Abstract
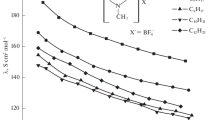
An investigation is performed of the electrical conductivity of a number of dicationic ionic liquids based on 3‑methylpyridinium and inorganic anions in acetonitrile. The Lee–Wheaton procedure is used to calculate constants Ka of ion association, the limiting molar electrical conductivity (λ0), and the Gibbs energy of association (ΔG) in solutions. It is shown that the nature and size of the anion are a key influence on the association of the studied ionic liquids. It is established that dicationic 3-methylpyridinium salts with bromide anions are more associated in solution than corresponding hexafluorophosphates or tetrafluoroborates.





Similar content being viewed by others
REFERENCES
J. P. Hallett and T. Welton, Chem. Rev. 111, 3508 (2011). https://www.doi.org/10.1021/cr1003248
T. Torimoto, T. Tsuda, K. I. Okazaki, and S. Kuwabata, Adv. Mater. 22, 1196 (2010). https://www.doi.org/10.5772/52597
D. D. Patel and J. M. Lee, Chem. Rec. 12, 329 (2012). https://www.doi.org/10.1002/tcr.201100036
A. P. Abbott and K. J. McKenzie, Phys. Chem. Chem. Phys. 8, 4265 (2006). https://www.doi.org/10.1039/B607329H
L. Gre, E. Paillard, G. T. Kim, et al., Int. J. Mol. Sci. 15, 8122 (2014). https://www.doi.org/10.3390/ijms15058122
H. Sakaebe, H. Matsumoto, and K. Tatsumi, Electrochim. Acta 53, 1048 (2007). https://www.doi.org/10.1016/j.electacta.2007.02.054
O. N. Kalugin, Iu. V. Voroshylova, A. V. Riabchunova, et al., Electrochim. Acta 105, 188 (2013). https://doi.org/10.1016/j.electacta.2013.04.140
O. E. Zhuravlev, L. I. Voronchikhina, and K. P. Gerasimova, Russ. J. Gen. Chem. 86, 2606 (2016). https://www.doi.org/10.1134/S1070363216120069
V. L. Chumak, M. R. Maksimyuk, T. V. Neshta, et al., Vost.-Evrop. Zh. Pered. Tekhnol. 62 (2/5), 59 (2013).
W. H. Lee and R. J. Wheaton, J. Chem. Soc. Faraday Trans. II 74, 743 (1978). https://www.doi.org/10.1039/F29787400743
W. H. Lee, and R. J. Wheaton, J. Chem. Soc. Faraday Trans. II 74, 1456 (1978). https://www.doi.org/10.1039/F29787401456
W. H. Lee, and R. J. Wheaton, J. Chem. Soc. Faraday Trans. II 75, 1128 (1979). https://www.doi.org/10.1039/f29797501128
A. D. Pethybridge and S. S. Taba, J. Chem. Soc. Faraday Trans. I 76, 368 (1980). https://www.doi.org/10.1039/F19807600368
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem. 14, 1347 (1993). https://www.doi.org/10.1002/jcc.540141112
A. A. Granovsky, PC GAMESS/Firefly version 8.1. http://classic.chem.msu.su/gran/gamess/index.html.
N. V. Sastry, N. M. Vaghela, P. M. Macwan, et al., J. Colloid Interface Sci. 137, 52 (2012). https://www.doi.org/10.1016/j.jcis.2011.12.077
R. Jan, G. M. Rather, and M. A. Bhat, J. Solution Chem. 42, 738 (2013). https://www.doi.org/10.1007/s10953-013-9999-4
C. G. Hanke, N. A. Atamas, and R. M. Lynden-Bell, Green Chem. 4, 107 (2002). https://doi.org/10.1039/b109179b
O. E. Zhuravlev, Russ. J. Phys. Chem. A 95, 298 (2021). https://www.doi.org/10.1134/S0036024421020308
B. Ramsauer, M. M. Meier, R. Neueder, et al., J. Acta Chim. Slov. 56, 30 (2009).
D. Das, B. Das, and D. K. Hazra, J. Solut. Chem. 32, 77 (2003). https://www.doi.org/10.1023/A:1022648916138
Y. Gao, L. Zhang, Y. Wang, et al., J. Phys. Chem. B 114, 2828 (2010). https://www.doi.org/10.1021/jp910528m
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuravlev, O.E. Effect of the Structure of Dicationic Pyridinium Ionic Liquids on Processes of Ionic Association and the Electrical Conductivity of Their Solutions in Acetonitrile. Russ. J. Phys. Chem. 95, 2503–2508 (2021). https://doi.org/10.1134/S0036024421120244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421120244