Abstract
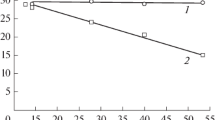
A model is developed for describing the transport of charged colloidal particles in an evaporating sessile droplet on the electrified metal substrate in the presence of a solvent flow. The model takes into account the electric charge of colloidal particles and small ions produced by electrolytic dissociation of the active groups on the colloidal particles and solvent molecules. We employ a system of self-consistent Poisson and Nernst–Planck equations for electric potential and average concentrations of colloidal particles and ions with the appropriate boundary conditions. The fluid dynamics, temperature distribution and evaporation process are described with the Navier–Stokes equations, equations of heat conduction and vapor diffusion in air, respectively. The developed model is used to carry out a first-principles numerical simulation of charged silica colloidal particle transport in an evaporating aqueous droplet. We find that electric double layers can be destroyed by a sufficiently strong fluid flow.
Graphic Abstract








Similar content being viewed by others
References
D. Zang, S. Tarafdar, Y.Y. Tarasevich, M.D. Choudhury, T. Dutta, Evaporation of a droplet: From physics to applications. Phys. Rep. 804, 1 (2019)
K.S. Kolegov, L.Y. Barash, Applying droplets and films in evaporative lithography. Adv. Coll. Interface. Sci. 285, 102271 (2020)
R.G. Larson, Transport and deposition patterns in drying sessile droplets. AIChE J. 60, 1538 (2014)
W. Han, Z. Lin, Learning from coffee rings: Ordered structures enabled by controlled evaporative self-assembly. Angew. Chem. Int. Ed. 51, 1534 (2012)
M. Parsa, S. Harmand, K. Sefiane, Mechanisms of pattern formation from dried sessile drops. Adv. Coll. Interface. Sci. 254, 22 (2018)
N.D. Patil, R. Bhardwaj, Recent developments on colloidal deposits obtained by evaporation of sessile droplets on a solid surface. J. Indian Inst. Sci. 99, 143 (2019)
J. Wang, L. Wang, Y. Song, L. Jiang, Patterned photonic crystals fabricated by inkjet printing. J. Mater. Chem. C 1, 6048 (2013)
T. Brugarolas, F. Tu, D. Lee, Directed assembly of particles using microfluidic droplets and bubbles. Soft Matter 9, 9046 (2013)
X. Zhong, A. Crivoi, F. Duan, Sessile nanofluid droplet drying. Adv. Coll. Interface. Sci. 217, 13 (2015)
V. Lotito, T. Zambelli, Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Coll. Interface. Sci. 246, 217 (2017)
S. Tarafdar, Y. Y. Tarasevich, M. Dutta Choudhury, T. Dutta, D. Zang, Droplet drying patterns on solid substrates: From hydrophilic to superhydrophobic contact to levitating drops, Adv. Condens. Matter Phys. 2018, 5214924 ( 2018)
B.B. Patel, Y. Diao, Multiscale assembly of solution-processed organic electronics: the critical roles of confinement, fluid flow, and interfaces. Nanotechnology 29, 044004 (2018)
K.S. Kolegov, L.Y. Barash, Joint effect of advection, diffusion, and capillary attraction on the spatial structure of particle depositions from evaporating droplets. Phys. Rev. E 100, 033304 (2019)
Z. Lin, Evaporative self-assembly of ordered complex structures ( World Scientific, 2012) p. 396
P. Innocenzi, L. Malfatti, P. Falcaro, Water droplets to nanotechnology (Royal Society of Chemistry, 2013) p. 184
R. Bhardwaj, X. Fang, P. Somasundaran, D. Attinger, Self-assembly of colloidal particles from evaporating droplets: Role of dlvo interactions and proposition of a phase diagram. Langmuir 26, 7833 (2010)
V.R. Dugyala, M.G. Basavaraj, Control over coffee-ring formation in evaporating liquid drops containing ellipsoids. Langmuir 30, 8680 (2014)
V.L. Morales, J.-Y. Parlange, M. Wu, F.J. Pérez-Reche, W. Zhang, W. Sang, T.S. Steenhuis, Surfactant-mediated control of colloid pattern assembly and attachment strength in evaporating droplets. Langmuir 29, 1831 (2013)
A. Crivoi, X. Zhong, F. Duan, Crossover from the coffee-ring effect to the uniform deposit caused by irreversible cluster-cluster aggregation. Phys. Rev. E 92, 032302 (2015)
M. Anyfantakis, Z. Geng, M. Morel, S. Rudiuk, D. Baigl, Modulation of the coffee-ring effect in particle/surfactant mixtures: the importance of particle-interface interactions. Langmuir 31, 4113 (2015)
D.M. Kuncicky, O.D. Velev, Surface-guided templating of particle assemblies inside drying sessile droplets. Langmuir 24, 1371 (2008)
T.A.H. Nguyen, M.A. Hampton, A.V. Nguyen, Evaporation of nanoparticle droplets on smooth hydrophobic surfaces: The inner coffee ring deposits. J. Phys. Chem. C 117, 4707 (2013)
Q. Yan, L. Gao, V. Sharma, Y.-M. Chiang, C.C. Wong, Particle and substrate charge effects on colloidal self-assembly in a sessile drop. Langmuir 24, 11518 (2008)
E. Homede, A. Zigelman, L. Abezgauz, O. Manor, Signatures of van der waals and electrostatic forces in the deposition of nanoparticle assemblies. J. Phys. Chem. Lett. 9, 5226 (2018)
E. Homede, O. Manor, Deposition of nanoparticles from a volatile carrier liquid. J. Colloid Interface Sci. 562, 102 (2020)
A. Zigelman, O. Manor, The deposition of colloidal particles from a sessile drop of a volatile suspension subject to particle adsorption and coagulation. J. Colloid Interface Sci. 509, 195 (2018)
C.L. Moraila-Martínez, M.A. Cabrerizo-Vílchez, M.A. Rodríguez-Valverde, The role of the electrostatic double layer interactions in the formation of nanoparticle ring-like deposits at driven receding contact lines. Soft Matter 9, 1664 (2013)
D. Noguera-Marín, C.L. Moraila-Martínez, M.A. Cabrerizo-Vílchez, M.A. Rodríguez-Valverde, In-plane particle counting at contact lines of evaporating colloidal drops: effect of the particle electric charge. Soft Matter 11, 987 (2015)
H. Lee, S. Fu, C. Tso, C.Y. Chao, Study of residue patterns of aqueous nanofluid droplets with different particle sizes and concentrations on different substrates. Int. J. Heat Mass Transf. 105, 230 (2017)
N. Bridonneau, M. Zhao, N. Battaglini, G. Mattana, V. Thévenet, V. Noël, M. Roché, S. Zrig, F. Carn, Self-assembly of nanoparticles from evaporating sessile droplets: Fresh look into the role of particle/substrate interaction. Langmuir 36, 11411 (2020)
M. Anyfantakis, D. Baigl, B.P. Binks, Evaporation of drops containing silica nanoparticles of varying hydrophobicities: Exploiting particle-particle interactions for additive-free tunable deposit morphology. Langmuir 33, 5025 (2017)
R. Williams, A.M. Goodman, Wetting of thin layers of sio2 by water. Appl. Phys. Lett. 25, 531 (1974)
H. Park, J. Lee, T. Kim, Comparison of the Nernst-Planck model and the Poisson-Boltzmann model for electroosmotic flows in microchannels. J. Colloid Interface Sci. 315, 731 (2007)
M.G. Kurnikova, R.D. Coalson, P. Graf, A. Nitzan, A lattice relaxation algorithm for three-dimensional Poisson-Nernst-Planck theory with application to ion transport through the gramicidin a channel. Biophys. J . 76, 642 (1999)
H. Wang, A. Thiele, L. Pilon, Simulations of cyclic voltammetry for electric double layers in asymmetric electrolytes: A generalized modified Poisson-Nernst-Planck model. J. Phys. Chem. C 117, 18286 (2013)
J.J. López-García, J. Horno, C. Grosse, Ionic size, permittivity, and viscosity-related effects on the electrophoretic mobility: A modified electrokinetic model. Phys. Rev. Fluids 4, 103702 (2019)
P.B. Warren, Non-faradaic electric currents in the Nernst-Planck equations and nonlocal diffusiophoresis of suspended colloids in crossed salt gradients. Phys. Rev. Lett. 124, 248004 (2020)
P.B. Warren, S. Shin, H.A. Stone, Diffusiophoresis in ionic surfactants: effect of micelle formation. Soft Matter 15, 278 (2019)
S. Shin, Diffusiophoretic separation of colloids in microfluidic flows. Phys. Fluids 32, 101302 (2020)
N. Rivas, S. Frijters, I. Pagonabarraga, J. Harting, Mesoscopic electrohydrodynamic simulations of binary colloidal suspensions. J. Chem. Phys. 148, 144101 (2018)
H. Hu, R.G. Larson, Analysis of the effects of marangoni stresses on the microflow in an evaporating sessile droplet. Langmuir 21, 3972 (2005)
L.Y. Barash, T.P. Bigioni, V.M. Vinokur, L.N. Shchur, Evaporation and fluid dynamics of a sessile drop of capillary size. Phys. Rev. E 79, 046301 (2009)
R.D. Deegan, O. Bakajin, T.F. Dupont, G. Huber, S.R. Nagel, T.A. Witten, Capillary flow as the cause of ring stains from dried liquid drops. Nature 389, 827 (1997)
R.D. Deegan, O. Bakajin, T.F. Dupont, G. Huber, S.R. Nagel, T.A. Witten, Contact line deposits in an evaporating drop. Phys. Rev. E 62, 756 (2000)
H. Hu, R.G. Larson, Evaporation of a sessile droplet on a substrate. J. Phys. Chem. B 106, 1334 (2002)
L. D. Landau, E. M. Lifshitz, L. P. Pitaevskii, Course of Theoretical Physics VIII: Electrodynamics of continuous media ( Pergamon Press, 1984)
S.R. Maduar, A.V. Belyaev, V. Lobaskin, O.I. Vinogradova, Electrohydrodynamics near hydrophobic surfaces. Phys. Rev. Lett. 114, 118301 (2015)
T.M. Squires, S.R. Quake, Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 77, 977 (2005)
M.A. Al-Muzaiqer, K.S. Kolegov, N.A. Ivanova, V.M. Fliagin, Nonuniform heating of a substrate in evaporative lithography. Phys. Fluids 33, 092101 (2021)
Y.A. Budkov, A.I. Frolov, M.G. Kiselev, N.V. Brilliantov, Surface-induced liquid-gas transition in salt-free solutions of model charged colloids. J. Chem. Phys. 139, 194901 (2013)
Acknowledgements
The work of Y.A.B. and L.Y.B was supported by Grant No. 18-71-10061 from the Russian Science Foundation. The work of Y.A.B. was finished within the Project Teams framework of MIEM HSE.
Author information
Authors and Affiliations
Contributions
Y.A.B. and L.Y.B. developed the model. S.V.Z. and A.L.K. performed the simulations and the data analysis. All the authors were involved in preparing the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Appendices
Appendix A: Basic equations in the case of axial symmetry
In this appendix, we will write the basic equations in cylindrical coordinates r, z taking into account the axial symmetry.
Equation (1) in (r, z) coordinates takes the form
The Nernst–Planck equation (5) in the (r, z) coordinates can be written as follows:
In the same way, we rewrite Eq. (7) for concentrations of ions, \(n_i (r, z, t)\) (\(i=\pm \)),
In our simulation, we deal with an incompressible liquid, so that \(\nabla \cdot \mathbf {v}=0\).
The boundary conditions for the electrostatic potential have the following form:
where R is the radius of the substrate and \(\theta \) is the contact angle. The boundary conditions for the concentrations take the following form:
Appendix B: Temperature distribution inside the droplet
The field of temperature, \(T(\mathbf {r},t)\), inside the droplet can be obtained from the numerical solution of the heat transfer equation taking into account the liquid motion
where \(\kappa \) is the thermal diffusivity of the liquid. The boundary conditions for this equation have the following form: \(\partial T / \partial r = 0\) at \(r = 0\); \(T = T_0\) at \(z = 0\); \(\partial T / \partial {\mathbf {n}} = -Q_0(r)/k\) at the droplet free boundary, where k is the thermal conductivity of the liquid; \(Q_0(r) = LJ_s(r)\) is the heat flow with the specific heat of vaporization, L, and the vaporization flux density, \(J_s(r)\), which can be calculated by the following interpolation formula [44]
where \(J_0 (\theta )\) can be determined by the following expressions [45]:
We note that in our case, it is not necessary to solve the heat transfer equation inside the substrate, because we assume that the substrate connects with a thermostat, fixing its temperature \(T_0\).
Note that the droplet surface of the spherical segment shape can be parameterized by the following equations:
where \(\varphi \) is the angle between the normal vector and the vertical axis z.
Appendix C: Velocity distribution inside the droplet for the Marangoni flux case
In the axial symmetry case, the Navier–Stokes equations can be rewritten in terms of vorticity, \(\gamma (r,z)={\partial u_r}/{\partial z}-{\partial u_z}/{\partial r}\), and stream function, \(\psi \), as follows: [42]:
where the stream function is related to the velocity components via the following relations \({\partial \psi }/{\partial z}=ru_r\), \({\partial \psi }/{\partial r}=-ru_z\).
The boundary conditions: \(\gamma =0\) at \(r=0\), \(\gamma =\partial u_r/\partial z\) at \(z=0\); \(\gamma =\eta ^{-1}{d\sigma }/ds+2u_\tau d\varphi /ds\) at the droplet boundary; \(\psi =0\) at all the boundaries: droplet boundary, axis of symmetry (\(r=0\)) and substrate-fluid interface (\(z=0\)). Here, \({d\sigma }/{ds}=\sigma ^{\prime }\partial T/\partial s\) is the derivative of the surface tension with respect to the arc length along the droplet surface, where the distribution of temperature discussed above is taken into account; \(\sigma ^{\prime }=\partial \sigma /\partial T\) is derivative of surface tension with respect to the temperature. Variable \(\varphi \) is the angle between the normal vector and the vertical axis z, so that \(\partial T/\partial s = \cos \varphi \cdot \partial T/\partial r - \sin \varphi \cdot \partial T/\partial z\), and the tangent component of velocity is \(u_\tau = u_r \cos \varphi -u_z \sin \varphi \). For the spherical shape of the droplet, described by equations (B6), the arc length s is described by \(\varphi \) via the relation \(s = R\varphi / \sin \theta \). In particular, at the contact line \(s_{max} = R\theta / \sin \theta \).
Appendix D: Boundary conditions for Navier–Stokes equations for the capillary fluid flow case
The boundary conditions for Navier–Stokes equations discussed in Appendix C were derived with the assumption that \(u_n = 0\). That is why the capillary flow was not taken into account in this derivation. In order to take into account the capillary fluid flow, it is necessary to rewrite the boundary conditions in a more general form. For the vorticity at the droplet boundary, we have
where \(u_n = u_r \sin \varphi + u_z \cos \varphi = -\partial \psi / (r\partial s)\). For the stream function \(\psi =0\) at the axis of the droplet symmetry and the droplet-substrate interface. However, at the droplet boundary, we have
As it follows from the mass conservation law in an infinitely small cylindrical volume near the droplet surface, on the droplet surface, the following relation
is fulfilled, where n is the liquid density.
Note that \(m = n\pi h_0^2 (R-h_0/3)\), where \(h_0 = R(1-\cos \theta )/\sin \theta \), i.e., \(m = \frac{1}{3}\pi R^3n (3-\tan (\theta /2))\tan ^2(\theta /2)\). Therefore,
Thus, using the relation
one can calculate \(d\theta /dt\). Further, using equations (B6), we get
Knowing \(\partial h(r,t)/\partial t\), we obtain \(u_n\) on the droplet boundary using Eq. (D3). Knowing \(u_n\), we can use boundary conditions (D1) and (D2).
Rights and permissions
About this article
Cite this article
Zavarzin, S.V., Kolesnikov, A.L., Budkov, Y.A. et al. Influence of fluid flows on electric double layers in evaporating colloidal sessile droplets. Eur. Phys. J. E 45, 24 (2022). https://doi.org/10.1140/epje/s10189-022-00178-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/s10189-022-00178-2