Abstract
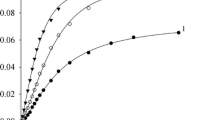
The vanadate anion in the presence of pyrazine-2-carboxylic acid (PCA) was found to effectively catalyze the oxidation of isopropanol to acetone with hydrogen peroxide. The electronic spectra of solutions and the kinetics of oxidation were studied. The conclusion was drawn that the rate-determining stage of the reaction was the decomposition of the vanadium(V) diperoxo complex with PCA, and the particle that induced the oxidation of isopropanol was the hydroxyl radical. Supposedly, the HO· radical detached a hydrogen atom from isopropanol, and the Me2 C· (OH) radical formed reacted with HOO· to produce acetone and hydrogen peroxide. The electronic spectra of solutions in isopropanol and acetonitrile and the dependences of the initial rates of isopropanol oxidation without a solvent and cyclohexane oxidation in acetonitrile on the initial concentration of hydrogen peroxide were compared. The conclusion was drawn that hydroxyl radicals appeared in the oxidation of alkanes in acetonitrile in the decomposition of the vanadium diperoxo complex rather than the monoperoxo derivative, as was suggested by us earlier.
Similar content being viewed by others
References
G. B. Shul’pin, D. Attanasio, and L. Suber, J. Catal. 142, 147 (1993).
G. B. Shul’pin and G. Süss-Fink, J. Chem. Soc., Perkin Trans. 2, p. 1459 (1995).
G. B. Shul’pin, R. S. Drago, and M. Gonzalez, Izv. Akad. Nauk, Ser. Khim., p. 2514 (1996) [Russ. Chem. Bull. 45, 2386 (1996)].
G. B. Shul’pin, M. C. Guerreiro, and U. Schuchardt, Tetrahedron 52, 13051 (1996).
M. C. Guerreiro, U. Schuchardt, and G. B. Shul’pin, Izv. Akad. Nauk, Ser. Khim., p. 780 (1997) [Russ. Chem. Bull. 46, 749 (1997)].
A. E. Shilov and G. B. Shul’pin, Chem. Rev. 97, 2879 (1997).
G. V. Nizova, G. Süss-Fink, and G. B. Shul’pin, Tetrahedron 53, 3603 (1997).
U. Schuchardt, M. C. Guerreiro, and G. B. Shul’pin, Izv. Akad. Nauk, Ser. Khim., p. 253 (1998) [Russ. Chem. Bull. 47, 247 (1998)].
G. Süss-Fink, G. V. Nizova, S. Stanislas, and G. B. Shul’pin, J. Mol. Catal. A: Chem. 130, 163 (1998).
G. B. Shul’pin, Y. Ishii, S. Sakaguchi, and T. Iwahama, Izv. Akad. Nauk, Ser. Khim., p. 896 (1999) [Russ. Chem. Bull. 48, 887 (1999)].
G. Süss-Fink, S. Stanislas, G. B. Shul’pin, et al., J. Chem. Soc., Dalton Trans., p. 3169 (1999).
A. Shilov and G. B. Shul’pin, Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes (Kluwer, Dordrecht, 2000).
Yu. N. Kozlov, G. V. Nizova, and G. B. Shul’pin, Zh. Fiz. Khim. 75, 865 (2001) [Russ. J. Phys. Chem. 75, 770 (2001)].
G. B. Shul’pin, Y. N. Kozlov, G. V. Nizova, et al., J. Chem. Soc., Perkin Trans. 2, p. 1351 (2001).
G. B. Shul’pin, J. Mol. Catal. A: Chem. 189, 39 (2002).
M. H. C. de la Cruz, Y. N. Kozlov, E. R. Lachter, and G. B. Shul’pin, New J. Chem. 27, 634 (2003).
G. B. Shul’pin, C. R. Acad. Sci., Ser. II: Chim. 6, 163 (2003).
Y. N. Kozlov, G. V. Nizova, and G. B. Shul’pin, J. Mol. Catal. A: Chem. 227, 247 (2005).
R. Z. Khaliullin, A. T. Bell, and M. Head-Gordon, J. Phys. Chem. B 109, 17984 (2005).
A. Butler, M. J. Clague, and G. E. Meister, Chem. Rev. 94, 625 (1994).
E. P. Talsi and K. V. Shalyaev, J. Mol. Catal. 92, 245 (1994).
V. Conte, F. Di Furia, and G. Licini, Appl. Catal., A 157, 335 (1997).
M. Bonchio, O. Bortolini, M. Carraro, et al., J. Inorg. Biochem. 80, 191 (2000).
M. Bonchio, O. Bortolini, V. Conte, and S. Primon, J. Chem. Soc., Perkin Trans. 2, p. 763 (2001).
C. Li, P. Zheng, J. Li, et al., Angew. Chem., Int. Ed. Engl. 42, 5063 (2003).
M. Sam, J. H. Hwang, G. Chanfreau, and M. M. Abu-Omar, Inorg. Chem. 43, 8447 (2004).
Yu. N. Kozlov, L. Gonzalez-Cuervo, G. Süss-Fink, and G. B. Shul’pin, Zh. Fiz. Khim. 77, 652 (2003) [Russ. J. Phys. Chem. 77, 575 (2003)].
G. B. Shul’pin, G. V. Nizova, Y. N. Kozlov, et al., Adv. Synth. Catal. 346, 317 (2004).
Farhataziz and A. B. Ross, Selected Specific Rates of Reactions of Transients from Water in Aqueous Solution. III. Hydroxyl Radical and Perhydroxyl Radical and Their Radical Ions (National Bureau of Standards, Washington, D.C., 1977), No. NSRDS-NBS 59.
E. S. Rudakov, L. K. Volkova, and V. P. Tret’yakov, Soobshch. Kinet. Katal. 16, 333 (1981).
T. V. Khar’kova, I. L. Arest-Yakubovich, and V. V. Lipes, Kinet. Katal. 30, 954 (1989).
L. M. Dorfman and G. E. Adams, Reactivity of the Hydroxyl Radical in Aqueous Solutions (National Bureau of Standards, Washington, D.C., 1973), NSRDS-NBS 46, p. 24.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.B. Romakh, Yu.N. Kozlov, G. Suss-Fink, G.B. Shul’pin, 2007, published in Zhurnal Fizicheskoi Khimii, 2007, Vol. 81, No. 8, pp. 1389–1397.
Rights and permissions
About this article
Cite this article
Romakh, V.B., Kozlov, Y.N., Süss-Fink, G. et al. The kinetics and mechanism of oxidation of isopropanol with the hydrogen peroxide-vanadate ion-pyrazine-2-carboxylic acid system. Russ. J. Phys. Chem. 81, 1221–1229 (2007). https://doi.org/10.1134/S0036024407080080
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0036024407080080