Abstract
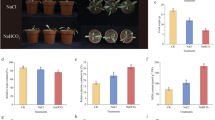
Plant exposure to stress results in the decomposition of their cell membrane phospholipids, and therefore it can elevate the level of EA (ethanolamine) in the cell, and this elevated level of EA induces an alarm response that activates cellular resistance and tolerance mechanisms. In the present study, in vitro cultured tobacco plants (Nicotiana rustica L.) were pretreated with ethanolamine (EA) before salt treatment. After 3 weeks of salt treatment (200 mM NaCl), the plants pretreated with exogenous EA showed the elevated levels of SOD, CAT and APX activity compared with unpretreated plants. Furthermore, total antioxidant capacity, fresh and dry weight and the content of photosynthetic pigments were also increased. In contrast, H2O2 content decreased under similar conditions. According to the results of this study, it can be suggested that EA pretreatment increased salt tolerance of tobacco plants at least partly by stimulation of antioxidative responses.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- EA:
-
ethanolamine
- FRAP:
-
ferric reducing ability of plasma
- NBT:
-
nitro blue tetrazolium
- SOD:
-
superoxide dismutase
References
Epstein, E., Norlyn, J., Rush, D., Kings, R., and Kelly, D., Saline culture of crops: a genetic approach, Science, 1980, vol. 210, pp. 399–404.
Ren, S., Weeda, S., Li, H., Whitehead, B., Guo, Y., Atalay, A., and Parry, J., Salt tolerance in soybean WF-7 is partially regulated by ABA and ROS signaling and involves withholding toxic Clions from aerial tissues, Plant Cell Rep., 2012, vol. 31, pp. 1527–1533.
Asada, K., The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1999, vol. 50, pp. 601–639.
Gogorcena, Y., Iturbe-Ormaetexe, I., Escuredo, P.R., and Becana, M., Antioxidant defenses against activated oxygen in pea nodules subjected to water stress, J. Plant Physiol., 1995, vol. 108, pp. 753–758.
Bergmann, H., Lippmann, B., Leinhos, V., Tiroke, S., and Machelett, B., Activation of stress resistance in plants and consequence for product quality, J. Appl. Bot., 1999, vol. 73, pp. 153–161.
Bowler, C., van Montagu, M., and Inze, D., Superoxide dismutase and stress tolerance, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1992, vol. 43, pp. 83–116.
Mascher, R., Lippmann, B., Holzinger, S., and Bergmann, H., Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants, Plant Sci., 2002, vol. 163, pp. 961–969.
Fridovich, I., Superoxide radical: an endogenous toxicant, Annu. Rev. Pharmacol. Toxicol., 1983, vol. 23, pp. 239–257.
Mascher, R., Nagy, E., Lippmann, B., Hornelein, S., Fischer, S., Scheiding, W., Neagoe, A., and Bergmann, H., Improvement of tolerance to paraquat and drought in barley (Hordeum vulgare L.) by exogenous 2-aminoethanol: effects on superoxide dismutase activity and chloroplast ultra structure, Plant Sci., 2005, vol. 168, pp. 691–698.
Bergmann, H., Machelett, B., and Leinhos, V., Effect of natural amino alcohols on the yield of essential amino acids and the amino acid pattern in stressed barley, Amino Acids, 1994, vol. 7, pp. 327–331.
Leinhos, V. and Bergmann, H., Effect of amino alcohol application, rhizobacteria and mycorrhiza inoculation on the growth, the content of protein and phenolics and protein pattern of drought stressed lettuce (Lactuca sativa L. ‘Amerikanischer Brauner’), J. Appl. Bot., 1995, vol. 69, pp. 153–156.
Bergmann, H., Rost, S., and Machelett, B., Improvement of drought tolerance and changes of glycine betaine or proline accumulation in Hordeum vulgare L., by choline and 2-aminoethanol treatments, J. Appl. Bot., 2002, vol. 76, pp. 87–95.
Eckert, H., Reissmann, P., and Bergmann, H., Metabolism of [14C]-monoethanolamine in Hordeum vulgare, Biochem. Physiol. Pflanzen., 1988, vol. 183, pp. 15–25.
Lippmann, B., Leinhos, V., and Bergmann, H., Influence of auxin producing rhizobacteria on root morphology and nutrient accumulation of crops. I. Changes in root morphology and nutrient accumulation in maize (Zea mays L.) caused by inoculation with indole-3-acetic acid (IAA) producing Pseudomonas and Acinetobacter strains or IAA applied exogenously, J. Appl. Bot., 1995, vol. 69, pp. 31–36.
Rajaeian, S., Heidari, R., and Ehsanpour, A., Effect of 2-aminoethanol pretreatment on the antioxidant enzyme activity in Zea mays under oxidative stress, Russ. J. Plant Physiol., 2011, vol. 58, pp. 45–50.
Murashige, T. and Skoog, F., A revised medium for rapid growth and bioassays with tobacco cultures, Physiol. Plant., 1962, vol. 159, pp. 473–479.
Lichtenthaler, H. and Wellburn, A., Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents, Biochem. Soc. Trans., 1983, vol. 11, pp. 591–592.
Benzie, F. and Strain, J., The ferric reducing ability of plasma (FARP) as a measure of antioxidant power: the FARP assay, Anal. Biochem., 1996, vol. 239, pp. 70–76.
Szöllösi, R. and Varga, I.Sz., Total antioxidant power in some species of Labiatae (Adaptation of FRAP method), Acta Biol. Szeged, 2002, vol. 46, pp. 124–127.
Loreto, F. and Velikova, V., Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes, Plant Physiol., 2001, vol. 127, pp. 1781–1787.
Nakano, Y. and Asada, A., Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Beauchamp, C. and Fridovich, I., Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, vol. 44, pp. 276–287.
Weretilnyk, E., Bednarek, S., McCue, K., Rhodes, D., and Hanson, A., Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons, Planta, 1989, vol. 178, pp. 342–352.
Leinhos, V., Tiroke, S., and Bergmann, H., Influence of osmotic stress and aminoalcohol treatment on protein content, protein patterns and growth of germinating barley, Angew. Bot., 1996, vol. 70, pp. 199–204.
Mascher, R., Fischer, S., Scheiding, W., Neagoe, A., and Bergmann, H., Exogenous 2-aminoethanol can diminish paraquat induced oxidative stress in barley (Hordeum vulgare L.), Plant Growth Regul., 2005, vol. 45, pp. 103–112.
Asada, K., Ascorbate peroxidase — a hydrogen peroxide-scavenging enzyme in plants, Physiol. Plant., 1992, vol. 85, pp. 235–241.
Willekens, H., Inze, D., van Montagu, M., and van Camp, W., Catalase in plants, Mol. Breed., 1995, vol. 1, pp. 207–228.
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Montagu, M., and Inze, D., Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants, EMBO J., 1997, vol. 16, pp. 4806–4816.
Hernández, J.A., Jiménez, A., Mullineaux, P., and Sevilla, F., Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defences, Plant Cell Environ., 2000, vol. 23, pp. 853–862.
Kogan, M., Kristoff, G., Benavides, M., and Tomaro, M., Effect of pretreatment with ethanolamine on the response of Helianthus annuus L. to salt stress, Plant Growth Regul., 2000, vol. 38, pp. 87–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
This text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Rajaeian, S.O., Ehsanpour, A.A. Physiological responses of tobacco plants (Nicotiana rustica) pretreated with ethanolamine to salt stress. Russ J Plant Physiol 62, 246–252 (2015). https://doi.org/10.1134/S1021443715020156
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443715020156