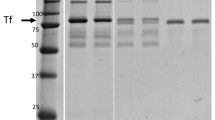
Abstract
A number of single-domain antibodies (nanobodies) obtained previously to major marker blood proteins were tested as tools to preprocess urine samples from patients with bladder cancer. Nanobody-based tools demonstrated unique possibilities for noninvasive diagnostic studies along with other conventional methods, such as electrophoresis and, in prospect, mass spectrometric analysis. A testing of 22 samples from bladder cancer patients showed that the development of bladder cancer is accompanied by an increase in the urine contents of major blood proteins, including those known as potential bladder cancer biomarkers. New nanobody-based immunosorbents allow both specific enrichment and specific removal of particular antigenic proteins and subproteomes associated with them from a biological fluid. The isolation of immune complexes from the urine of a particular patient is of particular interest. An initial study of the complexes showed not only increased contents of IgA and IgG at advanced stages of the disease, but also many other components, which provide potential biomarkers of the pathological process in a particular patient. It is intended to use the approaches proposed in this work in a future larger-scale study of urine samples from patients with bladder cancer at different stages of the disease in order to identify new promising biomarkers of bladder cancer.







Similar content being viewed by others
REFERENCES
IARC. 2021. Cancer Today. Estimated number of new cases in 2020, worldwide, both sexes, all ages. https://gco.iarc.fr/today/online-analysis-table.
https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/#3
American Cancer Society. 2020. What is bladder cancer? (https://www.cancer.org/cancer/bladder-cancer/ about/what-is-bladder-cancer.html. Accessed September 2020.
van Rhijn B.W., Burger M., Lotan Y., Solsona E., Stief C.G., Sylvester R.J., Witjes J.A., Zlotta A.R. 2009. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur. Urol. 56 (3), 430‒442. https://doi.org/10.1016/j.eururo.2009.06.028
Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. 2016. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur. Urol. 70 (1), 106‒119.
Santoni G., Morelli M.B., Amantini C. Battelli N. 2018. Urinary markers in bladder cancer: an update. Front. Oncol. 7 (8), 362.
Blick C.G.T., Nazir S.A., Mallett S., Turney B.W., Onwu N.N., Roberts I.S.D., Jeremy P. Crew J.H., Cowan N.C. 2011. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int. 110, 84–94.
Tillib S.V., Ivanova T.I., Vasilev L.A. 2010. Fingerprint analysis of “nanoantibody” selection by phage display using two variants of helper phages. Acta Nat. 2, 100–108.
Tillib S., Ivanova T.I., Vasilev L.A., Rutovskaya M.V., Saakyan S.A., Gribova I.Y., Tutykhina I.L., Sedova E.S., Lysenko A.A., Shmarov M.M., Logunov D.Y., Naroditsky B.S., Gintsburg A.L. 2013. Formatted single-domain antibodies can protect mice against infection with influenza virus (H5N2). Antiviral Res. 97, 245‒254.
Tillib S.V., Privezentseva M.E., Ivanova T.I., Efimov G.A., Gursky Y.G., Georgiev G.P., Goldman I.L., Sadchikova E.R. 2014. Single-domain antibody-based ligands for immunoaffinity separation of recombinant human lactoferrin from the goat lactoferrin of trasgenic goat milk. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 949–950, 48–57.
Tillib S.V., Vyatchanin A.S., Muildermans S. 2014. Molecular analysis of heavy chain-only antibodies of Camelus bactrianus. Biochemistry (Moscow). 79, 1382–1390.
Goryainova O.S., Ivanova T.I., Rutovskaya M.V., Tillib S.V. 2017. A method for the parallel and sequential generation of single-domain antibodies for the proteomic analysis of human blood plasma. Mol. Biol. (Moscow). 51 (6), 855–864.
Tillib S.V. 2020. Prospective applications of single-domain antibodies in biomedicine. Mol. Biol. (Moscow). 54 (3), 317–326.
Tillib S.V., Ivanova T.I., Lysyuk E.Yu., Larin S.S., Kibardin A.V., Korobko E.V., Vikhreva P.N., Gnuchev N.V., Georgiev G.P., Korobko I.V. 2012. Nanoantibodies for detection and blocking of bioactivity of human vascular endothelial growth factor A(165). Biochemistry (Moscow). 77, 659–665.
Garas M.N., Tillib S.V., Zubkova O.V., Rogozhin V.N., Ivanova T.I., Vasil’ev L.A., Logunov D.Yu., Shmarov M.M., Tutykhina I.L., Esmagambetov I.B., Gribova I.Yu., Bandelyuk A.S., Naroditsky B.S., Gintsburg A.L. 2014. Target-specific gene delivery using recombinant pseudoadenoviral particles capable of efficiently binding to nanoantibodies. Acta Nat. 6 (2), 102‒113.
Marshall T., Williams K.M. 1998. Clinical analysis of human urinary proteins using high resolution electrophoretic methods. Electrophoresis. 19, 1752‒1770.
Wessel D., Fluegge U.I. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem, 138, 141‒143.
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680‒685.
Floege J., Boddeker M., Stolte H., Koch K.M. 1990. Urinary IgA, secretory IgA and secretory component in women with recurrent urinary tract infections. Nephron. 56, 50‒55.
Machii R., Kubota R., Hiratsuka N., Sugimoto K., Masudo R., Kurihara Y., Kobayashi S., Shiba K. 2004. Urinary protein fraction in healthy subjects using cellulose acetate membrane electrophoresis followed by colloidal silver staining. J. Clin. Lab. Anal. 18, 231–236.
Huang C., Ren J., Ji F., Muyldermans S., Jia L. 2020. Nanobody-based high-performance immunosorbent for selective beta 2-microglobulin purification from blood. Acta Biomater. 107, 232–241.
Betkerur V., Baumgartner G., Talluri K., Sharifi R., Nagubadi S., Guinan P. 1981. Urinary immunoglobulin A in the diagnosis of bladder cancer. J. Surg. Oncol. 16, 215‒217.
Khalifa A., Fathi O., Mousa M.A., el Magraby H. 1987. Immunoglobulin A and alpha 2-macroglobulin as tumor markers in bladder cancer. Chemioter. 6 (Suppl. 2), 736‒737.
Li C., Li H., Zhang T., Li J., Liu L., Chang J. 2014. Discovery of Apo-A1 as a potential bladder cancer biomarker by urine proteomics and analysis. Biochem. Biophys. Res. Commun. 446, 1047‒1052.
Dong F., Shen Y., Xu T., Wang X., Gao F., Zhong S., Chen S., Shen Z. 2018. Effectiveness of urine fibronectin as a non-invasive diagnostic biomarker in bladder cancer patients: a systematic review and meta-analysis. World J. Surg. Oncol. 16, 61.
Matsumura E., Kosuge N., Nakanishi S., Suda T., Sugawa A., Fujimura T., Miyagi R., Yoshimi N., Saito S. 2020. Urine lactoferrin as a potential biomarker reflecting the degree of malignancy in urothelial carcinoma of the bladder. Tohoku J. Exp. Med. 252 (3), 225‒244.
Funding
This work was supported by the Russian Science Foundation (project no. 20-14-00305).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies of biological fluids were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study. The study was approved by the Ethics Committee at the Institute of Gene Biology (Approval dated January 18, 2021) and the Ethics Committee at the Hertsen Moscow Oncology Research Institute.
Additional information
Translated by T. Tkacheva
Rights and permissions
About this article
Cite this article
Tillib, S.V., Goryainova, O.S., Sachko, A.M. et al. Single-Domain Antibodies Used to Pretreat the Human Urinary Proteome in Cancer Biomarker Testing. Mol Biol 56, 616–627 (2022). https://doi.org/10.1134/S0026893322040124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893322040124