Abstract
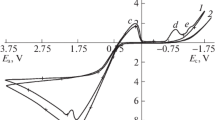
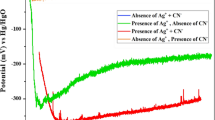
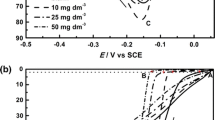
Regularities of silver dissolution in acid thiocarbamide electrolytes are studied. The kinetics of the process is shown to be severely affected by the admixture of hydrogen sulfide molecules that form upon inserting sodium sulfide or accumulate in electrolyte with the passage of time elapsed since its preparation. Catalytic effect increases with increasing length of time of the electrode’s contact with solution prior to the beginning of experiment or following an increase in the concentration of sulfide ions. Experiments with the surface renewed in the course of potential scans show that the catalytic effect is connected with the adsorption of sulfide ions on an interface. At large values of the surface coverage with sulfide ions, the dissolution rate increases so much that the dissolution process starts to be limited largely by the process of supply of thiocarbamide molecules toward the electrode surface.
Similar content being viewed by others
References
Kudryavtsev, N.T., Elektroliticheskie pokrytiya metallami (Electroplating with Metals), Moscow: Khimiya, 1979.
Lacconi, G.T. and Macagno, V.A., Electrochim. Acta, 1994, vol. 39, p. 2607.
Nikolaeva, V.A., Kharin, A.N., and Plugotorenko, F.N., in Issledovaniya po elektroosazhdeniyu i rastvoreniyu metallov (Studies on Electrodeposition and Dissolution of Metals), Frumkin, A.N. and Kabanov, B.N., Eds., Moscow: Nauka, 1971, p. 140.
Lodeishchikov, V.V., Shamis, L.A., Kakovskii, I.A., and Khmel’nitskaya, O.D., Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., 1975, vol. 18, p. 77.
Lodeishikov, V.V., Panchenko, A.F., and Khmel’nitskaya, O.D., Gidrometallurgiya zolota (Hydrometallurgy of Gold), Moscow: Nauka, 1980, p. 26.
Mineev, G.G. and Panchenko, A.F., Rastvoriteli zolota i serebra v gidrometallurgii (Solvents of Gold and Silver for Hydrometallurg), Moscow: Metallurgiya, 1994.
Bruckard, W.J., Sparrow, G.J., and Woodcock, J.T., Hydrometallurgy, 1993, vol. 33, p. 17.
Balaz, P., Ficeriova, J., and Leon, C.V., Hydrometallurgy, 2003, vol. 70, p. 113.
Bek, R.Yu. and Shuraeva, L.I., Elektrokhimiya, 1997, vol. 33, p. 636.
Gao X., Zhang Yun, and Weaver, M.J., Langmuir, 1992, vol. 8, p. 668.
Bek, R.Yu., Shevtsova, O.N., and Shuraeva, L.I., Elektrokhimiya, 2005, vol. 41, p. 1363.
Bek, R.Yu. and Lavrova, T.A., Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1971, no. 4, issue 2, p. 102.
Zelinskii, A.G and Bek, R.Yu., Elektrokhimiya, 1985, vol. 21, p. 66.
Kenzin, V.I., Novitskii, S.P., Bek, R.Yu., and Polumordvinov, I.S., Zavod. Lab., 1993, no. 8, p. 12.
Aleksandrova, T.P., Ovchinnikova, S.N., Vais, A.A., and Bek, R.Yu., Zh. Anal. Khim., 1999, vol. 54, p. 732.
Berthon, G. and Luca, C., Bull. Soc. Chim. Fr., 1969, p. 432.
Pawelka, F.G., Z. Electrochem., 1924, vol. 30, p. 180.
Bek, R.Yu. and Shuraeva, L.I., Elektrokhimiya, 2006, vol. 42, p. 340.
Vetter, K.J., Elektrochemische Kinetik, Berlin: Springer, 1961.
Bek, R.Yu., Elektrokhimiya, 2002, vol. 38, p. 1366.
Gubailovskii, V.V., Tsvetn. Metall., 1972, vol. 45, no. 5, p. 36.
Li, J. and Miller, J.D., Hydrometallurgy, 2002, vol. 63, p. 215.
Gornostaeva, T.D., Khmel’nitskaya, O.D., Panchenko, A.F., and Lodeishchikov, V.V., Zh. Neorg. Khim., 1986, vol. 31, p. 115.
Pilipenko, A.T. and Lisetskaya, G.S., Ukr. Khim. Zh., 1953, vol. 19, p. 81.
Fyfe, W.S., J. Chem. Soc., 1955, p. 1032.
Nazarova, L.V. and Prizhilevskaya, V.I., Zh. Neorg. Khim., 1967, vol. 12, p. 3051.
Shul’man, V.M. and Savel’eva, Z.A., Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim., 1970, no. 4, issue 9, p. 124.
Shevtsova, O.N., Bek, R.Yu., Zelinskii, A.G., and Vais, A.A., Elektrokhimiya, 2006, vol.42, p. 279.
Bek, R.Yu., Shevtsova, O.N., and Shuraeva, L.I., Elektrokhimiya, 2006, vol. 42, p. 751.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © R.Yu. Beck, L.I. Shuraeva, S.N. Ovchinnikova, A.A. Vais, 2007, published in Elektrokhimiya, 2007, Vol. 43, No. 3, pp. 305–312.
Rights and permissions
About this article
Cite this article
Beck, R.Y., Shuraeva, L.I., Ovchinnikova, S.N. et al. Silver dissolution in acid thiocarbamide solutions containing sulfide ions. Russ J Electrochem 43, 288–295 (2007). https://doi.org/10.1134/S102319350703007X
Received:
Issue Date:
DOI: https://doi.org/10.1134/S102319350703007X