Abstract
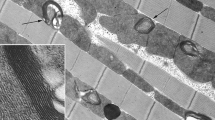
Morphometric analysis of mitochondria in skeletal muscles and heart of 6- and 60-month-old naked mole rats (Heterocephalus glaber) revealed a significant age-dependent increase in the total area of mitochondrial cross-sections in studied muscle fibers. For 6- and 60-month-old animals, these values were 4.8 ± 0.4 and 12.7 ± 1.8%, respectively. This effect is mainly based on an increase in the number of mitochondria. In 6-month-old naked mole rats, there were 0.23 ± 0.02 mitochondrial cross-sections per μm2 of muscle fiber, while in 60-month-old animals this value was 0.47 ± 0.03. The average area of a single mitochondrial cross-section also increased with age in skeletal muscles–from 0.21 ± 0.01 to 0.29 ± 0.03 μm2. Thus, naked mole rats show a drastic enlargement of the mitochondrial apparatus in skeletal muscles with age due to an increase in the number of mitochondria and their size. They possess a neotenic type of chondriome accompanied by specific features of mitochondrial functioning in the state of oxidative phosphorylation and a significant decrease in the level of matrix adenine nucleotides.
Similar content being viewed by others
References
Bennett, N. C., and Faulkes, C. G. (2000) The Evolution of Sociality in African Mole-Rats, Cambridge University Press, Cambridge, UK.
Bennett, N. C., and Faulkes, C. G. (2000) Social Organization in African Mole Rats, Cambridge University Press, Cambridge, UK.
Brett, R. A. (1991) The Population Structure of Naked Mole Rat Colonies, Princeton University Press, Princeton, NJ.
Buffenstein, R., Park, R., Hanes, M., and Antwohl, J. E. (2012) in The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents (Suckow, M. A., Stevens, K. A., and Wilson, R. P., eds.) Elsevier, London, pp. 1055–1074.
Jarvis, J. U. (1981) Eusociality in a mammal: cooperative breeding in naked mole-rat colonies, Science, 212, 571–573.
Sherman, P. W., Jarvis, J. U., and Alexander, R. D. (1991) The Biology of the Naked Mole-Rat, Princeton University Press, Princeton, NJ.
Lacey, E. A., Patton, J. L., and Cameron, G. N. (2000) Life Underground: The Biology of Subterranean Rodents, University of Chicago Press, Chicago, IL.
Delaney, M. A., Nagy, L., Kinsel, M. J., and Treuting, P. M. (2013) Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population, Vet. Pathol., 50, 607–621.
Buffenstein, R. (2000) Ecological and Physiological Responses to Underground Habitats, University of Chicago Press, Chicago, IL.
Larson, J., and Park, T. J. (2009) Extreme hypoxia tolerance of naked mole rat brain, Neuroreport, 20, 1634–1637.
Maina, J. N., Gebreegziabher, Y., Woodley, R., and Buffenstein, R. (2001) Effects of change in environmental temperature and natural shifts in carbon dioxide and oxygen concentrations on the lungs of captive naked mole rats (Heterocephalus glaber): a morphological and morphometric study, J. Zool., 253, 371–382.
Park, T. J., Lu, Y., Juttner, R., Smith, E. S., Hu, J., Brand, A., Wetzel, C., Milenkovic, N., Erdmann, B., Heppenstall, P. A., Laurito, C. E., Wilson, S. P., and Lewin, G. R. (2008) Selective inflammatory pain insensitivity in the African naked mole rat (Heterocephalus glaber), PLoS Biol., 6, e13.
Buffenstein, R. (2008) Negligible senescence in the longest living rodent, the naked mole rat: insights from a successfully aging species, J. Comp. Physiol. B, 178, 439–445.
Harman, D. (1956) Aging: a theory based on free radical and radiation chemistry, J. Gerontol., 11, 298–300.
Lenaz, G. (2001) The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology, IUBMB Life, 52, 159–164.
Andreyev, A. Yu., Kushnareva, Yu. E., and Starkov, A. A. (2005) Mitochondrial metabolism of reactive oxygen species, Biochemistry (Moscow), 70, 200–214.
Honda, H. M., Korge, P., and Weiss, J. N. (2005) Mitochondria and ischemia/reperfusion injury, Ann. N. Y. Acad. Sci., 1047, 248–258.
Zweier, J. L., and Talukder, M. A. (2006) The role of oxidants and free radicals in reperfusion injury, Cardiovasc. Res., 70, 181–190.
Yellon, D. M., and Hausenloy, D. J. (2007) Myocardial reperfusion injury, N. Engl. J. Med., 357, 1121–1135.
Eltzschig, H. K., and Eckle, T. (2011) Ischemia and reperfusion − from mechanism to translation, Nat. Med., 17, 1391–1401.
Borutaite, V., Toleikis, A., and Brown, G. C. (2013) In the eye of the storm: mitochondrial damage during heart and brain ischemia, FEBS J., 280, 4999–5014.
Chouchani, E. T., Pell, V. R., Gaude, E., Aksentijevic, D., Sundier, S. Y., Robb, E. L., Logan, A., Nadtochiy, S. M., Ord, E. N., Smith, A. C., Eyassu, F., Shirley, R., Hu, C. H., Dare, A. J., James, A. M., Rogatti, S., Hartley, R. C., Eaton, S., Costa, A. S., Brookes, P. S., Davidson, S. M., Duchen, M. R., Saeb-Parsy, K., Shattock, M. J., Robinson, A. J., Work, L. M., Frezza, C., Krieg, T., and Murphy, M. P. (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS, Nature, 515, 431–435.
Harman, D. (1972) The biologic clock: the mitochondria? J. Am. Geriatr. Soc., 20, 145–147.
Miquel, J., Economos, A. C., Fleming, J., and Johnson, J. E. (1980) Mitochondrial role in cell aging, Exp. Gerontol., 15, 575–591.
Skulachev, V. P. (1997) Aging is a specific biological function rather than the result of a disorder in complex living systems: biochemical evidence in support of Weismann’s hypothesis, Biochemistry (Moscow), 62, 1191–1195.
Skulachev, V. P. (1999) Phenoptosis: programmed death of an organism, Biochemistry (Moscow), 64, 1418–1426.
Skulachev, V. P. (2001) Phenomena of programmed death. Mitochondria, cells and organs: role of reactive oxygen species, Soros. Obraz. Zh., 7, 4–10.
Labinskyy, N., Csiszar, A., Orosz, Z., Smith, K., Rivera, A., Buffenstein, R., and Ungvari, Z. (2006) Comparison of endothelial function, O2 – and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice, Am. J. Physiol. Heart. Circ. Physiol., 291, H2698–2704.
Lambert, A. J., Boysen, H. M., Buckingham, J. A., Yang, T., Podlutsky, A., Austad, S. N., Kunz, T. H., Buffenstein, R., and Brand, M. D. (2007) Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms, Aging Cell, 65, 607–618.
Csiszar, A., Labinskyy, N., Orosz, Z., Xiangmin, Z., Buffenstein, R., and Ungvari, Z. (2007) Vascular aging in the longest-living rodent, the naked mole rat, Am. J. Physiol. Heart. Circ. Physiol., 293, H919–927.
Vays, V. B., Eldarov, C. M., Vangely, I. M., Kolosova, N. G., Bakeeva, L. E., and Skulachev, V. P. (2014) Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure, Aging (Albany, NY), 6, 140–148.
Carter, H. N., Chen, C. C. W., and Hood, D. A. (2015) Mitochondria, muscle health, and exercise with advancing age, Physiology, 30, 208–223.
Del Campo, A., Jaimovich, E., and Tevy, M. F. (2016) Mitochondria in the aging muscles of flies and mice: new perspectives for old characters, Oxid. Med. Cell. Longev., 2016, 9057593.
Onyango, D. W., and Oduorokelo, D. (1993) Ultrastructural study of the testis of non-breeding naked mole-rat (Heterocephalus glaber Ruppell), Ann. Anat., 175, 447–452.
Brovko, L., Romanova, N. A., and Ugarova, N. N. (1994) Bioluminescent assay of bacterial intracellular AMP, ADP, and ATP with the use of a coimmobilized three-enzyme reagent (adenylate kinase, pyruvate kinase, and firefly luciferase), Anal. Biochem., 220, 410–414.
Reynolds, E. S. (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy, J. Cell Biol., 17, 208–212.
Bakeeva, L. E., Chentsov, Y. S., and Skulachev, V. P. (1981) Ontogenesis of mitochondrial reticulum in rat diaphragm muscle, Eur. J. Cell. Biol., 25, 175–181.
Bakeeva, L. E. (2015) Age-related changes in ultrastructure of mitochondria. Effect of SkQ1, Biochemistry (Moscow), 80, 1582–1588.
Glagolev, A. A. (1941) Geometric Methods of Quantitative Analysis of Aggregates under the Microscope [in Russian], Gosgeolizdat, Moscow.
McCallister, B. D., and Brown, A. L. (1965) A quantitative study of myocardial mitochondria in experimental cardiac hypertrophy, Lab. Invest., 14, 692–700.
McCallister, L. P., and Page, E. (1973) Effects of thyroxin on ultrastructure of rat myocardial cells: a stereological study, J. Ultrastruct. Res., 42, 136–55.
McCallister, L. P., Page, E., and Power, B. (1971) Stereological measurements of cardiac ultrastructures implicated in excitation–contraction coupling, Proc. Natl. Acad. Sci. USA, 68, 1465–1466.
Weibel, E. R. (1979) Stereological Methods. Vol. 1. Practical Methods for Biological Morphometry, Academic Press, London.
Sachs, H. G., Colgan, J. A., and Lazarus, M. L. (1977) Ultrastructure of the aging myocardium: a morphometric approach, Am. J. Anat., 150, 63–71.
Frenzel, H., and Feimann, J. (1984) Age-dependent structural changes in the myocardium of rats. A quantitative light- and electron-microscopic study on the right and left chamber wall, Mech. Ageing Dev., 27, 29–41.
Maina, J. N. (1988) Morphology and morphometry of the normal lung of the adult vervet monkey (Cercopithecus aethiops), Am. J. Anat., 183, 258–267.
Maina, J. N. (2002) Some recent advances on the study and understanding of the functional design of the avian lung: morphological and morphometric perspectives, Biol. Rev. Camb. Philos. Soc., 77, 97–152.
Maina, J. N., and King, A. S. (1987) A morphometric study of the lung of a Humboldt penguin (Sphenicus humboldti), Anat. Histol. Embryol., 16, 293–297.
Maina, J. N., and Nathaniel, C. (2001) A qualitative and quantitative study of the lung of an ostrich Struthio camelus, J. Exp. Biol., 204, 2313–2330.
Maina, J. N., and Van Gils, P. (2001) Morphometric characterization of the airway and vascular systems of the lung of the domestic pig Sus scrofa: comparison of the airway, arterial and venous systems, Comp. Biochem. Physiol. A Mol. Integr. Physiol., 130, 781–798.
Aprille, J. R., and Asimakis, G. K. (1980) Postnatal-development of rat-liver mitochondria-state-3 respiration, adenine nucleotide translocase activity, and the net accumulation of adenine nucleotides, Arch. Biochem. Biophys., 201, 564–575.
Korshunov, S. S., Skulachev, V. P., and Starkov, A. A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria, FEBS Lett., 416, 15–18.
Marzetti, E., Hwang, J. C. Y., Lees, H. A., Wohlgemuth, S. E., Dupont-Versteegden, E. E., Carter, C. S., Bernabei, R., and Leeuwenburgha, C. (2010) Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy, Biochim. Biophys. Acta, 1800, 235–244.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Original Russian Text © S. Holtze, C. M. Eldarov, V. B. Vays, I. M. Vangeli, M. Yu. Vysokikh, L. E. Bakeeva, V. P. Skulachev, T. B. Hildebrandt, 2016, published in Biokhimiya, 2016, Vol. 81, No. 12, pp. 1703–1712.
Rights and permissions
About this article
Cite this article
Holtze, S., Eldarov, C.M., Vays, V.B. et al. Study of age-dependent structural and functional changes of mitochondria in skeletal muscles and heart of naked mole rats (Heterocephalus glaber). Biochemistry Moscow 81, 1429–1437 (2016). https://doi.org/10.1134/S000629791612004X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629791612004X