-
PDF
- Split View
-
Views
-
Cite
Cite
Karishma Shah, He Cai, Jennifer C E Lane, Gary S Collins, Nigel K Arden, Dominic Furniss, Stephanie R Filbay, Prognostic factors for finger interphalangeal joint osteoarthritis: a systematic review, Rheumatology, Volume 60, Issue 3, March 2021, Pages 1080–1090, https://doi.org/10.1093/rheumatology/keaa735
Close - Share Icon Share
Abstract
Radiographic hand OA affects one in five adults. Symptomatic hand OA can result in functional impairment, pain and reduced quality of life. A prevalent form of hand OA is IP joint OA, however prognostic factors for IP joint OA remain poorly understood. This systematic review aimed to identify prognostic factors for IP joint OA, and to summarize the diagnostic criteria for IP joint OA in prognostic studies.
EMBASE, MEDLINE, Scopus and The Cochrane Library were searched from inception until 19 February 2020 (PROSPERO CRD42019116782). Eligible studies described diagnostic criteria defining IP joint OA, and assessed potential prognostic factors for IP joint OA. Risk of bias was assessed using a modified Quality in Prognosis Studies (QUIPS) tool and a best evidence synthesis was used.
Eighteen studies were included (risk of bias: eight high, three moderate, seven low). All defined OA radiographically, and three studies incorporated clinical symptoms into their definition of OA. Forty-nine potential prognostic factors were assessed. Eight were prognostic: older age in women, female gender (both moderate evidence); family history of Heberden’s nodes, Kashin–Beck disease, older age in men, dental occupation in men, finger fracture, parity (all limited evidence). Higher BMI in women (limited evidence) was prognostic for symptomatic radiographic OA. No prognostic factors for symptomatic OA were identified.
IP joint OA is most commonly defined radiographically, yet criteria were heterogeneous. Eight prognostic factors for radiographic IP joint OA and one for symptomatic radiographic IP joint OA were identified, all with limited or moderate evidence. Further studies on causality and on prognostic pathways are needed.
Studies assessing hand IP joint OA most commonly diagnose OA using a radiographic criteria.
There is moderate evidence that older age in women and female gender are prognostic factors.
Nodes in family, Kashin–Beck disease, older age in men, male dentists, fracture and parity have limited evidence as prognosticators.
Introduction
Radiographic OA in the hand and wrist are amongst the most prevalent types of OA in the general population [1]. This year, OA is expected to be the fourth leading cause of worldwide disability [2]. Currently, treatment of hand OA is difficult and relies on symptomatic management, as there are no disease-modifying drugs. However, the efficacies of such symptomatic treatments are often limited by other comorbidities and by drug toxicities [3–5]. With few effective treatment options available, clinical trials evaluating new drugs and non-pharmacological therapies are needed. Guidelines for randomized control trials in hand OA advise recruiting patients with established hand OA [6]. However, recruiting patients with an increased risk of developing hand OA could facilitate the identification of new drug therapies to delay or prevent the development of OA [6], and excluding these participants can limit the ability to detect a therapeutic treatment effect [7]. If it were possible to predict which people are at highest risk of developing hand OA, suitable participants could be recruited to future trials. Furthermore, if prognostic factors for hand OA were recognized in clinical settings, clinicians could implement preventative strategies.
Diagnosing OA in the hand can be challenging, as IP joint OA is thought to be a different subset of disease to OA at the first CMC joint, and is therefore considered to have different aetiology and pathology [8]. There is also known disassociation between radiographic signs and symptoms [9]. Research to date has mainly focused on defining CMC joint OA and understanding its aetiology, whilst prognostic factors for the presence of IP joint OA, and diagnostic criteria used to describe IP joint OA, are not yet well established. Therefore, the aim of this systematic review was to identify prognostic factors for the presence of IP joint OA, and describe the diagnostic criteria used to define IP joint OA in prognostic studies.
Methods
This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [10]. The protocol was prospectively registered on PROSPERO (CRD42019116782).
Search strategy
A specialist healthcare librarian (Elinor Harriss) assisted in developing the search strategies, which retrieved articles including terms related to the hand and fingers, terms related to OA, and terms related to development and incidence, in the abstract, title or as MeSH terms (Supplementary Data S1, available at Rheumatology online). Searches were undertaken in four electronic databases: (i) MEDLINE by Ovid, (ii) EMBASE by Ovid, (iii) Scopus and (iv) The Cochrane Library, and search terms were amended as necessary for each database (Supplementary Data S1, available at Rheumatology online). The searches were initially completed on 17 October 2018 and duplicates were removed (Supplementary Data S2, available at Rheumatology online). All articles were imported into Rayyan QCRI Tool [11], and two groups of reviewers (group 1: K.S., group 2: H.C. and J.C.E.L.) independently screened titles and abstracts. Potentially eligible articles, including articles with insufficient title and/or abstract information, proceeded to full text review. The reference lists of included articles were also reviewed by two independent reviewers for additional relevant studies, though no additional articles were identified this way. For articles where the full text was not available, a request was sent directly to the authors with a 4-month response policy. Any inter-rating disagreement was discussed at a meeting between the reviewers, and any further disagreement was resolved through discussion with a third reviewer (S.R.F.). The searches were updated on 19 February 2020.
Inclusion criteria
For inclusion, papers must have (i) investigated the association between a factor and the presence of IP joint OA, (ii) described the diagnostic criteria, either radiographic and/or symptomatic, used to define IP joint OA, (iii) measured IP joint [i.e. distal IP joint (DIPJ) and/or proximal IP joint (PIPJ)] OA separate from first CMC joint OA, (iv) described the association between one or more potential prognostic factor and the presence of IP joint OA, and (v) allowed for a time delay between the presence of the factor and the presence of OA, allowing time for OA to develop. We included cross-sectional studies if the prognostic factor was historical (for example, a history of a medical condition), as long as the prognostic factor did not change over time.
Exclusion criteria
Excluded article types were case reports, brief reports, abstracts, reviews and letters to the editor. Ineligible articles included animal, cell and cadaver studies, studies of inflammatory arthritis, and articles not published in English. Articles assessing genetic factors were excluded. Finally, articles for which a full text was not accessible and not provided from the author within 4 months of correspondence were excluded.
Risk of bias
Risk of bias of included studies was assessed independently by two reviewers (K.S. and H.C.), using a modified Quality in Prognosis Studies (QUIPS) tool [12] (supplementary Table S1, available at Rheumatology online).We excluded the ‘confounding factors’ domain, as it is difficult to define a confounder in the context of prognostic factor studies, and confounders themselves can be considered to be known prognostic factors [13]. An overall score of ‘low’, ‘moderate’ or ‘high’ was given to each of the six domains (supplementary Table S1, available at Rheumatology online), and then an overall risk of bias for each study was calculated. A study was defined as having an overall high risk of bias if one or more domains was of high risk of bias [14–17]; an overall moderate risk of bias if three or more domains were of moderate risk of bias (none high risk of bias) [14–16]; and an overall low risk of bias if all domains were low risk of bias, or one or two domains were moderate risk of bias [18]. Any discrepancies between reviewers were discussed at a consensus meeting, and any further disagreement was resolved by discussion with a third reviewer (S.R.F.).
Data extraction
All data were extracted by one reviewer (K.S.) and inputted to a Microsoft Excel spreadsheet, and independently cross-checked by a second reviewer (H.C.). Extracted data included participant demographics, study characteristics, the factor(s) assessed, the diagnostic criteria used to define IP joint OA and the effect measure. Data were extracted from all time points reported in each study.
Best evidence synthesis
All potential prognostic factors assessed for IP joint OA were identified separately for radiographic, radiographic symptomatic and symptomatic diagnostic criteria. Subgroup analyses for DIPJ and PIPJ OA were performed. In the absence of heterogeneity, a meta-analysis to pool factors investigated across multiple studies was planned, and quality of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework [19].
If studies were heterogeneous with regards to study populations, potential prognostic factors analysed, effect measures and the methods used to define IP joint OA, a narrative summary was used. The association between a potential prognostic factor and the presence of IP joint OA was categorized as: (i) a prognostic factor (positive association/second), (ii) not a prognostic factor (negative association/second or no statistical association) or (iii) mixed evidence (associations in different directions). A best evidence synthesis method was used to summarize the data, assessed in the following sequential order [20–24]. If multiple analyses were performed within one study, the consistent findings approach below was applied to the analyses to determine whether the study itself showed consistent or mixed evidence, after which the study was used in calculating the overall best evidence synthesis.
Consistent findings (≥75% of studies showing associations in the same direction) for either a positive or negative/no association.
Mixed evidence: inconsistent findings (<75% of studies showing associations in the same direction).
If findings were consistent, the strength of evidence was assessed:
Strong evidence: more than two studies with low risk of bias.
Moderate evidence: one study with low risk of bias and one other study, or more than two studies with moderate or high risk of bias.
Limited evidence with low risk of bias: one study with low risk of bias.
Limited evidence: one or two studies with moderate or high risk of bias.
Results
Search strategy
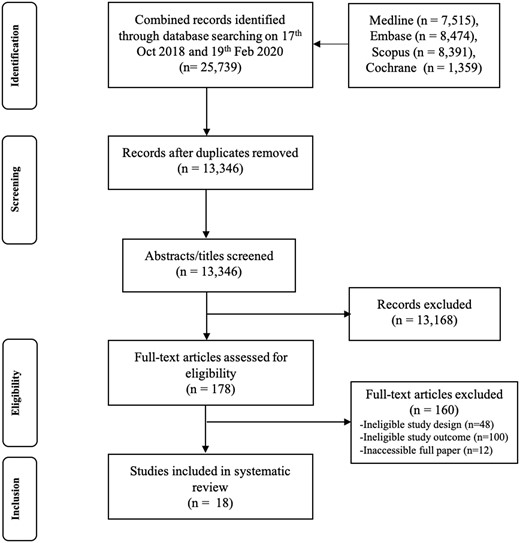
The results from the search in 2018 and the updated search in 2020 are combined in the PRISMA figure (Fig. 1). In total, the searches produced 25 739 abstracts, and after the removal of duplicates 13 346 remained. After title and abstract screening, 178 full texts were reviewed, and 18 articles met the inclusion criteria. No further articles were included from screening the reference lists of other reviews.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart of study selection
Study characteristics
Data were extracted from 18 studies (supplementary Table S2, available at Rheumatology online) [25–42]. Four studies were prospective cohort studies [26, 31, 35, 38], 11 studies were cross-sectional [27–30, 32–34, 36, 37, 39, 41] and 3 studies were case–control studies [25, 40, 42] Five studies included only women [28, 30, 34, 35, 37]. Of the studies that reported mean age of participants, Fu et al. [40] included the youngest participants [age of cases: mean (s.d.) 39.2 (3.6) years, age of controls: 38.7 (3.1) years], whilst Cho et al. included the oldest participants [72 (5) years] [41].
Risk of bias
Eight studies had an overall high risk of bias [25, 26, 29–31, 33, 38, 39], three had an overall moderate risk of bias [27, 35, 41] and seven had an overall low risk of bias [28, 32, 34, 36, 37, 39, 42] (Table 1). ‘Study participation’ was of high risk of bias in the studies by Jones et al. [29] and Fu et al. [40], as they did not describe the source population and it is unclear whether there was 80% participation from eligible individuals. Lehto et al. [25] and Haara et al. [31] did not describe ‘study attrition’. Risk of bias for the ‘prognostic factor measurement’ domain was high in the studies by Cooley et al. [30] and Kalichman et al. [33], as comprehensive descriptions of the factors were not provided; whilst in the study by Biermasz et al. [38], a continuous variable was converted to a categorical variable. Chaisson et al. [26] was found to be of high risk of bias in the ‘outcome measurement domain’ as the radiographs were not interpreted with blinding, and some participants had missing radiographic data.
Risk of bias for included studies
| Study . | Biasesa . | Overall risk of bias . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| Lehto et al. [25] | Moderate | High | Moderate | Low | Moderate | High |
| Chaisson et al. [26] | Moderate | Moderate | Low | High | Moderate | High |
| Cvijetić et al. [27] | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Yoshida et al. [28] | Moderate | Low | Low | Moderate | Low | Low |
| Jones et al. [29] | High | Low | Moderate | Moderate | Low | High |
| Cooley et al. [30] | Moderate | Low | High | Moderate | Low | High |
| Haara et al. [31] | Low | High | Moderate | Low | Low | High |
| Kessler et al. [32] | Moderate | Low | Moderate | Low | Low | Low |
| Kalichman et al. [33] | Moderate | Low | High | Moderate | Moderate | High |
| Solovieva et al. [34] | Moderate | Low | Moderate | Low | Low | Low |
| Szoeke et al. [35] | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Dahaghin et al. [36] | Moderate | Low | Moderate | Low | Low | Low |
| Ding et al. [37] | Moderate | Low | Moderate | Low | Low | Low |
| Biermasz et al. [38] | Moderate | Low | High | Low | Low | High |
| Hoeven et al. [39] | Moderate | Low | Moderate | Low | Low | Low |
| Fu et al. [40] | High | Low | Moderate | Moderate | Moderate | High |
| Cho et al. [41] | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Gregson et al. [42] | Moderate | Moderate | Low | Low | Low | Low |
| Study . | Biasesa . | Overall risk of bias . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| Lehto et al. [25] | Moderate | High | Moderate | Low | Moderate | High |
| Chaisson et al. [26] | Moderate | Moderate | Low | High | Moderate | High |
| Cvijetić et al. [27] | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Yoshida et al. [28] | Moderate | Low | Low | Moderate | Low | Low |
| Jones et al. [29] | High | Low | Moderate | Moderate | Low | High |
| Cooley et al. [30] | Moderate | Low | High | Moderate | Low | High |
| Haara et al. [31] | Low | High | Moderate | Low | Low | High |
| Kessler et al. [32] | Moderate | Low | Moderate | Low | Low | Low |
| Kalichman et al. [33] | Moderate | Low | High | Moderate | Moderate | High |
| Solovieva et al. [34] | Moderate | Low | Moderate | Low | Low | Low |
| Szoeke et al. [35] | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Dahaghin et al. [36] | Moderate | Low | Moderate | Low | Low | Low |
| Ding et al. [37] | Moderate | Low | Moderate | Low | Low | Low |
| Biermasz et al. [38] | Moderate | Low | High | Low | Low | High |
| Hoeven et al. [39] | Moderate | Low | Moderate | Low | Low | Low |
| Fu et al. [40] | High | Low | Moderate | Moderate | Moderate | High |
| Cho et al. [41] | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Gregson et al. [42] | Moderate | Moderate | Low | Low | Low | Low |
Biases from Modified Quality in Prognosis Studies (QUIPS) tool: 1: study participation; 2: study attrition; 3: prognostic factor measurement; 4: outcome measure; 5: statistical analysing and reporting.
Risk of bias for included studies
| Study . | Biasesa . | Overall risk of bias . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| Lehto et al. [25] | Moderate | High | Moderate | Low | Moderate | High |
| Chaisson et al. [26] | Moderate | Moderate | Low | High | Moderate | High |
| Cvijetić et al. [27] | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Yoshida et al. [28] | Moderate | Low | Low | Moderate | Low | Low |
| Jones et al. [29] | High | Low | Moderate | Moderate | Low | High |
| Cooley et al. [30] | Moderate | Low | High | Moderate | Low | High |
| Haara et al. [31] | Low | High | Moderate | Low | Low | High |
| Kessler et al. [32] | Moderate | Low | Moderate | Low | Low | Low |
| Kalichman et al. [33] | Moderate | Low | High | Moderate | Moderate | High |
| Solovieva et al. [34] | Moderate | Low | Moderate | Low | Low | Low |
| Szoeke et al. [35] | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Dahaghin et al. [36] | Moderate | Low | Moderate | Low | Low | Low |
| Ding et al. [37] | Moderate | Low | Moderate | Low | Low | Low |
| Biermasz et al. [38] | Moderate | Low | High | Low | Low | High |
| Hoeven et al. [39] | Moderate | Low | Moderate | Low | Low | Low |
| Fu et al. [40] | High | Low | Moderate | Moderate | Moderate | High |
| Cho et al. [41] | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Gregson et al. [42] | Moderate | Moderate | Low | Low | Low | Low |
| Study . | Biasesa . | Overall risk of bias . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| Lehto et al. [25] | Moderate | High | Moderate | Low | Moderate | High |
| Chaisson et al. [26] | Moderate | Moderate | Low | High | Moderate | High |
| Cvijetić et al. [27] | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Yoshida et al. [28] | Moderate | Low | Low | Moderate | Low | Low |
| Jones et al. [29] | High | Low | Moderate | Moderate | Low | High |
| Cooley et al. [30] | Moderate | Low | High | Moderate | Low | High |
| Haara et al. [31] | Low | High | Moderate | Low | Low | High |
| Kessler et al. [32] | Moderate | Low | Moderate | Low | Low | Low |
| Kalichman et al. [33] | Moderate | Low | High | Moderate | Moderate | High |
| Solovieva et al. [34] | Moderate | Low | Moderate | Low | Low | Low |
| Szoeke et al. [35] | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Dahaghin et al. [36] | Moderate | Low | Moderate | Low | Low | Low |
| Ding et al. [37] | Moderate | Low | Moderate | Low | Low | Low |
| Biermasz et al. [38] | Moderate | Low | High | Low | Low | High |
| Hoeven et al. [39] | Moderate | Low | Moderate | Low | Low | Low |
| Fu et al. [40] | High | Low | Moderate | Moderate | Moderate | High |
| Cho et al. [41] | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Gregson et al. [42] | Moderate | Moderate | Low | Low | Low | Low |
Biases from Modified Quality in Prognosis Studies (QUIPS) tool: 1: study participation; 2: study attrition; 3: prognostic factor measurement; 4: outcome measure; 5: statistical analysing and reporting.
Diagnostic criteria used to define IP joint OA
All studies used radiography in their definition of OA [25–42], including one study which specified a symptomatic radiographic definition [37] (Table 2). A version of the Kellgren Lawrence (KL) atlas [43–45] was most commonly used [25–28, 31, 33, 34, 36–41]. Five studies used the Altman, also known as the Osteoarthritis Research Society International (OARSI) [46] atlas [29, 30, 32, 35, 42]. Ding et al. was the only study to include a definition of radiographic symptomatic OA, which was defined as ‘KL grade ≥2 with ‘mild’ pain during the last 30 days in ≥1 DIPJ’ [37]. Two studies used a non-radiographic symptomatic definition of OA, by assessing the presence of Heberden’s nodes [29, 30]. Jones et al. [29] defined symptomatic IP joint OA as the ‘total number of DIPJs with Heberden’s nodes’, whilst Cooley et al. [30] defined it as ‘Heberden’s nodes in ≥1 DIPJ’.
Diagnostic criteria used to define the presence of finger IP joint OA in included studies
| Study . | Diagnostic criteria used to define the presence of finger IP joint OA . |
|---|---|
| Lehto et al. [25] | Outcome 1: total number of IP joints with KL grade ≥2a Outcome 2: KL grade ≥2 in DIPJ of the index finger used for pinch power |
| Chaisson et al. [26] | Total number of IP joints with KL grade ≥2 at follow-up from 0/1 at baseline of the right hand onlya |
| Cvijetić et al. [27] | KL grade ≥2 (no further information given)a |
| Yoshida et al. [28] | KL grade ≥2 of the second and third fingersa |
| Jones et al. [29] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Cooley et al. [30] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Haara et al. [31] | KL grade ≥2 in ≥2 symmetrical DIPJs |
| Kessler et al. [32] | Using the Kessler hand scale, Altman JSN grade ≥2 in ≥2 IP joints or JSN grade 1 with sclerosis or OP grade ≥2 in ≥2 IP joints |
| Kalichman et al. [33] | KL sum score for IP jointsa |
| Solovieva et al. [34] | Outcome 1: KL grade ≥2 in ≥1 IP joints Outcome 2: KL grade ≥2 in ≥1 IP joints of ring and little fingers |
| Szoeke et al. [35] | Outcome 1: Altman [46] OP grade ≥2 in ≥1 IP jointa Outcome 2: Altman [46] JSN grade ≥2 in ≥1 IP jointa |
| Dahaghin et al. [36] | KL grade ≥2 in ≥1 IP jointa |
| Ding et al. [37] | KL grade ≥2 in ≥1 DIPJ |
| Biermasz et al. [38] | Sum KL score in IP jointsa |
| Hoeven et al. [39] | KL grade ≥2 in ≥1 IP jointsa |
| Fu et al. [40] | Outcome 1: OARSI OP grade ≥1 in ≥1 IP joint of right handa Outcome 2: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 3: OARSI JSN grade ≥1 in ≥1 IP joint of right handa Outcome 4: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 5: KL grade ≥2 in ≥1 IP joint of right handa Outcome 6: KL grade 4 in ≥1 IP joint of right handa |
| Cho et al. [41] | KL grade ≥2 in ≥1 IP jointsa |
| Gregson et al. [42] | Outcome 1: Altman OP grade ≥1 in ≥1 DIPJ of dominant hand Outcome 2: Altman OP grade ≥ 2in ≥1 DIPJ of dominant hand Outcome 3: Altman JSN grade ≥1 in ≥1 DIPJ of dominant hand Outcome 4: Altman JSN grade ≥2 in ≥1 DIPJ of dominant hand Outcome 5: presence of sub-chondral sclerosis, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand Outcome 6: presence of malalignment, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand |
| Study . | Diagnostic criteria used to define the presence of finger IP joint OA . |
|---|---|
| Lehto et al. [25] | Outcome 1: total number of IP joints with KL grade ≥2a Outcome 2: KL grade ≥2 in DIPJ of the index finger used for pinch power |
| Chaisson et al. [26] | Total number of IP joints with KL grade ≥2 at follow-up from 0/1 at baseline of the right hand onlya |
| Cvijetić et al. [27] | KL grade ≥2 (no further information given)a |
| Yoshida et al. [28] | KL grade ≥2 of the second and third fingersa |
| Jones et al. [29] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Cooley et al. [30] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Haara et al. [31] | KL grade ≥2 in ≥2 symmetrical DIPJs |
| Kessler et al. [32] | Using the Kessler hand scale, Altman JSN grade ≥2 in ≥2 IP joints or JSN grade 1 with sclerosis or OP grade ≥2 in ≥2 IP joints |
| Kalichman et al. [33] | KL sum score for IP jointsa |
| Solovieva et al. [34] | Outcome 1: KL grade ≥2 in ≥1 IP joints Outcome 2: KL grade ≥2 in ≥1 IP joints of ring and little fingers |
| Szoeke et al. [35] | Outcome 1: Altman [46] OP grade ≥2 in ≥1 IP jointa Outcome 2: Altman [46] JSN grade ≥2 in ≥1 IP jointa |
| Dahaghin et al. [36] | KL grade ≥2 in ≥1 IP jointa |
| Ding et al. [37] | KL grade ≥2 in ≥1 DIPJ |
| Biermasz et al. [38] | Sum KL score in IP jointsa |
| Hoeven et al. [39] | KL grade ≥2 in ≥1 IP jointsa |
| Fu et al. [40] | Outcome 1: OARSI OP grade ≥1 in ≥1 IP joint of right handa Outcome 2: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 3: OARSI JSN grade ≥1 in ≥1 IP joint of right handa Outcome 4: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 5: KL grade ≥2 in ≥1 IP joint of right handa Outcome 6: KL grade 4 in ≥1 IP joint of right handa |
| Cho et al. [41] | KL grade ≥2 in ≥1 IP jointsa |
| Gregson et al. [42] | Outcome 1: Altman OP grade ≥1 in ≥1 DIPJ of dominant hand Outcome 2: Altman OP grade ≥ 2in ≥1 DIPJ of dominant hand Outcome 3: Altman JSN grade ≥1 in ≥1 DIPJ of dominant hand Outcome 4: Altman JSN grade ≥2 in ≥1 DIPJ of dominant hand Outcome 5: presence of sub-chondral sclerosis, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand Outcome 6: presence of malalignment, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand |
DIPJ: distal IP joint; JSN: joint space narrowing; KL: Kellgren Lawrence; OARSI: Osteoarthritis Research Society International (synonymous with the Altman atlas); OP: osteophyte; PIPJ: proximal IP joint. aDIPJs and PIPJs assessed separately.
Diagnostic criteria used to define the presence of finger IP joint OA in included studies
| Study . | Diagnostic criteria used to define the presence of finger IP joint OA . |
|---|---|
| Lehto et al. [25] | Outcome 1: total number of IP joints with KL grade ≥2a Outcome 2: KL grade ≥2 in DIPJ of the index finger used for pinch power |
| Chaisson et al. [26] | Total number of IP joints with KL grade ≥2 at follow-up from 0/1 at baseline of the right hand onlya |
| Cvijetić et al. [27] | KL grade ≥2 (no further information given)a |
| Yoshida et al. [28] | KL grade ≥2 of the second and third fingersa |
| Jones et al. [29] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Cooley et al. [30] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Haara et al. [31] | KL grade ≥2 in ≥2 symmetrical DIPJs |
| Kessler et al. [32] | Using the Kessler hand scale, Altman JSN grade ≥2 in ≥2 IP joints or JSN grade 1 with sclerosis or OP grade ≥2 in ≥2 IP joints |
| Kalichman et al. [33] | KL sum score for IP jointsa |
| Solovieva et al. [34] | Outcome 1: KL grade ≥2 in ≥1 IP joints Outcome 2: KL grade ≥2 in ≥1 IP joints of ring and little fingers |
| Szoeke et al. [35] | Outcome 1: Altman [46] OP grade ≥2 in ≥1 IP jointa Outcome 2: Altman [46] JSN grade ≥2 in ≥1 IP jointa |
| Dahaghin et al. [36] | KL grade ≥2 in ≥1 IP jointa |
| Ding et al. [37] | KL grade ≥2 in ≥1 DIPJ |
| Biermasz et al. [38] | Sum KL score in IP jointsa |
| Hoeven et al. [39] | KL grade ≥2 in ≥1 IP jointsa |
| Fu et al. [40] | Outcome 1: OARSI OP grade ≥1 in ≥1 IP joint of right handa Outcome 2: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 3: OARSI JSN grade ≥1 in ≥1 IP joint of right handa Outcome 4: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 5: KL grade ≥2 in ≥1 IP joint of right handa Outcome 6: KL grade 4 in ≥1 IP joint of right handa |
| Cho et al. [41] | KL grade ≥2 in ≥1 IP jointsa |
| Gregson et al. [42] | Outcome 1: Altman OP grade ≥1 in ≥1 DIPJ of dominant hand Outcome 2: Altman OP grade ≥ 2in ≥1 DIPJ of dominant hand Outcome 3: Altman JSN grade ≥1 in ≥1 DIPJ of dominant hand Outcome 4: Altman JSN grade ≥2 in ≥1 DIPJ of dominant hand Outcome 5: presence of sub-chondral sclerosis, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand Outcome 6: presence of malalignment, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand |
| Study . | Diagnostic criteria used to define the presence of finger IP joint OA . |
|---|---|
| Lehto et al. [25] | Outcome 1: total number of IP joints with KL grade ≥2a Outcome 2: KL grade ≥2 in DIPJ of the index finger used for pinch power |
| Chaisson et al. [26] | Total number of IP joints with KL grade ≥2 at follow-up from 0/1 at baseline of the right hand onlya |
| Cvijetić et al. [27] | KL grade ≥2 (no further information given)a |
| Yoshida et al. [28] | KL grade ≥2 of the second and third fingersa |
| Jones et al. [29] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Cooley et al. [30] | Outcome 1: presence of JSN or OP, as defined by the Altman atlas, in ≥1 DIPJ Outcome 2: sum score of JSN and OP, as defined by the Altman atlas, in all DIPJs |
| Haara et al. [31] | KL grade ≥2 in ≥2 symmetrical DIPJs |
| Kessler et al. [32] | Using the Kessler hand scale, Altman JSN grade ≥2 in ≥2 IP joints or JSN grade 1 with sclerosis or OP grade ≥2 in ≥2 IP joints |
| Kalichman et al. [33] | KL sum score for IP jointsa |
| Solovieva et al. [34] | Outcome 1: KL grade ≥2 in ≥1 IP joints Outcome 2: KL grade ≥2 in ≥1 IP joints of ring and little fingers |
| Szoeke et al. [35] | Outcome 1: Altman [46] OP grade ≥2 in ≥1 IP jointa Outcome 2: Altman [46] JSN grade ≥2 in ≥1 IP jointa |
| Dahaghin et al. [36] | KL grade ≥2 in ≥1 IP jointa |
| Ding et al. [37] | KL grade ≥2 in ≥1 DIPJ |
| Biermasz et al. [38] | Sum KL score in IP jointsa |
| Hoeven et al. [39] | KL grade ≥2 in ≥1 IP jointsa |
| Fu et al. [40] | Outcome 1: OARSI OP grade ≥1 in ≥1 IP joint of right handa Outcome 2: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 3: OARSI JSN grade ≥1 in ≥1 IP joint of right handa Outcome 4: OARSI OP grade ≥3 in ≥1 IP joint of right handa Outcome 5: KL grade ≥2 in ≥1 IP joint of right handa Outcome 6: KL grade 4 in ≥1 IP joint of right handa |
| Cho et al. [41] | KL grade ≥2 in ≥1 IP jointsa |
| Gregson et al. [42] | Outcome 1: Altman OP grade ≥1 in ≥1 DIPJ of dominant hand Outcome 2: Altman OP grade ≥ 2in ≥1 DIPJ of dominant hand Outcome 3: Altman JSN grade ≥1 in ≥1 DIPJ of dominant hand Outcome 4: Altman JSN grade ≥2 in ≥1 DIPJ of dominant hand Outcome 5: presence of sub-chondral sclerosis, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand Outcome 6: presence of malalignment, as defined by the Altman atlas, in ≥1 DIPJ of dominant hand |
DIPJ: distal IP joint; JSN: joint space narrowing; KL: Kellgren Lawrence; OARSI: Osteoarthritis Research Society International (synonymous with the Altman atlas); OP: osteophyte; PIPJ: proximal IP joint. aDIPJs and PIPJs assessed separately.
Potential prognostic factors for IP joint OA
In total, 64 potential prognostic factors for IP joint OA were investigated across the 18 studies (Table 3). Due to heterogeneity in study populations, the methods used to measure the factors and to measure OA, and difference in statistical analyses, meta-analyses could not be performed and a best evidence descriptive synthesis was used.
Potential prognostic factors assessed for their association with the presence of IP joint OA
| Study . | Potential prognostic factor assessed for association with interphalangeal joint OA . |
|---|---|
| Lehto et al. [25] | Dental occupation in men; dental occupation in women; pinch power: stronger |
| Chaisson et al. [26] | Grip strength in men: higher; grip strength in women: higher |
| Cvijetić et al. [27] | Age in men: older; age in women: older; BMI in men: higher; BMI in women: higher; menopause years: longer; blood pressure (systolic) in men: higher; blood pressure (diastolic) in men: higher; blood pressure (systolic) in women: higher; blood pressure (diastolic) in women: higher; smoking |
| Yoshida et al. [28] | Japanese ethnicity in women |
| Jones et al. [29] | BMI: higher; BMI: lower; history of finger fracture; mechanical stress during work; physical activity |
| Cooley et al. [30] | Breastfed ever; hysterectomy; menarche age: older; menstruation years: longer; menopause age: older; number of children: higher; oral contraceptive use; oral contraceptive duration: longer |
| Haara et al. [31] | BMI: higher; education: longer; female gender; physical exertion at work; smoking |
| Kessler et al. [32] | BMI: higher; diabetes; female gender; hypertension; physical exertion at work |
| Kalichman et al. [33] | Age in men: older; age in women: older; female gender |
| Solovieva et al. [34] | Age in women: older; BMI in women: higher; family history of Heberden’s nodes in women; mechanical stress during work in women |
| Szoeke et al. [35] | Age in women: older; BMI in women: higher; hormonal therapy: no use; physical activity in women; smoked (never) in women |
| Dahaghin et al. [36] | BMI: higher |
| Ding et al. [37] | BMI in women: higher |
| Biermasz et al. [38] | IGF-1: higher |
| Hoeven et al. [39] | Atherosclerosis |
| Fu et al. [40] | Adult Kashin–Beck disease |
| Cho et al. [41] | Female gender |
| Gregson et al. [42] | Bone mass: higher |
| Study . | Potential prognostic factor assessed for association with interphalangeal joint OA . |
|---|---|
| Lehto et al. [25] | Dental occupation in men; dental occupation in women; pinch power: stronger |
| Chaisson et al. [26] | Grip strength in men: higher; grip strength in women: higher |
| Cvijetić et al. [27] | Age in men: older; age in women: older; BMI in men: higher; BMI in women: higher; menopause years: longer; blood pressure (systolic) in men: higher; blood pressure (diastolic) in men: higher; blood pressure (systolic) in women: higher; blood pressure (diastolic) in women: higher; smoking |
| Yoshida et al. [28] | Japanese ethnicity in women |
| Jones et al. [29] | BMI: higher; BMI: lower; history of finger fracture; mechanical stress during work; physical activity |
| Cooley et al. [30] | Breastfed ever; hysterectomy; menarche age: older; menstruation years: longer; menopause age: older; number of children: higher; oral contraceptive use; oral contraceptive duration: longer |
| Haara et al. [31] | BMI: higher; education: longer; female gender; physical exertion at work; smoking |
| Kessler et al. [32] | BMI: higher; diabetes; female gender; hypertension; physical exertion at work |
| Kalichman et al. [33] | Age in men: older; age in women: older; female gender |
| Solovieva et al. [34] | Age in women: older; BMI in women: higher; family history of Heberden’s nodes in women; mechanical stress during work in women |
| Szoeke et al. [35] | Age in women: older; BMI in women: higher; hormonal therapy: no use; physical activity in women; smoked (never) in women |
| Dahaghin et al. [36] | BMI: higher |
| Ding et al. [37] | BMI in women: higher |
| Biermasz et al. [38] | IGF-1: higher |
| Hoeven et al. [39] | Atherosclerosis |
| Fu et al. [40] | Adult Kashin–Beck disease |
| Cho et al. [41] | Female gender |
| Gregson et al. [42] | Bone mass: higher |
IGF-1: Insulin-like growth factor-1.
Potential prognostic factors assessed for their association with the presence of IP joint OA
| Study . | Potential prognostic factor assessed for association with interphalangeal joint OA . |
|---|---|
| Lehto et al. [25] | Dental occupation in men; dental occupation in women; pinch power: stronger |
| Chaisson et al. [26] | Grip strength in men: higher; grip strength in women: higher |
| Cvijetić et al. [27] | Age in men: older; age in women: older; BMI in men: higher; BMI in women: higher; menopause years: longer; blood pressure (systolic) in men: higher; blood pressure (diastolic) in men: higher; blood pressure (systolic) in women: higher; blood pressure (diastolic) in women: higher; smoking |
| Yoshida et al. [28] | Japanese ethnicity in women |
| Jones et al. [29] | BMI: higher; BMI: lower; history of finger fracture; mechanical stress during work; physical activity |
| Cooley et al. [30] | Breastfed ever; hysterectomy; menarche age: older; menstruation years: longer; menopause age: older; number of children: higher; oral contraceptive use; oral contraceptive duration: longer |
| Haara et al. [31] | BMI: higher; education: longer; female gender; physical exertion at work; smoking |
| Kessler et al. [32] | BMI: higher; diabetes; female gender; hypertension; physical exertion at work |
| Kalichman et al. [33] | Age in men: older; age in women: older; female gender |
| Solovieva et al. [34] | Age in women: older; BMI in women: higher; family history of Heberden’s nodes in women; mechanical stress during work in women |
| Szoeke et al. [35] | Age in women: older; BMI in women: higher; hormonal therapy: no use; physical activity in women; smoked (never) in women |
| Dahaghin et al. [36] | BMI: higher |
| Ding et al. [37] | BMI in women: higher |
| Biermasz et al. [38] | IGF-1: higher |
| Hoeven et al. [39] | Atherosclerosis |
| Fu et al. [40] | Adult Kashin–Beck disease |
| Cho et al. [41] | Female gender |
| Gregson et al. [42] | Bone mass: higher |
| Study . | Potential prognostic factor assessed for association with interphalangeal joint OA . |
|---|---|
| Lehto et al. [25] | Dental occupation in men; dental occupation in women; pinch power: stronger |
| Chaisson et al. [26] | Grip strength in men: higher; grip strength in women: higher |
| Cvijetić et al. [27] | Age in men: older; age in women: older; BMI in men: higher; BMI in women: higher; menopause years: longer; blood pressure (systolic) in men: higher; blood pressure (diastolic) in men: higher; blood pressure (systolic) in women: higher; blood pressure (diastolic) in women: higher; smoking |
| Yoshida et al. [28] | Japanese ethnicity in women |
| Jones et al. [29] | BMI: higher; BMI: lower; history of finger fracture; mechanical stress during work; physical activity |
| Cooley et al. [30] | Breastfed ever; hysterectomy; menarche age: older; menstruation years: longer; menopause age: older; number of children: higher; oral contraceptive use; oral contraceptive duration: longer |
| Haara et al. [31] | BMI: higher; education: longer; female gender; physical exertion at work; smoking |
| Kessler et al. [32] | BMI: higher; diabetes; female gender; hypertension; physical exertion at work |
| Kalichman et al. [33] | Age in men: older; age in women: older; female gender |
| Solovieva et al. [34] | Age in women: older; BMI in women: higher; family history of Heberden’s nodes in women; mechanical stress during work in women |
| Szoeke et al. [35] | Age in women: older; BMI in women: higher; hormonal therapy: no use; physical activity in women; smoked (never) in women |
| Dahaghin et al. [36] | BMI: higher |
| Ding et al. [37] | BMI in women: higher |
| Biermasz et al. [38] | IGF-1: higher |
| Hoeven et al. [39] | Atherosclerosis |
| Fu et al. [40] | Adult Kashin–Beck disease |
| Cho et al. [41] | Female gender |
| Gregson et al. [42] | Bone mass: higher |
IGF-1: Insulin-like growth factor-1.
Radiographic IP joint OA
Forty-nine potential prognostic factors were assessed for their association with radiographic IP joint OA (all effect measures shown in supplementary Table S3, available at Rheumatology online) (Table 4). Eight prognostic factors were identified: older age in women {moderate evidence: multiple regression β = 0.39 and β = 0.35 [27], likelihood method (effect measure not given) P < 0.001 and P < 0.001 [33], odds ratio (OR) 1.12 [95% confidence interval (CI) 1.07, 1.18] and 1.12 (1.07, 1.67) [34], relative risk 1.0 (0.9, 1.2), 0.9 (0.7, 1.2), 1.0 (0.9, 1.2), 0.9 (0.1, 1.1) [35]}; female gender [moderate evidence: OR 2.85 (2.28, 3.57) [31], OR 1.3 (0.9, 1.9) [32], one-way multivariate analysis of covariance (MANCOVA) (effect size not given) P = 0.011 and P = 0.019 (33), OR 3.5 (2.2, 5.8) and 2.1 (1.6–2.9) [41]]; family history of Heberden’s nodes in women [limited evidence in low risk of bias study: OR 1.94 (1.15, 3.28) and 1.75 (1.04, 2.93) [34]]; adult Kashin–Beck disease [limited evidence: Fisher’s exact test (effect size not given) P < 0.05 for all analyses [40]]; older age in men [limited evidence: multiple regression β = 0.22 and β = 0.35 [27], likelihood method (effect measure not given) P < 0.001 and P < 0.014 [33]]; dental occupation in men [limited evidence: (effect measure and size not given) P = 0.02 [25]]; history of finger fracture [limited evidence: OR 2.42 (1.22, 4.83) [29]]; and parity [limited evidence: OR 7.78 (1.25, 50.0) [30]] (Table 4). Mixed evidence was found for higher BMI [29, 31, 32, 36], higher BMI in men [27] and higher Insulin-like growth factor-1 [38], whilst 39 factors were found not be prognostic for IP joint OA (effect measures shown in supplementary Table S3, available at Rheumatology online) (Table 4).
Best evidence synthesis for potential prognostic factors assessed for their association with the presence of radiographic finger IP joint OA
| Association . | Evidence . | Factor assessed for association with radiographic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | Older age in womenf,k [β = 0.39 and 0.35 [27], LL P < 0.001 and P < 0.001 [33], OR 1.12 (1.07, 1.18) and 1.12 (1.07, 1.67) [34], RR 1.0 (0.9, 1.2), 0.9 (0.7, 1.2), 1.0 (0.9, 1.2), 0.9 (0.1, 1.1) [35]] Female gendera,h [OR 2.85 (2.28, 3.57) [31], OR 1.3 (0.9, 1.9) [32], MANCOVA P = 0.011 and P = 0.019 (33), OR 3.5 (2.2, 5.8) and 2.1 (1.6, 2.9) [41]] | |
| Limited with low r isk of bias | Family history of Heberden’s nodes in women [OR 1.94 (1.15, 3.28) and 1.75 (1.04, 2.93) [34]] | |
| Limited | Adult Kashin–Beck diseaseb,h (FET P < 0.05 for all analyses [40]) Older age in menb,h (β = 0.22 and 0.35 [27], LL P < 0.001 and P < 0.014 [33]) Dental occupation in menb (mP = 0.02 [25]) | |
| Not a prognostic factor | Strong | |
| Moderate | Higher BMI in womenc,j [34]l [27, 35, 37] | |
| Limited with low risk of bias | Atherosclerosis in mend,i [39], atherosclerosis in womenf,i [39], higher bone massd [42], diabetesd [32], hypertensiond [32], mechanical stress in women [34], physical exertion at workd [31, 32] | |
| Limited | Higher diastolic blood pressure in mene,j [27], higher systolic blood pressure in mene,j [27], higher diastolic blood pressure in womene,j [27], higher systolic blood pressure in womene,j [27], lower BMIe [31], ever breastfede [30], dental occupation in womene [25], longer educatione [31], higher grip strength in mene,k [26], higher grip strength in womene,j [26], no use of hormonal therapyf,j [30], hysterectomye [30], Japanese ethnicityj [28], older age at menarchee [30], longer years of menstruatione [30], older age at menopausee [30], longer years of menopausee,j [27], mechanical stress during worke [29], higher number of childrene [30], oral contraception usee [30], longer duration of oral contraception usee [30], physical activitye,j [29, 35], physical exertion in mene [31], physical exertion in womene [31], stronger pinch powere [25], smokinge [29], smoking in mene,j [29, 31], Smoking in womene,j [29, 31], never smoked in womene,j [35] | |
| Mixed evidence | Higher BMIf,g [29, 31, 32, 36], higher BMI in menb,j [27]l, higher IGF-1f,k [38]l | |
| Association . | Evidence . | Factor assessed for association with radiographic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | Older age in womenf,k [β = 0.39 and 0.35 [27], LL P < 0.001 and P < 0.001 [33], OR 1.12 (1.07, 1.18) and 1.12 (1.07, 1.67) [34], RR 1.0 (0.9, 1.2), 0.9 (0.7, 1.2), 1.0 (0.9, 1.2), 0.9 (0.1, 1.1) [35]] Female gendera,h [OR 2.85 (2.28, 3.57) [31], OR 1.3 (0.9, 1.9) [32], MANCOVA P = 0.011 and P = 0.019 (33), OR 3.5 (2.2, 5.8) and 2.1 (1.6, 2.9) [41]] | |
| Limited with low r isk of bias | Family history of Heberden’s nodes in women [OR 1.94 (1.15, 3.28) and 1.75 (1.04, 2.93) [34]] | |
| Limited | Adult Kashin–Beck diseaseb,h (FET P < 0.05 for all analyses [40]) Older age in menb,h (β = 0.22 and 0.35 [27], LL P < 0.001 and P < 0.014 [33]) Dental occupation in menb (mP = 0.02 [25]) | |
| Not a prognostic factor | Strong | |
| Moderate | Higher BMI in womenc,j [34]l [27, 35, 37] | |
| Limited with low risk of bias | Atherosclerosis in mend,i [39], atherosclerosis in womenf,i [39], higher bone massd [42], diabetesd [32], hypertensiond [32], mechanical stress in women [34], physical exertion at workd [31, 32] | |
| Limited | Higher diastolic blood pressure in mene,j [27], higher systolic blood pressure in mene,j [27], higher diastolic blood pressure in womene,j [27], higher systolic blood pressure in womene,j [27], lower BMIe [31], ever breastfede [30], dental occupation in womene [25], longer educatione [31], higher grip strength in mene,k [26], higher grip strength in womene,j [26], no use of hormonal therapyf,j [30], hysterectomye [30], Japanese ethnicityj [28], older age at menarchee [30], longer years of menstruatione [30], older age at menopausee [30], longer years of menopausee,j [27], mechanical stress during worke [29], higher number of childrene [30], oral contraception usee [30], longer duration of oral contraception usee [30], physical activitye,j [29, 35], physical exertion in mene [31], physical exertion in womene [31], stronger pinch powere [25], smokinge [29], smoking in mene,j [29, 31], Smoking in womene,j [29, 31], never smoked in womene,j [35] | |
| Mixed evidence | Higher BMIf,g [29, 31, 32, 36], higher BMI in menb,j [27]l, higher IGF-1f,k [38]l | |
Effect measures and sizes shown in brackets for prognostic factors only. β: multiple regression (95% CI not given); DIPJ: distal IP joint; FET: Fisher’s exact test (effect size not given); IGF-1: Insulin growth factor-1; LL: likelihood method (effect sizes not given); MANCOVA: one-way multivariate analysis of covariance (effect size not given); OR: odds ratio (95% CI); PIPJ: proximal IP joint; RR: relative risk (95% CI).
DIPJ: prognostic factor (moderate evidence);
DIPJ: prognostic factor (limited evidence);
DIPJ: not a prognostic factor (moderate evidence);
DIPJ: not a prognostic factor (limited evidence with low risk of bias);
DIPJ: not a prognostic factor (limited evidence);
DIPJ: mixed evidence,
PIPJ: prognostic factor (limited evidence with low risk of bias);
PIPJ: prognostic factor (limited evidence);
PIPJ: not a prognostic factor (limited evidence with low risk of bias);
PIPJ: not a prognostic factor (limited evidence);
PIPJ: mixed evidence; lmixed evidence within study;
effect measure and size not given.
Best evidence synthesis for potential prognostic factors assessed for their association with the presence of radiographic finger IP joint OA
| Association . | Evidence . | Factor assessed for association with radiographic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | Older age in womenf,k [β = 0.39 and 0.35 [27], LL P < 0.001 and P < 0.001 [33], OR 1.12 (1.07, 1.18) and 1.12 (1.07, 1.67) [34], RR 1.0 (0.9, 1.2), 0.9 (0.7, 1.2), 1.0 (0.9, 1.2), 0.9 (0.1, 1.1) [35]] Female gendera,h [OR 2.85 (2.28, 3.57) [31], OR 1.3 (0.9, 1.9) [32], MANCOVA P = 0.011 and P = 0.019 (33), OR 3.5 (2.2, 5.8) and 2.1 (1.6, 2.9) [41]] | |
| Limited with low r isk of bias | Family history of Heberden’s nodes in women [OR 1.94 (1.15, 3.28) and 1.75 (1.04, 2.93) [34]] | |
| Limited | Adult Kashin–Beck diseaseb,h (FET P < 0.05 for all analyses [40]) Older age in menb,h (β = 0.22 and 0.35 [27], LL P < 0.001 and P < 0.014 [33]) Dental occupation in menb (mP = 0.02 [25]) | |
| Not a prognostic factor | Strong | |
| Moderate | Higher BMI in womenc,j [34]l [27, 35, 37] | |
| Limited with low risk of bias | Atherosclerosis in mend,i [39], atherosclerosis in womenf,i [39], higher bone massd [42], diabetesd [32], hypertensiond [32], mechanical stress in women [34], physical exertion at workd [31, 32] | |
| Limited | Higher diastolic blood pressure in mene,j [27], higher systolic blood pressure in mene,j [27], higher diastolic blood pressure in womene,j [27], higher systolic blood pressure in womene,j [27], lower BMIe [31], ever breastfede [30], dental occupation in womene [25], longer educatione [31], higher grip strength in mene,k [26], higher grip strength in womene,j [26], no use of hormonal therapyf,j [30], hysterectomye [30], Japanese ethnicityj [28], older age at menarchee [30], longer years of menstruatione [30], older age at menopausee [30], longer years of menopausee,j [27], mechanical stress during worke [29], higher number of childrene [30], oral contraception usee [30], longer duration of oral contraception usee [30], physical activitye,j [29, 35], physical exertion in mene [31], physical exertion in womene [31], stronger pinch powere [25], smokinge [29], smoking in mene,j [29, 31], Smoking in womene,j [29, 31], never smoked in womene,j [35] | |
| Mixed evidence | Higher BMIf,g [29, 31, 32, 36], higher BMI in menb,j [27]l, higher IGF-1f,k [38]l | |
| Association . | Evidence . | Factor assessed for association with radiographic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | Older age in womenf,k [β = 0.39 and 0.35 [27], LL P < 0.001 and P < 0.001 [33], OR 1.12 (1.07, 1.18) and 1.12 (1.07, 1.67) [34], RR 1.0 (0.9, 1.2), 0.9 (0.7, 1.2), 1.0 (0.9, 1.2), 0.9 (0.1, 1.1) [35]] Female gendera,h [OR 2.85 (2.28, 3.57) [31], OR 1.3 (0.9, 1.9) [32], MANCOVA P = 0.011 and P = 0.019 (33), OR 3.5 (2.2, 5.8) and 2.1 (1.6, 2.9) [41]] | |
| Limited with low r isk of bias | Family history of Heberden’s nodes in women [OR 1.94 (1.15, 3.28) and 1.75 (1.04, 2.93) [34]] | |
| Limited | Adult Kashin–Beck diseaseb,h (FET P < 0.05 for all analyses [40]) Older age in menb,h (β = 0.22 and 0.35 [27], LL P < 0.001 and P < 0.014 [33]) Dental occupation in menb (mP = 0.02 [25]) | |
| Not a prognostic factor | Strong | |
| Moderate | Higher BMI in womenc,j [34]l [27, 35, 37] | |
| Limited with low risk of bias | Atherosclerosis in mend,i [39], atherosclerosis in womenf,i [39], higher bone massd [42], diabetesd [32], hypertensiond [32], mechanical stress in women [34], physical exertion at workd [31, 32] | |
| Limited | Higher diastolic blood pressure in mene,j [27], higher systolic blood pressure in mene,j [27], higher diastolic blood pressure in womene,j [27], higher systolic blood pressure in womene,j [27], lower BMIe [31], ever breastfede [30], dental occupation in womene [25], longer educatione [31], higher grip strength in mene,k [26], higher grip strength in womene,j [26], no use of hormonal therapyf,j [30], hysterectomye [30], Japanese ethnicityj [28], older age at menarchee [30], longer years of menstruatione [30], older age at menopausee [30], longer years of menopausee,j [27], mechanical stress during worke [29], higher number of childrene [30], oral contraception usee [30], longer duration of oral contraception usee [30], physical activitye,j [29, 35], physical exertion in mene [31], physical exertion in womene [31], stronger pinch powere [25], smokinge [29], smoking in mene,j [29, 31], Smoking in womene,j [29, 31], never smoked in womene,j [35] | |
| Mixed evidence | Higher BMIf,g [29, 31, 32, 36], higher BMI in menb,j [27]l, higher IGF-1f,k [38]l | |
Effect measures and sizes shown in brackets for prognostic factors only. β: multiple regression (95% CI not given); DIPJ: distal IP joint; FET: Fisher’s exact test (effect size not given); IGF-1: Insulin growth factor-1; LL: likelihood method (effect sizes not given); MANCOVA: one-way multivariate analysis of covariance (effect size not given); OR: odds ratio (95% CI); PIPJ: proximal IP joint; RR: relative risk (95% CI).
DIPJ: prognostic factor (moderate evidence);
DIPJ: prognostic factor (limited evidence);
DIPJ: not a prognostic factor (moderate evidence);
DIPJ: not a prognostic factor (limited evidence with low risk of bias);
DIPJ: not a prognostic factor (limited evidence);
DIPJ: mixed evidence,
PIPJ: prognostic factor (limited evidence with low risk of bias);
PIPJ: prognostic factor (limited evidence);
PIPJ: not a prognostic factor (limited evidence with low risk of bias);
PIPJ: not a prognostic factor (limited evidence);
PIPJ: mixed evidence; lmixed evidence within study;
effect measure and size not given.
In the subgroup analysis for DIPJ OA, 44 potential prognostic factors were assessed. Compared with the analysis of all IP joints, differences were found for: female gender, which showed strong evidence for being a prognostic factor [OR 2.85 (2.28–3.57) [31], OR 1.3 (0.9–1.9) [32], one-way MANCOVA (effect size not given) P = 0.019 (33), OR 2.1 (1.6–2.9) [41]]; and higher BMI in men, which showed limited evidence for being a prognostic factor [multiple regression β = 0.25 [27]]. Older age in women [27, 33, 35], atherosclerosis in women [39] and no use of hormonal therapy [30] showed mixed evidence (effect measures shown in supplementary Table S3, available at Rheumatology online) (Table 4).
In the subgroup analysis for PIPJ OA, 23 potential prognostic factors were investigated. When compared with all IP joint, different results were found for: female gender, which showed limited evidence for being a prognostic factor [one-way MANCOVA (effect size not given) P = 0.011 [33], OR 3.5 (2.2–5.8) [41]]; and higher BMI in men [27] and higher BMI in women [27, 35], which both showed limited evidence for not being prognostic factors; higher grip strength in men [26] and older age in women [27, 33–35] showed mixed results (effect measures shown in supplementary Table S3, available at Rheumatology online) (Table 4).
Radiographic symptomatic IP joint OA
Higher BMI in women was the only potential prognostic factor assessed, and it was found to have limited evidence in one low risk of bias study for being a prognostic factor [37] (effect measures shown in supplementary Table S4, available at Rheumatology online). The definition of OA presence used by Ding et al. [37] was based on the DIPJs, so no subgroup analyses were performed.
Symptomatic IP joint OA
Fourteen potential prognostic factors were assessed for their association with the presence of symptomatic IPJ OA [29, 30] (effect measures shown in supplementary Table S5, available at Rheumatology online). Each potential prognostic factor was assessed by one high risk of bias study, and no factor was found to be prognostic for IP joint OA (Table 5). All definitions of symptomatic IP joint OA included only the DIPJ, so subgroup analysis could not be performed.
Best evidence synthesis for potential prognostic factors assessed for their association with the presence of symptomatic finger IP joint OA
| Association . | Evidence . | Factor assessed for association with symptomatic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | ||
| Not a prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | Higher BMI [28], ever breastfed [29], history of finger fracture [28], hysterectomy [29], mechanical stress during work [28], older age at menarche [29], longer years of menstruation [29], older age at menopause [29], higher number of children [29], oral contraception use [29], longer duration of oral contraception use [29], parity [29], physical activity [28], smoking [28] | |
| Mixed evidence | ||
| Association . | Evidence . | Factor assessed for association with symptomatic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | ||
| Not a prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | Higher BMI [28], ever breastfed [29], history of finger fracture [28], hysterectomy [29], mechanical stress during work [28], older age at menarche [29], longer years of menstruation [29], older age at menopause [29], higher number of children [29], oral contraception use [29], longer duration of oral contraception use [29], parity [29], physical activity [28], smoking [28] | |
| Mixed evidence | ||
Best evidence synthesis for potential prognostic factors assessed for their association with the presence of symptomatic finger IP joint OA
| Association . | Evidence . | Factor assessed for association with symptomatic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | ||
| Not a prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | Higher BMI [28], ever breastfed [29], history of finger fracture [28], hysterectomy [29], mechanical stress during work [28], older age at menarche [29], longer years of menstruation [29], older age at menopause [29], higher number of children [29], oral contraception use [29], longer duration of oral contraception use [29], parity [29], physical activity [28], smoking [28] | |
| Mixed evidence | ||
| Association . | Evidence . | Factor assessed for association with symptomatic IP joint OA . |
|---|---|---|
| Prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | ||
| Not a prognostic factor | Strong | |
| Moderate | ||
| Limited with low risk of bias | ||
| Limited | Higher BMI [28], ever breastfed [29], history of finger fracture [28], hysterectomy [29], mechanical stress during work [28], older age at menarche [29], longer years of menstruation [29], older age at menopause [29], higher number of children [29], oral contraception use [29], longer duration of oral contraception use [29], parity [29], physical activity [28], smoking [28] | |
| Mixed evidence | ||
Discussion
We identified 18 studies in the literature that assessed prognostic factors for the presence of IP joint OA [25–42]. All studies defined OA radiographically [25–42]. Symptomatic radiographic OA was also defined by one study [37], and symptomatic OA assessing Heberden’s nodes was also defined by two studies [29, 30]. Sixty-four potential risk factors were investigated in the literature [25–42]. Of these, eight were prognostic factors for radiographic IP joint OA, with evidence ranging from moderate to limited [25, 27, 29, 31–35, 40, 41], and one prognostic factor (limited evidence) for symptomatic radiographic OA was found [37]. Few differences were seen in the DIPJ and PIPJ subgroup analyses.
Of the radiographic definitions, the KL atlas, or a modified version of it, was most commonly used [43–45]. The 2008 EULAR Standing Committee on International Clinical Studies Including Therapeutic Trials (ESCISIT) for hand OA listed recommendations for diagnosing hand OA [1]. They also identified radiographic definitions as the most common method of diagnosing hand OA. A systematic review assessing the use of radiography in studies of hand OA found that the KL atlas in particular was the most commonly used radiographic scoring method for hand OA [47]. It described the KL atlas as being a composite sum score across all hand joints [47]. In our review, KL sum score was used as a diagnostic criteria in only two studies [33, 38]. However, these studies did not use a specific cut-off sum score above which OA was diagnosed. It is possible that Kalichman et al. [33] and Biermasz et al. [38] included IP joints with a KL grade of 1 when counting the KL sum score. However, a KL grade of 1 describes possible or doubtful joint space narrowing with possible osteophyte formation, and is often considered not to represent OA in the joint [43–45]. Kalichman et al. [33] used this definition when reporting older age in women as a prognostic factor for radiographic IP joint OA. Despite four studies assessing the association between older age in women and IP joint OA, this could have skewed the results, and therefore they should be interpreted with caution [27, 33–35]. More commonly, studies included in this review used a threshold of KL grade ≥2 in a specific number of IP joints as the definition of OA being present [25, 27, 28, 31, 34, 36, 37, 39, 41, 48]. However, there was also no consensus on how the KL atlas was applied between studies. The lack of harmonization between studies in defining the presence of radiographic OA prevented meta-analyses, and further work is needed to identify methods of harmonization between datasets, to allow for the pooling of data.
Diagnostic criteria to define radiographic symptomatic OA was used by one study [37], Our results found that higher BMI in women was a prognostic factor for symptomatic radiographic IP joint OA, but not for radiographic OA [27, 34, 35, 37]. However, only one study assessed symptomatic radiographic IP joint OA, and it was of high risk of bias, resulting in limited evidence [37]. Diagnostic criteria to define symptomatic OA were used by two studies [29, 30]. No prognostic factors were identified for symptomatic OA, despite 14 factors being investigated [29, 30]. In the literature, there is poor correlation between radiographic and symptomatic hand OA [9]. All of the factors investigated for an association with symptomatic OA were also investigated for an association with radiographic OA. Twelve of these were found to have no association with either radiographic or symptomatic OA, suggesting that although diagnostic criteria for radiographic and symptomatic OA might not be well correlated, their aetiology and pathogenesis might be [29, 30]. Additional and more robust studies are required to validate the prognostic factor findings for symptomatic radiographic and symptomatic IP joint OA. Similarly, further research is needed to better understand the relationship between radiographic and symptomatic IP joint OA, particularly as patients more commonly seek medical investigations or management because of clinical symptoms rather than radiographic signs of IP joint OA. Studies investigating prognostic factors for symptomatic IP joint OA are likely to have more meaningful implications for patients than those investigating radiographic IP joint OA.
The work from the 2008 EULAR ESCISIT for hand OA identified risk factors for hand OA [1]. They used a Delphi consensus of 21 OA experts, and both their results and ours identified female gender as a prognostic factor [1, 31–33, 41]. Zhang et al. [1] performed a literature review and reported age >40 years as a risk factor. We found older age in men and women were both prognostic factors, with all studies investigating these factors reporting a mean age of participants of >40 years [27, 33–35]. Zhang et al. [1] identified obesity as a prognostic factor; we found only higher BMI in women was prognostic for symptomatic radiographic OA [37]. They also identified family history as a risk factor, whilst we identified only a family history of Heberden’s nodes as a prognostic factor; they identified prior injury, whilst we found this was specifically finger fracture; and they listed occupation, whilst we found only dental occupation in men to be prognostic [1, 25, 29, 34]. Additionally, they listed higher bone density and menopausal status as risk factors, though we were not able to replicate these recommendations [1, 30, 42]. However, Zhang et al. [1] did not include a systematic review and therefore might not have fully assessed all associations and lack of associations between factors and OA. Furthermore, they used hand OA as an outcome, despite some evidence suggesting IP joint and base of thumb OA might be different subsets [49] and have different prognostic factors [1, 6]. Our results suggest that older aged women [27, 33–35] and females [31–33, 41] are at increased risk of developing radiographic IP joint OA; meanwhile, older age in men [27, 33], men who are dentists [25], people with a history of finger fracture [29], those with adult Kashin–Beck disease [40] and women with a family history of Heberden’s nodes [34] or a history of parity [30] may also be at increased risk, though the evidence is limited. Our subgroup analyses suggest that DIPJ and PIPJ OA have similar prognostic factors, and should be combined in future analyses. However, further studies are required to validate these findings. Prognostic pathway studies are also needed to understand the relationship between risk factors and whether multiple factors in co-existence with each other moderate the risk of radiographic IP joint OA being present [50].
To our knowledge, this is the first systematic review to identify prognostic factors for the presence of IP joint OA. A strength of this review is the exclusion of first CMC joint OA, and the analysis of radiographic IP joint OA separate to symptomatic IP joint OA. Only one study diagnosed OA using a symptomatic radiographic definition [37], and only two studies diagnosed symptomatic OA [29, 30]. Additional studies using non-radiographic definitions of IP joint OA are needed. Primary studies did not assess prognostic pathways and no prognostic models for IP joint OA were identified, highlighting that further work is needed to understand prognostic pathways and to better inform clinical decision making [50]. This review is limited by the majority of studies being of moderate or high risk of bias [25–27, 29–31, 33, 35, 38, 39, 41]. A meta-analysis could not be performed due to heterogeneity in methodology between studies and differences in effect measures between studies. Harmonization between datasets is required to enable pooling of results.
Acknowledgements
We would like to thank Elinor Harriss for her assistance in developing the search strategies and for running the searches and de-duplicating the results.
Funding: This work was supported by the Centre for Sport, Exercise and Osteoarthritis Research Vs Arthritis (Grant reference 21595). This work was supported by a British Society for Surgery of the Hand Research Fellowship to K.S. This work was supported by a China Scholarship Council to H.C. This work was supported by a Versus Arthritis Clinical Research Fellowship (Grant reference 21605), and a Medical Research Council Doctoral Training Fellowship (Grant reference MR/K501256/1) to J.C.E.L. This work was supported by the National Institute for Health Research Oxford Biomedical Research Centre to G.S.C. and D.F. This work was supported by the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis to N.K.A. This work was supported by the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis (Grant reference 21595) to S.R.F. The study sponsors had no involvement in the study design, data collection, analysis or interpretation, or in writing the manuscript.
Dislosure statement: N.K.A. receives personal fees from Pfizer/Lily for consultancy and a grant from Merck, outside the submitted work. All authors certify they have no commercial association that might pose a conflict of interest in connection with the submitted article.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
References
GRADE Working Group. Quality of evidence. In: Schünemann H, Brożek J, Guyatt G, Oxman A, eds. GRADE handbook for grading quality of evidence and strength of recommendations [e-book]. Cochrane Training;
The epidemiology of chronic rheumatism, atlas of standard radiographs.




Comments