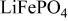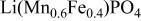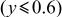Abstract
The room temperature (x, y) two-dimensional phase diagram of the olivine-type solid-solution, 

 orthorhombic,
orthorhombic,  is determined. The x-dependent changes in the unit cell dimensions at various fixed Mn contents y are analyzed in detail. The manganese substitution for iron in the octahedral 4c sites induces 1, the two-phase
is determined. The x-dependent changes in the unit cell dimensions at various fixed Mn contents y are analyzed in detail. The manganese substitution for iron in the octahedral 4c sites induces 1, the two-phase  redox region with a potential of 4.1 V vs.
redox region with a potential of 4.1 V vs.  2, a partial conversion of the form of the
2, a partial conversion of the form of the  redox reaction (3.4 V vs.
redox reaction (3.4 V vs.  from two-phase to single-phase; and 3, phase instability at the composition region close to the point (x, y) = (0, 1) caused by the Jahn-Teller effect of
from two-phase to single-phase; and 3, phase instability at the composition region close to the point (x, y) = (0, 1) caused by the Jahn-Teller effect of  The conversion, 2, is complete at around
The conversion, 2, is complete at around  The phase instability, 3, makes the Mn-rich phase
The phase instability, 3, makes the Mn-rich phase  unsuitable for battery applications. The local lattice deformation around
unsuitable for battery applications. The local lattice deformation around  is severe enough to induce significant selective damping in the extended X-ray absorption fine structure for
is severe enough to induce significant selective damping in the extended X-ray absorption fine structure for  © 2001 The Electrochemical Society. All rights reserved.
© 2001 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Since the demonstration of reversible electrochemical lithium insertion-extraction for  in 1997,1 lithium transition metal phosphates with an ordered olivine structure,
in 1997,1 lithium transition metal phosphates with an ordered olivine structure, 
 Ni, Mn, Fe, Cu), have attracted much attention as promising new cathode materials for rechargeable lithium batteries.2
3
4
5
6
7
8
9
10 The initially present single lithium ion,
Ni, Mn, Fe, Cu), have attracted much attention as promising new cathode materials for rechargeable lithium batteries.2
3
4
5
6
7
8
9
10 The initially present single lithium ion,  per transition metal ion,
per transition metal ion,  can be extracted in the first charge process, compensating for the oxidation of
can be extracted in the first charge process, compensating for the oxidation of  to
to  and transferred to the carbon anode through the nonaqueous electrolyte
and transferred to the carbon anode through the nonaqueous electrolyte
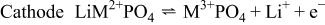
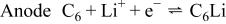
The oxidation (charge) of  to
to  induces a reduction in the unit cell volume. This shrinkage compensates for the volume expansion of the carbon anodes in the charge process and contributes to efficient use of volume in a practical lithium-ion cell. The opposite movement of lithium ions and electrons occurs during the discharge process, while the transition metal M is reduced from trivalent to divalent.
induces a reduction in the unit cell volume. This shrinkage compensates for the volume expansion of the carbon anodes in the charge process and contributes to efficient use of volume in a practical lithium-ion cell. The opposite movement of lithium ions and electrons occurs during the discharge process, while the transition metal M is reduced from trivalent to divalent.
The  crystal has an orthorhombic unit cell
crystal has an orthorhombic unit cell  space group
space group  which accommodates four units of
which accommodates four units of  11 As a typical example,
11 As a typical example,  has unit-cell dimensions of
has unit-cell dimensions of 
 and
and  7 Graphic representations of the crystal structure have been given in many references.1
3
4
11 Both the Li and M atoms are in octahedral sites with Li located in the 4a and M in the 4c positions. The oxygen atoms are nearly hexagonal closed-packed and the M atoms occupy zigzag chains of corner-shared octahedra running parallel to the c axis in alternate
7 Graphic representations of the crystal structure have been given in many references.1
3
4
11 Both the Li and M atoms are in octahedral sites with Li located in the 4a and M in the 4c positions. The oxygen atoms are nearly hexagonal closed-packed and the M atoms occupy zigzag chains of corner-shared octahedra running parallel to the c axis in alternate  planes. These chains are bridged by corner- and edge-sharing
planes. These chains are bridged by corner- and edge-sharing  polyanions to form a host structure with strong three-dimensional bonding. The
polyanions to form a host structure with strong three-dimensional bonding. The  ions in 4a sites form continuous linear chains of edge-shared octahedra running parallel to the c axis in the other
ions in 4a sites form continuous linear chains of edge-shared octahedra running parallel to the c axis in the other  planes, which makes two-dimensional motion possible.
planes, which makes two-dimensional motion possible.
The charge-discharge reaction of the presently used materials, such as the layered rock salt systems,  (space group,
(space group,  and a spinel framework system
and a spinel framework system  (space groups:
(space groups:  are all based on the
are all based on the  couple in edge-shared
couple in edge-shared  octahedra in the closed-packed oxygen array which generates ca. 4 V12
13
14
15
octahedra in the closed-packed oxygen array which generates ca. 4 V12
13
14
15
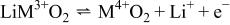
Therefore, the presence of large tetrahedral polyanions  corner-shared
corner-shared  octahedra, and the use of the
octahedra, and the use of the  redox couple are distinctive features of olivine class cathodes. The
redox couple are distinctive features of olivine class cathodes. The  linkage in the structure induces the superexchange interaction that tunes the
linkage in the structure induces the superexchange interaction that tunes the  redox energy to useful levels (3.4, 4.1, and 4.8 V for
redox energy to useful levels (3.4, 4.1, and 4.8 V for 
 and
and  respectively).1
5 The strong
respectively).1
5 The strong  covalency stabilizes the antibonding
covalency stabilizes the antibonding 
 state of primarily cationic origin to generate an appropriately high voltage, which is called the "inductive effect."1
6 The stable nature of the olivine-type structure having a
state of primarily cationic origin to generate an appropriately high voltage, which is called the "inductive effect."1
6 The stable nature of the olivine-type structure having a  polyanion with a strong P-O covalent bond provides not only excellent cycle-life but also a safe system. When the battery is fully charged, the reactivity to the combustion reaction with the organic electrolyte is low.6 Safety issues are of paramount importance in the design of consumer batteries, and this makes olivine-type materials particularly attractive as cathodes for lithium-ion battery systems.
polyanion with a strong P-O covalent bond provides not only excellent cycle-life but also a safe system. When the battery is fully charged, the reactivity to the combustion reaction with the organic electrolyte is low.6 Safety issues are of paramount importance in the design of consumer batteries, and this makes olivine-type materials particularly attractive as cathodes for lithium-ion battery systems.
The energy density of olivine-type  is equal to that of presently used materials, based on the theoretical charge-discharge capacity of ca. 170 mAh/g obtained from the
is equal to that of presently used materials, based on the theoretical charge-discharge capacity of ca. 170 mAh/g obtained from the  one-electron redox reaction of
one-electron redox reaction of 
 6 and the high voltage of >3.4 V vs.
6 and the high voltage of >3.4 V vs.  However, the inherent low conductivity for both electrons and lithium ions must be overcome by optimized powder engineering to realize uniformly small particles, or utilization is limited by slow diffusion of these charge carriers. We have recently observed a reversible capacity of >160 mAh/g at room temperature for optimized
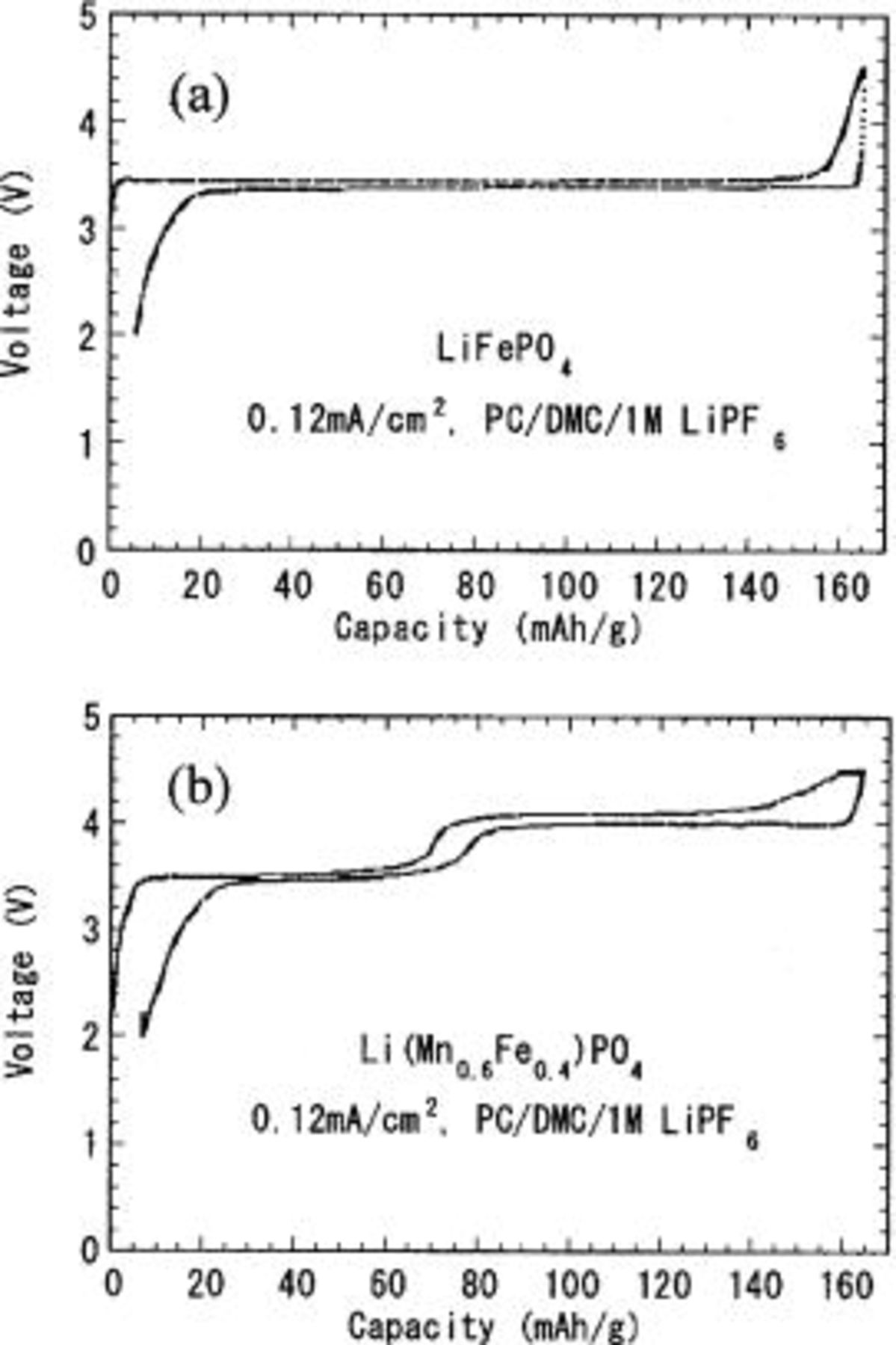
However, the inherent low conductivity for both electrons and lithium ions must be overcome by optimized powder engineering to realize uniformly small particles, or utilization is limited by slow diffusion of these charge carriers. We have recently observed a reversible capacity of >160 mAh/g at room temperature for optimized  powder sintered at the moderate temperatures of 500-600°C,6 as shown in Fig. 1a. In the optimization process, we identified two main obstacles to achieving optimum charge/discharge performance: (i) undesirable particle growth at higher temperatures and (ii) the presence of residual
powder sintered at the moderate temperatures of 500-600°C,6 as shown in Fig. 1a. In the optimization process, we identified two main obstacles to achieving optimum charge/discharge performance: (i) undesirable particle growth at higher temperatures and (ii) the presence of residual  at lower temperatures in the sintering and/or milling process.6
at lower temperatures in the sintering and/or milling process.6
In the olivine-type  family,
family, 
 and their solid solution system,
and their solid solution system,  1 look promising because they operate at 3.4-4.1 V vs.
1 look promising because they operate at 3.4-4.1 V vs.  This is providential, because it is not so high as to decompose the organic electrolyte but is not so low as to sacrifice energy density. The positions of redox couples were confirmed in our laboratory by first-principle calculations.6 The use of
This is providential, because it is not so high as to decompose the organic electrolyte but is not so low as to sacrifice energy density. The positions of redox couples were confirmed in our laboratory by first-principle calculations.6 The use of  is of particular interest because the position of the
is of particular interest because the position of the  couple, 4.1 V vs.
couple, 4.1 V vs.  1 is compatible with present lithium-ion batteries and generates high energy density. However, it has been shown that the capacity at 4.1 V is not achieved without Fe coexisting with Mn at the octahedral 4c site1 Padhi et al. have performed systematic experiments on the electrochemical charge and discharge characteristics of
1 is compatible with present lithium-ion batteries and generates high energy density. However, it has been shown that the capacity at 4.1 V is not achieved without Fe coexisting with Mn at the octahedral 4c site1 Padhi et al. have performed systematic experiments on the electrochemical charge and discharge characteristics of 
 0.50, 0.75, 1.0) and reported that the width of the 4.1 V plateau
0.50, 0.75, 1.0) and reported that the width of the 4.1 V plateau  relative to that of the 3.4 V plateau
relative to that of the 3.4 V plateau  increases as Mn content y is increased, but that the total capacity rapidly decreases at
increases as Mn content y is increased, but that the total capacity rapidly decreases at  .1
.1
In our study, the strong electron  lattice interaction (Jahn-Teller effect) in the charged state
lattice interaction (Jahn-Teller effect) in the charged state  and the resultant limit to lattice distortion have been suggested to be intrinsic obstacles to generating the charge-discharge of the Mn-rich phase
and the resultant limit to lattice distortion have been suggested to be intrinsic obstacles to generating the charge-discharge of the Mn-rich phase  7 The solubility limit in the
7 The solubility limit in the  binary system lies in the composition range of
binary system lies in the composition range of  For this reason,
For this reason, 
 was initially chosen as a promising composition for the 4 V cathode material and a capacity equal to the theoretical value (170 mAh/g), more than half of which generates 4 V, was demonstrated using the optimized powder.7 See Fig. 1b. The charge-discharge reaction mechanism of
was initially chosen as a promising composition for the 4 V cathode material and a capacity equal to the theoretical value (170 mAh/g), more than half of which generates 4 V, was demonstrated using the optimized powder.7 See Fig. 1b. The charge-discharge reaction mechanism of 
 was also investigated in detail.8 We found a flat two-phase region with an open-circuit voltage (OCV) of 4.1 V (region I:
was also investigated in detail.8 We found a flat two-phase region with an open-circuit voltage (OCV) of 4.1 V (region I: 
 and an s curved single-phase region with
and an s curved single-phase region with  (region II:
(region II: 
 8 This is significantly different from the
8 This is significantly different from the 
 in which the whole
in which the whole  reaction proceeds in a two-phase manner
reaction proceeds in a two-phase manner  with a flat voltage profile at 3.4 V.1
with a flat voltage profile at 3.4 V.1
Our principal concern at this stage is why the  redox reaction in the same olivine-type host structure proceeds in a single-phase mechanism when 60% of the manganese coexists in the same octahedral 4c sites,
redox reaction in the same olivine-type host structure proceeds in a single-phase mechanism when 60% of the manganese coexists in the same octahedral 4c sites, 
 8 while there is a two-phase reaction over the entire compositional range of
8 while there is a two-phase reaction over the entire compositional range of 
 1 In this paper, the (x, y) two-dimensional phase diagram of the olivine-type solid-solution,
1 In this paper, the (x, y) two-dimensional phase diagram of the olivine-type solid-solution, 

 is determined. We show below the results of a survey of the phase change in the (x, y) plane and how the manganese substitution for iron changes the charge-discharge reaction mechanism, with special consideration of the two-phase to single-phase transformation of the
is determined. We show below the results of a survey of the phase change in the (x, y) plane and how the manganese substitution for iron changes the charge-discharge reaction mechanism, with special consideration of the two-phase to single-phase transformation of the  redox reaction. The severe local lattice deformation around the Jahn-Teller active
redox reaction. The severe local lattice deformation around the Jahn-Teller active  which induces phase instability around
which induces phase instability around  is also highlighted.
is also highlighted.
Experimental
The  (six samples:
(six samples:  0.2, 0.4, 0.6, 0.8, 1.0) compounds were prepared by solid-state reaction of
0.2, 0.4, 0.6, 0.8, 1.0) compounds were prepared by solid-state reaction of 

 and
and  They were dispersed into acetone, then thoroughly mixed, and reground by ballmilling. After evaporating the acetone, the olivine phase was synthesized in purified
They were dispersed into acetone, then thoroughly mixed, and reground by ballmilling. After evaporating the acetone, the olivine phase was synthesized in purified  gas flow (800 cm3/min) to prevent the formation of trivalent compounds as impurities. The mixture was first decomposed at 280°C for 3 h to disperse the gases and firmly reground again, then sintered for 24 h at 600°C.
gas flow (800 cm3/min) to prevent the formation of trivalent compounds as impurities. The mixture was first decomposed at 280°C for 3 h to disperse the gases and firmly reground again, then sintered for 24 h at 600°C.
Chemical oxidation to obtain  was performed by reacting
was performed by reacting  with nitronium tetrafluoroborate
with nitronium tetrafluoroborate  in acetonitrile.16 The redox potential of
in acetonitrile.16 The redox potential of  is ca. 5.1 V vs.
is ca. 5.1 V vs.  and is effective for oxidizing
and is effective for oxidizing  with redox potentials of 3.4 V
with redox potentials of 3.4 V  and 4.1 V
and 4.1 V  vs.
vs.  1
7
8
16 The reaction is written as
1
7
8
16 The reaction is written as
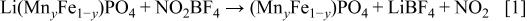
To ensure a complete reaction, the amount of  added was double the amount estimated from Reaction 1. After 8.5 g of
added was double the amount estimated from Reaction 1. After 8.5 g of  was dissolved into 300 mL acetonitrile, 5 g of
was dissolved into 300 mL acetonitrile, 5 g of  was added and the mixture was stirred for 24 h at room temperature under bubbling purified Ar gas. The product was filtered and washed several times with acetonitrile to remove impurities before it was dried under vacuum at 70°C.
was added and the mixture was stirred for 24 h at room temperature under bubbling purified Ar gas. The product was filtered and washed several times with acetonitrile to remove impurities before it was dried under vacuum at 70°C.
Lithiated samples of 
 were prepared by reacting
were prepared by reacting  with various amounts of LiI (High Purity Chemicals, 99.9%) in acetonitrile. The hygroscopic LiI powder was treated in a dry atmosphere, and the ratio of acetonitrile to
with various amounts of LiI (High Purity Chemicals, 99.9%) in acetonitrile. The hygroscopic LiI powder was treated in a dry atmosphere, and the ratio of acetonitrile to  was set at 200 mL to 0.2 g. The solution was stirred for 24 h at room temperature, and the products were filtered and washed several times with acetonitrile to remove impurities before they were dried under vacuum at 70°C. The chemistry of LiI as a reducing agent in acetonitrile has been studied previously in detail.17 The oxidation of
was set at 200 mL to 0.2 g. The solution was stirred for 24 h at room temperature, and the products were filtered and washed several times with acetonitrile to remove impurities before they were dried under vacuum at 70°C. The chemistry of LiI as a reducing agent in acetonitrile has been studied previously in detail.17 The oxidation of  to
to  occurs in two reversible steps
occurs in two reversible steps
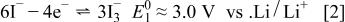
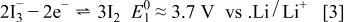
Reaction 2 was performed successively to form  with a redox potential of 2.9 V.18 In the present case, for the 4.1 V region of
with a redox potential of 2.9 V.18 In the present case, for the 4.1 V region of 
 both steps 2 and 3 shift completely to the right at room temperature, and the reaction can be assumed to incorporate all of the
both steps 2 and 3 shift completely to the right at room temperature, and the reaction can be assumed to incorporate all of the  in acetonitrile supplied by LiI into
in acetonitrile supplied by LiI into  8 whereas, for the 3.5 V region of
8 whereas, for the 3.5 V region of 
 only Reaction 1 was induced and excess LiI was added to produce the desired composition.8 All the products were analyzed using inductively coupled plasma spectroscopy-atomic emission spectroscopy (ICPS-AES, Shimazu Co., ICPS-8000) to determine their exact composition.
only Reaction 1 was induced and excess LiI was added to produce the desired composition.8 All the products were analyzed using inductively coupled plasma spectroscopy-atomic emission spectroscopy (ICPS-AES, Shimazu Co., ICPS-8000) to determine their exact composition.
All of the characterizations by X-ray powder diffraction (XRD), Mössbauer spectroscopy, and X-ray absorption spectroscopy (XAS) were based on chemically prepared samples of 

 to ensure the data is for clean samples. X-ray powder diffraction (RINT-2500v, Rigaku Co.) with
to ensure the data is for clean samples. X-ray powder diffraction (RINT-2500v, Rigaku Co.) with  radiation was used to identify the phases and analyze the structure. The lattice constants were calculated using the least squares method with a Si standard. The diffraction profiles were measured in the slow-scan mode (0.5°/min). The
radiation was used to identify the phases and analyze the structure. The lattice constants were calculated using the least squares method with a Si standard. The diffraction profiles were measured in the slow-scan mode (0.5°/min). The  Mössbauer spectra in transmission geometry were collected using a
Mössbauer spectra in transmission geometry were collected using a  γ-ray source. Velocity calibration was made with α-Fe data at room temperature. The sample thickness was adjusted so that the Fe content was ca. 8 mg/cm2.
γ-ray source. Velocity calibration was made with α-Fe data at room temperature. The sample thickness was adjusted so that the Fe content was ca. 8 mg/cm2.
X-ray absorption measurements were performed with the Industrial Consortium Beamline BL16B2 in SPring-8 using synchrotron radiation from the electron storage ring at an electron energy of 8 GeV. A white X-ray beam was monochromatized by a Si(111) double-crystal monochromator. A half-cylindrical Rh-coated total-reflection mirror was used to eliminate the harmonics and converge the beam in the horizontal direction. X-ray absorption spectra were taken in the transmission mode near the Mn and Fe K edges on samples diluted in boron nitride powder at proportions calculated to give an edge jump in  of ca. 1. The intensities of incident and transmitted X-ray beams were measured using ionization chambers filled with 100% nitrogen gas and nitrogen gas mixed with 15% Ar gas, respectively. The data was analyzed using the REX1 program (Rigaku Co.)
of ca. 1. The intensities of incident and transmitted X-ray beams were measured using ionization chambers filled with 100% nitrogen gas and nitrogen gas mixed with 15% Ar gas, respectively. The data was analyzed using the REX1 program (Rigaku Co.)
Results and Discussion
Samples with small Mn content  .—
.—
The compositional analysis from ICP-AES revealed that for the samples with  the amount of residual lithium in the oxidized products from Reaction 1 for 24 h is less than 0.02 mol per formula unit. In addition, the amount of lithium after partial lithiation by LiI for 24 h based on Reactions 2 and 3 was close to the expected values. Therefore, for our samples with
the amount of residual lithium in the oxidized products from Reaction 1 for 24 h is less than 0.02 mol per formula unit. In addition, the amount of lithium after partial lithiation by LiI for 24 h based on Reactions 2 and 3 was close to the expected values. Therefore, for our samples with  having uniformly small particle size, 24 h is long enough for the reaction to go to completion. The delithiation-lithiation reactions were confirmed by X-ray diffraction to be topotactic; the structure of the host
having uniformly small particle size, 24 h is long enough for the reaction to go to completion. The delithiation-lithiation reactions were confirmed by X-ray diffraction to be topotactic; the structure of the host 
 was changed only by atomic displacements with no diffusive rearrangement. The X-ray diffraction (XRD) profiles for
was changed only by atomic displacements with no diffusive rearrangement. The X-ray diffraction (XRD) profiles for 

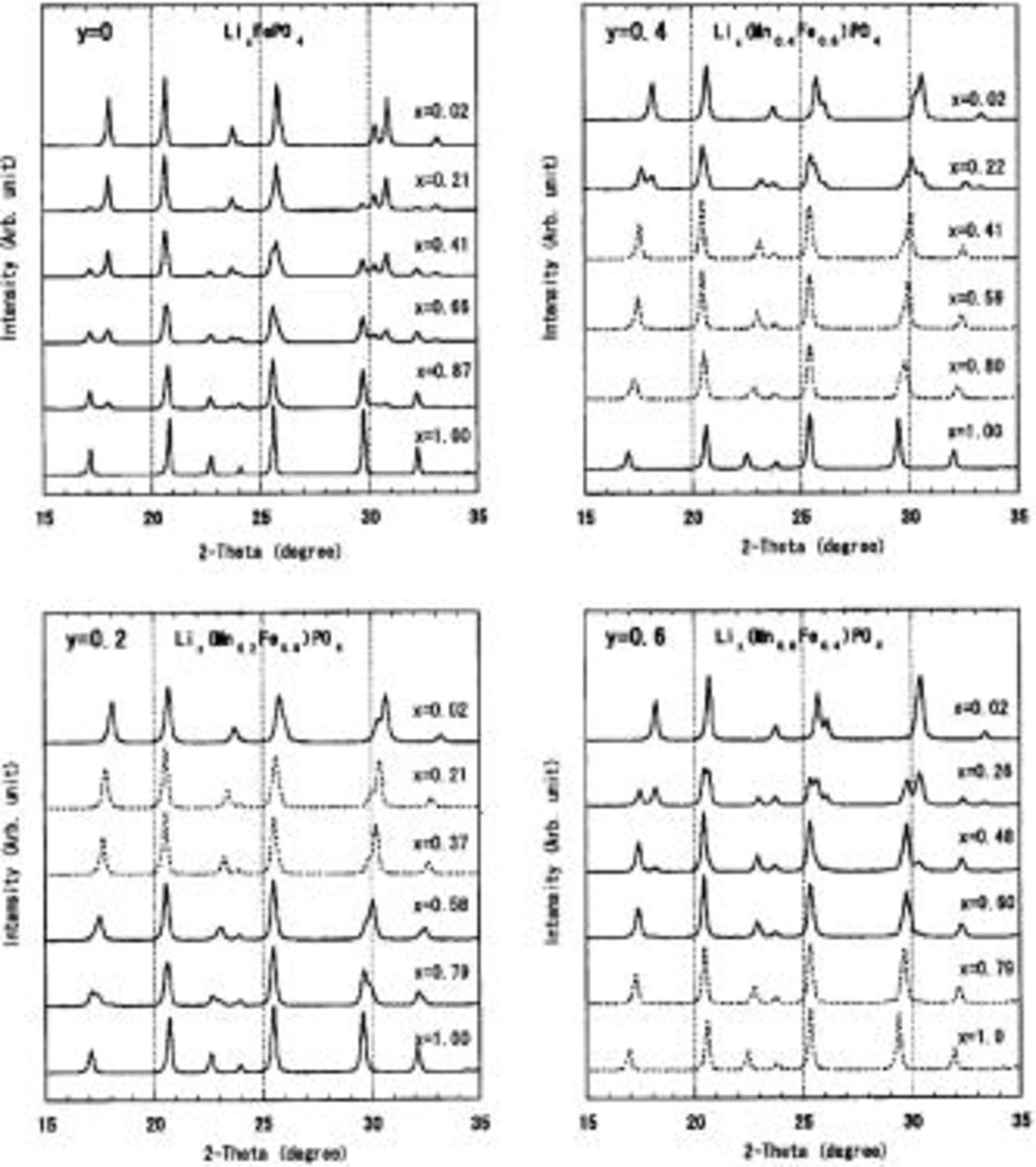
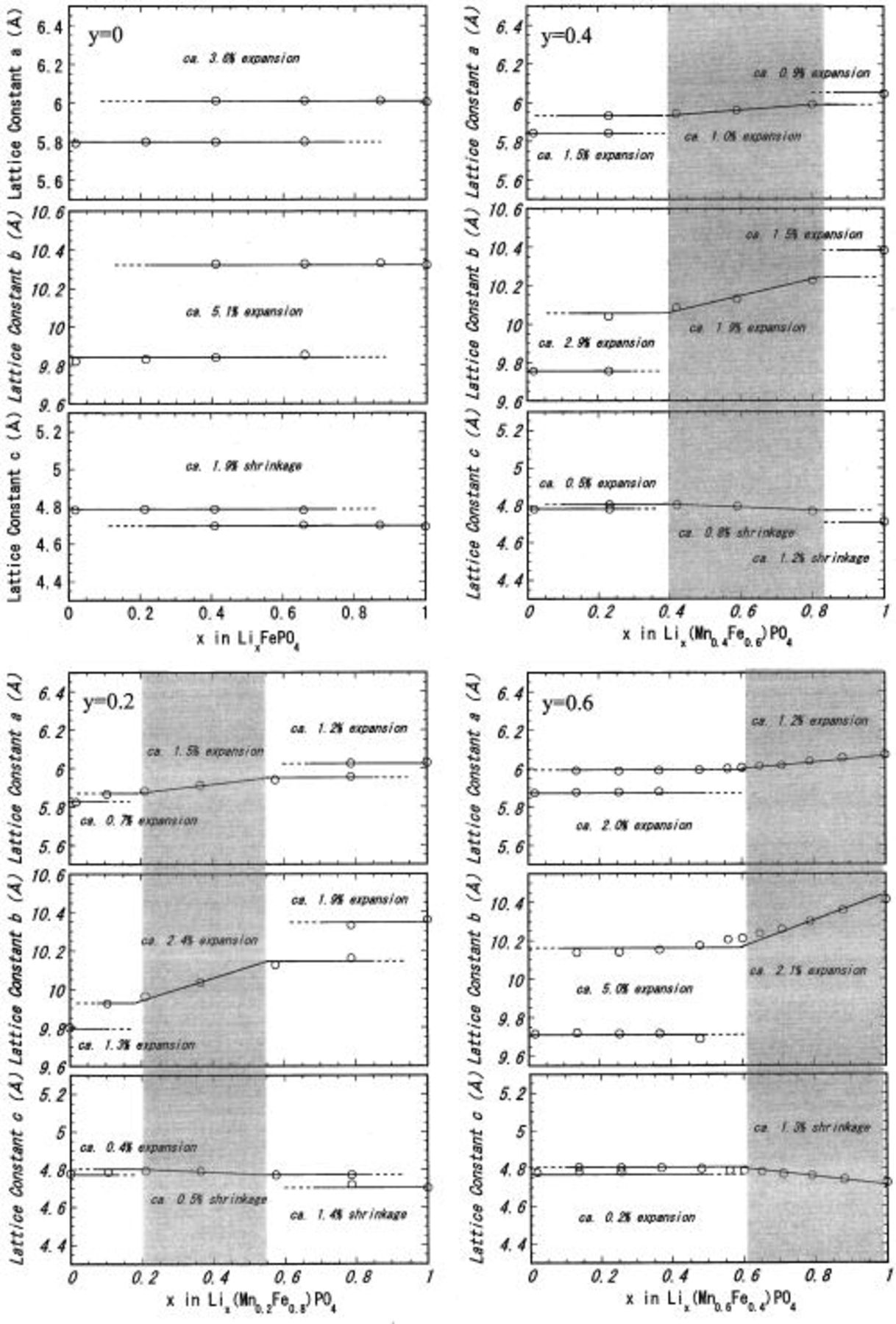
 are shown in Fig. 2. Variations in the three lattice constants of the orthorhombic Pmnb lattice a, b, and c with the lithium composition x are summarized in Fig. 3.
are shown in Fig. 2. Variations in the three lattice constants of the orthorhombic Pmnb lattice a, b, and c with the lithium composition x are summarized in Fig. 3.
Figure 2. XRD patterns measured at fixed y values  0.2, 0.4, 0.6) with various Li composition x for
0.2, 0.4, 0.6) with various Li composition x for 

 Broken lines are for the single-phase region and solid lines for the two-phase region.
Broken lines are for the single-phase region and solid lines for the two-phase region.
Figure 3. Variations in the three lattice constants of the orthorhombic Pmnb lattice a, b, c with lithium composition x at fixed y values  0.2, 0.4, 0.6) for
0.2, 0.4, 0.6) for 

 The single-phase regions are shaded to show the general tendency. The lines are for visual guidance.
The single-phase regions are shaded to show the general tendency. The lines are for visual guidance.
A typical two-phase reaction proceeds in 
 1 The Mn substitution for Fe in the octahedral 4c sites results in (i) the formation of a two-phase
1 The Mn substitution for Fe in the octahedral 4c sites results in (i) the formation of a two-phase  region at
region at  (4.1 V vs.
(4.1 V vs.  where all of the three orthorhombic lattice constants show discrete elongation by Li insertion, and (ii) a partial conversion of the reaction for the
where all of the three orthorhombic lattice constants show discrete elongation by Li insertion, and (ii) a partial conversion of the reaction for the  region of
region of  (3.4 V vs.
(3.4 V vs.  from two-phase to single-phase, where the a and b lattice constants increase, and the c lattice constant decreases. As a result of this two-phase to single-phase conversion in
from two-phase to single-phase, where the a and b lattice constants increase, and the c lattice constant decreases. As a result of this two-phase to single-phase conversion in  and the growth of the
and the growth of the  region, the two-phase region in
region, the two-phase region in  disappears at
disappears at  The tendency for changes in unit cell dimensions (lithium insertion induces an elongation of the a and b axis and a slight contraction of the c axis) is common for all samples with any Mn content y.
The tendency for changes in unit cell dimensions (lithium insertion induces an elongation of the a and b axis and a slight contraction of the c axis) is common for all samples with any Mn content y.
The change in the valence state of Fe was analyzed using Mössbauer spectroscopy, and showed the  and
and  redox reactions in the compositional regions
redox reactions in the compositional regions  and
and  respectively. The boundaries at
respectively. The boundaries at  were distinct. A typical example, the spectrum for
were distinct. A typical example, the spectrum for 
 can be seen in a previous report.8 One of the advantages of Mössbauer spectroscopy is its ability to detect short-range symmetry and the local electronic state, and hence to identify the number of phases, including amorphous, nanoparticle, and/or ions with different valence states.6
7
8 However, no X-ray indiscernible nonolivine phase (amorphous or nanoparticle) was detected for any sample with
can be seen in a previous report.8 One of the advantages of Mössbauer spectroscopy is its ability to detect short-range symmetry and the local electronic state, and hence to identify the number of phases, including amorphous, nanoparticle, and/or ions with different valence states.6
7
8 However, no X-ray indiscernible nonolivine phase (amorphous or nanoparticle) was detected for any sample with  This, in turn, strongly suggests that the reaction is exclusively topotactic.
This, in turn, strongly suggests that the reaction is exclusively topotactic.
Samples with large Mn content  .—
.—
The (local) lattice distortion induced by the Jahn-Teller effect of  19 makes
19 makes  unstable when the
unstable when the  content y is large. For the samples with
content y is large. For the samples with  reversible topotactic delithiation-lithiation reactions of
reversible topotactic delithiation-lithiation reactions of  occurred within the limited Li composition x but had very slow kinetics; the phases close to the point (x, y) = (0, 1) are not stable enough to maintain the olivine framework.7 As demonstrated in our previous reports, the fully delithiated forms,
occurred within the limited Li composition x but had very slow kinetics; the phases close to the point (x, y) = (0, 1) are not stable enough to maintain the olivine framework.7 As demonstrated in our previous reports, the fully delithiated forms,  and
and  could not be isolated in a stable equilibrium state.7
could not be isolated in a stable equilibrium state.7
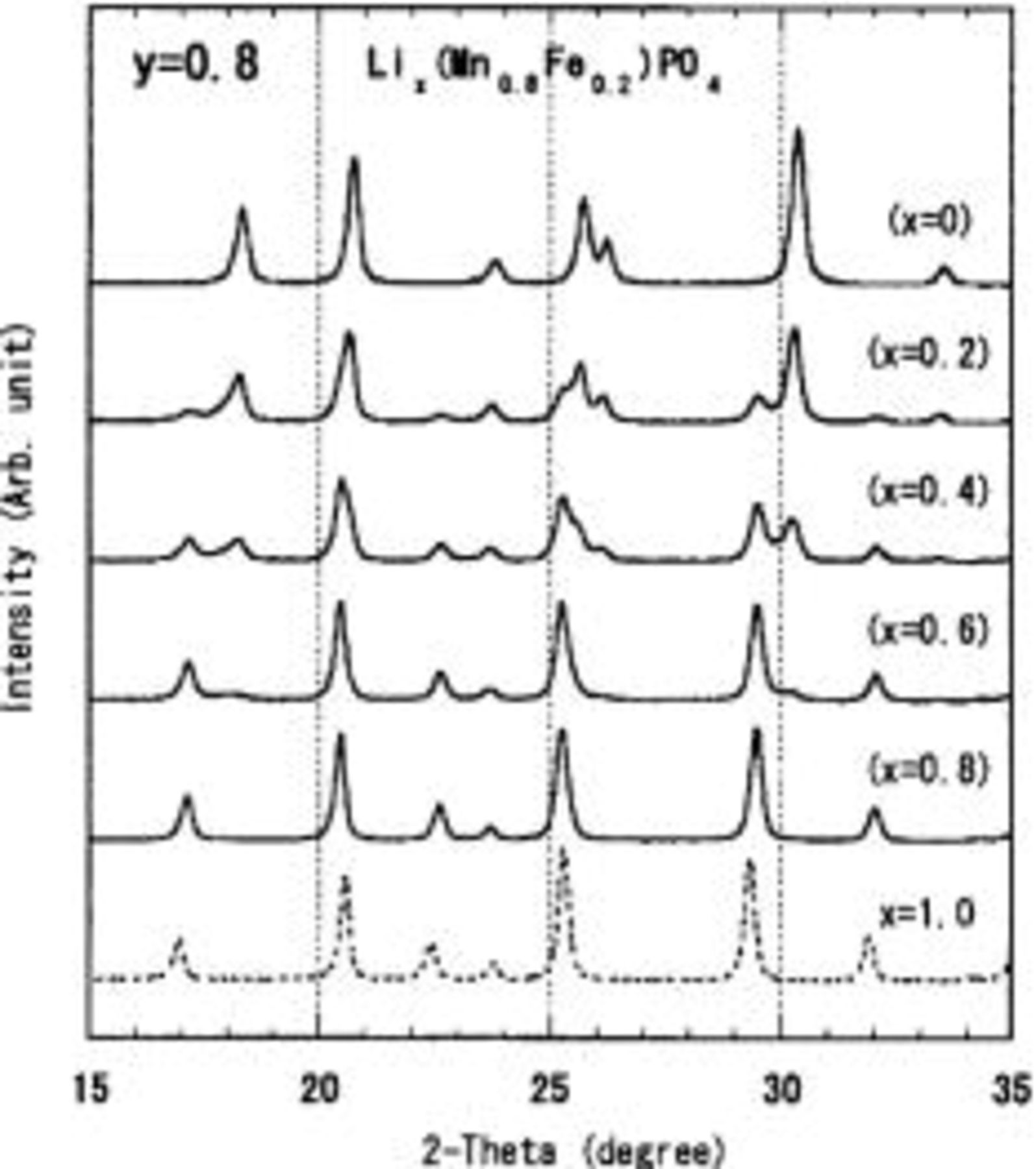
Although an accurate compositional analysis of Li content x in the olivine phase was not possible, the overall trend of the reaction within the limited lithium composition x is dominated by the two-phase character of  XRD profiles measured for
XRD profiles measured for  with nominal composition
with nominal composition  0.2, 0.4, 0.6, 0.8, 1.0, are shown in Fig. 4. These lithium compositions are, of course, not accurate because they are based on the assumption that LiI reacts with pure
0.2, 0.4, 0.6, 0.8, 1.0, are shown in Fig. 4. These lithium compositions are, of course, not accurate because they are based on the assumption that LiI reacts with pure  which includes an X-ray indiscernible nonolivine phase.7 However, we can conclude from Fig. 4 that the reaction mechanism is dominated by the two-phase reaction of
which includes an X-ray indiscernible nonolivine phase.7 However, we can conclude from Fig. 4 that the reaction mechanism is dominated by the two-phase reaction of  For
For 
 the situation was almost the same.
the situation was almost the same.
Figure 4. XRD patterns measured for 
 These samples include the X-ray indiscernible nonolivine phase, which could be detected only by using Mössbauer spectroscopy. The compositions x in parenthesis are based on the assumption that LiI reacts with pure
These samples include the X-ray indiscernible nonolivine phase, which could be detected only by using Mössbauer spectroscopy. The compositions x in parenthesis are based on the assumption that LiI reacts with pure 
Local structures.—
For short-range characterization, EXAFS provides independent information on the local structures (coordination number, bond length, etc.) around Fe and Mn. Of particular interest here is the local structure around  in the charged state,
in the charged state,  In our previous paper,7 we reported the existence of
In our previous paper,7 we reported the existence of  -induced anisotropic lattice distortion in the
-induced anisotropic lattice distortion in the  binary system,
binary system,  and the resultant equilibrium solubility-limit in the compositional range
and the resultant equilibrium solubility-limit in the compositional range  However, the analysis was based on the average long-range dimensional change of the orthorhombic lattice found using XRD. In phospho-olivines, the edge-shared arrangement of the oxygen-octahedra
However, the analysis was based on the average long-range dimensional change of the orthorhombic lattice found using XRD. In phospho-olivines, the edge-shared arrangement of the oxygen-octahedra 
 as well as the localized character of the 3d electrons will enhance the selective local lattice distortion around the Jahn-Teller active
as well as the localized character of the 3d electrons will enhance the selective local lattice distortion around the Jahn-Teller active  ions.
ions.
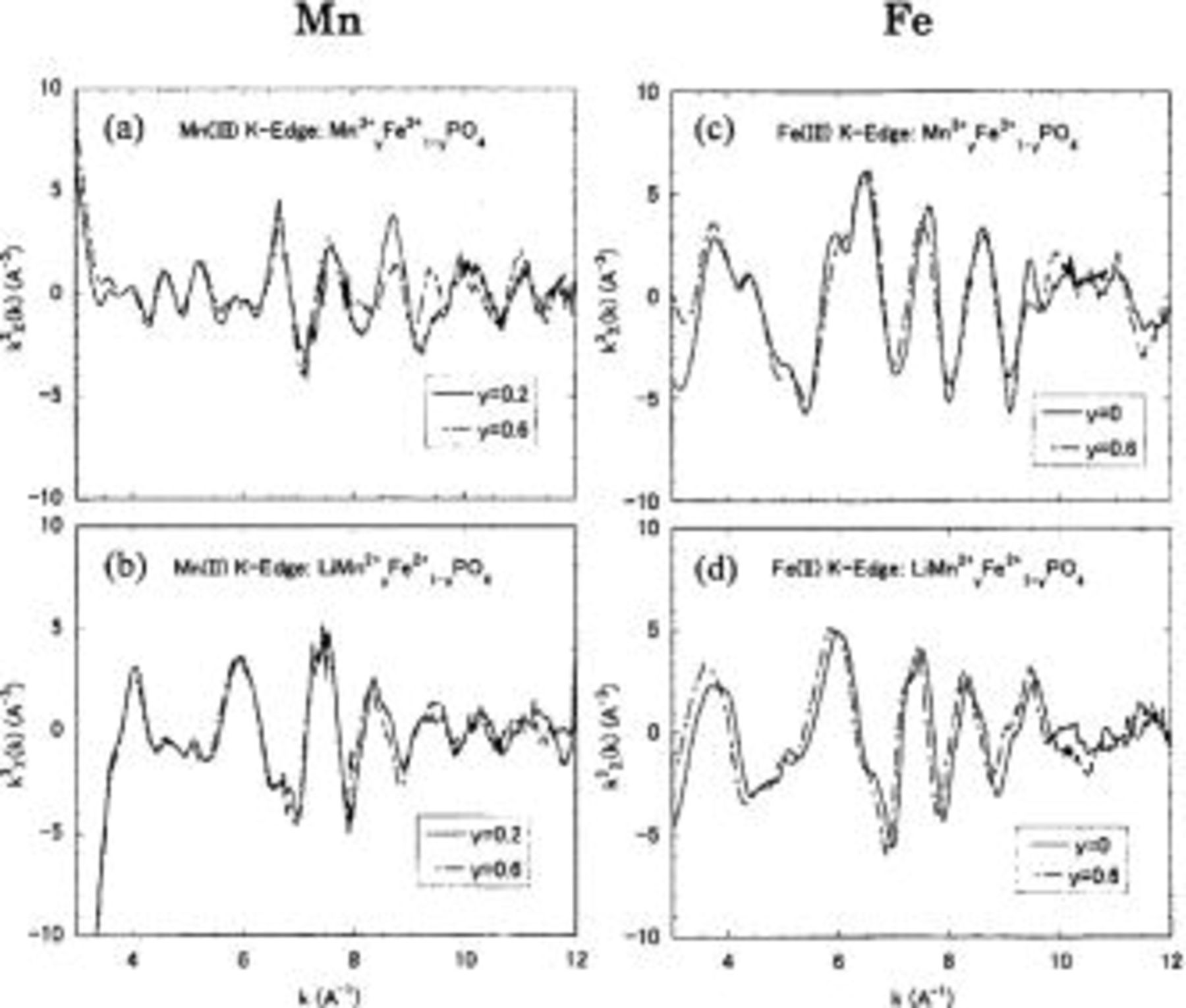
The normalized extended X-ray absorption fine structure (EXAFS) χ weighted by  as shown in Fig. 5, are consistent with this. The EXAFS, are extracted from the absorption spectra after the pre-edge background is subtracted,
as shown in Fig. 5, are consistent with this. The EXAFS, are extracted from the absorption spectra after the pre-edge background is subtracted,  and are normalized by the absorption spectrum obtained assuming that absorbing atoms are isolated,
and are normalized by the absorption spectrum obtained assuming that absorbing atoms are isolated,  The function
The function  was determined using the cubic spline method as follows:
was determined using the cubic spline method as follows:  was separated into several parts;
was separated into several parts;  was independently estimated in each part and was smoothly connected at the boundary between the neighboring parts. The energy, E, is converted to the photoelectron wavenumber,
was independently estimated in each part and was smoothly connected at the boundary between the neighboring parts. The energy, E, is converted to the photoelectron wavenumber,  where
where  is the threshold energy of the absorption edge and m is the free-electron mass. The difference in amplitude is evident for
is the threshold energy of the absorption edge and m is the free-electron mass. The difference in amplitude is evident for  of
of  the significant damping suggests the enhancement of local disorder by the Jahn-Teller effect of
the significant damping suggests the enhancement of local disorder by the Jahn-Teller effect of  20
21
22
23
24 In addition, the spectrum shapes for
20
21
22
23
24 In addition, the spectrum shapes for  and
and  (Fig. 5c and d) are similar, while those for
(Fig. 5c and d) are similar, while those for  and
and  are completely different (Fig. 5a and b). These experimental results strongly suggest that, in the process of oxidation (charging) of
are completely different (Fig. 5a and b). These experimental results strongly suggest that, in the process of oxidation (charging) of  to
to  changes in the atomic position are induced mainly around
changes in the atomic position are induced mainly around 
Figure 5. Normalized EXAFS χ weighted by  on the K edge of (a)
on the K edge of (a)  in
in  (b)
(b)  in
in  (c)
(c)  in
in  and (d)
and (d)  in
in  measured at 300 K. (a) and (c) were charged; (b) and (d) were discharged.
measured at 300 K. (a) and (c) were charged; (b) and (d) were discharged.
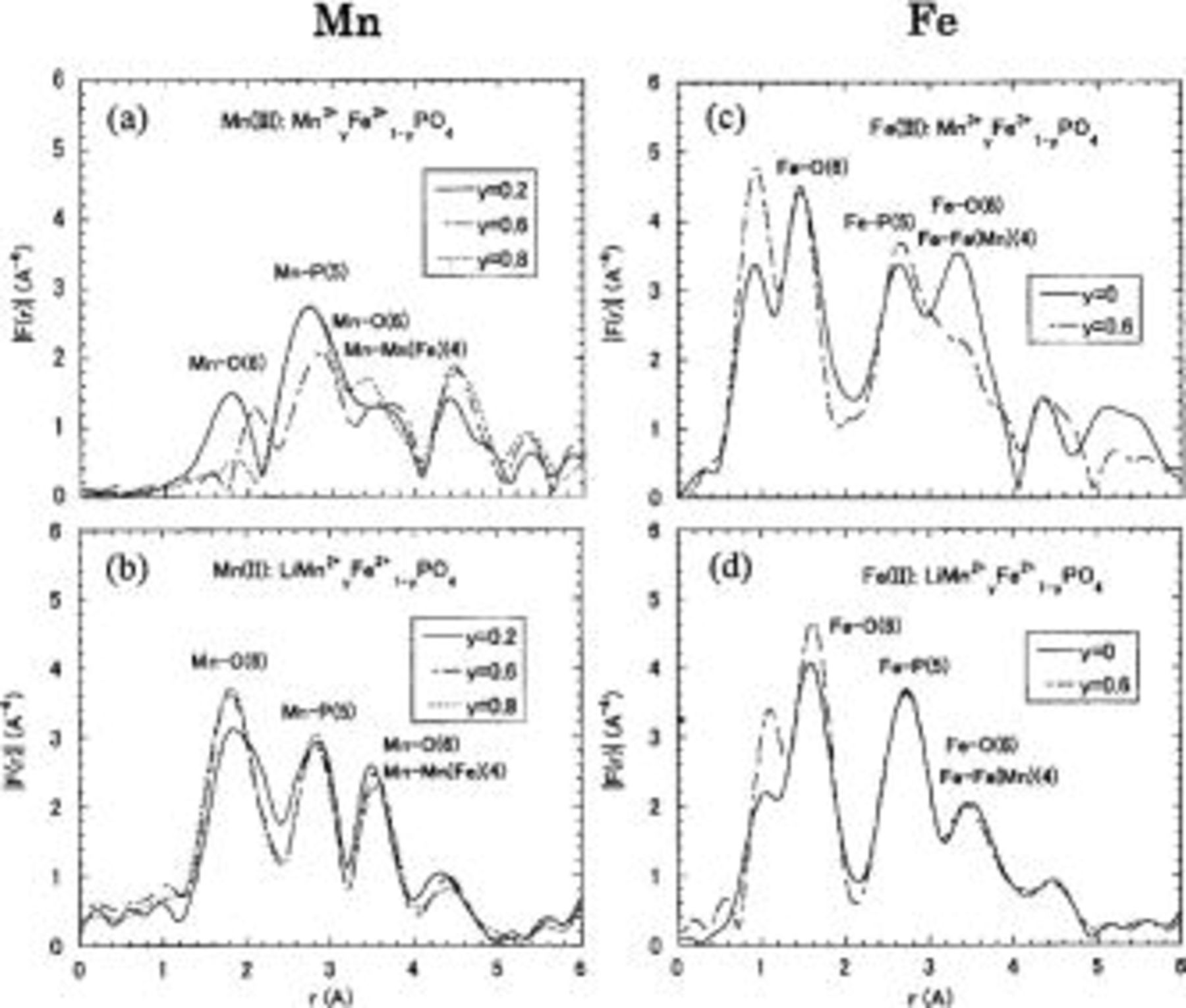
The Fourier transform of 
 shows a radial atomic distribution-like function in real space about the emitting metal ions (Fig. 6). Note that these peaks are at shorter distances than the actual interatomic distance, because the phase shift in the electron-scattering process is not considered. Attention should also be paid to the peak around Fe-O at ca. 1.1 A, which is a false peak not indicating any actual chemical bonding. A nonessential ghost peak in the short bond-length region often appears, it is dependent on the unrestricted
shows a radial atomic distribution-like function in real space about the emitting metal ions (Fig. 6). Note that these peaks are at shorter distances than the actual interatomic distance, because the phase shift in the electron-scattering process is not considered. Attention should also be paid to the peak around Fe-O at ca. 1.1 A, which is a false peak not indicating any actual chemical bonding. A nonessential ghost peak in the short bond-length region often appears, it is dependent on the unrestricted  processing in the REX1 program. However, all other peaks were well defined, as is shown in Fig. 6. The numbers in parentheses in this figure are the coordination numbers for the emitting metal ions. As is shown in Fig. 6c and d,
processing in the REX1 program. However, all other peaks were well defined, as is shown in Fig. 6. The numbers in parentheses in this figure are the coordination numbers for the emitting metal ions. As is shown in Fig. 6c and d,  for
for  and
and  have similar distributions, indicating the local environments are almost identical.
have similar distributions, indicating the local environments are almost identical.
Figure 6. Fourier transform of  of (a)
of (a)  in
in  (b)
(b)  in
in  (c)
(c)  in
in  and (d)
and (d)  in
in  measured at 300 K. (a) and (c) were charged; (b) and (d) were discharged.
measured at 300 K. (a) and (c) were charged; (b) and (d) were discharged.
On the other hand, the amplitude of the  peak in
peak in  is unusually low compared to the
is unusually low compared to the  peak in
peak in  and is significantly reduced as the
and is significantly reduced as the  content y increases (Fig. 6a and b). The peak height is related to the backscattering of photoelectrons by the coordinated atoms; the atoms arranged in coherent coordination shells have a larger contribution.22 When the distances between the absorber atom and the coordination atoms are not uniform, as in the case of a distorted oxygen octahedron, the related peak shows a broadening and an apparent decrease in the peak height due to interference between the real and imaginary part of the spectrum.23 Consequently, the local lattice distortion around the Jahn-Teller active
content y increases (Fig. 6a and b). The peak height is related to the backscattering of photoelectrons by the coordinated atoms; the atoms arranged in coherent coordination shells have a larger contribution.22 When the distances between the absorber atom and the coordination atoms are not uniform, as in the case of a distorted oxygen octahedron, the related peak shows a broadening and an apparent decrease in the peak height due to interference between the real and imaginary part of the spectrum.23 Consequently, the local lattice distortion around the Jahn-Teller active  is much more severe than that expected from the XRD data, and this situation promotes phase destabilization close to the point
is much more severe than that expected from the XRD data, and this situation promotes phase destabilization close to the point 
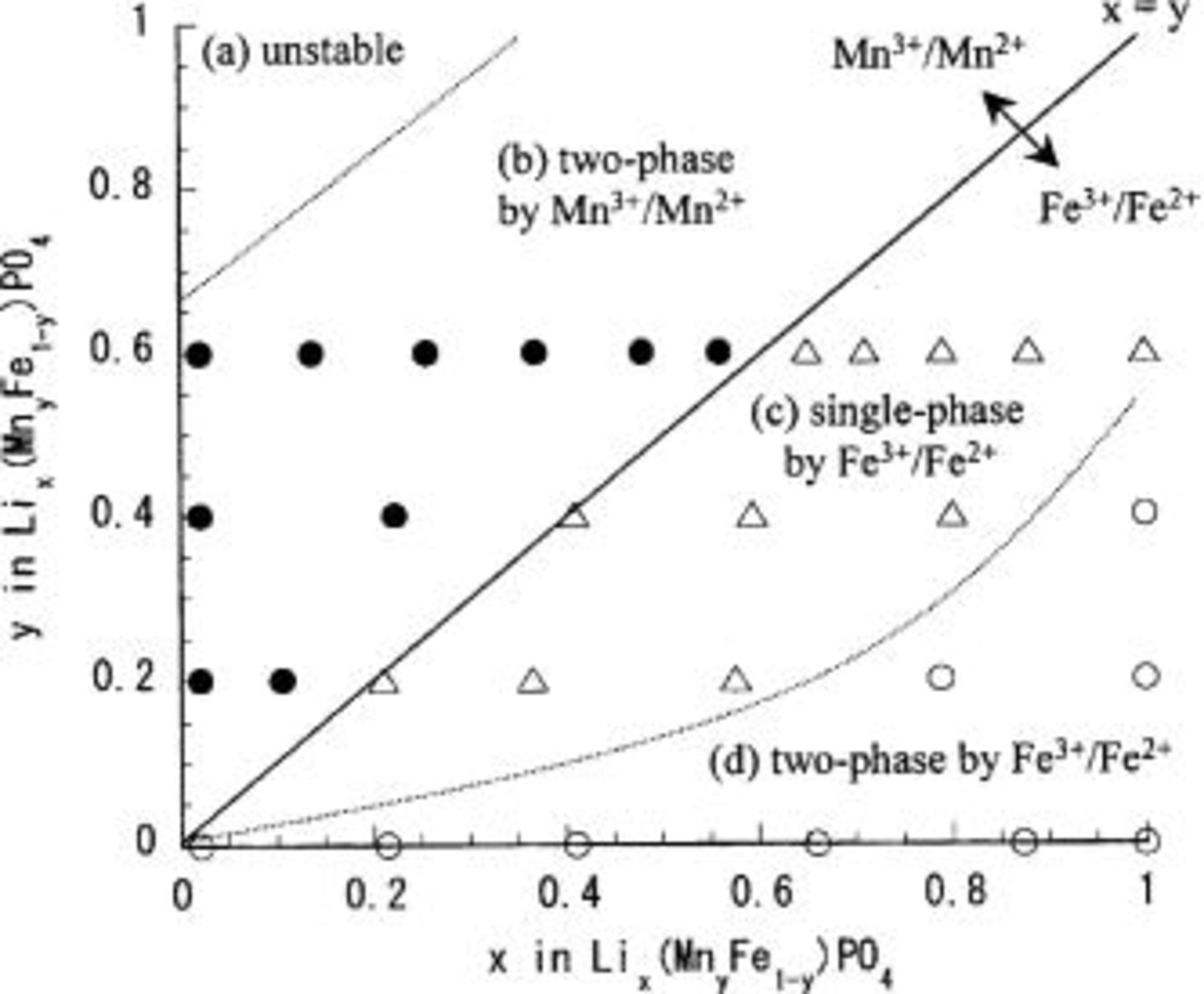
(x, y) Two-dimensional phase diagram.—
Based on the results of the preceding three sections the (x, y) two-dimensional phase diagram of the 

 system shown in Fig. 7 establishes some general trends for the phase change. The (x, y) phase map in Fig. 7 is divided into four areas: (a) the unstable region close to the point
system shown in Fig. 7 establishes some general trends for the phase change. The (x, y) phase map in Fig. 7 is divided into four areas: (a) the unstable region close to the point  (b) the two-phase region caused by
(b) the two-phase region caused by 
 (d) the two-phase region caused by
(d) the two-phase region caused by  (a part of
(a part of  and (c) the single-phase region caused by
and (c) the single-phase region caused by  connecting (b) and (d).
connecting (b) and (d).
Figure 7. The (x, y) two-dimensional phase diagram of the 
 system obtained using XRD and Mössbauer spectroscopy. Information is simply given on the single-phase or two-phase matter together with the valence states of Mn and Fe. The map is divided into four areas: (a) the unstable region close to the point
system obtained using XRD and Mössbauer spectroscopy. Information is simply given on the single-phase or two-phase matter together with the valence states of Mn and Fe. The map is divided into four areas: (a) the unstable region close to the point  (b) the two-phase region by
(b) the two-phase region by  (closed circles;
(closed circles;  (d) the two-phase region by
(d) the two-phase region by  (open circles; a part of
(open circles; a part of  and (c) the single-phase region by
and (c) the single-phase region by  connecting (b) and (d) (open triangles).
connecting (b) and (d) (open triangles).
It is noteworthy that the  redox reaction (region of
redox reaction (region of  proceeds entirely in a two-phase manner. This can be readily understood by the first-order transition between the Jahn-Teller active
proceeds entirely in a two-phase manner. This can be readily understood by the first-order transition between the Jahn-Teller active  phase with cooperative elastic deformation and the Jahn-Teller-inactive
phase with cooperative elastic deformation and the Jahn-Teller-inactive  phase. Another important aspect is that the single-phase region (c) appears only in
phase. Another important aspect is that the single-phase region (c) appears only in  in Mn/Fe solid solution system but not in
in Mn/Fe solid solution system but not in  The origin of the appearance of this single-phase region c is not clear, but it is reasonable to speculate conceptually that the random distribution of divalent manganese in region c may dilute the weak cooperative interaction that discretely adjusts the framework to lithium insertion/extraction and makes the inherent
The origin of the appearance of this single-phase region c is not clear, but it is reasonable to speculate conceptually that the random distribution of divalent manganese in region c may dilute the weak cooperative interaction that discretely adjusts the framework to lithium insertion/extraction and makes the inherent  redox reaction in
redox reaction in 
 a two-phase type.
a two-phase type.
The phase diagram in Fig. 7 leads us to the intuitive idea that 
 is not entirely inactive in the electrochemical charge-discharge reaction at 4.1 V vs.
is not entirely inactive in the electrochemical charge-discharge reaction at 4.1 V vs.  and may be useful as a stable yet compatible 4 V cathode for
and may be useful as a stable yet compatible 4 V cathode for 
 and
and  However, our electrochemical investigations of
However, our electrochemical investigations of  gave negative results even though the uniform small particles were carefully prepared.
gave negative results even though the uniform small particles were carefully prepared.  showed an open-circuit voltage of 4.1 V, but with much smaller capacity than the theoretical value of 170 mAh/g and very large polarization in the galvanostatic charge/discharge mode. In comparing
showed an open-circuit voltage of 4.1 V, but with much smaller capacity than the theoretical value of 170 mAh/g and very large polarization in the galvanostatic charge/discharge mode. In comparing  with
with  as practical 4 V cathodes, several intrinsic obstacles in
as practical 4 V cathodes, several intrinsic obstacles in  must be overcome, such as the competitive materials cost, the limited capacity, the much slower reaction kinetics, the need to synthesize in inert gas with a divalent source, and a much lower true density (3.4 g/cm3
must be overcome, such as the competitive materials cost, the limited capacity, the much slower reaction kinetics, the need to synthesize in inert gas with a divalent source, and a much lower true density (3.4 g/cm3  vs. 4.2 g/cm3
vs. 4.2 g/cm3  Thus, at the moment, olivine-type
Thus, at the moment, olivine-type  as well as Mn-rich
as well as Mn-rich 
 remain far away from a practical application.
remain far away from a practical application.
Conclusion
A room-temperature (x, y) two-dimensional phase diagram of the olivine-type solid-solution, 

 orthorhombic, D: Pmnb) is presented. The manganese substitution for iron in the octahedral 4c sites induces 1, the two-phase
orthorhombic, D: Pmnb) is presented. The manganese substitution for iron in the octahedral 4c sites induces 1, the two-phase  redox region
redox region  with a potential of 4.1 V vs.
with a potential of 4.1 V vs.  2, a partial conversion of the reaction form in the
2, a partial conversion of the reaction form in the  redox region
redox region  3.4 V vs.
3.4 V vs.  from two-phase to single-phase; and 3, phase instability in the composition region close to the point
from two-phase to single-phase; and 3, phase instability in the composition region close to the point  caused by the Jahn-Teller effect of
caused by the Jahn-Teller effect of  Conversion 2 is complete at around
Conversion 2 is complete at around  and the phase instability 3 makes the Mn-rich phase
and the phase instability 3 makes the Mn-rich phase  unsuitable for battery applications. The local lattice deformation around
unsuitable for battery applications. The local lattice deformation around  is severe enough to induce significant selective damping in the EXAFS for
is severe enough to induce significant selective damping in the EXAFS for 
Acknowledgments
The authors thank K. Ishikawa for ICP measurements. Helpful and enlightening discussions with Dr. S. C. Chung and Dr. M. Hosoya are greatly appreciated.
Sony Corporation assisted in meeting the publication costs of this article.