Abstract
The problem of studying the relationship of infraslow electrical activity with neural networks has gained importance due to the new facts on the modulation mechanisms of these networks. The aim of this study was to explore the correlation between the brain direct current (DC) potential level and the resting-state neural connectivity in patients with chronic cerebral ischaemia (CCI). In 55 patients with CCI (mean age 66.3 ± 0.85 years), Millivolt scale brain DC potentials were recorded using non-polarizable electrodes were compared with resting state brain functional MRI (fMRI) connectivity. The bipolar DC potential difference recorded between the sagittal frontal and occipital areas was found to be associated with different connectivity of the brain networks. We examined the fMRI connectivity associated with the bipolar difference of the DC potential level between the frontal and occipital areas. The positive difference in the DC potential was associated with higher connectivity of cerebellum with the frontal and occipitotemporal regions. A small number of connections, corresponding to the negative difference in the DC potential level, were present between the cerebellum and the occipital cortex, mainly in the left hemisphere. The results suggest the that in patients with CCI, the infraslow electrical brain activity has a modulating effect on the neural networks and individual functional connections.
Similar content being viewed by others
INTRODUCTION
A significant number of studies have investigated the relationship between the BOLD (blood-oxygen-level-dependent) signals detected in fMRI and the electroencephalographic characteristics in traditional frequency ranges, but the link between the BOLD signal and infraslow electrical brain activity (<0.1–0.5 Hz) has only recently become a focus of research. The term “infraslow electrical activity” has multiple meanings. In this article, we will consider very slow electrical activity in the millivolt range. There are several equivalent terms used to describe this kind of slow activity in the Russian and English literature: DC potentials, shift of DC potentials, millivolt-scale of DC shifts, DC EEG shift, etc. In Russian, the terms very slow activity, DC potential level, etc. are often used. These terms will be primarily used in this article [1–4]. The origin of this infraslow electrical activity recorded from the scalp electrodes is not directly related to neuronal activity, although there are hypotheses about the possible contribution of cortical apical dendrites to the very slow potentials of the millivolt range [5]. A proven source of DC potential level are vascular potentials sensitive to the acid–base balance on both sides of the blood-brain barrier (BBB). The end products of cerebral energy metabolism mainly affect the acid–base balance [1–4, 6–8]. The intensity of the BOLD signal depends on this balance, since it affects haemoglobin’s affinity for oxygen (Bohr effect) [9]. It has also recently been shown that the DC potential level depends on the concentration of corpuscular haemoglobin in blood [10]. The synchronized dynamic of the BOLD signal between different brain regions is caused by the simultaneous release of oxygen, followed by its entry into neurons, which is accompanied by haemoglobin’s transition from the oxidized to the reduced form. These processes play a part in neural network modulation. Some authors suggest that variations in infraslow electrical activity reflect the contribution of neural network to these processes, since oxygen’s entry into neurons leads to the alterations of acid–base balance [8]. In addition, there are energy processes that are not synchronized with other regions, due to the brain oxygen intake, whose resting function is to maintain the basic activity of neurons.
Another factor affecting the DC potential level is blood flow velocity. Theoretical hypothesis about this phenomenon is based on the Helmholtz–Smoluchowski equation, which allows to calculate the zeta potential, as well as the facts about change in the electrical characteristics of vessels and capillaries which accompany vascular wall deformation during blood flow. These views on the origin of the DC potential level have been previously described in more detail [10].
The intensity of the energy expenditures is reflected in the infraslow electrical activity and affects the BOLD signal, which suggests that the involvement of resting-state brain networks may vary at different levels of the brain DC potentials [1–3]. This is observed in normal ageing and in various diseases, such as chronic cerebral ischaemia (CCI). CCI is a widespread and socially significant vascular disease, with dementia being a possible unfavourable outcome [11].
In 1979, N.A. Aladzhalova [12] suggested that the brain’s control system is modulated by the infraslow electrical brain activity. With the advent of fMRI technology, it has now become possible to test this hypothesis, namely, to assess the relationship between the brain DC potential level and the brain networks connectivity. The purpose of this study was to test this hypothesis. It is also important because there are both a widespread decrease in brain networks connectivity and the alteration in infraslow electrical brain activity in CCI. Identifying the link between these characteristics will contribute to timely detection of pathological decreases in connectivity, the role of which in various functions, including cognitive, is significant.
METHODS
The study involved 20 men and 35 women with CCI, from 50 to 85 years old, with a mean age of 66.3 ± 0.85 years. Cognitive impairment is a feature of this disease, and patients differed from each other mainly in the quantitative characteristics of their memory impairment, daily living activity, irritability, brainstem symptoms, etc. The main underlying causes of CCI in these patients were atherosclerosis, hypertension (including hypertensive disease) and venous insufficiency. Study inclusion criteria: vascular encephalopathy stage I and II (initial manifestation and subcompensation); patients who did not require constant assistance with everyday activities. Exclusion criteria: dementia equivalent to one or more points on the Clinical Dementia Rating Scale [13], a history of acute cerebrovascular injury or traumatic brain injury, severe cardiac or metabolic (type 2 diabetes mellitus) disease, renal failure or uncompensated thyroid disease. All patients were right-handed; the method for determining handedness has been previously described [14].
All subjects underwent T2*-weighted resting-state fMRI of the brain to obtain a BOLD signal, using the Magnetom Verio magnetic resonance tomograph (Siemens, Germany) with a 3.0 T magnetic field. The subjects were instructed to relax as much as possible, to lie quietly with their eyes closed (to exclude stimulation of the visual system) and not to think about anything in particular. MRI data pre-processing was performed using the SPM12 program in the MATLAB environment. The CONN-18b application in the SPM-12 toolbox was used to study connectivity [15].
Duplex ultrasound. We assessed the linear and volume velocity of systolic and diastolic blood flow in the right and left internal carotid arteries (ICA), middle cerebral and brachial arteries. Colour duplex ultrasound was performed on a Toshiba Viamo ultrasound machine. The type and value of systolic linear and volumetric blood flow velocity and the peripheral resistance index in the arteries were evaluated, using the standard method with a linear sensor with a frequency of 5.0–12.0 MHz.
Infraslow electrical brain activity (brain DC potential level) was recorded in patients with vascular encephalopathy on the 5-channel “Neuroenergocartograph” complex using non-polarizable silver chloride electrodes. The active electrodes were placed over the scalp according to the 10 × 20 scheme, while the reference electrode was placed on the right wrist. Electrode placement: along the sagittal inferior frontal (Fpz), central (Cz), and occipital (Oz) areas; as well as temporal areas (Td, Ts). Recording was carried out after measuring and eliminating electrodes and skin artefacts. The bipolar difference in the DC potential level between the sagittal frontal and occipital areas (dFO) was evaluated. All patients were divided into two groups with negative and positive value of this difference (groups 1 and 2).
Resting-state fMRI was used to study the neural connectivity in patients in a state of relaxed wakefulness. Connectivity was compared between the two patient groups, who differed in their infraslow electrical activity characteristics (bipolar difference in the DC potential level in the frontal and occipital areas, see above). The existing connectivity between two brain areas is numerically equal to the regression coefficient of the BOLD signals in these areas. The difference in connectivity between the group 1 and group 2 was estimated using a difference of standardized regression coefficient, adjusted for multiple comparisons (FDR) [15]. The diagram of connectivity analysis is shown in Fig. 1.
A diagram of the neural connectivity analysis at different DC potential levels in the frontal and occipital areas. FO—bipolar difference of the DC potential level between the frontal and occipital areas. 1, Conn, FO < 0—connectivity of brain networks in the group1 with CCI (with negative bipolar difference in the DC potential between the frontal and occipital areas). 2, Conn, FO > 0—the same for group 2 (with positive bipolar difference in the DC potential between the frontal and occipital areas). 3, Conn, dFO—the set of connectivity differences (group 1 minus group 2). The set of connectivity differences (3) includes connectivity with a plus or minus sign. When subtracting, the resulting differences have a plus or minus sign: a plus sign when the connectivity values in group 1 are significantly (pFDR < 0.05) higher than the same connectivity in group 2: Fig. 1, 3a (+Conn, dFO > 0), and a minus sign when the connectivity values in group 1 are significantly (pFDR < 0.05) lower than the same connectivity in group 2: Fig. 1, 3b (–Conn, dFO < 0). If the organization of these positive and negative connectivity in brain networks in group 1 and group 2 is significantly different (pFDR < 0.05), this means that the brain DC potentials are directly or indirectly related to connectivity modulation. The characteristics of positive and negative difference of connectivity in the groups will be analysed separately in the Results section.
The analysis of variance was also performed using the Statistica-12 package. We calculated the means and their errors, the Pearson and Spearman correlation coefficients, the partial correlation coefficients, as well as the normality of distribution using the Shapiro–Wilk test.
RESULTS
The patterns of functional links, corresponding to the connectivity differences (pFDR < 0.05) in the group 1 of CCI patients (with the difference of brain DC potentials between frontal and occipital areas dFO<0) and the group 2 of the CCI patients (with the difference of brain DC potentials between frontal and occipital areas dFO > 0) are presented in the Fig. 2.
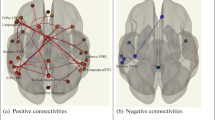
The patterns of functional links, corresponding to connectivity difference in the CCI patients’ groups with different bipolar DC potential level between frontal and occipital areas. Legend: L and R—left and right hemisphere. (a) 1. Salience network of the rostral prefrontal cortex: 1, 2, 3—Superior frontal gyrus L, R. 4—Orbitofrontal cortex R. 5—Pars triangularis of the inferior frontal gyrus R. 6—Articulation network of the inferior frontal gyrus R. 7—Temporal pole R. 8—Pars opercularis of the inferior frontal gyrus R. 9—Posterior part of the inferior temporal gyrus R. 10—Language network of the superior temporal gyrus R. 11—Occipitotemporal part of the inferior temporal gyrus L. 12. Occipitotemporal part of the middle cerebral gyrus L. 13—Cerebellum (1) L. 14—Vermis (7). 1—Brain stem. 2—Vermis (10). 3—Cerebellum (9) R. 4—Cerebellum (1) L. 5—Posterior cerebellar network. 6—Medial visual network. 7—Cuneate cortex R. The other symbols are the same as in Fig. 2.
The numbers in parentheses after the words Cerebellum and Vermis correspond to the specific segments of these structures.
Figure 2 shows that the patterns of functional links associated with bipolar DC potential level is different, which implies a relationship between infraslow electrical activity and the connectivity of brain networks.
The mean sagittal differences in the DC potential level in the two groups were –9.4 ± 1.1 and 8.0 ± 1.3 mV. At the same time, the sagittal difference in the DC potential level between the midline frontal and occipital leads was statistically significantly related to the ICA blood flow characteristics and age. The Table 1 below shows the one-way ANOVA analysis characteristics, which indicate that the two patient groups with a different sign of the sagittal difference of the DC potential level, show differences in the blood flow volumetric and linear velocity in the ICA, as well as a difference in age.
The red colour shows the statistically significant differences in the parameters between the two groups. dFO—sagittal difference of the DC potential level; Is—mean systolic blood flow rate in the right and left ICA; VVIr—volumetric blood flow rate in the right ICA. N—number of subjects, F—Fisher’s exact test; p—level of significance of the difference between the two groups.
To avoid the influence of blood flow velocity and age on the connectivity patterns, two experimental patient groups were selected with 13 subjects in each group. The patients included in these groups had a different sign of the sagittal difference in the DC potential level, and the mean difference in the DC potential level in both groups were statistically significantly different (–10.9 ± 1.8 and 8.7 ± 1.6 mV, p < 0.00005), while the differences in age and blood flow characteristics were not significant (p > 0.05).
Since difference in the DC potential level between these two groups was statistically significant, while the differences in mean age and blood flow were not significant, we could assume that the difference in infraslow electrical activity and the connectivity parameters in these groups are interrelated (Figs. 3a, 3b). The pattern of functional links, corresponding to the positive values of connectivity differences, linked various regions of the cerebellum and occipital cortex to the frontal areas Fig. 3a. A small number of connections, corresponding to the negative difference in the DC potential level, were present between the cerebellum and the occipital cortex, mainly in the left hemisphere. Furthermore, the left temporal areas were connected to the dorsal attention network in the right hemisphere, Fig. 3b.
The patterns of functional links, corresponding to connectivity difference in the CCI patients’ groups with different bipolar DC potential level between frontal and occipital areas, but with similar age and blood flow characteristics. (a) Connectivity pattern corresponding to the positive values of the connectivity difference; (b) the same, but with negative values of the connectivity difference. All connectivities shown in Fig. (a) and (b) were significantly different from zero with pFDR < 0.05.Legend, (a) 1, 2—Frontal pole L, R. 3, 4—Frontoparietal network in the lateral prefrontal cortex L, R. 5, 6—Lower frontal gyrus L, R. 7, 8—Language network in the lower frontal gyrus L, R. 9—Lower frontal gyrus L. 10—Temporal pole, L. 11—Middle frontal gyrus Ll. 12, 15—Superior frontal gyrus L, R. 13, 14—Salience network in the upper cingulate cortex L, R. 16—Frontal orbital cortex R. 17—Middle frontal gyrus R. 18—Inferior frontal gyrus, pars opercularis R. 19—Temporal fusiform cortex, anterior part L. 20—Posterior middle temporal gyrus R; 21—Occipitotemporal fusiform cortex L. 22—Cerebellar vermis (4–5, 9, 10). 23—Posterior parietal cortex R. 24—Angular gyrus L. 25—Frontoparietal network in the posterior parietal cortex L. 26, 27—Cerebellum (6), R, L. 28, 29—Anterior cerebellar network L, R; 30, 31—Cerebellum (1, 2), R. 32—Cerebellum (2), L. 33—Vermis (7); 34—Posterior cerebellar network. Legend, (b) 1—Anterior middle temporal gyrus, L. 2—Anterior part of the inferior temporal gyrus, L; 3—Dorsal attention network. Frontal ocular field, R. 4—Cerebellum (9), L. 5—Occipitotemporal fusiform cortex L. 6—Cerebellum (6), L. 7—Cerebellum (2), L; 8—Lateral visual network. The other symbols are the same as in Fig. 2.
The Fig. 3 shows that the targets and sources of connectivity are found predominantly at two levels: the frontal regions and at the level of the cerebellum and the occipital lobe. This distribution corresponds approximately to the projections of the DC potentials recording electrodes (in the frontal and occipital areas), Fig. 3a. The predominant connectivity pattern indicates the synchronization of areas located on the anterior and posterior regions of the brain. If the sign of the difference was negative (Fig. 3b), this significantly reduced the synchronization between the frontal areas and the cerebellum and occipital lobe. It was thus shown that the sign of the sagittal difference in the DC potential level is associated with fMRI connectivity. If the DC potential level is higher in the occipital area compared to the frontal, the connectivity values are higher, mainly between the cerebellum and the frontal, frontotemporal and frontopariеtal areas of the brain. If the difference between the DC potential level in the occipital and frontal areas is the reverse, a significant decrease in connectivity between these areas is observed.
DISCUSSION
This study revealed that a change in the sagittal difference of the DC potential level is associated with different resting-state brain networks connectivity. The concept of vascular and neural network is helpful to explain the association between the brain DC potentials and neural network connectivity, as the BBB structures, along with glial, nerve and other cells, form a single, interdependent, structural and functional organization that makes up the neurovascular network [16].
This study has shown that DC potentials related neural networks connect cerebellum with cerebral hemispheres. It was previously demonstrated that the afferent and efferent cerebellar pathways are connected with different parts of cortex and subcortex. PET studies showed that in cerebellar stroke, hypometabolic foci occur in the frontal, temporal, parietal and occipital regions of the cortex, as well as in subcortical regions [17]. The main finding of this study is that the millivolt range brain DC potentials (DC potential level) are associated with synchronization of BOLD signals in brain networks. Two aspects of this association can be considered. The first is the relationship of the DC potential level with the BOLD signal, which can be explained by the link between the DC potential level and the acid−base balance on both sides of the BBB. This, in turn, affects haemoglobin’s ability to release oxygen, which is necessary for normal neuronal function [9]. Therefore, the causal relationship between the DC potential level and the BOLD signal seems understandable. On the other hand, it is not fully understood why a particular gradient direction of the DC potential level may influence the synchronization of the BOLD signals between two regions? This may be due to asymmetry in the afferent and efferent cerebellar pathways entering various neural networks. Activation of these networks may create a fronto−occipital DC potential gradient. A similar interpretation was suggested in the recent TMS studies, with electrodes located over the forehead and occipital region. It turned out that changing the direction of direct current changed the neural activation in the motor cortex and cerebellum. Moreover, there are two unequal cerebellar networks, activated by different current directions [18]. In addition, others studies on transcranial direct current stimulation have reported that this procedure changes the connectivity and synchronized activity of various brain regions [19]. Can the gradient created by the DC potential level be considered as a kind of analogue of transcranial direct current stimulation? This question requires further investigations.
Therefore, the ideas of N.A. Aladzhalova [12] that we had mentioned in the Introduction may be, to some extent, relevant today. Although a more common point of view is that the DC potential level is a biomarker of various conditions affecting the connectivity of different brain regions, than as a regulatory factor.
The obtained results can be useful in research and clinical practice to assess brain networks connectivity using electrophysiological registration of the DC potential level. Numerous results of correlation between infraslow electrical brain activity and cognitive function, metabolic characteristics and various blood flow parameters have been gathered to date [1]. The link between infraslow electrical brain activity and neural networks connectivity makes it possible to explain these phenomena in a new way.
CONCLUSION
A mechanism modulating cerebral connectivity associated with infraslow electrical brain activity was described in this study, which explains the correlation between infraslow electrical brain activity and the organization of various resting-state networks. An association was found between the fronto-occipital gradient of the brain DC potential and several neural networks. This correlation is probably related to a link between brain DC potentials and the brain’s acid-base balance, which, in turn, can affect the supply of oxygen to the brain. The practical aspect of this work is quite interesting, it makes it possible the electrophysiological evaluation of the state of neural networks. We found that the bipolar difference in the DC potential level between the frontal and occipital areas is associated with significant differences in the patterns of cerebral connectivity. Further studies may provide opportunities of real-time neural network monitoring based on infraslow electrical brain activity.
REFERENCES
Fokin, V.F. and Ponomareva, N.V., Energeticheskaya fiziologiya mozga (Energetic Physiology of the Brain), Moscow: Antidor, 2003.
Nita, D.A., Vanhatalo, S., Lafortune, F.-D., Voipio, J., Kaila, K., and Amzica, F., Nonneuronal origin of CO2-related DC EEG shifts: an in vivo study in the cat, J. Neurophysiol., 2004, vol. 92, pp. 1011–1022.
Voipio, J., Tallgren, P., Heinonen, E., Vanhatalo, S., and Kaila, K., Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator, J. Neurophysiol., 2003, vol. 89, pp. 2208–2214.
Grooms, J.K., Thompson, G.J., Pan, W.J., Billings, J., Schumacher, E.H., Epstein, C.M., and Keilholz, S.D., Infraslow EEG and dynamic resting state network activity, Brain Connect., 2017, vol. 7, no. 5, pp. 265–280. https://doi.org/10.1089/brain.2017.0492
He, B.J. and Raichle, M.E., The fMRI signal, slow cortical potential and consciousness, Trends Cognit. Sci., 2009, vol. 13, no. 7, pp. 302–309. https://doi.org/10.1016/j.tics.2009.04.004
Palva, J.M. and Palva, S., Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series, NeuroImage, 2012, vol. 62, pp. 2201–2211. https://doi.org/10.1016/j.neuroimage.2012.02.060
Hiltunen, T., Kantola, J., Abou Elseoud, A., Lepola, P., Suominen, K., Starck, T., et al., Infra-slow EEG fluctuations are correlated with resting-state network dynamics in fMRI, J. Neurosci., 2014, vol. 34, pp. 356–362. https://doi.org/10.1523/JNEUROSCI.0276-13.2014
Li, Q., Liu, G., Yuan, G., Wang, G., Wu, Z., and Zhao, X., DC shifts-fMRI: a supplement to event-related fMRI, Front. Comput. Neurosci., 2019, vol. 13, p. 37. https://doi.org/10.3389/fncom.2019.00037
Pittman, R.N., Regulation of Tissue Oxygenation, Ch. 4: Oxygen Transport, San Rafael, CA: Morgan Claypool Life Sci., 2011. https://www.ncbi.nlm.nih.gov/books/NBK54103/
Fokin, V.F., Ponomareva, N.V., Medvedev, R.B., Tanashyan, M.M., and Shabalina, A.A., The effect of the cerebral gas transport system on slow-wave brain activity in patients with vascular encephalopathy, Ann. Klin. Eksp. Nevrol., 2017, vol. 11, no. 4, pp. 29–35.
Suslina, Z.A., Illarioshkin, S.N., and Piradov, M.A., Neurology and neuroscience: development prognosis, Ann. Klin. Eksp. Nevrol., 2007, vol. 1, no. 1, pp. 5–9.
Aladzhalova, N.A., Psikhofiziologicheskie aspekty sverkhmedlennoi ritmicheskoi aktivnosti golovnogo mozga (Psychophysiological Aspects of Very Slow-Wave Rhythmic Activity of the Brain), Moscow: Nauka, 1979.
Dooneief, G., Marder, K., Tang, M.-X., and Stern, Y., The Clinical Dementia Rating scale: community-based validation of “profound” and “terminal” stages, Neurology, 1996, vol. 46, no. 6, pp. 1746–1749. https://doi.org/10.1212/WNL.46.6.1746
Fokin, V.F. and Ponomareva, N.V., Technology for the study of cerebral asymmetry, in Nevrologiya XXI veka: diagnosticheskie, lechebnye i issledovatel’skie tekhnologii. Rukovodstvo dlya vrachei. Tom 3. Sovremennye issledovatel’skie tkehnoloii v eksperimental’noi nevrologii (Neurology of the 21st Century: Diagnostic, Treatment, and Research Technologies: Guide for Physicians, Vol. 3: Modern Research Technologies in Neurology), Moscow: ATMO, 2015, pp. 350–375.
Whitfield-Gabrieli, S. and Nieto-Castanon, A., Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks, Brain Connect., 2012, vol. 2, no. 3, pp. 125–141. https://doi.org/10.1089/brain.2012.0073
Zhang, J., Badaut, J., Tang, J., et al., The vascular neural network—a new paradigm in stroke pathophysiology, Nat. Rev. Neurol., 2012, vol. 8, pp. 711–716. https://doi.org/10.1038/nrneurol.2012.210
Shmyrev, V.I., Rudas, M.S., and Pereverzev, I.V., Metabolic disorders in cerebellar stroke (correlation with positron emission tomography data), Klin. Byull., 2009, no. 3, pp. 7–10.
Spampinato, D.A., Celnik, P.A., and Rothwell, J.C., Cerebellar-motor cortex connectivity: One or two different networks? J. Neurosci., 2020, vol. 40, no. 21, pp. 4230–4239. https://doi.org/10.1523/JNEUROSCI.2397-19.2020
Liu, A., Vöröslakos, M., Kronberg, G., Henin, S., Krause, M.R., Huang, Y., Opitz, A., Mehta, A., Pack, C.C., Krekelberg, B., Berényi, A., Parra, L.C., Melloni, L., Devinsky, O., and Buzsáki, G., Immediate neurophysiological effects of transcranial electrical stimulation, Nat. Commun., 2018, vol. 9, no. 1, p. 5092. PMCID: PMC6269428.https://doi.org/10.1038/s41467-018-07233-730504921
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ponomareva, N.V., Fokin, V.F., Medvedev, R.B. et al. Correlation between Infraslow-Electrical Brain Activity and Resting-State Functional MRI Connectivity in Patients with Chronic Cerebral Ischaemia. Hum Physiol 47, 847–853 (2021). https://doi.org/10.1134/S0362119721080107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119721080107