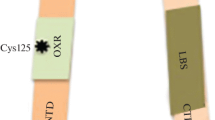
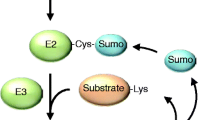
Abstract
This review is devoted to the analysis of proteins of the sestrin family and their role in the response of cells to stress. The paper describes the structure and functions of sestrins and their key role in the regulation of mTOR kinase and metabolism. In addition, the functions of sestrins in the regulation of aging and age-related diseases are considered in detail in this review.






Similar content being viewed by others
REFERENCES
Abe, T., Makino, N., Furukawa, T., et al., Identification of three commonly deleted regions on chromosome arm 6q in human pancreatic cancer, Genes, Chromosomes Cancer, 1999, vol. 25, no. 1, pp. 60–64.
Andrysik, Z., Galbraith, M.D., Guarnieri, A.L., et al., Identification of a core TP53 transcriptional program with highly distributed tumor suppressive activity, Genome Res., 2017, vol. 27, no. 10, pp. 1645–1657.
Bae, S.H., Sung, S.H., Oh, S.Y., et al., Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage, Cell Metab., 2013, vol. 17, no. 1, pp. 73–84.
Bar-Peled, L., Chantranupong, L., Cherniack, A.D., et al., A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1, Science, 2013, vol. 340, no. 6136, pp. 1100–1106.
Ben-Sahra, I., Dirat, B., Laurent, K., et al., Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death, Cell Death Differ., 2013, vol. 20, no. 4, pp. 611–619.
Brace, L.E., Vose, S.C., Stanya, K., et al., Increased oxidative phosphorylation in response to acute and chronic DNA damage, N.P.J. Aging Mech. Dis., 2016, vol. 2, art. ID 16022.
Bruning, A., Rahmeh, M., and Friese, K., Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation, Mol. Oncol., 2013, vol. 7, no. 6, pp. 1012–1018.
Bryk, R., Lima, C. D., Erdjument-Bromage, H., et al., Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein, Science, 2002, vol. 295, no. 5557, pp. 1073–1077.
Budanov, A.V., Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling, Antioxid. Redox Signaling, 2011, vol. 15, no. 6, pp. 1679–1690.
Budanov, A.V., Sestrins link tumor suppressors with the AMPK-MTOR signaling network, in Protein Phosphorylation in Human Health, Huang, C., Ed., Nvi Sad: InTech, 2012, pp. 51–96.
Budanov, A.V., SESTRINs regulate mTORC1 via RRAGs: the riddle of GATOR, Mol. Cell. Oncol., 2015, vol. 2, no. 3, art. ID e997113.
Budanov, A.V. and Karin, M., p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling, Cell, 2008, vol. 134, no. 3, pp. 451–460.
Budanov, A.V., Lee, J.H., and Karin, M., Stressin’ sestrins take an aging fight, EMBO Mol. Med., 2010, vol. 2, no. 10, pp. 388–400.
Budanov, A.V., Shoshani, T., Faerman, A., et al., Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability, Oncogene, 2002, vol. 21, no. 39, pp. 6017–6031.
Budanov, A.V., Sablina, A.A., Feinstein, E., et al., Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD, Science, 2004, vol. 304, no. 5670, pp. 596–600.
Byun, J.K., Choi, Y.-K., Kim, J.-H., et al., A positive feedback loop between Sestrin2 and mTORC2 is required for the survival of glutamine-depleted lung cancer cells, Cell Rep., 2017, vol. 20, no. 3, pp. 586–599.
Carvalho, B., Seruca, R., Buys, C.H.C.M., and Kok, K., Novel expressed sequences obtained by means of a suppression subtractive hybridisation analysis from the 6q21 region that is frequently deleted in gastric cancer, Eur. J. Cancer, 2002, vol. 38, no. 8, pp. 1126–1132.
Chantranupong, L., Wolfson, R.L., Orozco, J.M., et al., The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1, Cell Rep., 2014, vol. 9, no. 1, pp. 1–8.
Chen, C.C., Jeon, S.M., Bhaskar, P.T., et al., FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor, Dev. Cell, 2010, vol. 18, no. 4, pp. 592–604.
Chen, K.B., Xuan, Y., Shi, W.-J., et al., Sestrin2 expression is a favorable prognostic factor in patients with non-small cell lung cancer, Am. J. Transl. Res., 2016, vol. 8, no. 4, pp. 1903–1909.
Chen, Y.-S., Chen, S.-D., Wu, C.-L., et al., Induction of sestrin2 as an endogenous protective mechanism against amyloid beta-peptide neurotoxicity in primary cortical culture, Exp. Neurol., 2014, vol. 253, pp. 63–71.
Cornu, M., Albert, V., and Hall, M.N., mTOR in aging, metabolism, and cancer, Curr. Opin. Genet. Dev., 2013, vol. 23, no. 1, pp. 53–62.
Cuadrado, A., Rojo, A.I., Wells, G., et al., Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases, Nat. Rev. Drug Discovery, 2019, vol. 18, no. 4, pp. 295–317.
Dalina, A.A., Kovaleva, I.E., and Budanov, A.V., Sestrins are gatekeepers in the way from stress to aging and disease, Mol. Biol., 2018, vol. 52, no. 6, pp. 948–962.
D’Autreaux, B. and Toledano, M.B., ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis, Nat. Rev. Mol. Cell Biol., 2007, vol. 8, no. 10, pp. 813–824.
Ding, B., Parmigiani, A., Yang, C., and Budanov, A.V., Sestrin2 facilitates death receptor-induced apoptosis in lung adenocarcinoma cells through regulation of XIAP degradation, Cell Cycle, 2015, vol. 14, no. 20, pp. 3231–3241.
Ding, B., Parmigiani, A., Divakaruni, A.S., et al., Sestrin2 is induced by glucose starvation via the unfolded protein response and protects cells from non-canonical necroptotic cell death, Sci. Rep., 2016, vol. 6, art. ID 22538.
Doonan, F., Wallace, D.M., O’Driscoll, C., and Cotter, T.G., Rosiglitazone acts as a neuroprotectant in retinal cells via up-regulation of sestrin-1 and SOD-2, J. Neurochem., 2009, vol. 109, no. 2, pp. 631–643.
Ebnoether, E., Ramseier, A., Cortada, M., et al., Sesn2 gene ablation enhances susceptibility to gentamicin-induced hair cell death via modulation of AMPK/mTOR signaling, Cell Death Discovery, 2017, vol. 3, art. ID 17024.
Eid, A.A., Lee, D.-Y., Roman, L.J., et al., Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression, Mol. Cell Biol., 2013, vol. 33, no. 17, pp. 3439–3460.
Eijkelenboom, A. and Burgering, B.M., FOXOs: signalling integrators for homeostasis maintenance, Nat. Rev. Mol. Cell Biol., 2013, vol. 14, no. 2, pp. 83–97.
Fischer, M., Census and evaluation of p53 target genes, Oncogene, 2017, vol. 36, no. 28, pp. 3943–3956.
Gan, X., Wang, J., Su, B., and Wu, D., Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate, J. Biol. Chem., 2011, vol. 286, no. 13, pp. 10998–11002.
Garaeva, A.A., Kovaleva, I.E., Chumakov, P.M., and Evstafieva, A.G., Mitochondrial dysfunction induces SESN2 gene expression through Activating Transcription Factor 4, Cell Cycle, 2016, vol. 15, no. 1, pp. 64–71.
Green, D.R., Galluzzi, L., and Kroemer, G., Mitochondria and the autophagy-inflammation-cell death axis in organismal aging, Science, 2011, vol. 333, no. 6046, pp. 1109–1112.
Hagenbuchner, J., Kuznetsov, A., Hermann, M., et al., FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3, J. Cell Sci., 2012, vol. 125, no. 5, pp. 1191–1203.
Hatano, N., Nishikawa, N.S., McElgunn, C., et al., A comprehensive analysis of loss of heterozygosity caused by hemizygous deletions in renal cell carcinoma using a subtraction library, Mol. Carcinog., 2001, vol. 31, no. 3, pp. 161–170.
Heidler, J., Fysikopoulos, A., Wempe, F., et al., Sestrin-2, a repressor of PDGFRβ signalling, promotes cigarette-smoke-induced pulmonary emphysema in mice and is upregulated in individuals with COPD, Dis. Models Mech., 2013, vol. 6, no. 6, pp. 1378–1387.
Hou, Y.S., Guan, J.-J., Xu, H.-D., et al., Sestrin2 protects dopaminergic cells against rotenone toxicity through AMPK-dependent autophagy activation, Mol. Cell Biol., 2015, vol. 35, no. 16, pp. 2740–2751.
Hwang, H.-J., Jung, T.W., Choi, J.-H., et al., Knockdown of sestrin2 increases pro-inflammatory reactions and ER stress in the endothelium via an AMPK dependent mechanism, Biochim. Biophys. Acta, 2017, vol. 1863, no. 6, pp. 1436–1444.
Jegal, K.H., Park, W., Cho, S.S., et al., Activating transcription factor 6-dependent sestrin 2 induction ameliorates ER stress-mediated liver injury, Biochim. Biophys. Acta, 2017, vol. 1864, no. 7, pp. 1295–1307.
Jeong, W., Bae, S.H., Toledano, M.B., and Rhee, S.G., Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression, Free Radical Biol. Med., 2012, vol. 53, no. 3, pp. 447–456.
Johnson, M.R., Behmoaras, J., Bottolo, L., et al., Systems genetics identifies Sestrin 3 as a regulator of a proconvulsant gene network in human epileptic hippocampus, Nat. Commun., 2015, vol. 6, art. ID 6031.
Johnson, S.C., Rabinovitch, P.S., and Kaeberlein, M., mTOR is a key modulator of ageing and age-related disease, Nature, 2013, vol. 493, no. 7432, pp. 338–345.
Kallenborn-Gerhardt, W., Lu, R., Syhr, K.M.J., et al., Antioxidant activity of sestrin 2 controls neuropathic pain after peripheral nerve injury, Antioxid. Redox Signaling, 2013, vol. 19, no. 17, pp. 2013–2023.
Kamata, H., Honda, S.-I., Maeda, S., et al., Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases, Cell, 2005, vol. 120, no. 5, pp. 649–661.
Kastenhuber, E.R. and Lowe, S.W., Putting p53 in context, Cell, 2017, vol. 170, no. 6, pp. 1062–1078.
Kim, H., An, S., Ro, S.H., et al., Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains, Nat. Commun., 2015, vol. 6, art. ID 10025.
Kim, J.-R., Lee, S.-R., Chung, H.L., et al., Identification of amyloid beta-peptide responsive genes by cDNA microarray technology: involvement of RTP801 in amyloid beta-peptide toxicity, Exp. Mol. Med., 2003, vol. 35, no. 5, pp. 403–411.
Kim, M.G., Yang, J.H., Kim, K.M., et al., Regulation of Toll-like receptor-mediated Sestrin2 induction by AP-1, Nrf2, and the ubiquitin-proteasome system in macrophages, Toxicol. Sci., 2015, vol. 144, no. 2, pp. 425–435.
Kim, M.J., Bae, S.H., Ryu, J.-C., et al., SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages, Autophagy, 2016, vol. 12, no. 8, pp. 1272–1291.
Kimball, S.R., Gordon, B.S., Moyer, J.E., et al., Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation, Cell Signaling, 2016, vol. 28, no. 8, pp. 896–906.
Kourtis, N. and Tavernarakis, N., Cellular stress response pathways and ageing: intricate molecular relationships, EMBO J., 2011, vol. 30, no. 13, pp. 2520–2531.
Kruiswijk, F., Labuschagne, C.F., and Vousden, K.H., p53 in survival, death and metabolic health: a lifeguard with a licence to kill, Nat. Rev. Mol. Cell Biol., 2015, vol. 16, no. 7, pp. 393–405.
Kinnula, V.L., Paakko, P., and Soini, Y., Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung, FEBS Lett., 2004, vol. 569, nos. 1–3, pp. 1–6.
Kopnin, P.B., Agapova, L.S., Kopnin, B.P., and Chumakov, P.M., Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability, Cancer Res., 2007, vol. 67, no. 10, pp. 4671–4678.
Lane, D.P., Cancer. p53, guardian of the genome, Nature, 1992, vol. 358, no. 6381, pp. 15–16.
Lanna, A., Gomes, D.C.O., Muller-Durovic, B., et al., A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging, Nat. Immunol., 2017, vol. 18, pp. 354–363.
Lee, J.H., Budanov, A.V., Park, E.J., et al., Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies, Science, 2010, vol. 327, no. 5970, pp. 1223–1228.
Lee, J.H., Budanov, A.V., Talukdar, S., et al., Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3, Cell Metab., 2012, vol. 16, no. 3, pp. 311–321.
Lee, J.H., Budanov, A.V., and Karin, M., Sestrins orchestrate cellular metabolism to attenuate aging, Cell Metab., 2013, vol. 18, no. 6, pp. 792–801.
Lehmann, S., Ogawa, S., Raynaud, S.D., et al., Molecular allelokaryotyping of early-stage, untreated chronic lymphocytic leukemia, Cancer, 2008, vol. 112, no. 6, pp. 1296–1305.
Leister, I., Weith, A., Brüderlein, S., et al., Human colorectal cancer: high frequency of deletions at chromosome 1p35, Cancer Res., 1990, vol. 50, no. 22, pp. 7232–7235.
Levine, A.J., p53, the cellular gatekeeper for growth and division, Cell, 1997, vol. 88, no. 3, pp. 323–331.
Li, D.D., Sun, T., Wu, X.-Q., et al., The inhibition of autophagy sensitises colon cancer cells with wild-type p53 but not mutant p53 to topotecan treatment, PLoS One, 2012, vol. 7, no. 9, art. ID e45058.
Liu, S.Y., Lee, Y.J., and Lee, T.C., Association of platelet-derived growth factor receptor beta accumulation with increased oxidative stress and cellular injury in sestrin 2 silenced human glioblastoma cells, FEBS Lett., 2011, vol. 585, no. 12, pp. 1853–1858.
Lopez-Otin, C., Blasco, M.A., Partridge, L., et al., The hallmarks of aging, Cell, 2013, vol. 153, no. 6, pp. 1194–1217.
Majd, S., Power, J.H., and Grantham, H.J., Neuronal response in Alzheimer’s and Parkinson’s disease: the effect of toxic proteins on intracellular pathways, BMC Neurosci., 2015, vol. 16, art. ID 69.
Manning, B.D. and Toker, A., AKT/PKB signaling: navigating the network, Cell, 2017, vol. 169, no. 3, pp. 381–405.
Martindale, J.L. and Holbrook, N.J., Cellular response to oxidative stress: signaling for suicide and survival, J. Cell Physiol., 2002, vol. 192, no. 1, pp. 1–15.
Mizushima, N., Autophagy: process and function, Genes Dev., 2007, vol. 21, no. 22, pp. 2861–2873.
Mizumura, K., Cloonan, S.M., Nakahira, K., et al., Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD, J. Clin. Invest., 2014, vol. 124, no. 9, pp. 3987–4003.
Morrison, A., Chen, L., Wang, J., et al., Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart, FASEB J., 2015, vol. 29, no. 2, pp. 408–417.
Nagai, H., Negrini, M., Carter, S.L., et al., Detection and cloning of a common region of loss of heterozygosity at chromosome 1p in breast cancer, Cancer Res., 1995, vol. 55, no. 8, pp. 1752–1757.
Nogueira, V., Park, Y., Chen, C.C., et al., Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis, Cancer Cell, 2008, vol. 14, no. 6, pp. 458–470.
Oricchio, E., Katanayeva, N., Donaldson, M.C., et al., Genetic and epigenetic inactivation of SESTRIN1 controls mTORC1 and response to EZH2 inhibition in follicular lymphoma, Sci. Transl. Med., 2017, vol. 9, no. 396, pp. eaak9969.
Papadia, S., Soriano, F.X., Léveillé, F., et al., Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses, Nat. Neurosci., 2008, vol. 11, no. 4, pp. 476–487.
Park, H.-W., Park, H., Ro, S.-H., et al., Hepatoprotective role of Sestrin2 against chronic ER stress, Nat. Commun., 2014, vol. 5, art. ID 4233.
Parmigiani, A. and Budanov, A.V., Sensing the environment through sestrins: implications for cellular metabolism, Int. Rev. Cell Mol. Biol., 2016, vol. 327, pp. 1–42.
Parmigiani, A., Nourbakhsh, A., Ding, B., et al., Sestrins inhibit mTORC1 kinase activation through the GATOR complex, Cell Rep., 2014, vol. 9, no. 4, pp. 1281–1291.
Peeters, H., Debeer, P., Bairoch, A., et al., PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse, Hum. Genet., 2003, vol. 112, nos. 5–6, pp. 573–580.
Peng, M., Yin, N., and Li, M.O., Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling, Cell, 2014, vol. 159, no. 1, pp. 122–133.
Peng, M., Yin, N., and Li, M.O., SZT2 dictates GATOR control of mTORC1 signalling, Nature, 2017, vol. 543, no. 7645, pp. 433–437.
Polyak, K., Xia, Y., Zweier, J.L., et al., A model for p53-induced apoptosis, Nature, 1997, vol. 389, no. 6648, pp. 300–305.
Quan, N., Sun, W., Wang, L., et al., Sestrin2 prevents age-related intolerance to ischemia and reperfusion injury by modulating substrate metabolism, FASEB J., 2017, vol. 31, no. 9, pp. 4153–4167.
Reddy, K., Cusak, C.L., Nnah, I., et al., Dysregulation of nutrient sensing and CLEARance in presenilin deficiency, Cell. Rep., 2016, vol. 14, no. 9, pp. 2166–2179.
Ro, S.-H., Xue, X., Ramakrishnan, S.K., et al., Tumor suppressive role of sestrin2 during colitis and colon carcinogenesis, eLife, 2016, vol. 5, art. ID e12204.
Sablina, A.A., Budanov, A.V., Ilyinskaya, G.V., et al., The antioxidant function of the p53 tumor suppressor, Nat. Med., 2005, vol. 11, no. 12, pp. 1306–1313.
Sancak, Y., Peterson, T.R., Shaul, Y.D., et al., The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1, Science, 2008, vol. 320, no. 5882, pp. 1496–1501.
Sanli, T., Linher-Melville, K., Tsakiridis, T., and Singh, G., Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells, PLoS One, 2012, vol. 7, no. 2, art. ID e32035.
Sarbassov, D.D., Guertin, D.A., Ali, S.M., and Sabatini, D.M., Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex, Science, 2005, vol. 307, no. 5712, pp. 1098–1101.
Saveljeva, S., Cleary, P., Mnich, K., et al., Endoplasmic reticulum stress-mediated induction of SESTRIN 2 potentiates cell survival, Oncotarget, 2016, vol. 7, no. 11, pp. 12254–12266.
Saxton, R.A. and Sabatini, D.M., mTOR signaling in growth, metabolism, and disease, Cell, 2017, vol. 168, no. 6, pp. 960–976.
Saxton, R.A., Knockenhauer, K.E., Wolfson, R.L., et al., Structural basis for leucine sensing by the Sestrin2–mTORC1 pathway, Science, 2016, vol. 351, no. 6268, pp. 53–58.
Scheibye-Knudsen, M., Fang, E.F., Croteau, D.L., et al., Protecting the mitochondrial powerhouse, Trends Cell. Biol., 2015, vol. 25, no. 3, pp. 158–170.
Seo, K., Ki, S.H., and Shin, S.M., Sestrin2-AMPK activation protects mitochondrial function against glucose deprivation-induced cytotoxicity, Cell Signaling, 2015, vol. 27, no. 7, pp. 1533–1543.
Settembre, C., Fraldi, A., Medina, D.L., Ballabio, A., et al., Signals from the lysosome: a control centre for cellular clearance and energy metabolism, Nat. Rev. Mol. Cell Biol., 2013, vol. 14, no. 5, pp. 283–296.
Tao, R., Xiong, X., Liangpunsakul, S., Dong, X.C., et al., Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling, Diabetes, 2015, vol. 64, no. 4, pp. 1211–1223.
Thelander, E.F., Ichimura, K., Corcoran, M., et al., Characterization of 6q deletions in mature B cell lymphomas and childhood acute lymphoblastic leukemia, Leuk. Lymphoma, 2008, vol. 49, no. 3, pp. 477–487.
Tomasovic, A., Kurrle, N., Sürün, D., et al., Sestrin 2 protein regulates platelet-derived growth factor receptor β (Pdgfrβ) expression by modulating proteasomal and Nrf2 transcription factor functions, J. Biol. Chem., 2015, vol. 290, no. 15, pp. 9738–9752.
Tran, H., Brunet, A., Grenier, J.M., et al., DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein, Science, 2002, vol. 296, no. 5567, pp. 530–534.
Tsilioni, I., Filippidis, A.S., Kerenidi, T., et al., Sestrin-2 is significantly increased in malignant pleural effusions due to lung cancer and is potentially secreted by pleural mesothelial cells, Clin. Biochem., 2016, vol. 49, no. 9, pp. 726–728.
Velasco-Miguel, S., Buckbinder, L., Jean, P., et al., PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes, Oncogene, 1999, vol. 18, no. 1, pp. 127–137.
Walter, P. and Ron, D., The unfolded protein response: from stress pathway to homeostatic regulation, Science, 2011, vol. 334, no. 6059, pp. 1081–1086.
Wei, C.L., Wu, Q., Vega, V.B., et al., A global map of p53 transcription-factor binding sites in the human genome, Cell, 2006, vol. 124, no. 1, pp. 207–219.
Wei, J.L., et al., Decreased expression of sestrin 2 predicts unfavorable outcome in colorectal cancer, Oncol. Rep., 2015, vol. 33, no. 3, pp. 1349–1357.
Wempe, F., De-Zolt, S., Koli, K., et al., Inactivation of sestrin 2 induces TGF-β signaling and partially rescues pulmonary emphysema in a mouse model of COPD, Dis. Models Mech., 2010, vol. 3, nos. 3–4, pp. 246–253.
White, P.S., Kaufman, B.A., Marshall, H.N., and Brodeur, G.M., Use of the single-strand conformation polymorphism technique to detect loss of heterozygosity in neuroblastoma, Genes, Chromosomes Cancer, 1993, vol. 7, no. 2, pp. 102–108.
Wolfson, R.L., Chantranupong, L., Saxton, R.A., et al., Sestrin2 is a leucine sensor for the mTORC1 pathway, Science, 2016, vol. 351, no. 6268, pp. 43–48.
Wolfson, R.L. and Sabatini, D.M., The dawn of the age of amino acid sensors for the mTORC1 pathway, Cell Metab., 2017, vol. 26, no. 2, pp. 301–309.
Wolfson, R.L., Chantranupong, L., Wyant, G.A., et al., KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1, Nature, 2017, vol. 543, no. 7645, pp. 438–442.
Woo, H.A., Bae, S.H., Park, S., and Rhee, S.G., Sestrin 2 is not a reductase for cysteine sulfinic acid of peroxiredoxins, Antioxid. Redox Signaling, 2009, vol. 11, no. 4, pp. 739–745.
Wood, Z.A., Poole, L.B., and Karplus, P.A., Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling, Science, 2003, vol. 300, no. 5619, pp. 650–653.
Wullschleger, S., Loewith, R., and Hall, M.N., TOR signaling in growth and metabolism, Cell, 2006, vol. 124, no. 3, pp. 471–484.
Xu, C., Bailly-Maitre, B., and Reed, J.C., Endoplasmic reticulum stress: cell life and death decisions, J. Clin. Invest., 2005, vol. 115, no. 10, pp. 2656–2664.
Yang, J.H., Kim, K.M., Kim, M.G., et al., Role of sestrin2 in the regulation of proinflammatory signaling in macrophages, Free Radic. Biol. Med., 2015, vol. 78, pp. 156–167.
Yang, K., Xu, C., Zhang, Y., et al., Sestrin2 suppresses classically activated macrophages-mediated inflammatory response in myocardial infarction through inhibition of mTORC1 signaling, Front. Immunol., 2017, vol. 8, art. ID 728.
Yang, Y.-L., Loh, K.-S., Liou, B.-Yu., et al., SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans, Exp. Gerontol., 2013, vol. 48, no. 3, pp. 371–379.
Ye, J., Palm, W., Peng, M., et al., GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2, Gen. Dev., 2015, vol. 29, no. 22, pp. 2331–2336.
Yeh, S.H., Chen, P.J., Chen, H.L., et al., Frequent genetic alterations at the distal region of chromosome 1p in human hepatocellular carcinomas, Cancer Res., 1994, vol. 54, no. 15, pp. 4188–4192.
Yoshida, T., Mett, I., Bhunia, A.K., et al., Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema, Nat. Med., 2010, vol. 16, no. 7, pp. 767–773.
Zhang, C., Sun, W., Li, J., et al., Loss of sestrin 2 potentiates the early onset of age-related sensory cell degeneration in the cochlea, Neuroscience, 2017, vol. 361, pp. 179–191.
Zhao, B., Shah, P., Budanov, A.V., et al., Sestrin2 protein positively regulates AKT enzyme signaling and survival in human squamous cell carcinoma and melanoma cells, J. Biol. Chem., 2014, vol. 289, no. 52, pp. 35806–35814.
Zhao, B., Shah, P., Qiang, L. et al., Distinct role of Sesn2 in response to UVB-Induced DNA damage and UVA-induced oxidative stress in melanocytes, Photochem. Photobiol., 2017, vol. 93, no. 1, pp. 375–381.
Zeltukhin, A.O., Ilyinskaya, G.V., Budanov, A.V., and Chumakov, P.M., Some phenotypic characteristics of nematode Caenorhabditis elegans strain with defective functions of the sestrin (cSesn) gene, Biomed. Pharmacol. J., 2018, vol. 11, no. 2, pp. 759–767.
Zhou, D., Zhan, C., Zhong, Q., and Li, S., Upregulation of sestrin-2 expression via P53 protects against 1-methyl-4-phenylpyridinium (MPP+)neurotoxicity, J. Mol. Neurosci., 2013, vol. 51, no. 3, pp. 967–975.
Zighelboim, I., Goodfellow, P.J., Schmidt, A.P., et al., Differential methylation hybridization array of endometrial cancers reveals two novel cancer-specific methylation markers, Clin. Cancer Res., 2007, vol. 13, no. 10, pp. 2882–2889.
ACKNOWLEDGMENTS
The author thanks P.M. Chumakov for long-term cooperation and assistance in the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest. This article does not contain any studies involving humans and animals as subjects of study.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Budanov, A.V. The Role of Sestrins in the Regulation of the Cellular Response to Stress. Biol Bull Rev 12, 347–364 (2022). https://doi.org/10.1134/S2079086422040028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086422040028