Abstract
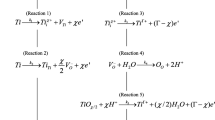
The effect of oxygen vacancies in the anodic oxide film on passive titanium on the kinetics of the oxygen electrode reaction has been studied by potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). Oxide films of different donor density were prepared galvanostatically at various current densities until a potential of 20.0 VSHE was achieved. The semiconductive properties of the oxide films were characterized using EIS and Mott-Schottky analysis, and the thickness was measured using ellipsometry. The film thickness was found to be almost constant at ∼44.7 ± 2.0 nm, but Mott-Schottky analysis of the measured high frequency interracial capacitance showed that the donor (oxygen vacancy) density in the n-type passive film decreased sharply with increasing oxide film formation rate (current density). Passive titanium surfaces covering a wide range of donor density were used as substrates for ascertaining relationships between the rates of oxygen reduction/evolution and the donor density. These studies show that the rates of both reactions are higher for passive films having higher donor densities. Possible explanations include enhancement of the conductivity of the film due to the vacancies facilitating charge transfer and the surface oxygen vacancies acting as catalytic sites for the reactions. The possible involvement of surface oxygen vacancies in the oxygen electrode reaction was explored by determining the kinetic order of the OER with respect to the donor concentration. The kinetic orders were found to be greater than zero, indicating that oxygen vacancies are involved as electrocatalytic reaction centers in both the oxygen evolution and reduction reactions.
Similar content being viewed by others
References
Henrich, V.E. and Cox, P.A., The Surface Science of Metal Oxides, Cambridge: Cambridge University, 1994.
Beck, T.J., Klust, A., Batzill, M., Diebold, U., Valentin, C.D., and Selloni, A., Phys. Rev. Lett., 2004, vol. 93, p. 36 104.
Schaub, R., Thostrup, P., Lopez, N., Laegsgaard, E., Stensgaard, I., Norskov, J.K., and Besenbacher, F., Phys. Rev. Lett., 2001, vol. 87, p. 266 104.
Luczak, F.J. and Landsman, D.A., US Patent, no. 4 677 092, 1987.
Ross, P.N. and Markovic, N., Hydrogen Fuel Cell and Infrastruct. Tech., Fiscal Year 2002 Prog. Rep., p. 429.
Macdonald, D.D., Technical Progress Report for The Fundamental Role of Nanoscale Oxide Films in the Oxidation of Hydrogen and the Reduction of Oxygen on Noble Metal Electrocatalysts, grant no. DE-FG02-01ER15238, 2004.
Gurney, R.W. and Condon, E.U., Phys. Rev. Lett., 1929, vol. 33, p. 127.
Gurney, R.W., Proc. Roy. Soc., 1931, vol. A134, p. 137.
Schultze, J.W. and Vetter, K.J., Electrochim. Acta, 1973, vol. 18, p. 889.
Vetter, K.J. and Schultze, J.W., Ber. Bunsen-Ges. Phys. Chem., 1973, vol. 77, p. 945.
Henrich, V.E., Prog. Surf. Sci., 1995, vol. 50, p. 77.
Henrich, V.E. and Cox, P.A., Appl. Surf. Sci., 1993, vol. 72, p. 277.
Lu, G., Linsebigler, A., and Yates, J.T., J. Phys. Chem., 1994, p. 11733.
Eriksen, S., Naylor, P.D., and Egdell, R.G., Spectrochim. Acta, Part A, 1987, vol. 43, p. 1535.
Gopel, W., Prog. Surf. Sci., 1985, vol. 20, p. 9.
Smith, J.R., Walsh, F.C., and Clarke, R.L., J. Appl. Electrochem., 1998, vol. 28, p. 1021.
Ellerbrock, D., Defect Characterization of Titanium Passive Films, Ph.D. Dissertation, Pennsylvania State Univ., University Park, PA, 1998.
Kofstad, P., Nonstoichiometry, Diffusion, and Electrical Conductivity in Binary Metal Oxides, New York: Wiley Interscience, 1972.
Balachandran, U. and Eror, N.G., J. Mater. Sci., 1988, vol. 23, p. 2676.
Millot, F., Blanchin, M.-G., Tetot, R., Marucco, J.-F., Poumellec, B., Picard, C., and Touzelin, B., Prog. Solid State Chem., 1987, vol. 17, p. 263.
Macdonald, D.D., J. Electrochem. Soc., 1992, vol. 139, p. 3434.
Macdonald, D.D., Pure Appl. Chem., 1999, vol. 71, p. 951.
James, W.J. and Straumanis, M.E., in Encyclopedia of Electrochemistry of the Elements, New York: Marcel Dckker, 1976.
Morrison, S.R., in Electrochemistry at Semiconductor and Oxidized Metal Electrodes, 1980.
Houlihan, J.F. and Mulay, L.N., Phys. Status Solidi B, 1974, vol. 61, p. 647.
Leitner, K., Schultze, J.W., and Slimming, U., J. Electrochem. Soc., 1986, vol. 133, p. 1561.
Marsh, J. and Gorse, D., Electrochim. Acta, 1998, vol. 43, p. 659.
Kudelka, S., Michaelis, A., and Schultze, J.W., Ber. Bunsen-Ges. Phys. Chem., 1995, vol. 99, p. 1020.
Sikora, J., Sikora, E., and Macdonald, D.D., Electrochim. Acta, 2000, vol. 45, p. 1875.
Torresi, R.M., Camara, O.R., Pauli, C.P.D., and Giordano, M.C., Electrochim. Acta, 1987, vol. 32, p. 1291.
Blackwookand, D.J. and Peter, L.M., Electrochim. Acta, 1989, vol. 34, p. 1505.
Ohtsuka, T. and Otsuki, T., Corros. Sci., 1998, vol. 40, p. 951.
Moffat, T.P., Yang, H., Fan, F.-R.F., and Bard, A.J., J. Electrochem. Soc., 1992, vol. 139, p. 3158.
Torresi, R.M., Camara, O.R., and Pauli, C.P.D., Electrochim. Acta, 1987, vol. 32, p. 1357.
Baez, V.B., Graves, J.E., and Pletcher, D., J. Electroanal. Chem., 1992, vol. 340, p. 273.
Ai, J., Chen, Y., Urquidi-Macdonald, M., and Macdonald, D.D., Private communication.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was submitted in honor of the many contributions to electrochemistry that have been made by Professor Boris Grafov.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Roh, B., Macdonald, D.D. Effect of oxygen vacancies in anodic titanium oxide films on the kinetics of the oxygen electrode reaction. Russ J Electrochem 43, 125–135 (2007). https://doi.org/10.1134/S1023193507020012
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1023193507020012