Abstract
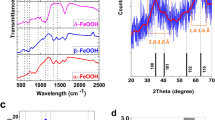
The catalytic activity of a colloidal catalyst based on iron(III) oxides in decomposition of H2O2 is studied. The catalyst is obtained by hydrolysis followed by peptization of FeCl3 · 6H2O salt in water in the presence of 1% ethanol. The structure, composition, and size of colloidal particles of the catalyst are studied by the methods of Mössbauer spectroscopy, X-ray fluorescence, X-ray diffraction, and transmission electron microscopy. The obtained catalyst is based on α-Fe2O3 crystals with an admixture of other crystalline structures of iron oxides and carbon-containing compounds. The activity of the catalyst with respect to H2O2 decomposition undergoes nonlinear and nonmonotonic variations and its particle size enlarges beginning from 1 to 3 nm with increasing initial concentration of FeCl3 · 6H2O. The catalyst obtained under optimal conditions exhibits high activity corresponding to the most efficient agents of H2O2 decomposition.
Similar content being viewed by others
References
Dondur, V., Radic, N., Grbic, B., and Drofenic, M., Mater. Sci. Forum, 2006, vol.518, p. 85.
Shin, E.J., Miser, D.E., Chan, W.G., and Hajaligol, M.R., Appl. Catal. B, 2005, vol.61, p. 79.
Arena, F., Gatti, G., Stievano, L., Martra, G., Coluccia, S., Frusteri, F., Spadaro, I., and Parmaliana, A., Catal. Today, 2006, vol.117, p. 75.
Lin, S.S. and Gurol, M.D., Environ. Sci. Technol., 1998, vol.32, p. 1417.
Cuzzola, A., Bertini, M., and Salvadori, P., Appl. Catal., vol.36, p. 231.
Kwan, W.P. and Voelker, B.M., Environ. Sci. Technol., 2002, vol.36, p. 1467.
Zelmanov, G. and Semiat, R., Water Res., 2008, vol.42, p. 492.
Kirk, T.K., in Microbial Degradation of Organic Compounds, Gibson, D.T., Ed., New York: Marcel Dekker, 1984, vol.13, p. 399.
Boeran, W., Ralph, J., and Baucher, M., Annu. Rev. Plant Biol., 2003, vol.54, p. 519.
Flaig, W., Geohim. Cosmochim. Acta, 1964, vol.28, p. 1523.
Kirk, T.K. and Farrell, R.L., Annu. Rev. Microbiol., 1987, vol.41, p. 465.
Kersten, P.J. and Kirk, T.K., J. Bacteriol., 1987, vol.169, p. 2195.
Latifoglu, A. and Kilic, A., Fresenius Environ. Bull., 2002, vol.11, p. 894.
Lesin, V.I., Koksharov, Yu.A., and Khomutov, G.B., Neftekhimiya, 2010, vol.50, p. 114.
Lesin, V.I., Koksharov, Yu.A., and Khomutov, G.B., Neft. Khoz., 2009, no. 3, p. 95.
Lesin, V.I., Koksharov, Yu.A., and Khomutov, G.B., Georesursy Geoenerg. Geopolit., 2010, no. 1. www.oilgasjournal.ru
Lesin, V.I., Neft. Khoz., 2004, no. 1, p. 68.
Lesin, V.I., Neftepromysl. Delo, 2008, no. 1, p. 43.
Denisov, E.T. and Afanas’ev, I.B., Oxidation and Antioxidants in Organic Chemistry and Biology, Boca Raton: CRC, 2005.
Lipid Oxidation Pathways, vol.2, Kamal-Eldin, A. and Min, D.B., Eds., Champaign: AOCS, 2008.
Chou, S.S. and Huang, C.P., Appl. Catal. A, 1999, vol.185, p. 237.
Lesin, V.I., Pisarenko, L.M., and Kasaikina, O.T., Pat. RF 2425715, 2011.
Kanevskii, V.M., Vlasov, V.P., Lesin, V.I., and Muslimov, A.E., Abstracts of Papers, XIII nats. konf. po rostu kristallov (NKRK-2008) (XIII Natl. Conf. on Crystal Growth), Moscow, 2008, p. 442.
Kanevskii, V.M., Vlasov, V.P., Lesin, V.I., and Muslimov, A.E., Abstracts of Papers, XIII nats. konf. po rostu kristallov (NKRK-2008) (XIII Natl. Conf. on Crystal Growth), Moscow, 2008, p. 444.
Hermanek, M., Zboril, R., Medrik, I., Pechousek, J., and Gregor, C., J. Am. Chem. Soc., 2007, vol.129, p. 10929.
Suzdalev, I.P., Buravtsev, V.N., Maksimov, Yu.M., Imshennik, V.N., Novichikhin, S.V., Matveev, V.V., and Plachinda, A.S., Ross. Khim. Zh., 2001, vol.45, no. 3, p. 66.
Suzdalev, I.P., Maksimov, Yu.M., Imshennik, V.N., Novichikhin, S.V., Matveev, V.V., Tret’yakov, Yu.D., Lukashin, A.V, Eliseev, A.A., Malygin, A.A., and Sosnov, E.A., 2006, vol.1, nos. 1–2, p. 134.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Lesin, L.M. Pisarenko, O.T. Kasaikina, 2012, published in Kolloidnyi Zhurnal, 2012, vol.74, No. 1, pp. 90–95.
Rights and permissions
About this article
Cite this article
Lesin, V.I., Pisarenko, L.M. & Kasaikina, O.T. Colloidal catalysts based on iron(III) oxides. 1. Decomposition of hydrogen peroxide. Colloid J 74, 85–90 (2012). https://doi.org/10.1134/S1061933X12010103
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X12010103