Abstract
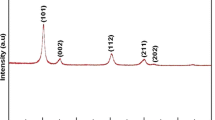
Compounds Mn0.5Ti2(PO4)3 and Mn0.5Zr2(PO4)3 and Mn0.5+2xZr2 – x(PO4)3 (0 < x ≤0.35) solid solution were prepared by two variants of the sol-gel method using inorganic and organic reagents and were characterized using X-ray diffraction and IR spectroscopy. Mn0.5Ti2(PO4)3, a compound with an NaZr2(PO4)3 (NZP) structure, is formed at 600°C and is stable up to 950°C. Mn0.5Zr2(PO4)3 has dimorphism; its low-temperature phase having the Sc2(WO4)3 (SW) structure was prepared at 650°C, and the high-temperature NZP phase, at 1200°C. Mn0.5 + 2xZr2−x(PO4)3 solid solution crystallizes in an SW-type structure; it is thermally unstable at temperatures above 900°C. The thermal stability of samples decays as x rises. p ]The numbers of the stretching and bending vibrations in an \({\rm{PO}}_4^{3 - }\) ion in the IR spectra of NZP and SW ortho-phosphates agree with factor-group analysis for space group R3̅ and P21/n. Structure refinement was carried out for the low-temperature Mn0.5Zr2(PO4)3 phase (space group P21/n, a = 8.861(3) Å, b = 8.869(2) Å, c = 12.561(3) Å, β = 89.51(2)°) and for the solid solution. The basis of the structures is a framework built of corner-sharing tetrahedra PO4 and octahedra ZrO6 or (Mn,Zr)O6. The framework interstices are occupied by cations Mn2+ in tetrahedral oxygen coordination. A comparative crystal-chemical analysis of the morpho-tropic series of M0.5Zr2(PO4)3 phosphates (M stands for a metal in the oxidation state +2) elucidated a relationship between structural features.
Similar content being viewed by others
References
V. I. Pet’kov, A. I. Orlova, G. I. Dorokhova, and Ya. V. Fedotova, Crystallogr. Repts. 45, 36 (2000).
V. I. Pet’kov, V. S. Kurazhkovskaya, A. I. Orlova, and M. L. Spiridonova, Crystallogr. Repts. 47, 736 (2002).
E. A. Asabina, I. O. Glukhova, V. I. Pet’kov, et al., Russ. J. Gen. Chem. 87, 684 (2017). doi https://doi.org/10.1134/S1070363217040041
S. Barth, R. Olazcuaga, P. Gravereau, et al., Mater. Lett. 16, 96 (1993). https://doi.org/10.1016/0167-577X(93)90031-R.
R. Olazcuaga, G. Le Flem, A. Boireau, and J. L. Soubey-roux, Adv. Mater. Res. 1–2, 177 (1994). https://doi.org/10.4028/www.scientific.net/AMR.1-2.177.
V. I. Pet’kov, E. V. Zhilkin, E. A. Asabina, and E.Yu. Borovikova, Russ. J. Inorg. Chem. 59, 1087 (2014). doi https://doi.org/10.1134/S003602361410012X
R. Olazcuaga and J. M. Dance, “Le Flem G. Et Al,” J. Solid State Chem. 143, 224 (1999). https://doi.org/10.1006/jssc.1998.8097.
J. Derouet, L. Beaury, P. Porcher, et al., J. Solid State Chem. 143, 230 (1999). https://doi.org/10.1006/jssc.1998.8098.
R. Essehli, B. El Bali, S. Benmokhtar, et al., Mater. Res. Bull. 44, 1502 (2009). https://doi.org/10.1016/j.materresbull.2009.02.013.
M. Schöneborn and R. Glaum, Z. Anorg. Allg. Chem. 634, 1843 (2008). https://doi.org/10.1002/zaac.200800186.
S. Benmokhtar, A. El Jazouli, A. Aatiq, et al., J. Solid State Chem. 180, 2004 (2007). doi https://doi.org/10.1016/j.jssc.2007.04.014
A. Aatiq, M. Menetrier, A. El Jazouli, and C. Delmas, Solid State Ionics 150, 391 (2002). doi https://doi.org/10.1016/S0167-2738(02)00135-2
S. Senbhagaraman, RowT. N. Guru, and A. M. Umarji, J. Mater. Chem. 3, 309 (1993). doi https://doi.org/10.1039/JM9930300309
A. El Bouari and A. El Jazouli, Phosphorus Res. Bull 15, 136 (2004). doi https://doi.org/10.3363/prb1992.15.0_136
D. A. Woodcock, P. Lightfoot, and R. I. Smith, J. Mater. Chem. 9, 2631 (1999). doi https://doi.org/10.1039/A903489G
A. El Jazouli, J. L. Soubeyroux, J. M. Dance, and G. Le Flem, J. Solid State Chem. 65, 351 (1986). doi https://doi.org/10.1016/0022-4596(86)90107-6
J.-P. Chaminade, A. El Bouari, A. El Jazouli, et al., Acta Crystallogr., Sect. A 61, C325 (2005). doi https://doi.org/10.1107/50108767305086186
A. Mouline, M. Alami, R. Brochu, et al., Mater. Res. Bull. 35, 899 (2000). doi https://doi.org/10.1016/S0025-5408(00)00277-4
R. Brochu, M. El-Yacoubi, M. Louer, et al., Mater. Res. Bull. 32, 15 (1997). doi https://doi.org/10.1016/S0025-5408(96)00162-6
J. Alamo and J. L. Rodrigo, Solid State Ionics 63–65, 678 (1993). doi https://doi.org/10.1016/0167-2738(93)90178-6
A. El Yacoubi, A. Mouline, M. Alami, et al., Phys. Chem. News 44, 76 (2008).
E. R. Gobechiya, Yu. K. Kabalov, V. I. Pet’kov, and M. V. Sukhanov, Crystallorg. Repts. 49, 741 (2004).
V. I. Pet’kov, A. I. Orlova, and D. A. Kapranov, Russ. J. Inorg. Chem. 43, 1429 (1998).
V. I. Petkov and A. I. Orlova, J. Therm. Anal. Calom. 54, 71 (1998). https://doi.org/10.1023/A:1010156616525.
M. Kinoshita, A. N. Fitch, Y. Piffard, et al., Eur. J. Solid State Inorg. Chem. 28, 683 (1991).
E. R. Gobechiya, M. V. Sukhanov, V. I. Pet’kov, and Yu. K. Kabalov, Crystallorg. Repts. 53, 53 (2008). doi https://doi.org/10.1134/S1063774508010069
A. El Jazouli, M. Alami, R. Brochu, et al., J. Solid State Chem. 71, 444 (1987). doi https://doi.org/10.1016/0022-4596(87)90253-2
K. Nomura, S. Ikeda, H. Masuda, and H. Einaga, Solid Electrolyte. Chem. Lett. 22, 893 (1993). doi https://doi.org/10.1246/cl.1993.893
K. Nomura, S. Ikeda, K. Ito, and H. Einaga, Bull. Chem. Soc. Jpn. 65, 3221 (1992). https://doi.org/10.1246/bcsj.65.3221.
V. I. Pet’kov, A. I. Orlova, G. N. Kazantsev, et al., J. Therm. Anal. Calom. 66, 623 (2001). https://doi.org/10.1023/A:1013145807987.
V. Pet’kov, E. Asabina, V. Loshkarev, and M. Sukha-nov, J. Nucl. Mater. 471, 122 (2016). doi https://doi.org/10.1016/j.jnucmat.2016.01.016
S. N. Ienealem, S. G. Gul’yanova, T. K. Chekhlova, et al., Zh. Fiz. Khim. 74, 2273 (2000).
M. Sukhanov, V. Pet’lkov, M. Ermilova, et al., Phosphorus Res. Bull. 19, 90 (2005). https://doi.org/10.3363/prb1992.19.0_90.
I. Shchelokov, E. Asabina, M. Sukhanov, et al., Solid State Sci. 10, 513 (2008). doi https://doi.org/10.1016/j.solidstate-sciences.2007.12.005
A. I. Pylinina, I. I. Mikhalenko, M. M. Ermilova, et al., Russ. J. Phys. Chem. A. 84, 400 (2010). doi https://doi.org/10.1134/S0036024410030106
H. M. Rietveld, Acta Crystallogr. 22, 151 (1967).
Y. I. Kim and F. Izumi, J. Ceram. Soc. Jpn. 102, 401 (1994). doi https://doi.org/10.2109/jcersj.102.401
F. Izumi, The Rietveld Method, Ed. by C. A. Young (Oxford Univ. Press, Oxford, 1993).
V. I. Pet’kov, M. V. Sukhanov, A. S. Shipilov, et al., Inorg. Mater. 50, 263 (2014). doi https://doi.org/10.1134/S0020168514030091
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © V.I. Pet’kov, D.A. Lavrenov, M.V. Sukhanov, A.M. Koval’skii, E.Yu. Borovikova, 2019, published in Zhurnal Neorganicheskoi Khimii, 2019, Vol. 64, No. 2, pp. 137–145.
Rights and permissions
About this article
Cite this article
Pet’kov, V.I., Lavrenov, D.A., Sukhanov, M.V. et al. Sol—Gel Synthesis and Structure Formation of Manganese Zirconium (Titanium) Phosphates. Russ. J. Inorg. Chem. 64, 170–178 (2019). https://doi.org/10.1134/S0036023619020165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619020165