Abstract
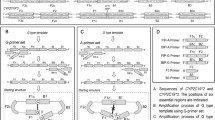
This study tested a method designed to correctly identify single nucleotide polymorphisms in DNA sequences that are capable of forming a hairpin. Fragments of the angiotensin (AGT) and cytochrome (CYP2C19) genes with rs699 (T>C) and rs4986893 (G>A), respectively, were chosen as examples. DNA probes complementary to the polymorphic sites formed hairpin structures with a loop of 6 nt in the case of rs699 (AGT) or 4 nt in the case of rs4986893 (CYP2C19). Fluorophore-labeled target DNA was obtained via two-round multiplex PCR with simultaneous incorporation of a fluorescent label in the second round. When target DNA was hybridized to a corresponding pair of probes immobilized in gel pads of a biochip, dramatically low, if any, fluorescent signals were detected from the pads. A replacement of one nucleotide in the DNA probes prevented the formation of intramolecular structures, as was confirmed by melting curves. However, the DNA probes completely lost their complementarity to target DNA as a result of the replacement. To restore the complementary interaction with the DNA probe, corresponding nucleotide replacements were introduced in target DNA via site-directed mutagenesis. The approach significantly increased the specific fluorescent signals from biochip pads, thus allowing correct genotyping of rs699 and rs4986893.






Similar content being viewed by others
REFERENCES
X. Weng, in Encyclopedia of Microfluidics and Nanofluidics (Springer, New York, 2015), pp. 1–8.
J. H. Monserud and D. K. Schwartz, ACS Nano 8, 4488 (2014).
Y. A. Jakubek and D. J. Cutler, BMC Genom. 13, 737 (2012).
D. Erickson, D. Li, and U. J. Krull, Anal. Biochem. 317, 186 (2003).
A. Munir, H. Waseem, M. R. Williams, et al., Microarrays 6 (2), E9 (2017).
D. Gryadunov, E. Dementieva, V. Mikhailovich, et al., Expert Rev. Mol. Diagn. 11 (8), 839 (2011).
D. Gryadunov, F. Nicot, M. Dubois, et al., J. Clin. Microbiol. 48 (11), 3910 (2010).
T. V. Nasedkina, N. A. Guseva, O. A. Gra, et al., Mol. Diagn. Ther. 13 (2), 91 (2009).
C. R. Lee, J. A. Goldstein, and J. A. Pieper, Pharmacogenet. Genomics 12 (3), 251 (2002).
J. A. Johnson, K. E. Caudle, L. Gong, et al., Clin. Pharmacol. Ther. 102, 397 (2017).
K. E. Caudle, A. E. Rettie, M. Whirl-Carrillo, et al., Clin. Pharmacol. Ther. 96, 542 (2014).
S. A. Scott, K. Sangkuhl, C. M. Stein, et al., Clin. Pharmacol. Ther. 94, 317 (2013).
J. K. Hicks, J. R. Bishop, K. Sangkuhl, et al., Clin. Pharmacol. Ther. 98, 127 (2015).
A. Tornio and J. T. Backman, Adv. Pharmacol. 83, 3 (2018).
L. Kurland, U. Liljedahl, J. Karlsson, et al., Am. J. Hypertens. 17 (1), 8 (2004).
H. Schelleman, O. H. Klungel, J. C. Witteman, et al., Eur. J. Hum. Genet. 15 (4), 478 (2007).
O. A. Zasedateleva, V. A.Vasiliskov, S. A. Surzhikov, et al., Biotechnol. J. 9 (8), 1074 (2014).
http://unafold.rna.albany.edu.
C. Trapp, M. Schenkelberger, and A.Ott, BMC Biophys. 4, 20 (2011).
N. V. Sorokin, V. R. Chechetkin, M. A. Livshits, et al., J. Biomol. Struct. Dyn. 22 (6), 725 (2005).
N. V. Sorokin, D. Y. Yurasov, A. I. Cherepanov, et al., J. Biomol. Struct. Dyn. 24 (6), 571 (2007).
ACKNOWLEDGMENTS
This work was supported by the Studies and Developments in Priority Fields of the Research and Technology Complex of Russia from 2014 to 2020 federal program (agreement no. 14.604.21.0166, unique project identifier RFMEFI60417X0166).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by T. Tkacheva
Abbreviations: PCR, polymerase chain reaction; bp, base pair.
Rights and permissions
About this article
Cite this article
Ikonnikova, A.Y., Zasedateleva, O.A., Surzhikov, S.A. et al. Structural Destabilization of Intramolecular Duplexes Improves the Results of DNA Hybridization Analysis. BIOPHYSICS 63, 880–887 (2018). https://doi.org/10.1134/S000635091806012X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000635091806012X