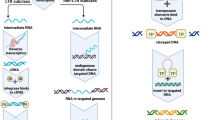
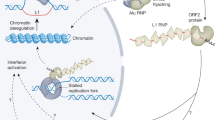
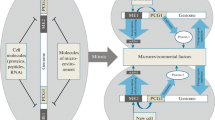
Abstract
The dysregulation of transposable elements plays a key role in human carcinogenesis. Physiological aging in humans is also caused by the deregulation of transposons. Moreover, aging is associated with the development of cancer. We present the results of an analysis of data on the presence of common epigenetic changes during aging and carcinogenesis, associated with changes in the expression of microRNAs derived from transposons. We find that aging is characterized by changes in the expression of 21 specific transposon-derived microRNAs associated with the development of malignant neoplasms. Before us, evidence similar to ours on the relationship between the mechanisms of aging and carcinogenesis at the epigenetic level has not been presented. We hypothesize that one of the key mechanisms of aging is an imbalance in the programmed activation of mobile genetic elements, which is reflected in changes in the body’s epigenetic regulation and leads to an increased risk of cancer. Since microRNA precursors can be translated with the formation of functional molecules, peptides used in gerontology can be considered as potential anticancer drugs.
Similar content being viewed by others
REFERENCES
Mustafin, R.N. and Khusnutdinova, E.K., The role of interactions between transposons and epigenetic factors in aging, Adv. Gerontol., 2017, no. 4, pp. 516–528.
Khavinson, V.Kh., Kuznik B.I., and Ryzhak, G.A., Peptide bioregulators: The new class of geroprotectors. Communication 1. Results of experimental studies, Usp. Gerontol., 2012, vol. 25, no. 4, pp. 696–708.
Khavinson, V.Kh., Therapeutic peptides: Past, present, future, Klin. Med., 2020, vol. 98, no. 3, pp. 165–177.
Andrenacci, D., Cavaliere, V., and Lattanzi, G., The role of transposable elements activity in aging and their possible involvement in laminopathic diseases, Ageing Res. Rev., 2020, vol. 57, p. 1000995.
Ahwazi, R.P., Kiani, M., Dinarvand, M., et al., Immobilization of HIV-1 Tat peptide on gold nanoparticles: A feasible approach for siRNA delivery, J. Cell Physiol., 2020, vol. 235, pp. 2049–2059.
Anwar, S.L., Wulaningsih, W., and Lehmann, U., Transposable elements in human cancer: Causes and consequences of deregulation, Int. J. Mol. Sci., 2017, vol. 18. Е974.
Attermann, A.S., Bjerregaard, A.M., Saini, S.K., et al., Human endogenous retroviruses and their implication for immunotherapeutics of cancer, Ann. Oncol., 2018, vol. 29, pp. 2183–2191.
Autio, A., Nevalainen, T., Mishra, B.H., et al., Effect of aging on the transcriptomic changes associated with the expression of the HERV-K (HML-2) provirus at 1q22, Immunol. Ageing, 2020, vol. 17, p. 11.
Baker, J.R., Vuppusetty, C., Colley, T., et al., MicroRNA-570 is a novel regulator of cellular senescence and inflammaging, FASEB J., 2019, vol. 33, pp. 1605–1616.
Behbahanipour, M., Peymani, M., Salari, M., et al., Expression profiling of blood microRNAs 885, 361, and 17 in the patients with the Parkinson’s disease: integrating interaction data to uncover the possible triggering age-related mechanisms, Sci. Rep., 2019, vol. 9, p. 13759.
Bermejo, A.V., Ragonnaud, E., Daradoumis, J., and Holst, P., Cancer associated endogenous retroviruses: Ideal immune target for adenovirus-based immunotherapy, Int. J. Mol. Sci., 2020, vol. 21, p. 4843.
Cardelli, M., The epigenetic alterations of endogenous retroelements in aging, Mech. Ageing Dev., 2018, vol. 174, pp. 30–46.
Chen, H., Zheng, X., Xiao, D., and Zheng, Y., Age-associated de-repression of retrotransposons in the Drosophila fat body, its potential cause and consequence, Aging Cell, 2016, vol. 15, pp. 542–552.
Chen, Y.F., Stampley, J.E., Irving, B.A., and Tammy, R.D., Chronic nucleoside reverse transcriptase inhibitors disrupt mitochondrial homeostasis and promote premature endothelial senescence, Toxicol. Sci., 2019, vol. 172, pp. 445–456.
Cho, J.H., Dimri, M., and Dimri, G.P., MicroRNA-31 is a transcriptional target of histone deacetylase inhibitors and a regulator of cellular senescence, J. Biol. Chem., 2015, vol. 290, pp. 10555–10567.
Couzigou, J.M., Andre, O., Guillotin, B., et al., Use of microRNA-encoded peptide miPEP172c to stimulate nodulation in soybean, New Phytol., 2016, vol. 211, pp. 379–381.
Dahiya, N., Sarachana, T., Kulkarni S. et al., MiR-570 interacts with mitochondrial ATPase subunit g(ATP5L) encoding mRNA in stored platelets, Platelets, 2017, vol. 28, pp. 74–81.
De Cecco, M., Criscione, S.W., Peckham, E.J., et al., Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements, Aging Cell, 2013, vol. 12, pp. 247–256.
De Cecco, M., Ito, T., Petrashen, A.P., et al., L1 drives IFN in senescent cells and promotes age-associated inflammation, Nature, 2019, vol. 566, pp. 73–78.
Dellago, H., Preschitz-Kammerhofer, B., Terlecki-Zaniewicz, L., et al., High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan, Aging Cell, 2013, vol. 12, pp. 446–458.
Dluzen, D.F., Kim, Y., Bastian, P., et al., MicroRNAs modulate oxidative stress in hypertension through PARP-1 regulation, Oxid. Med. Cell. Longev., 2017, vol. 2017, p. 3984280.
El Baidouri, M., Kim, K.D., Abernathy, B., et al., A new approach for annotation of transposable elements using small RNA mapping, Nucleic Acids Res., 2015, vol. 43. е84.
Elsner, D., Meusemann, K., and Korb, J., Longevity and transposon defense, the case of termite reproductives, Proc. Nat. Acad. Sci. U.S.A., 2018, vol. 115, pp. 5504–5509.
Erichsen, L., Beermann, A., Arauzo-Bravo, M.J., et al., Genome-wide hypomethylation of LINE-1 and Alu retroelements in cell-free DNA of blood is an epigenetic biomarker of human aging, Saudi. J. Biol. Sci., 2018, vol. 25, pp. 1220–1226.
Fang, J., Morsalin, S., Rao, V.N., and Reddy, E.S.P., Decoding of non-coding DNA and non-coding RNA: Pri-micro RNA-encoded novel peptides regulate migration of cancer cells, J. Pharm. Sci. Pharmacol., 2017, vol. 3, pp. 23–27.
Filshtein, T.J., Mackenzie, C.O., Dale, M.D., et al., Origin-based identification of microRNA targets, Mobile Genet. Elements, 2011, vol. 2, pp. 184–192.
Gregory, P.A., Bert, A.G., Paterson, E.L., et al., The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nat. Cell. Biol., 2008, vol. 10, pp. 593–601.
Guo, D., Ye, Y., Qi, J., et al., Age and sex differences in microRNAs expression during the process of thymus aging, Acta Biochim. Biophys. Sin. (Shanghai), 2017, vol. 49, рр. 409–419.
Haendeler, J., Hoffmann, J., Diehl, J.F., et al., Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells, Circ. Res., 2004, vol. 94, pp. 768–775.
Huang, J.Z., Chen, M., Chen, D., et al., A peptide encoded by a putative lncRNA HOXB-AS3 suppresses colon cancer growth, Mol. Cell, 2017, vol. 68, pp. 171–184.
Ipson, B.R., Fletcher, M.B., Espinoza, S.E., and Fisher, A.L., Identifying exosome-derived microRNAs as candidate biomarkers of frailty, J. Frailty Aging, 2018, vol. 7, pp. 100–103.
Juliano, C.E., Reich, A., Liu, N., et al., Piwi proteins and Piwi-interacting RNAs function in Hydra somatic stem cells, Proc. Nat. Acad. Sci. U.S.A., 2014, vol. 111, pp. 337–342.
Kang, M., Tang, B., Li, J., et al., Identification of miPEP133 as a novel tumor-suppressor microprotein encoded by miR-34a pri-miRNA, Mol. Cancer, 2020, vol. 19, p. 143.
KarakaUlah, G. and Yandim, C., Signature changes in the expressions of protein-coding genes, lncRNAs, and repeat elements in early and late cellular senescence, Turk. J. Biol., 2020, vol. 44, pp. 356–370.
Kurth, J., Krause, B.J., Schwarzenbock, S.M., et al., First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer, Europ. J. Nucl. Med. Mol. Imaging, 2020, vol. 47, pp. 123–135.
Lee, B.P., Buric, I., George-Pandeth, A., et al., MicroRNAs miR-203-3p, miR-664-3p and miR-708-5p are associated with median strain lifespan in mice, Sci. Rep., 2017, vol. 7, p. 44620.
Li, X., Song, Y., Liu, D., et al., MiR-495 promotes senescence of mesenchymal stem cells by targeting BMI-1, Cell. Physiol. Biochem., 2017, vol. 42, pp. 780–796.
Nidadavolu, L.S., Niedernhofer, L.J., and Khan, S.A., Identification of microRNAs dysregulated in cellular senescence driven by endogenous genotoxic stress, Aging (Albany N.Y.), 2013, vol. 5, рр. 460–473.
Niu, L., Lou, F., Sun, Y., et al., A micropeptide encoded by lncRNA MIR155HG suppresses autoimmune inflammation via modulating antigen presentation, Sci. Adv., 2020, vol. 6. eaaz2059.
Noren Hooten, N., Fitzpatrick, M., and Wood, W.H., 3rd, et al., Age-related changes in microRNA levels in serum, Aging (Albany N.Y.), 2013, vol. 5, рр. 725–740.
Oyama, Y., Onishi, H., Koga, S., et al., Patched 1-interacting peptide represses fibrosis in pancreatic cancer to augment the effectiveness of immunotherapy, J. Immunother., 2019, vol. 43, pp. 121–133. https://doi.org/10.1097/CJI.0000000000000305
Pal, S. and Tyler, J.K., Epigenetics and aging, Sci. Adv., 2016, vol. 2. e1600584.
Prel, A., Dozier, C., Combier, J.P., et al., Evidence that regulation of pri-miRNA/miRNA expression is not a general rule of miPEPs function in humans, Int. J. Mol. Sci., 2021, vol. 22, p. 3432.
Qin, S., Jin, P., Zhou, X., et al., The role of transposable elements in the origin and evolution of microRNAs in human, PLoS One, 2015, vol. 10. e0131365.
Raihan, O., Brishti, A., Molla, M.R., et al., The age-dependent elevation of miR-335-3p leads to reduced cholesterol and impaired memory in brain, Neuroscience, 2018, vol. 390, pp. 160–173.
Roberts, J.T., Cardin, S.E., and Borcehrt, G.M., Burgeoning evidence indicates that microRNAs were initially formed from transposable element sequences, Mobile Genet. Elements, 2014, vol. 4. e29255.
Robertson, P.A., Romero, M.A., Osburn, S.C., et al., Skeletal muscle LINE-1 ORF1 mRNA is higher in older humans but decreases with endurance exercise and is negatively associated with higher physical activity, J. Appl. Physiol., 2019, vol. 127, no. 4, pp. 895–904.
Rodriguez-Martin, B., Alvarez, E.G., Baez-Ortega, A., et al., Pan-cancer analysis of whole genomes identifies driver rearrangements promoted by LINE-1 retrotransposition, Nat. Genet., 2020, vol. 52, pp. 306–319.
Sataranatarajan, K., Feliers, D., Mariappan, M.M., et al., Molecular events in matrix protein metabolism in the aging kidney, Aging Cell, 2012, vol. 11, pp. 1065–1073.
Schwertz, H. and Rondina, M.T., Do platelets line up for aging? Aging (Albany N.Y.), 2018, vol. 10, рр. 3054–3055.
Seipel, K., Yanze, N., and Schmid, V., The germ line and somatic stem cell gene cniwi in the jellyfish Podocoryne carnea, Int. J. Dev. Biol., 2004, vol. 48, pp. 1–7.
Shahsavari, S., Shaghaghi, Z., Abedi, S.M., and Hosseinimehr, S.J., Evaluation of 99mTc-HYNIC-(Ser)3-LTWPWY peptide for glioblastoma tumor imaging, Int. J. Radiat. Biol., 2019, vol. 12, pp. 1–23. https://doi.org/10.1080/09553002.2020.1704906
Shi, X., Shi, C., Ye, W., et al., Targeted fluorescence imaging and biological effects of peptide conjugated quantum dots on pancreatic cancer cells, J. Nanosci. Nanotchnol., 2020, vol. 20, pp. 1351–1357.
Simon, M., Meter, M.V., Ablaeva, J., et al., LINE1 derepression in aged wild-type and SIRT6-deficient mice derives inflammation, Cell Metab., 2019, vol. 29, pp. 871–885.
Smith-Vikos, T., Liu, Z., Parsons, C., et al., A serum miRNA profile of human longevity: Findings from the Baltimore Longitudinal Study of Aging (BLSA), Aging (Albany N.Y.), 2016, vol. 8, рр. 2971–2987.
Sultana, T., Zamborlini, A., Crostofari, G., and Lesage, P., Integration site selection by retroviruses and transposable elements in eukaryotes, Nat. Rev. Genet., 2017, vol. 18, pp. 292–308.
Tempel, S., Pollet, N., and Tahi, F., NcRNA classifier: a tool for detection and classification of transposable element sequences in RNA hairpins, BMC Bioinformatics, 2012, vol. 13, pp. 246–258.
Terlecki-Zaniewicz, L., Lammermann, I., Latreille, J., et al., Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype, Aging (Albany N.Y.), 2018, vol. 10, рр. 1103–1132.
Testa, U., Pelosi, E., Castelli, G., and Labbaye, C., miR-146 and miR-155: two key modulators of immune response and tumor development, Noncoding RNA, 2017, vol. 3, p. 22.
Ukai, T., Sato, M., Akutsu, H., et al., MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism, J. Orthop. Res., 2012, vol. 30, pp. 1915–1922.
Vazquez, B.N., Thackray, J.K., Simonet, N.G., et al., SIRT7 mediates L1 elements transcriptional repression and their association with the nuclear lamina, Nucleic Acids Res., 2019, vol. 47, pp. 7870–7885.
Wang, J., Geesman, G.J., Hostikka, S.L., et al., Inhibition of activated pericentromeric SINE/Alu repeat transcription in senescent human adult stem cells reinstates self-renewal, Cell Cycle, 2011, vol. 10, pp. 3016–3030.
Wang, J., Zhu, S., Meng, N., et al., NcRNA-encoded peptides or proteins and cancer, Mol. Ther., 2019, vol. 27, pp. 1718–1725.
Wei, G., Qin, S., Li, W., et al., MDTE DB: a database for microRNAs derived from transposable element, IEEE/ACM Trans. Comput. Biol. Bioinform., 2016, vol. 13, pp. 1155–1160.
Wong, N.W., Chen, Y., Chen, S., and Wang, X., OncomiR: an online resource for exploring pan-cancer microRNA dysregulation, Bioinformatics, 2018, vol. 34, pp. 713–715.
Yamada, K., Kaneko, H., Shimizu, H., et al., Lamivudine inhibits Alu RNA-induced retinal pigment epithelium degeneration via anti-inflammatory and anti-senescence activities, Transl. Vis. Sci. Technol., 2020, vol. 9, p. 1. https://doi.org/10.1167/tvst.9.8.1
Yuan, Z., Sun, X., Liu, H., and Xie, J., MicroRNA genes derived from repetitive elements and expanded by segmental duplication events in mammalian genomes, PLoS One, 2011, vol. 6. e17666.
Yuan, S., Shi, Y., Guo, K., and Tang, S., Nucleoside reverse transcriptase inhibitors (NRTIs) induce pathological pain through Wnt5a-mediated neuroinflammation in aging mice, J. Neuroimmune Pharmacol., 2018, vol. 13, pp. 230–236.
Zhang, H., Yang, H., Zhang, C., et al., Investigation of microRNA expression in human serum during the aging process, J. Geront. A Biol. Sci. Med. Sci., 2015, vol. 70, pp. 102–109.
Zhang, T., Brinkley, T.E., Liu, K., et al., Circulating miRNAs as biomarkers of gait speed responses to aerobic exercise training in obese older adults, Aging (Albany N.Y.), 2017, vol. 9, рр. 900–913.
Zhang, Z.K., Li, J., Guan, D., et al., A newly identified lncRNA MaR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration, J. Cachexia Sarcopenia Muscle, 2018, vol. 9, pp. 613–626.
Zhang, J., Wei, F., Ding, L., et al., MicroRNA-1976 regulates degeneration of the sinoatrial node by targeting Cav1.2 and Cav1.3 ion channels, J. Mol. Cell. Cardiol., 2019, vol. 134, pp. 74–85.
Zheng, D., Sabbagh, J.J., Blair, L.J., et al., MicroRNA-511 binds to FKBP5 mRNA, which encodes a chaperone protein, and regulates neuronal differentiation, J. Biol. Chem., 2016, vol. 291, pp. 17 897–17 906.
Zuberi, M., Mir, R., Das, J., et al., Expression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features, Clin. Transl. Oncol., 2015, vol. 17, pp. 779–787.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by P. Kuchina
Rights and permissions
About this article
Cite this article
Mustafin, R.N. Interrelation of MicroRNAs and Transposons in Aging and Carcinogenesis. Adv Gerontol 12, 264–277 (2022). https://doi.org/10.1134/S2079057022030092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057022030092