Abstract
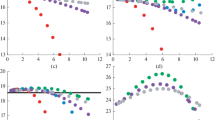
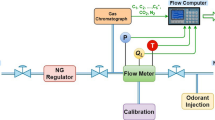
Natural gas (NG) is a mixture of 21 elements and widely used in the industries and domestics. Knowledge of its thermodynamic properties is essential for designing appropriate process and equipments. In this study, the detailed numerical procedures for computing most thermodynamic properties of natural gas are discussed based on the AGA8 equation of state (EOS) and thermodynamics relationships. To validate the procedures, the numerical values are compared with available measured values. The validations show that the average absolute percent deviation (AAPD) for density calculations is 0.0831%, for heat capacity at the constant pressure is 0.87%, for heat capacity at the constant volume is 1.13%, for Joule-Thomson coefficient is 1.93%, for speed of sound is 0.133%, and for enthalpy is 1.06%. Furthermore, in this work, the new procedures are presented for computing the entropy and internal energy. Due to lack of experimental data for these properties, the validation is done for pure methane. The validation shows that AAPD is 0.078% and 0.0133% for internal energy and entropy, respectively.
Similar content being viewed by others
References
McCarty, R.D., Extended Corresponding States as a Tool for the Prediction of the Thermodynamic Properties of Mixtures, Int. J. Thermophys., 1986, vol. 7, no. 4.
Estela-Uribe, J.F. and Trusler, J.P., Extended Corresponding States Equation of State for Natural Gas Systems, Fluid Phase Equilibria, 2001, vols. 183–184, pp. 21–29.
Estela-Uribe, J.F., De Mendozaa, A., and Trusler, J.P., Extended Corresponding StatesModel for Fluids and Fluid Mixtures II. Application toMixtures and Natural Gas Systems, Fluid Phase Equilibria, 2004, vol. 216, pp. 59–84.
Jaeschke, M., Audibert, S., van Caneghem, P., Humphreys, A.E., Janssen-van Rosmalen, R., Pellei, Q., Michels, J.P., Schouten, J.A., and ten Seldam, C.A., 1989, MGERG-88, High Accuracy Compressibility Factor for Natural Gases and Similar Mixtures by Use of a Truncated Virial Equation, GERG Tech. Monogr., vol. TM2.
Kunz, O., Klimeck, R., Wagner, W., and Jaeschke, M., The GERG-2004 Wide-Range Equation of State for Natural Gases and Other Mixtures, Fortschritt-Berichte VDI, ser. 6, no. 557, VDI Verlag.
Redlich, O. and Kwong, J.N., On the Thermodynamics of Solutions: V. An Equation of State: Fugacities of Gaseous Solutions, Chem. Rev., 1949, vol. 44, pp. 233–244.
Soave, G., Equilibrium Constants from a Modified Redlich-Kwong Equation of State, Chem. Eng. Sci., 1972, vol. 27, pp. 1197–1203.
Peng, D.-Y. and Robinson, D.B., A New Two-Constant Equation of State, Ind. Eng. Chem. Fundam., 1976, vol. 15, pp. 59–64.
Valderrama, J.O., A Generalized Patel-Teja Equation of State for Polar and Non-Polar Fluids and Their Mixtures, J. Chem. Eng. Japan, 1990, vol. 23, pp. 87–91.
Anderko, A., Cubic and Generalized van der Waals Equation, Equations of State for Fluids and Fluid Mixtures, Sengers, J.V., Kayser, R.F., Peters, C.J., and White, H.J., Eds., Amsterdam: Elsevier, 2000.
Neubauer, B., Tavitian, B., Boutin, A., and Ungerer, P., Molecular Simulations on Volumetric Properties of Natural Gas, Fluid Phase Equilibria, 1999, vol. 161, pp. 45–62.
Elsharkawy, A.D, Yousef, S.Kh., Hashem, S., and Alikhan, A.A., Compressibility Factor for Gas Condensates, Energy Fuels, 2001, vol. 15, pp. 807–816.
Elsharkawy, A.M., Efficient Methods for Calculations of Compressibility, Density and Viscosity of Natural Gases, Fluid Phase Equilibria, 2004, vol. 218. pp. 1–13.
Wendland, M., Saleh, B., and Fischer, J., Accurate Thermodynamic Properties from the BACKONE Equation for the Processing of Natural Gas, Energy Fuels, 2004, vol. 18, pp. 938–951.
Nasrifar, Kh. and Bolland, O., Prediction of Thermodynamic Properties of Natural Gas Mixtures Using 10 Equations of State Including a New Cubic Two-Constant Equation of State, J. Petroleum Sci. Eng., 2006, vol. 51, pp. 253–266.
Martinez, S.A. and Hall, R.K., Thermodynamic Properties of Light Synthetic Natural Gas Mixtures Using the RK-PR Cubic Equation of State, Ind. Eng. Chem. Res., 2006, vol. 45, pp. 3684–3692.
Huber, M.L., Smith, B.L., Ott, L.S., and Bruno, T.J., Surrogate Mixture Model for the Thermophysical Properties of Synthetic Aviation Fuel S-8: Explicit Application of the Advanced Distillation Curve, Energy Fuels, 2008, vol. 22, pp. 1104–1114.
AGA8-DC92 EoS (1992): Compressibility and Super Compressibility for Natural Gas and Other Hydrocarbon Gases, Transmission Measurement Committee Report, no. 8, AGA Catalog no. XQ 1285, Arlington, VA.
ISO-12213-2 (1997), Natural Gas-Calculation of Compression Factor, pt. 2: Calculation Using Molar Composition Analysis, ISO, Ref. no. ISO-12213-2:1997(E).
Marić, I., Galović, A., and Šmuc, T., Calculation of Natural Gas Isentropic Exponent, Flow Meas. Instrum., 2005, vol. 16, no. 1, pp. 13–20.
Marić, I., The Joule-Thomson Effect in Natural Gas Flow-Rate Measurements, Flow Meas. Instrum., 2005, vol. 16, pp. 387–95.
Marić, I., A Procedure for the Calculation of the Natural GasMolar Heat Capacity, the Isentropic Exponent, and the Joule-Thomson Coefficient, Flow Meas. Instrum., 2007, vol. 18, pp. 18–26.
Farzaneh-Gord, M., Khamforoush, A., Hashemi, Sh., and Pourkhadem, N.H., Computing Thermal Properties of Natural Gas by Utilizing AGA8 Equation of State, Int. J. Chem. Eng. Appl., 2010, vol. 1, no. 1.
Steven, C., Chapra, and Raymond, P., Numerical Method for Engineers, McGraw-Hill, 2005.
Capla, L., Buryan, P., Jedelsky, J., Rottner, M., Rottner, M., and Linek, J., Isothermal pVTMeasurements on GasHydrocarbon Mixtures Using a Vibrating-Tube Apparatus, J. Chem. Therm., 2002, vol. 34, pp. 657–667
Patil, P., Ejaz, S., Atilhan, M., Cristancho, D., Holste, J., and Hall, K.R., Accurate Density Measurements for a 91% Methane Natural Gas-LikeMixture, J. Chem. Therm., 2007, vol. 39, pp. 1157–1163.
Hwang, C.-A., Simon, P.P., Hou, H., Hall, K.R., Holste, J.C., and Marsh, K.N., Burnett and Pycnometric (p, V, T) Measurements for Natural GasMixtures, J. Chem. Therm., 1997, vol. 29, pp. 1455–1472.
DIPPR®801, Evaluated Standard Thermophysical Property Values, Design Institute for Physical Properties, 2004.
Mayrath, J.E. and Magee, J.W., Measurements ofMolar Heat Capacity at Constant Volume: C v,m{xCH6 + (1 − x)C2H6}, J. Chem. Therm., 1989, vol. 21, pp. 499–5I3.
Ernst, G., Keil, B., Wirbser, H., and Jaeschke, M., Flow-Calorimetric Results for the Massic Heat Capacity cp and the Joule-Thomson Coefficient of CH4, of 0.85CH4 + 0.15C2H6, and of aMixture Similar to Natural Gas, J. Chem. Therm., 2001, vol. 33, pp. 601–613.
Barreau, A., Janneteau, P., and Gaillard, K., Isobaric Heat Capacity of Natural Gases. Measurements and Modeling, Fluid Phase Equilibr., 1997, vol. 127, pp. 155–171.
Trusler, J.P., The Speed of Sound in (0.8CH4 +0.2C2H6) at Temperature between 200 K and 375 K, J. Chem. Therm., 1994, vol. 26, pp. 751–763.
Costa Gomes, M.F. and Trusler, J.P., The Speed of Sound in Two Methane-Rich Gas Mixtures at Temperatures between 250 K and 350 K and at Pressures up to 20 MPa, J. Chem. Therm., 1998, vol. 30, pp. 1121–1129.
Estela-Uribe, J.F, Trusler, J.P., Chamorro, C.R., Segovia, J.J., Martin, M.C., and Villamanan, M.A., Speeds of Sound in {(1 − x)CH4 + xN2} with x = (0.10001, 0.19999, and 0.5422) at Temperatures between 170 K and 400 K and Pressures up to 30 MPa, J. Chem. Therm., 2006, vol. 38, pp. 929–937.
Day, C., Stephan, M., and Oellrich, L.R., A New Flow Calorimeter for the Measurement of the Isobaric Enthalpy Increment and the Insenthalpic Joule-Thomson Effect Results for Methane and “Methane-Ethane,” J. Chem. Therm., 1997, vol. 29, pp. 949–971.
Ashton, G.J. and Haselden, G.G., Measurements of Enthalpy and Phase Equilibrium for Simulated Natural Gas Mixtures and Correlation of the Results by a Modified Starling Equation, Cryogenics, 1980.
Setzmann, U. and Wagner, W., A New Equation of State and Tables of Thermodynamic Properties for Methane Covering the Range from the Melting Line to 625 K at Pressures up to 1000 MPa, J. Phys. Chem. Ref. Data, 1991, vol. 20, no. 6, pp. 1061–1155.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farzaneh-Gord, M., Rahbari, H.R. Numerical procedures for natural gas accurate thermodynamic properties calculation. J. Engin. Thermophys. 21, 213–234 (2012). https://doi.org/10.1134/S1810232812040017
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1810232812040017