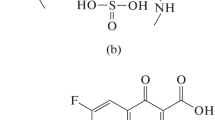
Abstract
Gentamicin and levofloxacin are encapsulated into bioresorbable polymer scaffolds and microparticles by the SCF methods: particles from gas saturated solutions (PGSS) and plasticization, with the subsequent foaming of amorphous polymers using supercritical carbon dioxide. The kinetics of the release of the incorporated drug substances into model physiological media are studied. It is shown that the use of the developed methods of SCF-encapsulation of drugs in bioresorbable carriers allows varying the size, shape, and morphology of the formed structures and, accordingly, the rate of release of the drugs into the physiological environments. In our opinion, this approach can be very promising for the development of components of new highly effective antibacterial prolonged action dosage forms.










Similar content being viewed by others
REFERENCES
I. D. Morris and A. M. Palmer, Drug News Persp. 18, 525 (2005).
Q. Ul-Ain, S. Sharma, and G. K. Khuller, Antimicrob. Agents Chemother. 47, 3005 (2003).
R. Pandey, A. Sharma, A. Zahoor, S. Sharma, G. K. Khuller, and B. Prasad, J. Antimicrob. Chemother. 52, 981 (2003).
R. S. Langer and N. A. Peppas, Biomaterials 2, 201 (1981).
S. Fredenberg, M. Wahlgren, M. Reslow, and A. Axelsson, Int. J. Pharm. 415, 34 (2011).
X. Li and B. R. Jasti, Design of Controlled Release Drug Delivery Systems (McGraw-Hill, New York, 2006).
K. Park, J. Control. Release 190, 3 (2014).
Y. Wang, W. Qu, and S. H. Choi, Am. Pharm. Rev. (2016).
E. J. Fraza and E. F. Schmitt, J. Biomed. Mater. Res. 1, 43 (1971).
H. Makadia and S. Siegel, Polymers 3, 1377 (2011).
J. M. Anderson and M. S. Shive, Adv. Drug Deliv. Rev. 64, 72 (2012).
A. M. Reed and D. K. Gilding, Polymer 22, 494 (1981).
F. Danhier, E. Ansorena, J. M. Silva, R. Coco, A. le Breton, and V. Prat, J. Control. Release 161, 505 (2012).
J. Szlek, A. Paclawsk, R. Lau, R. Jachowicz, and A. Mendyk, Int. J. Nanomed. 8, 4601 (2013).
F. Y. Han, K. J. Thurecht, A. K. Whittaker, and M. T. Smith, Front. Pharmacol. 7, 185 (2016).
N. Teekamp, L. F. Duque, H. W. Frijlink, W. L. Hinrichs, and P. Olinga, Expert Opinion Drug Deliv. 12, 1311 (2015).
G. Gasparini, R. G. Holdich, and S. R. Kosvintsev, Colloids Surf., B 75, 557 (2010).
F. Ito, H. Fujimori, H. Kawakami, K. Kanamura, and K. Makino, Colloids Surf., A 384, 368 (2011).
S. Feng, F. Lu, Y. Wang, and J. Suo, J. Appl. Polym. Sci. 132, 41943 (2015).
D. I. D. Cho and H. J. Yoo, J. Microelectromech. Syst. 24, 10 (2015).
S. P. Schwendeman, R. B. Shah, B. A. Bailey, and A. S. Schwendeman, J. Control. Release 190, 240 (2014).
C. Cai, S. Mao, O. Germershaus, A. Schaper, E. Rytting, and D. Chen, J. Microencapsul 26, 334 (2009).
A. Frank, S. K. Rath, F. Boey, and S. Venkatraman, Biomaterials 25, 813 (2004).
S. Mao, J. Xu, C. Cai, O. Germershaus, A. Schaper, and T. Kissel, Int. J. Pharm. 334, 137 (2007).
X. Huang and C. S. Brazel, J. Control. Release 73, 121 (2001).
C. R. de Azevedo, M. von Stosch, M. S. Costa, et al., Int. J. Pharm. 532, 229 (2017).
F. Esmaeili, M. Hosseini-Nasr, et al., Nanomedicine 3, 161 (2007).
D. Fang, M. Singkh, D. Kheigan, and M. Khora, RF Inventor’s Certificate No. 2257198 C2 (2004).
P. Marizza, L. Pontoni, T. Rindzevicius, et al., J. Supercrit. Fluids 107, 145 (2016).
B. S. Sekhon, J. Pharm. Tech. Res. 2, 810 (2010).
S. P. Cape, J. A. Villa, E. T. S. Huang, T. H. Yang, J. F. Carpenter, and R. E. Sievers, Pharm. Res. 25, 1967 (2008).
I. Pasquali, R. Bettini, and F. Giordano, Adv. Drug Deliv. Rev. 60, 399 (2008).
I. Pasquali and R. Bettini, Int. J. Pharm. 364, 176 (2008).
S. G. Kazarian, Polym. Sci., Ser. C 42, 78 (2000).
S. M. Howdle, M. S. Watson, M. J. Whitaker, V. K. Popov, et al., Chem. Commun., 109 (2001).
H. Tai, V. K. Popov, K. M. Shakesheff, and S. M. Howdle, Biochem. Soc. Trans. 35, 516 (2007).
S. E. Bogorodskii, L. I. Krotova, A. V. Mironov, and V. K. Popov, Russ.J. Phys. Chem. B 7, 916 (2013).
S. E. Bogorodski, L. I. Krotova, S. V. Kursakov, S. A. Minaeva, V. K. Popov, and V. I. Sevast’yanov, Russ.J. Phys. Chem. B 9, 1011 (2015).
E. V. Kudryashova, I. M. Deygen, K. V. Sukhoverkov, L. Yu. Filatova, N. L. Klyachko, A. M. Vorobei, O. I. Pokrovskiy, K. B. Ustinovich, O. O. Parenago, E. N. Antonov, A. G. Dunaev, L. I. Krotova, V. K. Popov, and A. M. Egorov, Russ.J. Phys. Chem. B 9, 1201 (2016).
E. N. Antonov and V. K. Popov, Russ. J. Phys. Chem. B 8, 980 (2014).
V. N. Bagratashvili, S. E. Bogorodskii, A. M. Egorov, L. I. Krotova, V. K. Popov, and V. I. Sevast’yanov, Russ. J. Phys. Chem. B 10, 1123 (2016).
USP 38-NF31/1092/ The Dissolution Procedure: Development and Validation, 1090-1097 (Rockville, MD, 2015).
M. K. Sedova, Cand. Sci. (Pharm. Sci.) Dissertation (Zakusov Inst. Pharmacology, Moscow, 2015).
F. M. Gumerov, Sub- and Supercritical Fluids in Polymer Processing (Fen, Kazan, 2000) [in Russian].
Funding
The study was supported by the Ministry of Science and Higher Education of the Russian Federation as part of a state order for FSRC “Crystallography and Photonics” of the Russian Academy of Sciences with respect to the development of SCF techniques for the formation of bioactive scaffold structures and by the Russian Foundation for Basic Research (no. 18-29-06062 mk) with respect to the development of pharmaceutical dosage forms of prolonged action.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. V. Makeeva
Rights and permissions
About this article
Cite this article
Antonov, E.N., Bogorodsky, S.E., Dunaev, A.G. et al. Development of Components of Prolonged Action Antibacterial Dosage Forms Using SCF Technologies. Russ. J. Phys. Chem. B 14, 1108–1115 (2020). https://doi.org/10.1134/S1990793120070027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793120070027