source via 8–15% partial melting (making the simplifying assumption that all incompatible elements will completely partition into the magma). As indicated by the key in the lower panel, the pattern within this cross-hatched region is the Tasmanian Dolerite average chilled margin composition [reported by Hergt et al. (1989a)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/41/7/10.1093_petrology_41.7.905/2/m_egd061.f4.jpeg?Expires=1716642055&Signature=mVMHkI1DCTxH1NcueUvUP3xDU8hSyYFUF60O1GXQiij-38T0l9LPxHnYNFuT6811qwcR8NHWVfBfUzNDhdjENQcbSkVJ6SBDKIGxMBDuKT348Qf7m47pEoUJXweZxM-x2tHhpi6AGMdyOYDj~5oL4iVGKyjKZUWFac39IsNdosaqW3nOmIVcFW0cA8ytseAjY2Ry5DCD4O8uOso3iyD6W~EVbHy5XGly07nQzJm6Ojv9seovZHHXj4oviKEMg5ZDqwrH~65Gr~W~or9xUjHvQVzq3TpGeRCixOedgiBrTM1CyhBsFbDttsR8HnpgFTTAOkTxZPOwna9Jm~~ghbNA~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
