Abstract
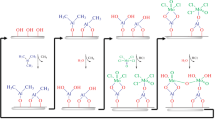
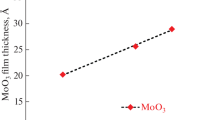
A study is performed of the thermal atomic layer deposition (ALD) of molybdenum oxide (MoOx) films using MoOCl4 and H2O and titanium–molybdenum oxide (TixMoyOz) thin films using TiCl4, MoOCl4, and H2O. Film growth is investigated via in situ quartz crystal microbalance (QCM) in the 115 to 180°C range of temperatures. ALD processes are considered for TixMoyOz films with different ratios of TiCl4–H2O and MoOCl4–H2O subcycles in a supercycle. The linear growth of a film upon an increase in the number of ALD cycles is in all cases established. The surface reactions of halides and H2O are shown to be of a self-limiting. The QCM data show the considered surface chemistry can be used for depositing thin MoOx and TixMoyOz films. Fields of potential application of these thin films a catalysis, electrochromic devices, lithium-ion batteries, antibacterial coatings and others.










Similar content being viewed by others
REFERENCES
W. Ren, Zh. Ai, F. Jia, et al., Appl. Catal. 69, 138 (2007). https://doi.org/10.1016/j.apcatb.2006.06.015
A. Fujishima and X. T. Zhang, C. R. Chim. 9, 750 (2006). https://doi.org/10.1016/j.crci.2005.02.055
R. Daghrir, P. Drogui, and D. Robert, Ind. Eng. Chem. Res. 52, 3581 (2013). https://doi.org/10.1021/ie303468t
C. W. Dunnill, A. Kafizas, and I. P. Parkin, Chem. Vap. Dep. 18 (4–6), 89 (2012). https://doi.org/10.1002/cvde.201200048
A. Vahl, S. Veziroglu, B. Henkel, et al., Materials 12, 2840 (2019). https://doi.org/10.3390/ma12172840
F. S. Al Mashary, J. F. Felix, S. O. Ferreira, et al., MSEB 259, 114578 (2020). https://doi.org/10.1016/j.mseb.2020.114578
A. A. Malygin, in Proceedings of the 3rd International Seminar of Atomic Layer Deposition, Russia, 2021 (2021), p. 13.
S. M. George, Chem. Rev. 110, 111 (2010). https://doi.org/10.1021/cr900056b
R. L. Puurunen, J. Appl. Phys. 97, 121301 (2005). https://doi.org/10.1063/1.1940727
A. I. Abdulagatov, A. M. Maksumova, D. K. Palchaev, M. Kh. Rabadanov, and I. M. Abdulagatov, Russ. J. Appl. Chem. 94, 890 (2021). https://doi.org/10.1134/S1070427221070053
Y. Xie, X. Zhao, Y. Chen, et al., J. Solid State Chem. 180, 3576 (2007). https://doi.org/10.1016/j.jssc.2007.10.023
L. Tian, A. Soum-Glaude, F. Volpi, et al., J. Vac. Sci. Technol. A 33, 01A141-1 (2015). https://doi.org/10.1116/1.4904025
A. Lee, J. A. Libera, R. Z. Waldman, et al., Adv. Sustainable Syst. 1, 1600041 (2017). https://doi.org/10.1002/adsu.201600041
V. Pore, M. Heikkilä, M. Ritala, et al., J. Photochem. Photobiol. 177, 68 (2006). https://doi.org/10.1016/j.jphotochem.2005.05.013
J. P. Niemela, H. Yamauchi, and M. Karppinen, Thin Solid Films 551, 19 (2014). https://doi.org/10.1016/j.tsf.2013.11.043
V. Pore, M. Ritala, M. Leskelä, et al., J. Mater. Chem. 17, 1361 (2007). https://doi.org/10.1039/B617307A
C. Y. Su, L. Ch. Wang, W. S. Liu, et al., ACS Appl. Mater. Interfaces 10, 33287 (2018). https://doi.org/10.1021/acsami.8b12299
V. Pore, T. Kivelä, M. Ritala, et al., Dalton Trans. 45, 6467 (2008). https://doi.org/10.1039/B809953G
J. H. Choi, S. H. Kwon, Y. K. Jeong, et al., J. Electrochem. Soc. 158, B749 (2011). https://doi.org/10.1149/1.3582765
J.-G. Huang, X-T. Guo, B. Wang, et al., J. Spectrosc. 2015, 681850 (2015). https://doi.org/10.1155/2015/681850
H. Liu, T. Lv, Ch. Zhu, and Zh. Zhu, Sol. Energy Mater. Sol. Cells 153, 1 (2016). https://doi.org/10.1016/j.solmat.2016.04.013
J. Zhang, T. Huang, L. Zhang, and A. Yu, J. Phys. Chem. C 118, 25300 (2014). https://doi.org/10.1021/jp506401q
K. Galatsis, Y. X. Li, W. Wlodarski, et al., Sens. Actuators, B 3, 276 (2002).
S. I. Kol’tsov, Zh. Prikl. Khim. 42, 1023 (1969).
P. Dill, F. Pachel, C. Militzer, et al., J. Vac. Sci. Technol. A 39, 052406 (2021). https://doi.org/10.1116/6.0001193
L. Kavan, N. Tétreault, Th. Moehl, and M. Graetzel, J. Phys. Chem. C 118, 16408 (2014). https://doi.org/10.1021/jp030790+
X. Qi, Yu. Jiang, C. Detavernier, et al., J. Appl. Phys. 102, 083521 (2007). https://doi.org/10.1063/1.2798384
J. P. Niemela, G. Marin, and M. Karppinen, Semicond. Sci. Technol. 32, 093005 (2017). https://doi.org/10.1088/1361-6641/aa78ce
M. Diskus, O. Nilsen, and H. Fjellvå, J. Mater. Chem. 21, 705 (2011). https://doi.org/10.1039/C0JM01099E
T. L. Drake and P. C. Stair, J. Vac. Sci. Technol. A 34 (2016). https://doi.org/10.1116/1.4959532
T. Jurca, A. W. Peters, A. R. Mouat, et al., Dalton Trans. 46, 1172 (2017). https://doi.org/10.1039/C6DT03952A
M. F. J. Vos, M. Bacco, N. F. W. Thissen, et al., J. Vac. Sci. Technol. A 34, 01A103 (2016). https://doi.org/10.1116/1.4930161
J. N. Kvalvik, B. Jon, P.-A. Hansen, and O. Nilsen, J. Vac. Sci. Technol. A 38, 042406 (2020). https://doi.org/10.1116/6.0000219
M. Mattinen, P. J. King, L. Khriachtchev, et al., Mater. Today Chem. 9, 17 (2018). https://doi.org/10.1016/j.mtchem.2018.04.005
R. Aidan, A. R. Mouat, A. U. Mane, et al., Chem. Mater. 28, 1907 (2016). https://doi.org/10.1021/acs.chemmater.6b00248
C. E. Nanayakkara, A. Vega, G. Liu, et al., Chem. Mater. 28, 8591 (2016). https://doi.org/10.1021/acsami.1c06204
T. Fransen, O. Meer, P. Mars, et al., J. Phys. Chem. 80, 2103 (1976). https://doi.org/10.1021/j100560a010
L. Lietti, I. Nova, G. Ramis, et al., J. Catal. 187, 419 (1999). https://doi.org/10.1006/jcat.1999.2603
A. Marciel, M. Graca, A. Bastos, et al., Mater. 14, 821 (2021). https://doi.org/10.3390/ma14040821
U. K. Sen and S. Mitra, RSC Adv. 2, 11123 (2012). https://doi.org/10.1039/C2RA21373G
V. Guidi, G. Cardinali, L. Dori, et al., Sens. Actuators, B 49, 88 (1998). https://doi.org/10.1016/S0925-4005(98)00039-2
Sh. Shahram, V. O. Daniel, T. Fey, et al., Mater. Sci. Eng. C 58, 1064 (2016). https://doi.org/10.1016/j.msec.2015.09.069
V. Pershina and B. Fricke, Russ. J. Phys. Chem. 99, 144 (1995).
V. Pershina and B. Fricke, Russ. J. Phys. Chem. 100, 8748 (1996).
CRC Handbook of Chemistry and Physics, 102nd ed. (CRC, Taylor and Francis Group, 2021–2022).
J. W. Elam, M. D. Groner, and S. M. George, Rev. Sci. Instrum. 73, 2981 (2002). https://doi.org/10.1063/1.1490410
A. I. Kutchiev, Cand. Sci. (Chem.) Dissertation (St. Petersburg, 2006).
R. A. Wind and S. M. George, J. Phys. Chem. A 114, 1281 (2010). https://doi.org/10.1021/jp9049268
R. W. Wind, F. H. Fabreguette, Z. A. Sechrist, et al., J. Appl. Phys. 105, 074309 (2009). https://doi.org/10.1063/1.3103254
A. A. Malygin, A. N. Volkova, S. I. Kol’tsov, and V. B. Alekskovskii, Russ. J. Gen. Chem. 42, 2373 (1972).
A. A. Malygin, A. N. Volkova, S. I. Kol’tsov, and V. B. Alekskovskii, Russ. J. Gen. Chem. 43, 1436 (1973).
A. A. Malygin, Russ. J. Gen. Chem. 72, 575 (2002).
A. I. Efimov, L. P. Belokurova, I. V. Vasil’kova, and V. P. Chechev, Properties of Inorganic Compounds, The Handbook (Khimiya, Leningrad, 1983), p. 392 [in Russian].
A. J. M. Mackus, J. R. Schneider, C. MacIsaac, et al., Chem. Mater. 31, 1142 (2019). https://doi.org/10.1021/acs.chemmater.8b02878
S. M. George, Acc. Chem. Res. 53, 1151 (2020). https://doi.org/10.1021/cr900056b
Funding
This work was supported by the RF Ministry of Science and Higher Education as part of State Task no. FZNZ-2020-0002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Maksumova, A.M., Abdulagatov, I.M., Palchaev, D.K. et al. Studying the Atomic Layer Deposition of Molybdenum Oxide and Titanium–Molybdenum Oxide Films Using Quartz Crystal Microbalance. Russ. J. Phys. Chem. 96, 2206–2214 (2022). https://doi.org/10.1134/S0036024422100181
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422100181