Abstract
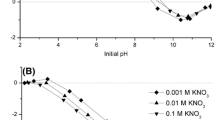
Hematite sample prepared by transformation of ferrihydrite was used to remove Cr(VI) from aqueous solution. Effects of initial concentration, contact time, pH, ionic strength, and temperature were investigated. The data were fitted to Langmuir, Freundlich, Dubinin-Kaganer-Radushkevich and Temkin models of adsorption. The results showed the best fit to the Langmuir isotherm. The increase of pH as well as that of increasing ionic strength resulted in the decrease of adsoiption of Cr(VI). Two kinetic models namely pseudo-first order and pseudo-second order were used to fit the experimental data. The thermodynamic parameters were found, and the results indicated spontaneous and exothermic nature of the process.
Similar content being viewed by others
References
Kotas, J. and Stasicka, Z., Environmental Pollution, 2000, vol. 107, p. 263.
Andersen, J.E.T., Analyt. Chim. Acta, 1998, vol. 361, p. 125.
Gad, C.S., Sci. Tot. Environ., 1989, vol. 89, p.149.
Lee, K.P., Ulrich, C.E., Geil, R.G., and Trochimowicz, H.J., Sci. Tot. Environ., 1989, vol. 86, p. 83.
Wang, X., Chen, X., Ma, X., Zheng, H., Ji, M., and Zhang, Z., Chem. Phys. Lett., 2004, vol. 384, p. 391.
Weng, C.H., Sharma, Y.C., and Chu, S.H., J. Hazard. Mat., 2008, vol. 155, p. 65.
Jianwei, L., Yanhui, Z., Yunqing, X., and Yu, L., Adv. Mater. Res., 2011, vol. 183–185, p. 1772.
Bajpai, S.K. and Armo, M.K., J. Macromol. Sci. A, 2009, vol. 46, p. 510.
Fang, J., Gu, Z., Gang, D., Liu, C., Ilton, E.S., and Deng, B., Environ. Sci. Technol., 2007, vol. 41, p. 4748.
Dobrowolski, R. and Stefaniak, E., Adsorption Sci. Technol., 2000, vol. 18, p. 97.
Ajouyed, O., Hurel, C., Ammari, M., Ben Allal, L., and Marmier, N., J. Hazard. Mater., 2010, vol. p. 616.
Singh, D.B., Gupta, G.S., Prasad, G., and Rupainwar, D.C., J. Environ. Sci. Health, 1993, vol. 28, p. 1813.
Brown, G.E., Chambers, S.A., Amonette, J.E., Rustad, J.R., Kendelewicz, T., Doyle, C.S., Grolimund, D., Foster-Mills, N.S., Joyce, S.A., and Thevuthasan, S., J. Conference Abstract, 2000, vol. 5, p. 253.
Cornell, R.M. and Schwertmann, U., The Iron Oxides: structure, properties, reactions, occurrences and uses, VCH, Weinheim, 1996, p. 395.
Kosmulski, M., J. Colloid Interface Sci., 2002, vol. 253, p. 77.
Gee, S.H., Hong, Y.K., Sur, J.C., Erikson, D.W., Park, M.H., and Jeffers, F., IEEE Trans. Magnetics, 2004, vol. 40, p. 2691.
Griffin, R.A., Au, A.K., and Frost, R.R., J. Environ. Sci. Health, 1977, vol. 12, p. 431.
Zeng, Y., Woo H., Lee, G., and Park, J., Micropor. Mesopor. Mater., 2010, vol. 130, p. 83.
Yusuf, A.M. and Nik Malek, N.A.N., J. Hazard. Mater., 2009, vol. 162, p. 1019.
Weng, C.H., Huang, C.P., Allen, H.E., and Sandas, P.F., J. Environ. Eng., 2001, vol. 127, p. 1124.
Namasivayam, C. and Yamuna, R.T., Chemosphere, 1995, vol. 30, p. 561.
Lagergren, S., Handlingar, 1898, vol. 24, p. 1.
Ho, Y. S., Ph. D Thesis, University of Birmingham, United Kingdom, 1995.
Mor, S., Ravindra, K., and Bishnoi, N.R., Biores. Technol., 2007, vol. 98, p. 954.
Tel, H., Atlas, Y., and Taner, M.S., J. Hazard. Mater., 2004, vol. B112, p. 225.
Fritzen, M.B., Souza, A.J., Silva, T.A.G, Souza, L., Nome, R.A., Fieldler, H.D., and Nome, F., J. Colloid Interface Sci., 2006, vol. 296, p. 465.
Barai, S.S., Das, S.N., Rath, P., and Chaudhury, G.R., Biochem. Eng. J., 2007, vol. 34, p. 69.
Hu, J., Chen, C., Zhu, X., and Wang, X., J. Hazard Mater. 2009, vol. 162, p. 1542.
Hu, J., Lo, I.M.C., Chen, G., Langmuir, 2005, vol. 21, p. 11173.
Baran, A., Bicak, E., and Baysal, S.H., and Onal, S. Bioresour. Technol., 2006, vol. 98, p. 661.
Sankararamakrishnan, N., Dixit, A., Iyengar, L., Sanghi, R. Bioresour. Technol., 2006, vol. 97, p. 2377.
Di, Z.C., Ding, J., Peng, X.J., Li, Y.H., Luan, Z.K., and Liang, J., Chemosphere, 2006, vol. 62, p. 861.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Adegoke, H.I., Adekola, F.A. Equilibrium sorption of hexavalent chromium from aqueous solution using synthetic hematite. Colloid J 74, 420–426 (2012). https://doi.org/10.1134/S1061933X12040035
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X12040035