Abstract
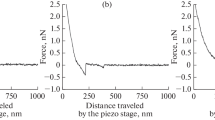
In order to investigate quantitatively the role of lipopolysaccharides (LPS) from outer bacterial membrane at the initial state of bacterium adhesion to a host cell membrane, a model system for single cell force spectroscopy was developed and used. The system comprised of an LPS-coated microsphere placed into optical trap and a J774 macrophage being approached the microsphere to initiate their binding and then moved back to rupture the bond. An “object shadow” phenomenon was discovered, manifested as large-scale variations of the signal of photodetector registering the trapped microsphere displacement, such variations emerging long before the actual interaction between the macrophage and microsphere. The theory and the measurements technique were developed for registration of the force required for detachment of bounded microsphere from the object investigated by means of optical tweezers under the “object shadow” conditions. Characteristic spectra of binding force between J774 macrophage and microspheres functionalized with various LPS, as well as LPS plus complementary antibodies preparations were obtained at the rate of detachment force application of 3–6 pN/s. Force spectrum characteristic of Yersinia pseudotuberculosis LPS possessing O-antigen had a maximum at ~14 pN with half-width of ~23 pN. The treatment of O-antigen with complementary antibodies resulted in transformation of this spectrum into a spectrum with maximum at ~10 pN and half-width of ~14 pN, being almost identical to the spectrum of Y. pestis LPS devoid of O-antigen, with a maximum at ~9 pN and half-width of ~13 pN. A possible mechanism of force spectra formation has been proposed under assumptions of nonspecific binding of O-antigen and probable receptor-type binding of LPS core region to the macrophage surface. The elastic modulus of macrophage envelope, as estimated using analysis of displacement of the contacting microsphere as an indenter, was ≈0.17 pN/nm.
Similar content being viewed by others
References
Ofek I, Hasty D.L., Doyle R.J. 2003. Basic concepts in bacterial adhesion. In: Bacterial Adhesion to Animal Cells and Tissues. Washington: ASM Press, pp. 1–18.
Mikula K.M., Kolodziejczyk R., Goldman A. 2013. Yersinia infection tools–characterization of structure and function of adhesins. Front. Cell Infect. Microbiol. 2, 169 (14).
Ribet D., Cossart P. 2015. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 17 (3), 173–183.
Viboud G.I., Bliska J.B. 2005. Yersinia outer proteins: Role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59, 69–89.
Pujol C., Bliska J.B. 2005. Turning Yersinia pathogenesis outside in: Subversion of macrophage function by intracellular yersiniae. Clin. Immunol. 114 (3), 216–226.
Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature. 449 (7164), 819–826.
Park B.S., Lee J.O. 2013. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exper. Mol. Med. 45 (e66), 1–9.
Krachler A.M., Orth K. 2013. Targeting the bacteriahost interface. Virulence. 4 (4), 284–294.
Rietschel E.Th., Kirikae T., Schade F.U., Mamat U., Schmidt G., Loppnow H., Ulmer A.J., Zähringer U., Seydel U., Di Padova F., Schreier, M., Brade, H. 1994. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 8 (2), 217–225.
Shoaf-Sweeney K.D., Hutkins R.W. 2009. Adherence, anti-adherence, and oligosaccharides preventing pathogens from sticking to the host. Adv. Food. Nutr. Res. 55, 101–161.
Doyle R.J. 2000. Contribution of the hydrophobic effect to microbial infection. Microbes Infect. 2 (4), 391–400.
Erridge C., Bennett-Guerrero E., Poxton I.R. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4 (8), 837–851.
Lu Q., Wang J., Faghihnejad A., Zeng H., Liu Y. 2011. Understanding the molecular interactions of lipopolysaccharides during E. coli initial adhesion with a surface forces apparatus. Soft Matter. 7 (19), 9366–9379.
Knirel, Y. A., Anisimov A. P. 2012. The LPS of the plague microbe Yersinia pestis: Structure, genetics and biological properties. Acta Naturae (Rus.). 4 (3), 49–61.
Lam J.S., Graham L.L., Lightfoot J. T. Dasgupta T., Beveridge T.J. 1992. Ultrastructural examination of the lipopolysaccharides of Pseudomonas aeruginosa strains and their isogenic rough mutants by freeze-substitution. J. Bacreriol. 174 (22), 7159–7167.
Strauss J., Burnham N.A., Camesano T.A. 2009. Atomic force microscopy study of the role of LPS Oantigen on adhesion of E. coli. J. Mol. Recognit. 22 (5), 347–355.
Leckband D., Israelachvili J. 2001. Intermolecular forces in biology. Quart. Rev. Biophys. 34 (2), 105–267.
Wright S.D., Jong M.T.C. 1986. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J. Exp. Med. 164 (6), 1876–1888.
Ofek I., Goldhar J., Keisari Y. Nonopsonic phagocytosis of microorganisms. 1995. Annu. Rev. Microbiol. 49, 239–276.
Jacques, M. 1996. Role of lipo-oligosaccharides and lipopolysaccharides in bacterial adherence. Trends Microbiol. 4 (10), 408–409.
Razatos A., Ong Y., Sharma M.M., Georgiou G. 1998. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc. Natl. Acad. Sci. USA. 95 (9), 11059–11064.
Targosz M., Labuda A., Czuba P., Biedron R., Strus M., Gamian A., Marcinkiewicz J., Szymonski M. 2006. Influence of macrophage activation on their capacity to bind bacterial antigens studied with atomic force microscopy. Nanomedicine. 2 (2), 82–88.
Vonna L., Wiedemann A., Aepfelbacher M., Sackmann E. 2007. Micromechanics of filopodia mediated capture of pathogens by macrophages. Eur. Biophys. J. 36 (2), 145–151.
Zidovska A., Sackmann E. 2011. On the mechanical stabilization of filopodia. Biophys. J. 100 (3), 1428–1437.
Wei M.T., Hua K.-F., Hsu J., Karmenyan A., Tseng K.Y., Wong C.H., Hsu H.-Y., Chiou A. 2007. The interaction of lipopolysaccharide with membrane receptors on macrophages pretreated with extract of Reishi polysaccharides measured by optical tweezers. Opt. Express. 15 (17), 11020–11032.
Bengoechea J.A., Najdenski H., Skurnik M. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52 (2), 451–469.
Murray G.L., Attridge S.R., Morona R. 2006. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J. Bacteriol. 188 (7), 2735–2739.
Byvalov A.A., Kononenko V.L., Konyshev I.V. 2017. Effect of lipopolysaccharide O-side chains on the adhesiveness of Yersinia pseudotuberculosis to J774 macrophages as revealed by optical tweezers. Appl. Biochem. Microbiol. 53 (2), 258–266. doi 10.1134/S0003683817020077
Achtman M., Morelli G., Zhu P., Wirth T. Diehl I, Kusecek B, Vogler A.J., Wagner D.M., Allender C.J., Easterday W.R., Chenal-Francisque V., Worsham P., Thomson N.R., Parkhill J., Lindler L.E., Carniel E, Keim P. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. USA. 101 (51), 17837–17842.
Skurnik M., Peippo A., Ervelä E. 2000. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Molec. Microbiol. 37 (2), 316–330.
Knirel Y.A., Kondakova A.N., Bystrova O.V., Lindner B., Shaikhutdinova R.Z., Dentovskaya S.V., Anisimov A.P. 2008. New features of Yersinia lipopolysaccharide structures as revealed by high-resolution electrospray ionization mass spectrometry. Adv. Sci. Lett. 1 (2), 192–198.
Westphal O., Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenolwater and further applications of the procedure. In: Methods in Carbohydrate chemistry. V. 5. Eds Whistler R.L., Wolfan M.L. New York: Acad. Press Inc., pp. 83–91.
Byvalov A.A., Dudina L.G., Lytvynets, S.G., Novikova O.D., Khomenko, V.A. Portnyagina, O.Yu., Ovodov Yu.S. 2014. The study of the surface antigenic epitopes of Yersinia pseudotuberculosis using monoclonal antibodies. Prikl. Biokhim. Mikrobiol. (Rus.). 50 (2), 203–210.
Wozniak A, Mameren J.V., Ragona S. 2009. Singlemolecule force spectroscopy using the NanoTracker optical tweezers platform: from design to application. Curr. Pharm. Biotechnol. 10, 467–473.
Molodtsov M.I., Grishchuk E.L., McIntosh J.R., Ataullakhanov F.I. 2007. A new type of biomechanical machine. Ross. Khim. Zh. (Rus.). 51 (1), 36–44.
Neuman K.C., Block S.M. 2004. Optical trapping. Rev. Sci. Instr. 75 (9), 2287–2809.
Collett D. 1994. Modelling survival data in medical research. Boca Raton: Chapman and Hall/CRC.
Wand M.P., Jones M.C. 1995. Kernel Smoothing. London: Chapman and Hal.
Busscher H.J., Weerkamp A.H. 1987. Specific and non-specific interactions in bacterial adhesion to solid substrata. FEMS Microbiol. Lett. 46 (2), 165–173.
Lower S.K. 2011. Atomic force microscopy to study intermolecular forces and bonds associated with bacteria. Adv. Exp. Med. Biol. 715, 285–299.
Lam J., Herant M., Dembo M., Heinrich V. 2009. Baseline mechanical characterization of J774 macrophages. Biophys. J. 96 (1), 248–254.
Evans E., Ritchie K. 1997. Dynamic strength of molecular adhesion bonds. Biophys. J. 72 (4), 1541–1555.
Chernyadev V.A., Byvalov A.A., Ananchenko B.A., Bushmeleva L.G., Lytvynets S.G. 2012. Morphological features of bacteria Yersinia pseudotuberculosis, grown under different temperature conditions. Izvestja Komi Nauchnogo Centra Uralskogo Otdelenija RAN (Rus.). 3 (11), 57–60.
Peula-Garcia J.M., Molina-Bolivar J.A., Velasco J., Rojas A., Galisteo-Gonzalez F. 2002. Interaction of bacterial endotoxine (lipopolysaccharide) with latex particles: Application to latex agglutination immunoassays. J. Colloid Interf. Sci. 245 (2), 230–236.
Petty R.P., Haefman D.G., McConnel H.M. 1981. Disappearance of macrophage surface folds after antibody- dependent phagocytosis. J. Cell Biol. 89 (3), 223–229.
Raucher D., Sheetz M.P. 1999. Characteristics of a membrane reservoir buffering membrane tension. Biophys. J. 77 (4), 1992–2002.
Skurnik M., Zhang L. 1996. Molecular genetics and biochemistry of Yersinia lipopolysaccharide. APMIS. 104, 849–872.
Lahtinen P., Brzezinska A., Skurnik M. 2003. Temperature and growth phase regulate the transcription of the O-antigen gene cluster of Yersinia enterocolitica O:3. In: The genus Yersinia: Entering the functional genomic era. Eds Skurnik M., Granfors K., Bengoechea J.A. New York: Kluwer Acad./Plenum Publ, pp. 289–292.
Skurnik M., Bengoechea J.A. 2009. Genetics and regulation of bacterial lipopolysaccharide synthesis. In: Bacterial polysaccharides–current innovations and future trends. Ed. Ulrich M. Norfolk: Caister Acad. Press, pp. 27–37.
Kerrigan A.M., Brown G.D. 2009. C-type lectins and phagocytosis. Immunobiol. 214 (7), 562–575.
Klena J., Zhang P., Schwartz O., Hull S., Chen T. 2005. The core lipopolysaccharide of Escherichia coli is a ligand for the dendritic-cell-specific intercellular adhesion molecule nonintegrin CD209 receptor. J. Bacteriol. 187 (5), 1710–1715.
Zhang P., Skurnik M., Zhang S.-S., Schwartz O., Kalyanasundaram R., Bulgheresi S., He J.J., Klena J.D., Hinnebusch B.J., Chen T. 2008. Human dendritic cellspecific intercellular adhesion molecule-grabbing nonintegrin (CD209) is a receptor for Yersinia pestis that promotes phagocytosis by dendritic cells. Infect. Immun. 76 (5), 2070–2079.
Rainho C.S., de Sá E.A., Jabur Gaziri L.C., Ostrensky Saridakis H., Felipe I. 1999. Modulation of lectinophagocytosis of Escherichia coli by variation of pH and temperature. FEMS Immunol. Med. Microbiol. 24 (1), 91–95.
Zamze S., Martinez-Pomares L., Jones H., Taylor P.R., Stillion R.J., Gordon S., Wong S.Y. 2002. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J. Biol. Chem. 277 (44), 41613–41623.
Reading P.C., Miller J.L., Anders E.M. 2000. Involvement of the mannose receptor in infection of macrophages by influenza virus. J. Virol. 74 (11), 5190–5197.
Protopopov V.V., Ustinov N.D. 1985. Lazernoje geterodinirovanie (Laser heterodyning). M.: Nauka Publisher.
Harada Y., Asakura T. 1996. Radiation forces on a dielectric sphere in the Rayleigh scattering regime. Optics Communic. 124 (5–6), 529–541.
Goncharenko A.M. 1977. Gaussovi puchki sveta (Gaussian beams of light). Minsk: Science and Technology.
van de Hulst H.C. 1981. Light scattering by small particles. New York: Dover Publications.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Byvalov, V.L. Kononenko, I.V. Konyshev, 2018, published in Biologicheskie Membrany, 2018, Vol. 35, No. 2, pp. 115–130.
Rights and permissions
About this article
Cite this article
Byvalov, A.A., Kononenko, V.L. & Konyshev, I.V. Single-Cell Force Spectroscopy of Interaction of Lipopolysaccharides from Yersinia pseudotuberculosis and Yersinia pestis with J774 Macrophage Membrane Using Optical Tweezers. Biochem. Moscow Suppl. Ser. A 12, 93–106 (2018). https://doi.org/10.1134/S1990747818020058
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747818020058