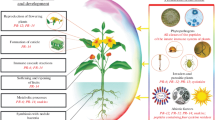
Abstract
Plant antimicrobial peptides represent one of the evolutionarily oldest innate immunity components providing the first line of host defense to pathogen attacks. This review is dedicated to a small, currently actively studied family of hevein-like peptides that can be found in various monocot and dicot plants. The review thoroughly describes all known pep- tides belonging to this family including data on their structures, functions, and antimicrobial activity. The main features allowing to assign these peptides to a separate family are given, and the specific characteristics of each peptide are described. Further, the mode of action for hevein-like peptides, their role in plant immune system, and the applications of these mol- ecules in biotechnology and medicine are considered.
Similar content being viewed by others
Abbreviations
- a.a.:
-
amino acid residue
- AMP:
-
antimicrobial peptide
References
Egorov, Ts. A., and Odintsova, T. I. (2012) Defense peptides of plant immune system, Bioorg. Khim., 38, 7–17.
Silverstein, K. A., Moskal, W. A., Jr., Wu, H. C., Underwood, B. A., Graham, M. A., Town, C. D., and VandenBosch, K. A. (2007) Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants, Plant J., 51, 262–280.
Slavokhotova, A. A., Shelenkov, A. A., and Odintsova, T. I. (2015) Prediction of Leymus arenarius (L.) antimicrobial peptides based on de novo transcriptome assembly, Plant Mol. Biol., 89, 203–214.
Slavokhotova, A. A., Shelenkov, A. A., Korostyleva, T. V., Rogozhin, E. A., Melnikova, N. V., Kudryavtseva, A. V., and Odintsova, T. I. (2017) Defense peptide repertoire of Stellaria media predicted by high throughput next generation sequencing, Biochimie, 135, 15–27.
Costa, F. T., Neto, S. M., Bloch, C., Jr., and Franco, O. L. (2007) Susceptibility of human pathogenic bacteria to antimicrobial peptides from sesame kernels, Curr. Microbiol., 55, 162–166.
Oard, S. V., and Enright, F. M. (2006) Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi, Plant Cell Rep., 25, 561–572.
Loeza-Angeles, H., Sagrero-Cisneros, E., Lara-Zarate, L., Villagomez-Gomez, E., Lopez-Meza, J. E., and Ochoa-Zarzosa, A. (2008) Thionin Thi2.1 from Arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity, Biotechnol. Lett., 30, 1713–1719.
Tam, J. P., Wang, S., Wong, K. H., and Tan, W. L. (2015) Antimicrobial peptides from plants, Pharmaceuticals (Basel), 8, 711–757.
Beintema, J. J. (1994) Structural features of plant chitinases and chitin-binding proteins, FEBS Lett., 350, 159–163.
Iseli, B., Boller, T., and Neuhaus, J. M. (1993) The N-terminal cysteine-rich domain of tobacco class I chitinase is essential for chitin binding but not for catalytic or antifungal activity, Plant Physiol., 103, 221–226.
Archer, B. L. (1960) The proteins of Hevea brasiliensis latex. 4.Isolation and characterization of crystalline hevein, Biochem. J., 75, 236–240.
Walujono, K., Scholma, R. A., Beintema, J. J., Mariono, A., and Hahn, A. M. (1975) Amino acid sequence of hevein, Proc. Int. Rubber Conference, Kuala Lumpur, Vol. 2, pp. 518–531.
Chapot, M. P., Peumans, W. J., and Strosberg, A. D. (1986) Extensive homologies between lectins from non-leguminous plants, FEBS Lett., 195, 231–234.
Peumans, W. J., De Ley, M., and Broekaert, W. F. (1983) An unusual lectin from stinging nettle (Urtica dioica) rhizomes, FEBS Lett., 177, 99–103.
Broekaert, W. F., Vanparijs, J., Leyns, F., Joos, H., and Peumans, W. J. (1989) A chitin-binding lectin from stinging nettle rhizomes with antifungal properties, Science, 245, 1100–1102.
Van Parijs, J., Broekaert, W. F., Goldstein, I. J., and Peumans, W. J. (1991) Hevein–an antifungal protein from rubber-tree (Hevea brasiliensis) latex, Planta, 183, 258–264.
Broekaert, I., Lee, H. I., Kush, A., Chua, N. H., and Raikhel, N. (1990) Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis), Proc. Natl. Acad. Sci. USA, 87, 7633–7637.
Lee, H. I., Broekaert, W. F., and Raikhel, N. V. (1991) Co-and post-translational processing of the hevein preproprotein of latex of the rubber tree (Hevea brasiliensis), J. Biol. Chem., 266, 15944–15948.
Soedjanaatmadja, U. M., Hofsteenge, J., Jeronimus-Stratingh, C. M., Bruins, A. P., and Beintema, J. J. (1994) Demonstration by mass spectrometry that pseudo-hevein and hevein have ragged C-terminal sequences, Biochim. Biophys. Acta, 1209, 144–148.
Gidrol, X., Chrestin, H., Tan, H. L., and Kush, A. (1994) Hevein, a lectin-like protein from Hevea brasiliensis (rubber tree) is involved in the coagulation of latex, J. Biol. Chem., 269, 9278–9283.
Pujade-Renaud, V., Sanier, C., Cambillau, L., Pappusamy, A., Jones, H., Ruengsri, N., Tharreau, D., Chrestin, H., Montoro, P., and Narangajavana, J. (2005) Molecular characterization of new members of the Hevea brasiliensis hevein multigene family and analysis of their promoter region in rice, Biochim. Biophys. Acta, 1727, 151–161.
Andersen, N. H., Cao, B., Rodriguezromero, A., and Arreguin, B. (1993) Hevein-NMR assignment and assessment of solution-state folding for the agglutinin-toxin motif, Biochemistry, 32, 1407–1422.
Rodriguez, A., Tablero, M., Barragan, B., Lara, P., Rangel, M., Arreguin, B., Possani, L., and Sorianogarcia, M. (1986) Crystallization of hevein–a protein from latex of Hevea brasiliensis (rubber tree), J. Crystal Growth, 76, 710–714.
Asensio, J. L., Canada, F. J., Siebert, H. C., Laynez, J., Poveda, A., Nieto, P. M., Soedjanaamadja, U. M., Gabius, H. J., and Jimenez-Barbero, J. (2000) Structural basis for chitin recognition by defense proteins: GlcNAc residues are bound in a multivalent fashion by extended binding sites in hevein domains, Chem. Biol., 7, 529–543.
Colombo, G., Meli, M., Canada, J., Asensio, J. L., and Jimenez-Barbero, J. (2004) Toward the understanding of the structure and dynamics of protein–carbohydrate interactions: molecular dynamics studies of the complexes between hevein and oligosaccharidic ligands, Carbohydr. Res., 339, 985–994.
Aboitiz, N., Vila-Perello, M., Groves, P., Asensio, J. L., Andreu, D., Canada, F. J., and Jimenez-Barbero, J. (2004) NMR and modeling studies of protein–carbohydrate interactions: synthesis, three-dimensional structure, and recognition properties of a minimum hevein domain with binding affinity for chitooligosaccharides, Chembiochem, 5, 1245–1255.
Mareska, V., Tvaroska, I., Kralova, B., and Spiwok, V. (2015) Molecular simulations of hevein/(GlcNAc)(3) complex with weakened OH/O and CH/pi hydrogen bonds: implications for their role in complex stabilization, Carbohydr. Res., 408, 1–7.
Chavez, M. I., Vila-Perello, M., Canada, F. J., Andreu, D., and Jimenez-Barbero, J. (2010) Effect of a serine-to-aspartate replacement on the recognition of chitin oligosaccharides by truncated hevein. A 3D view by using NMR, Carbohydr. Res., 345, 1461–1468.
Ownby, D. R. (2002) A history of latex allergy, J. Allergy Clin. Immunol., 110, S27–32.
Bousquet, J., Flahault, A., Vandenplas, O., Ameille, J., Duron, J. J., Pecquet, C., Chevrie, K., and Annesi-Maesano, I. (2006) Natural rubber latex allergy among health care workers: a systematic review of the evidence, J. Allergy Clin. Immunol., 118, 447–454.
Radauer, C., Adhami, F., Furtler, I., Wagner, S., Allwardt, D., Scala, E., Ebner, C., Hafner, C., Hemmer, W., Mari, A., and Breiteneder, H. (2011) Latex-allergic patients sensitized to the major allergen hevein and hevein-like domains of class I chitinases show no increased frequency of latex-associated plant food allergy, Mol. Immunol., 48, 600–609.
Chen, Z., Duser, M., Flagge, A., Maryska, S., Sander, I., Raulf-Heimsoth, M., and Baur, X. (2000) Identification and characterization of cross-reactive natural rubber latex and Ficus benjamina allergens, Int. Arch. Allergy Immunol., 123, 291–298.
Alenius, H., Kalkkinen, N., Lukka, M., Reunala, T., Turjanmaa, K., Makinen-Kiljunen, S., Yip, E., and Palosuo, T. (1995) Prohevein from the rubber tree (Hevea brasiliensis) is a major latex allergen, Clin. Exp. Allergy, 25, 659–665.
Blanco, C. (2003) Latex-fruit syndrome, Curr. Allergy Asthma Rep., 3, 47–53.
Wagner, S., and Breiteneder, H. (2002) The latex-fruit syndrome, Biochem. Soc. Trans., 30, 935–940.
Barre, A., Culerrier, R., Granier, C., Selman, L., Peumans, W. J., Van Damme, E. J., Bienvenu, F., Bienvenu, J., and Rouge, P. (2009) Mapping of IgE-binding epitopes on the major latex allergen Hev b2 and the cross-reacting 1,3-beta-glucanase fruit allergens as a molecular basis for the latex-fruit syndrome, Mol. Immunol., 46, 1595–1604.
Ganglberger, E., Radauer, C., Wagner, S., Riordain, G., Beezhold, D. H., Brehler, R., Niggemann, B., Scheiner, O., Jensen-Jarolim, E., and Breiteneder, H. (2001) Hev b8, the Hevea brasiliensis latex profilin, is a cross-reactive allergen of latex, plant foods and pollen, Int. Arch. Allergy. Immunol., 125, 216–227.
Schmidt, M. H., Raulf-Heimsoth, M., and Posch, A. (2002) Evaluation of patatin as a major cross-reactive allergen in latex-induced potato allergy, Ann. Allergy Asthma Immunol., 89, 613–618.
Beezhold, D. H., Hickey, V. L., Kostyal, D. A., Puhl, H., Zuidmeer, L., Van Ree, R., and Sussman, G. L. (2003) Lipid transfer protein from Hevea brasiliensis (Hev b12), a cross-reactive latex protein, Ann. Allergy Asthma Immunol., 90, 439–445.
Ferreira, F., Wallner, M., Breiteneder, H., Hartl, A., Thalhamer, J., and Ebner, C. (2002) Genetic engineering of allergens: future therapeutic products, Int. Arch. Allergy Immunol., 128, 171–178.
Banerjee, B., Wang, X., Kelly, K. J., Fink, J. N., Sussman, G. L., and Kurup, V. P. (1997) IgE from latex-allergic patients binds to cloned and expressed B cell epitopes of prohevein, J. Immunol., 159, 5724–5732.
Beezhold, D. H., Kostyal, D. A., and Sussman, G. L. (1997) IgE epitope analysis of the hevein preprotein; a major latex allergen, Clin. Exp. Immunol., 108, 114–121.
Drew, A. C., Eusebius, N. P., Kenins, L., De Silva, H. D., Suphioglu, C., Rolland, J. M., and O’Hehir, R. E. (2004) Hypoallergenic variants of the major latex allergen Hev b6.01 retaining human T lymphocyte reactivity, J. Immunol., 173, 5872–5879.
Karisola, P., Mikkola, J., Kalkkinen, N., Airenne, K. J., Laitinen, O. H., Repo, S., Pentikainen, O. T., Reunala, T., Turjanmaa, K., Johnson, M. S., Palosuo, T., Kulomaa, M. S., and Alenius, H. (2004) Construction of hevein (Hev b6.02) with reduced allergenicity for immunotherapy of latex allergy by comutation of six amino acid residues on the conformational IgE epitopes, J. Immunol., 172, 2621–2628.
Reyes-Lopez, C. A., Hernandez-Santoyo, A., Pedraza-Escalona, M., Mendoza, G., Hernandez-Arana, A., and Rodriguez-Romero, A. (2004) Insights into a conformational epitope of Hev b6.02 (hevein), Biochem. Biophys. Res. Commun., 314, 123–130.
Reyes-Lopez, C. A., Pedraza-Escalona, M., Mendoza, G., Hernandez-Santoyo, A., and Rodriguez-Romero, A. (2006) A single amino acid substitution on the surface of a natural hevein isoform (Hev b6.0202), confers different IgE recognition, FEBS Lett., 580, 2483–2487.
Pedraza-Escalona, M., Becerril-Lujan, B., Agundis, C., Dominguez-Ramirez, L., Pereyra, A., Riano-Umbarila, L., and Rodriguez-Romero, A. (2009) Analysis of B-cell epitopes from the allergen Hev b6.02 revealed by using blocking antibodies, Mol. Immunol., 46, 668–676.
Koo, J. C., Lee, S. Y., Chun, H. J., Cheong, Y. H., Choi, J. S., Kawabata, S., Miyagi, M., Tsunasawa, S., Ha, K. S., Bae, D. W., Han, C. D., Lee, B. L., and Cho, M. J. (1998) Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity, Biochim. Biophys. Acta, 1382, 80–90.
Fujimura, M., Minami, Y., Watanabe, K., and Tadera, K. (2003) Purification, characterization, and sequencing of a novel type of antimicrobial peptides, Fa-AMP1 and Fa-AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench.), Biosci. Biotechnol. Biochem., 67, 1636–1642.
Li, S. S., and Claeson, P. (2003) Cys/Gly-rich proteins with a putative single chitin-binding domain from oat (Avena sativa) seeds, Phytochemistry, 63, 249–255.
Broekaert, W. F., Marien, W., Terras, F. R., De Bolle, M. F., Proost, P., Van Damme, J., Dillen, L., Claeys, M., Rees, S. B., Vanderleyden, J., et al. (1992) Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins, Biochemistry, 31, 4308–4314.
Rivillas-Acevedo, L. A., and Soriano-Garcia, M. (2007) Isolation and biochemical characterization of an antifungal peptide from Amaranthus hypochondriacus seeds, J. Agric. Food Chem., 55, 10156–10161.
Lipkin, A., Anisimova, V., Nikonorova, A., Babakov, A., Krause, E., Bienert, M., Grishin, E., and Egorov, T. (2005) An antimicrobial peptide Ar-AMP from amaranth (Amaranthus retroflexus L.) seeds, Phytochemistry, 66, 2426–2431.
Kini, S. G., Nguyen, P. Q., Weissbach, S., Mallagaray, A., Shin, J., Yoon, H. S., and Tam, J. P. (2015) Studies on the chitin binding property of novel cysteine-rich peptides from Alternanthera sessilis, Biochemistry, 54, 6639–6649.
De Bolle, M. F., David, K. M., Rees, S. B., Vanderleyden, J., Cammue, B. P., and Broekaert, W. F. (1993) Cloning and characterization of a cDNA encoding an antimicrobial chitin-binding protein from amaranth, Amaranthus caudatus, Plant. Mol. Biol., 22, 1187–1190.
Nielsen, K. K., Nielsen, J. E., Madrid, S. M., and Mikkelsen, J. D. (1997) Characterization of a new antifungal chitin-binding peptide from sugar beet leaves, Plant Physiol., 113, 83–91.
Huang, X., Xie, W., and Gong, Z. (2000) Characteristics and antifungal activity of a chitin binding protein from Ginkgo biloba, FEBS Lett., 478, 123–126.
Rogozhin, E. A., Slezina, M. P., Slavokhotova, A. A., Istomina, E. A., Korostyleva, T. V., Smirnov, A. N., Grishin, E. V., Egorov, T. A., and Odintsova, T. I. (2015) A novel antifungal peptide from leaves of the weed Stellaria media L., Biochimie, 116, 125–132.
Slavokhotova, A. A., Shelenkov, A. A., Korostyleva, T. V., Rogozhin, E. A., Melnikova, N. V., Kudryavtseva, A. V., and Odintsova, T. I. (2017) Defense peptide repertoire of Stellaria media predicted by high throughput next generation sequencing, Biochimie, 135, 15–27.
Shukurov, R. R., Voblikova, V. D., Nikonorova, A. K., Komakhin, R. A., Komakhina, V. V., Egorov, T. A., Grishin, E. V., and Babakov, A. V. (2011) Transformation of tobacco and Arabidopsis plants with Stellaria media genes encoding novel hevein-like peptides increases their resistance to fungal pathogens, Transgenic Res., 21, 313–325.
Martins, J. C., Maes, D., Loris, R., Pepermans, H. A., Wyns, L., Willem, R., and Verheyden, P. (1996) H-NMR study of the solution structure of Ac-AMP2, a sugar binding antimicrobial protein isolated from Amaranthus caudatus, J. Mol. Biol., 258, 322–333.
Huang, R. H., Xiang, Y., Liu, X. Z., Zhang, Y., Hu, Z., and Wang, D. C. (2002) Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv., FEBS Lett., 521, 87–90.
Huang, R. H., Xiang, Y., Tu, G. Z., Zhang, Y., and Wang, D. C. (2004) Solution structure of Eucommia antifungal peptide: a novel structural model distinct with a five-disulfide motif, Biochemistry, 43, 6005–6012.
Xiang, Y., Huang, R. H., Liu, X. Z., Zhang, Y., and Wang, D. C. (2004) Crystal structure of a novel antifungal protein distinct with five disulfide bridges from Eucommia ulmoides Oliver at an atomic resolution, J. Struct. Biol., 148, 86–97.
Van den Bergh, K. P., Proost, P., Van Damme, J., Coosemans, J., Van Damme, E. J., and Peumans, W. J. (2002) Five disulfide bridges stabilize a hevein-type antimicrobial peptide from the bark of spindle tree (Euonymus europaeus L.), FEBS Lett., 530, 181–185.
Van den Bergh, K. P., Rouge, P., Proost, P., Coosemans, J., Krouglova, T., Engelborghs, Y., Peumans, W. J., and Van Damme, E. J. (2004) Synergistic antifungal activity of two chitin-binding proteins from spindle tree (Euonymus europaeus L.), Planta, 219, 221–232.
Odintsova, T. I., Vassilevski, A. A., Slavokhotova, A. A., Musolyamov, A. K., Finkina, E. I., Khadeeva, N. V., Rogozhin, E. A., Korostyleva, T. V., Pukhalsky, V. A., Grishin, E. V., and Egorov, T. A. (2009) A novel antifungal hevein-type peptide from Triticum kiharae seeds with a unique 10-cysteine motif, FEBS J., 276, 4266–4275.
Utkina, L. L., Zhabon, E. O., Slavokhotova, A. A., Rogozhin, E. A., Shiian, A. N., Grishin, E. V., Egorov, Ts. A., Odintsova, T. I., and Pukhal’skii, V. A. (2010) Heterologous expression of a synthetic gene encoding a novel hevein-type antimicrobial peptide of Leymus arenarius in Escherichia coli cells, Genetika, 46, 1645–1651.
Dubovskii, P. V., Vassilevski, A. A., Slavokhotova, A. A., Odintsova, T. I., Grishin, E. V., Egorov, T. A., and Arseniev, A. S. (2011) Solution structure of a defense peptide from wheat with a 10-cysteine motif, Biochem. Biophys. Res. Commun., 411, 14–18.
Andreev, Y. A., Korostyleva, T. V., Slavokhotova, A. A., Rogozhin, E. A., Utkina, L. L., Vassilevski, A. A., Grishin, E. V., Egorov, T. A., and Odintsova, T. I. (2012) Genes encoding hevein-like defense peptides in wheat: distribution, evolution, and role in stress response, Biochimie, 94, 1009–1016.
Muraki, M. (2002) The importance of CH/pi interactions to the function of carbohydrate binding proteins, Protein Pept. Lett., 9, 195–209.
Chavez, M. I., Andreu, C., Vidal, P., Aboitiz, N., Freire, F., Groves, P., Asensio, J. L., Asensio, G., Muraki, M., Canada, F. J., and Jimenez-Barbero, J. (2005) On the importance of carbohydrate–aromatic interactions for the molecular recognition of oligosaccharides by proteins: NMR studies of the structure and binding affinity of AcAMP2-like peptides with non-natural naphthyl and fluoroaromatic residues, Chemistry, 11, 7060–7074.
Koo, J. C., Lee, B., Young, M. E., Koo, S. C., Cooper, J. A., Baek, D., Lim, C. O., Lee, S. Y., Yun, D. J., and Cho, M. J. (2004) Pn-AMP1, a plant defense protein, induces actin depolarization in yeasts, Plant Cell Physiol., 45, 1669–1680.
Naumann, T. A., Wicklow, D. T., and Price, N. P. (2011) Identification of a chitinase-modifying protein from Fusarium verticillioides: truncation of a host resistance protein by a fungalysin metalloprotease, J. Biol. Chem., 286, 35358–35366.
Naumann, T. A., and Price, N. P. (2012) Truncation of class IV chitinases from Arabidopsis by secreted fungal proteases, Mol. Plant Pathol., 13, 1135–1139.
Caruso, C., Chilosi, G., Caporale, C., Leonardi, L., Bertini, L., Magro, P., and Buonocore, V. (1990) Induction of pathogenesis-related proteins in germinating wheat seeds infected with Fusarium culmorum, Plant Sci., 140, 87–97.
Slavokhotova, A. A., Naumann, T. A., Price, N. P., Rogozhin, E. A., Andreev, Y. A., Vassilevski, A. A., and Odintsova, T. I. (2014) Novel mode of action of plant defense peptides–hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases, FEBS J., 281, 4754–4764.
Kanrar, S., Venkateswari, J. C., Kirti, P. B., and Chopra, V. L. (2002) Transgenic expression of hevein, the rubber tree lectin, in Indian mustard confers protection against Alternaria brassicae, Plant Sci., 162, 441–448.
Lee, H. I., and Raikhel, N. V. (1995) Prohevein is poorly processed but shows enhanced resistance to a chitin-binding fungus in transgenic tomato plants, Braz. J. Med. Biol. Res., 28, 743–750.
Montoro, P., Lagier, S., Baptiste, C., Marteaux, B., Pujade-Renaud, V., Leclercq, J., and Alemanno, L. (2008) Expression of the HEV2.1 gene promoter in transgenic Hevea brasiliensis, Plant Cell Tissue Organ Cult., 94, 55–63.
Sunderasan, E. B., Badaruddin, B. E., Azharuddin, A., and Arokiaraj, P. (2012) Genetic transformation of Hevea brasiliensis with human atrial natriuretic factor, J. Rubber Res., 15, 255–264.
Sunderasan, E. S., Shuib, S. S., Badaruddin, B. E., Azharuddin, A., and Arokiaraj, P. (2010) Hevea genetic transformation for enhanced recombinant pharmaceutical production by the use of hevein promoter, in National Biotechnology Seminar, Kuala Lumpur, Malaysia.
Berthelot, K., Peruch, F., and Lecomte, S. (2016) Highlights on Hevea brasiliensis (pro)hevein proteins, Biochimie, 127, 258–270.
Koo, J. C., Chun, H. J., Park, H. C., Kim, M. C., Koo, Y. D., Koo, S. C., Ok, H. M., Park, S. J., Lee, S. H., Yun, D. J., Lim, C. O., Bahk, J. D., Lee, S. Y., and Cho, M. J. (2002) Over-expression of a seed specific hevein-like antimicrobial peptide from Pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants, Plant Mol. Biol., 50, 441–452.
Lee, O. S., Lee, B., Park, N., Koo, J. C., Kim, Y. H., Prasad, D. T., Karigar, C., Chun, H. J., Jeong, B. R., Kim, D. H., Nam, J., Yun, J. G., Kwak, S. S., Cho, M. J., and Yun, D. J. (2003) Pn-AMPs, the hevein-like proteins from Pharbitis nil confers disease resistance against phytopathogenic fungi in tomato, Lycopersicum esculentum, Phytochemistry, 62, 1073–1079.
De Bolle, M. F., Osborn, R. W., Goderis, I. J., Noe, L., Acland, D., Hart, C. A., Torrekens, S., Van Leuven, F., and Broekaert, W. F. (1996) Antimicrobial peptides from Mirabilis jalapa and Amaranthus caudatus: expression, processing, localization and biological activity in transgenic tobacco, Plant Mol. Biol., 31, 993–1008.
Liapkova, N. S., Loskutova, N. A., Maisurian, A. N., Mazin, V. V., Korableva, N. P., Platonova, T. A., Ladyzhenskaia, E. P., and Evsiunina, A. S. (2001) Isolation of genetically modified potato plant containing the gene of defensive peptide from Amaranthus, Prikl. Biokhim. Mikrobiol., 37, 349–354.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. A. Slavokhotova, A. A. Shelenkov, Ya. A. Andreev, T. I. Odintsova, 2017, published in Uspekhi Biologicheskoi Khimii, 2017, Vol. 57, pp. 209-244.
Rights and permissions
About this article
Cite this article
Slavokhotova, A.A., Shelenkov, A.A., Andreev, Y.A. et al. Hevein-like antimicrobial peptides of plants. Biochemistry Moscow 82, 1659–1674 (2017). https://doi.org/10.1134/S0006297917130065
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917130065