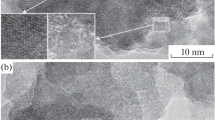
Abstract
Lead sulfide nanopowders have been prepared from aqueous solutions of lead acetate and sodium sulfide in the presence of sodium ethylenediaminetetraacetate or citrate. From microscopy and X-ray diffraction data purity and size of the formed nanoparticles are determined by the reagents concentrations, presence and nature of the complexing ligand, and the ratio of lead ions concentration to that of the ligand. PbS nanoparticles of the predefined size ranged from 5 to 55 nm can be prepared by controlled hydrochemical precipitation.
Similar content being viewed by others
References
Gusev, A.I., Nanomaterialy, nanostruktury, nanotekhnologii (Nanomaterials, Nanostructures, Nanotechnology), Moscow: Fizmatlit, 2009.
Rempel, A.A., Russ. Chem. Rev, 2007, vol. 76, no. 5, p. 435.
Dement’ev, A. and Gulbinas, V., Opt. Mater., 2009, vol. 31, no. 4, p. 647.
Patel, A.A., Wu, F., Zhang, J.Z., Torres-Martinez, C.L., Mehra, R.K., Yang, Y., and Risbud, S.H., J. Phys. Chem. (B), 2000, vol. 104, no. 49, p. 11598.
Martucci, A., Fick, J., Schell, J., Battaglin, G., and Guglielmi, M., J. Appl. Phys. 1999, vol. 86, no. 1, p. 79.
Kozhevnikova, N.S., Sadovnikov, S.I., and Rempel’, A.A., Pozharovzryvobezopasnost’, 2009, vol. 18, no. 5, p. 48.
Leontidis, E., Orphanou, M., Kyprianidou-Leondidou, T., Krumeich, F., and Caseri, W., Nano Lett., 2003, vol. 3, no. 4, p. 569.
Huang, Q. and Gao, L., Chem. Lett, 2004, vol. 33, no. 10, p. 1338.
Ma, Y., Qi, L., Ma, J., and Cheng, H., Cryst. Growth Design, 2004, vol. 4, no. 2, p. 351.
Ni, Y., Liu, H., Wang, F., Liang, Y., Hong, J., Ma, X., and Xu, Z., Cryst. Growth Design, 2004, vol. 4, no. 4, p. 759.
Fei Li, Xintang Huanga, Tao Kongb, Xueqin Liub, Qinghua Qinb, and Zhen Li., J. Alloys Compd, 2009, vol. 485, nos. 1–2, p. 554.
Li, C., Shi, G., Xu, H.Y., Guang, S.Y., Yin, R.H., and Song, Y.L., Mater. Lett, 2007, vol. 61, nos. 8–9, p. 1809.
Sharon, M., Ramaiah, K.S., Kumar, M., Neumann-Spallart, M., and Levy-Clement, C., J. Electroanal. Chem, 1997, vol. 436, nos. 1–2, p. 49.
Ding, T., Zhang, J.-R., Long, S., and Zhu, J.-J., Microelectr. Eng., 2003, vol. 66, nos. 1–4, p. 46.
Sun, J.-Q., Shen, X.P., Guo, L.-J., Chen, K.-M., and Liu, Q., Physica (E), 2009, vol. 41, no. 8, p. 1527.
Jusupov, R.A. and Mihajlov, O.V., Butlerov. Soobshch., 2004, vol. 5, no. 2, p. 22.
Parashar, V., Pandey, S.K., and Pandey, A.C., J. Optoelectr. Adv. Mat., 2009, vol. 11, no. 11, p. 1837.
Rempel, A.A., Kozhevnikova, N.S., Leenaers, A.J.G., and van den Berghe, S., J. Cryst. Growth., 2005, vol. 280, nos. 1–2, p. 300.
Jung-Hsuan Chen, Chuen-Guang Chao, Jong-Chyan Ou, and Tzeng-Feng Liu, Surf. Sci., 2007, vol. 601, no. 22, p. 5142.
Yonghong Ni, Fei Wang, Hongjiang Liu, Gui Yin, Jianming Hong, Xiang Ma, and Zheng Xu, J. Cryst. Growth., 2004, vol. 262, nos. 1–4, p. 399.
Kozhevnikova, N.S., Rempel’, A.A., and Sadovnikov, S.I., Russ. J. Gen. Chem., 2011, vol. 81, no. 10, p. 2062.
Piven, N.G., Shcherbak, L.P., Feichuk, P.I., Kalitchuk, S.M., Krylyuk, S.G., and Kobutyak, D.V., Kondens. Sredy i Mezhfaz. Gran., 2006, vol. 8, no. 4, p. 315.
Sadovnikov, S.I., Kozhevnikova, N.S., Pushin, V.G., and Rempel’, A.A., Inorg. Mater., 2012, vol. 48, no. 1, p. 21.
Noda, Y., Ohba, S., Sato, S., and Saito, Y., Acta Cryst. (B), 1983, vol. 39, no. 3, p. 312.
Noda, Y., Masumoto, K., Ohba, S., Saito, Y., Toriumi, K., Iwata, Y., and Shibuya, K., Acta Crystallogr. (C), 1987, vol. 43, no. 8, p. 1443.
Sadovnikov, S.I., Gusev, A.I., and Rempel’, A.A., JETP Lett., 2009, vol. 89, no. 5, p. 238.
Sadovnikov, S.I. and Rempel’, A.A., Fiz. Tverd. Tela., 2009, vol. 51, no. 11, p. 2237.
Kozhevnikova, N.S., Sadovnikov, S.I., Uritskaya, A.A., and Gusev, A.I., Russ. J. Gen. Chem., 2012, vol. 82, no. 4, p. 626.
Kozhevnikova, N.S., Sadovnikov, S.I., Urickaja, A.A., and Gusev, A.I., Izv. Vuzov, Ser. Khim. i Khim. Tekhnol., 2012, vol. 55, no. 3, p. 13.
Gusev, A.I. and Kurlov, A.S., Metallofiz. i Nov. Tekhnol., 2008, vol. 30, no. 5, p. 679.
X’Pert Plus Version 1.0. Program for Crystallography and Rietveld Analysis Philips Analytical, B., V. © Koninklijke Philips Electronics, N. V.
Match! Version 1.9a. Phase Identification from Powder Diffraction © Crystal Impact.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.I. Sadovnikov, A.I. Gusev, 2014, published in Zhurnal Obshchei Khimii, 2014, Vol. 84, No. 2, pp. 177–184.
Rights and permissions
About this article
Cite this article
Sadovnikov, S.I., Gusev, A.I. Preparation of nanocrystalline lead sulfide powder with controlled particles size. Russ J Gen Chem 84, 173–180 (2014). https://doi.org/10.1134/S1070363214020017
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363214020017