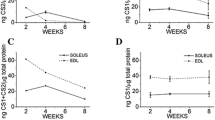
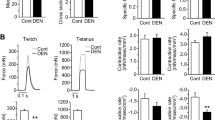
Abstract
The expression of genes responsible for the synthesis of essential proteins regulating the calcium-ion balance and ultrastructural characteristics of fast-twitch (m. extensor digitorum longus, EDL) and slow-twitch (m. soleus, SOL) skeletal muscles under prolonged exercise were studied in an experimental model of forced-swimming rats. A day after the end of the exercise, no significant changes in any of the five investigated genes were revealed in the SOL. A few triad elements (T-tubules and cisternae of sarcoplasmic reticulum) were revealed. A small number of excitation-contraction coupling (ECC) structures in the control and a slight increase in their amount after exercises were noticed. Polymorphism and mitochondrial defects within SOL muscles indicate the importance of these structures in the regulation of calcium balance. In EDL muscles, adaptation mechanisms are aimed mainly at pumping Ca2+ ions to the sarcoplasmic reticulum, where the main calcium buffer is calsequestrin. Expression of SERCA1 gene increased by an order of magnitude, and that of CASQ1 increased by three times. Electron microscopy showed a major role of triads in the maintenance of calcium homeostasis in the EDL muscles, as well as a greater destruction of these muscles compared to SOL after exhausting exercise. The high level of triads and a possible activation of the CICR (calcium-induced calcium release) mechanism in fast-twitch muscles can cause damage to them during exhausting exercise. Adaptation of SOL muscles is associated with structural rearrangements of the mitochondrial apparatus, while adaptation of the EDL muscles is caused by calcium removal from the sarcoplasm with Ca-ATPase and its retention in the sarcoplasmic reticulum by calsequestrin.
Similar content being viewed by others
Abbreviations
- ROS:
-
reactive oxygen species
- DHPR:
-
dihydropyridine receptors
- RT-qPCR:
-
real-time quantitative reverse transcription polymerase chain reaction
- RYR:
-
ryanodine receptor
- SR:
-
sarcoplasmic reticulum
- EMC:
-
electromechanical coupling
- CICR:
-
Ca2+-induced Ca2+ release
- DICR:
-
depolarization-induced Ca2+ release
- EDL:
-
m. extensor digitorum longus (fast (white) skeletal muscles)
- SOL:
-
m. soleus (slow (red) skeletal muscles)
References
Allen, D.G., Lamb, G.D., and Westerblad, H., Skeletal muscle fatigue: cellular mechanisms, Physiol. Rev., 2008, vol. 88, pp. 287–332.
Andersen, C.L., Jensen, J.L., and Orntoft, T.F., Normalization of real-time quantitative reverse transcription–PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets, Cancer Res., 2004, vol. 64, pp. 5245–5250.
Angelova, P.R., Baev, A.Y., Berezhnov, A.V., and Abramov, A.Y., Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death, Biochem. Soc. Trans., 2016, vol. 44, pp. 40–45.
Baumert, P., Lake, M.J., Stewart, C.E., Drust, B., and Erskine, R.M., Genetic variation and exercise-induced muscle damage: implications for athletic performance, injury and ageing, Eur. J. Appl. Physiol., 2016, vol. 116, pp. 1595–1625.
Cheng, A.J., Yamada, T., Rassier, D.E., Andersson, D.C., Westerblad, H., and Lanner, J.T., Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery, J. Physiol., 2016, vol. 594, no. 18, pp. 5149–5160.
Debold, E.P., Potential molecular mechanisms underlying muscle fatigue mediated by reactive oxygen and nitrogen species, Front. Physiol., 2015, vol. 6, pp. 239.
Durham, W.J., Aracena-Parks, P., Long, C., Rossi, A.E., Goonasekera, S.A., Boncompagni, S., Galvan, D.L., Gilman, C.P., Baker, M.R., Shirokova, N., Protasi, F., Dirksen, R., and Hamilton, S.L., RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 Knockin Mice, Cell, 2008, vol. 133, pp. 53–65.
Ean, B., Carson, B.P., Garcia-Roves, P.M., Chibalin, A.V., Sarsfield, F.M., Barron, N., McCaffrey, N., Moyna, N.M., Zierath, J.R., and O’Gorman, D.J., Exercise intensitydependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle, J. Physiol., 2010, vol. 588, pp. 1779–1790.
Endo, M., Calcium-induced calcium release in skeletal muscle, Physiol. Rev., 2009, vol. 89, pp. 1153–1176.
Ferraro, E., Giammarioli, A.M., Chiandotto, S., Spoletini, I., and Rosano, G., Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy, Antioxid. Redox. Signal., 2014, vol. 21, pp. 154–176.
Fredsted, A., Gissel, H., Madsen, K., and Clausen, T., Causes of excitation-induced muscle cell damage in isometric contractions: mechanical stress or calcium overload?, Am. J. Physiol. Regul. Integr. Comp. Physiol., 2007, vol. 292, pp. 2249–2258.
Georgiev, G.P. and Mant’eva, V.L., Informational and ribosomal RNA, of chromosome-nucleolar apparatus, methods of separating, and nucleotide composition, Biokhimiya, 1962, vol. 23, no. 5, pp. 949–953.
Györke, S. and Palade P., Calcium-induced calcium release in crayfish skeletal muscle, J. Physiol., 1992, vol. 457, pp. 195–210.
Györke, S. and Palade, P., Ca(2+)-dependent negative control mechanism for Ca(2+)-induced Ca2+ release in crayfish muscle, J. Physiol., 1994, vol. 476, pp. 315–322.
He, F., Li, J., Liu, Z., Chuang, C.C., Yang, W., and Zuo, L., Redox mechanism of reactive oxygen species in exercise, Front. Physiol., 2016, vol. 7, p. 486.
Hidalgo, C., Donoso, P., and Carrasco, M.A., The ryanodine receptors Ca2+ release channels: cellular redox sensors?, IUBMB Life, 2005, vol. 57, pp. 315–322.
Huang, C.C., Wang, T., Tung, Y.T., and Lin, W.T., Effect of exercise training on skeletal muscle SIRT1 and PGC-1α expression levels in rats of different age, Int. J. Med. Sci., 2016, vol. 13, pp. 260–270.
Janssens, S., Jonkers, R.A., Groen, A.K., Nicolay, K., van Loon, L.J., and Prompers, J.J., Effects of acute exercise on lipid content and dietary lipid uptake in liver and skeletal muscle of lean and diabetic rats, Am. J. Physiol. Endocrinol. Metab., 2015, vol. 309, pp. 874–883.
Kubasov, I.V., Arutunyan, R.S., and Matrosova, E.V., Transformation of the individual contractile responses within the tetanic train in fast-and slow-twitch rat skeletal muscles, J. Evol. Biochem. Physiol., 2016, vol. 52, no. 1, pp. 46–55.
Kubasov I.V., Arutunyan R.S., Matrosova E.V., and Kubasov, I.I., Characteristics of the individual contractile responses within the tetanic train in slow-twitch rat skeletal muscles during modulation of Ca2+ release from sarcoplasmic reticulum, J. Evol. Biochem. Physiol., 2016, vol. 52, no. 5, pp. 337–345.
Marty, I. and Fauré, J., Excitation–contraction coupling alterations in myopathies, J. Neuromuscul. Dis., 2016, vol. 3, pp. 443–453.
Matrosova, E.V., Kubasov, I.V., Novozhilov, A.V., Tavrovskaya, T.V., Korf, E.A., Aratunyan, R.S., and Goncharov, N.V., The effect of intense physical activities on mechanografic characteristics of the fast-and slow-twitch rat skeletal muscle, Ross. Fiziol. Zh. im. I.M. Sechenova, 2017, vol. 103, no. 4, pp. 000–000.
Michel, L.Y., Hoenderop, J.G., and Bindels, R.J., Calpain-3-mediated regulation of the Na+–Ca2+ exchanger isoform 3, Pflugers Arch., 2016, vol. 468, pp. 243–255.
Morissette, M.P., Susser, S.E., Stammers, A.N., O’ Hara, K.A., Gardiner, P.F., Sheppard, P., Moffatt, T.L., and Duhamel, T.A., Differential regulation of the fiber type-specific gene expression of the sarcoplasmic reticulum calcium-ATPase isoforms induced by exercise training, J. Appl. Physiol., 2014, vol. 117, pp. 544–555.
Nadeev, A.D., Zinchenko, V.P., Avdonin, P.V., and Goncharov, N.V., Toxic and signalling effects of the reactive oxygen species, Toxikol. Vestnik, 2014, vol. 2, pp. 22–27.
Nasledov, G.A., Katina, I.E., and Kobzeva, M.A., On the functioning of the electromechanical coupling in various stages of reduction of the contracture, Ross. Fiziol. Zh. im. I.M. Sechenova, 2004, vol. 90, no. 3, pp. 327–338.
Nasledov, G.A., Katina, I.E., and Zhitnikova Y.V., Changes in the functioning of the electromechanical connection during tetanic contraction, Neurosci. Behav. Physiol., 2007, vol. 37, pp. 153–159.
Nielsen, J., Cheng, A.J., Ørtenblad, N., and Westerblad, H., Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres, J. Physiol., 2014, vol. 592, pp. 2003–2012.
Novozhilov, A.V., Tavrovskaya, T.V., Voytenko, N.G., Maslova, M.N., Goncharov, N.V., and Morozov, V.I., The efficiency of the green tea extract in the experiment using two exercise models, Bull. Exp. Biol. Med., 2014, vol. 158, no. 9, pp. 327–332.
Pfaffl, M.W., Horgan, G.W., and Dempfle, L., Relative Expression Software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR, Nucleic Acids Res., 2002, vol. 30, pp. e36.
Pfaffl, M.W., Tichopad, A., Prgomet, C., and Neuvians, T.P., Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations, Biotechnol. Lett., 2004, vol. 26, pp. 509–515.
Prinz, W.A., Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics, J. Cell. Biol., 2014, vol. 205, pp. 759–769.
Rossi, A.E., Boncompagni, S., and Dirksen, R.T., Sarcoplasmic Reticulum-mitochondrial Symbiosis: Bidirectional Signaling In skeletal Muscle, Exerc. Sport. Sci. Rev., 2009, vol. 37, pp. 29–35.
Rubtsov, A.M., Ca-ATPase of the sarcoplasmic reticulum: molecular organization, functioning mechanism, and characteristics of regulation of activity, Usp. Biol. Nauk, 2005, vol. 45, pp. 235–268.
Schiaffino, S. and Reggiani, C., Fiber types in mammalian skeletal muscles, Physiol. Rev., 2010, vol. 91, pp. 1447–1531.
Silver N., Best, S., Jiang, J., and Thein, S.L., Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR, BMC Mol. Biol., 2006, vol. 7, p. 33.
Solesio, M.E., Demirkhanyan, L., Zakharian, E., and Pavlov, E.V., Contribution of inorganic polyphosphate towards regulation of mitochondrial free calcium, Biochim. Biophys. Acta, 2016, vol. 1860, pp. 1317–1325.
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F., Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes, Genome Biol., 2002, vol. 3, no. 7, pp. research0034.1–research0034.11. PMID: 12184808.
Witherspoon, J.W. and Meilleur, K.G., Review of RyR1 pathway and associated pathomechanisms, Acta. Neuropathol. Commun., 2016, vol. 4, p. 121.
Xie, F., Xiao, P., Chen, D., Xu, L., and Zhang, B., miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs, Plant. Mol. Biol., 2012, vol. 80, p. 75.
Yu, ZB., Tetanic contraction induces enhancement of fatigability and sarcomeric damage in atrophic skeletal muscle and its underlying molecular mechanisms, Zhongguo Ying Yong Sheng Li Xue Za Zhi, 2013, vol. 29, pp. 525–533.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Korf, I.V. Kubasov, M.S. Vonsky, A.V. Novozhilov, A.L. Runov, E.V. Kurchakova, E.V. Matrosova, T.V. Tavrovskaya, N.V. Goncharov, 2017, published in Tsitologiya, 2017, Vol. 59, No. 6, pp. 434–446.
Rights and permissions
About this article
Cite this article
Korf, E.A., Kubasov, I.V., Vonsky, M.S. et al. Ultrastructural and gene-expression changes in the calcium regulation system of rat skeletal muscles under exhausting exercise. Cell Tiss. Biol. 11, 371–380 (2017). https://doi.org/10.1134/S1990519X17050030
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X17050030