Abstract
Binary hydrous cobalt-nickel oxide with an amorphous structure (denoted as  anodically deposited from a
anodically deposited from a  solution with its Co:Ni ratio of 4:6 and pH 8.0 exhibited a very large pseudocapacitance of ca.
solution with its Co:Ni ratio of 4:6 and pH 8.0 exhibited a very large pseudocapacitance of ca.  in 1 M NaOH. The electrochemical reversibility of this material was examined at various charging-discharging currents in 1 M NaOH with different temperatures. This binary hydrous oxide exhibiting ideally pseudocapacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) has been demonstrated to be a potential candidate for the application of electrochemical supercapacitors. The morphology and rough nature of this deposit were demonstrated through means of atomic force microscopy. © 2002 The Electrochemical Society. All rights reserved.
in 1 M NaOH. The electrochemical reversibility of this material was examined at various charging-discharging currents in 1 M NaOH with different temperatures. This binary hydrous oxide exhibiting ideally pseudocapacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) has been demonstrated to be a potential candidate for the application of electrochemical supercapacitors. The morphology and rough nature of this deposit were demonstrated through means of atomic force microscopy. © 2002 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Energy storage systems delivering very high pulse power for limited time intervals with an acceptable capacitance are generally called supercapacitors. These electrochemical systems have been demonstrated to be promising devices for improving the service life of batteries.1 2 The supercapacitors usually consist of highly porous materials (e.g., active carbon) with very high specific surface area or electroactive materials with several oxidation states/structures within the potential window of solvent decomposition.1 3 4 The former devices are also called double-layer capacitors because of the storage of charge within the electrical double layer at the electrode-electrolyte interface. On the other hand, the latter are generally called pseudocapacitors since faradaic processes at/within the electroactive materials exhibit capacitive-like responses.
Amorphous hydrous oxides prepared by several techniques (e.g., sol-gel, electrochemical, and chemical precipitation methods) usually exhibit relatively large pseudocapacitance and good reversibility.3 4 5 6 7 8 Moreover, cheaper precursors as well as reliable and easy-control techniques for preparing the electroactive materials with good capacitive characteristics have attracted much attention. Because transition metal oxides (e.g., oxides of Co, Ni, Mn, Mo, V, Cr, W, Re, etc.) and conducting polymers have several oxidation states or structures that lead to redox transitions within the potential region of water decomposition,1 3 7 8 9 10 11 12 13 energy (i.e., pseudocapacitance) can be stored within the reversible redox transitions of these electroactive materials. Because the performance of an electrochemical (EC) supercapacitor is mainly determined by the electrochemical characteristics of the superficial electroactive species,1 3 4 5 6 the electrochemical reversibility as well as the pulse power property of these potential candidates should be examined systematically.
Hydrous cobalt and nickel oxides prepared by the sol-gel or electrochemical techniques have been proposed to be suitable materials for the application of EC supercapacitors.10 11 However, the sol-gel technique is relatively complicated and the electrochemical properties as well as electric conductivity of the electroactive materials may be affected by the introduction of binders (e.g., polyvinyldi- fluoride, PVDF) during the electrode preparation process. Accordingly, an alternative preparation method, which can directly coat hydrous oxides with high reversibility and pulse power density in the potential window of water decomposition, is worth seeking.
Nickel oxides can be anodically deposited from aqueous media14
15 and they are considered as suitable materials for batteries or electrochromic devices. In addition, the electrochemical reversibility of hydrous nickel oxide can be improved by the introduction of cobalt ions into the nickel oxide matrix.16 The purpose of this work is to present a suitable plating bath and operating procedures for fabricating an amorphous hydrous cobalt-nickel oxide (denoted hereafter as  ) deposit with ideally pseudocapacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) for EC supercapacitors.
) deposit with ideally pseudocapacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) for EC supercapacitors.
Experimental
The  deposits were electroplated directly onto
deposits were electroplated directly onto  graphite substrates (Nippon Carbon EG-NPL, N.C.K., Japan). These substrates were first abraded with ultrafine SiC paper, degreased with acetone and water, then etched in a 0.1 M HCl solution at room temperature (ca.
graphite substrates (Nippon Carbon EG-NPL, N.C.K., Japan). These substrates were first abraded with ultrafine SiC paper, degreased with acetone and water, then etched in a 0.1 M HCl solution at room temperature (ca.  ) for 10 min, and finally degreased with water in an ultrasonic bath. The exposed geometric area of these pretreated graphite supports is equal to
) for 10 min, and finally degreased with water in an ultrasonic bath. The exposed geometric area of these pretreated graphite supports is equal to  while the other surface areas were insulated with polytetrafluorene ethylene (PTFE) films. The plating solution, consisting of 4 mM
while the other surface areas were insulated with polytetrafluorene ethylene (PTFE) films. The plating solution, consisting of 4 mM  and 6 mM
and 6 mM  (Wako, EP, Japan) with an initial pH of 8.0 (adjusted with 0.1 M
(Wako, EP, Japan) with an initial pH of 8.0 (adjusted with 0.1 M  ), was stirred on a hot plate during the depositing process. The deposition was performed at 0.75 V for 4600 s. After deposition, the PTFE films were removed from the electrode. The electrode was doubly degreased with water. Note that during the degreasing process, the electrodes or substrates were first rinsed with a flow of pure water for ca. 60 s. These rinsed electrodes or substrates were then dipped into a beaker containing pure water (at room temperature) that is stirred by a hot plate for 5 min. Finally, the electrodes or substrates were dipped in a beaker with ultrasonic vibration for 5-15 min if ultrasonic cleaning was required. The average oxide loading of this hydrous oxide-coated electrode was
), was stirred on a hot plate during the depositing process. The deposition was performed at 0.75 V for 4600 s. After deposition, the PTFE films were removed from the electrode. The electrode was doubly degreased with water. Note that during the degreasing process, the electrodes or substrates were first rinsed with a flow of pure water for ca. 60 s. These rinsed electrodes or substrates were then dipped into a beaker containing pure water (at room temperature) that is stirred by a hot plate for 5 min. Finally, the electrodes or substrates were dipped in a beaker with ultrasonic vibration for 5-15 min if ultrasonic cleaning was required. The average oxide loading of this hydrous oxide-coated electrode was  obtained from the weight difference of the electrode without PTFE coating before and after oxide growth. These electrodes before and after oxide growth were dried by a cool airflow.
obtained from the weight difference of the electrode without PTFE coating before and after oxide growth. These electrodes before and after oxide growth were dried by a cool airflow.
The surface morphology of the deposit was examined via an atomic force microscope (AFM, Digital Instruments). The average composition of Ni-Co oxide was measured at three points by means of an energy-dispersive X-ray (EDX) spectroscope with standards coupled with a scanning electron microscope (SEM, JEOL JSM 35). The X-ray diffraction (XRD) pattern obtained from XRD analysis (Rigaku X-ray diffractometer using a Cu target) revealed an amorphous structure of the  deposit prepared in this work, which is not shown here. Electrochemical measurements for
deposit prepared in this work, which is not shown here. Electrochemical measurements for  deposits were performed by means of an electrochemical analyzer system, CHI 633A (CH Instruments, USA). All experiments were carried out in a three-compartment cell. An Ag/AgCl electrode (Argenthal, 3 M KCl, 0.207 V vs. SHE at
deposits were performed by means of an electrochemical analyzer system, CHI 633A (CH Instruments, USA). All experiments were carried out in a three-compartment cell. An Ag/AgCl electrode (Argenthal, 3 M KCl, 0.207 V vs. SHE at  ) was used as the reference and a platinum wire with an exposed area equal to
) was used as the reference and a platinum wire with an exposed area equal to  was employed as the counter electrode. A Luggin capillary, whose tip was set at a distance of 1-2 mm from the surface of the working electrode, was used to minimize errors due to iR drop in the electrolytes.
was employed as the counter electrode. A Luggin capillary, whose tip was set at a distance of 1-2 mm from the surface of the working electrode, was used to minimize errors due to iR drop in the electrolytes.
All solutions used in this work were prepared with 18 MΩ cm water produced by a reagent water system (Milli-Q SP, Japan), and all reagents not otherwise specified in this work were Merck, GR. In addition, the electrolyte, containing 1 M NaOH, used to study the capacitive behavior of  was degassed with purified nitrogen gas before voltammetric measurements and a nitrogen blanket was used during the measurements. The solution temperature was maintained at
was degassed with purified nitrogen gas before voltammetric measurements and a nitrogen blanket was used during the measurements. The solution temperature was maintained at  by means of a water thermostat (Haake DC3 and K20).
by means of a water thermostat (Haake DC3 and K20).
Results and Discussion
Typical linear sweep voltammograms (LSV) measured at  in 0.01 M
in 0.01 M  and 0.01 M
and 0.01 M  with initial pH of 8.0 are shown as curves 1 and 2 of Fig. 1 to find the suitable potentials of binary oxide deposition. On curve 1,
with initial pH of 8.0 are shown as curves 1 and 2 of Fig. 1 to find the suitable potentials of binary oxide deposition. On curve 1,  was oxidized and converted to nickel oxide at potentials positive of ca. 0.65 V. The limiting current of nickel oxide deposition reached ca.
was oxidized and converted to nickel oxide at potentials positive of ca. 0.65 V. The limiting current of nickel oxide deposition reached ca.  when potentials were more positive than 0.82 V. Moreover, oxygen evolution commenced at ca. 1.0 V. On curve 2, deposition of cobalt oxide from the chloride precursor commenced at ca. 0.38 V. In addition, deposition of Co oxide reached a limiting current of ca.
when potentials were more positive than 0.82 V. Moreover, oxygen evolution commenced at ca. 1.0 V. On curve 2, deposition of cobalt oxide from the chloride precursor commenced at ca. 0.38 V. In addition, deposition of Co oxide reached a limiting current of ca.  at ca. 0.5 V. The gradual increase in anodic currents at potentials more positive than ca. 0.8 V is attributable to oxygen evolution. From the above results and discussion, nickel and cobalt oxides can be simultaneously deposited onto the graphite substrate at potentials more positive than 0.65 V. In addition, cobalt oxide is more easily deposited by anodic deposition in comparison with nickel oxide. This phenomenon is probably due to the replacement of coordinated chloride by
at ca. 0.5 V. The gradual increase in anodic currents at potentials more positive than ca. 0.8 V is attributable to oxygen evolution. From the above results and discussion, nickel and cobalt oxides can be simultaneously deposited onto the graphite substrate at potentials more positive than 0.65 V. In addition, cobalt oxide is more easily deposited by anodic deposition in comparison with nickel oxide. This phenomenon is probably due to the replacement of coordinated chloride by  in the chloride precursor enhancing the deposition of Co oxide because the overpotential of oxide deposition from their chloride precursors should be higher than that from their chloro-hydroxyl precursors due to a change in chemical structures.6 From the LSV curves,
in the chloride precursor enhancing the deposition of Co oxide because the overpotential of oxide deposition from their chloride precursors should be higher than that from their chloro-hydroxyl precursors due to a change in chemical structures.6 From the LSV curves,  was anodically deposited at 0.75 V to obtain the binary oxide with a suitable composition (
was anodically deposited at 0.75 V to obtain the binary oxide with a suitable composition ( within the deposit when it was deposited from the bath containing 4 mM
within the deposit when it was deposited from the bath containing 4 mM  and 6 mM
and 6 mM  with initial pH 8.0).
with initial pH 8.0).
Figure 1. Linear sweep voltammograms measured at  in (1) 10 mM
in (1) 10 mM  and (2) 10 mM
and (2) 10 mM 
There is no direct evidence to explain the obvious difference in the limiting currents for the deposition of Co oxide and Ni oxide. A possible explanation is proposed in the following. From curve 2 in Fig. 1 and the potential-pH diagrams of Co,17 the Co species in the plating solution were likely oxidized to form  initially and then further oxidized to
initially and then further oxidized to  (or
(or  ) because Co oxide deposition commenced at ca. 0.38 V and two peaks were clearly found. In contrast, from the potential-pH diagrams of Ni17 and curve 1 in Fig. 1, the Ni species in the plating solution may be directly oxidized to form Ni(IV) because the Ni oxide deposition commenced at ca. 0.65 V. If this is the case, the electron transfer number of Ni oxide deposition (i.e., 2 per Ni atom) is much larger than that of Co oxide deposition (i.e., 2/3 per Co atom). Because
) because Co oxide deposition commenced at ca. 0.38 V and two peaks were clearly found. In contrast, from the potential-pH diagrams of Ni17 and curve 1 in Fig. 1, the Ni species in the plating solution may be directly oxidized to form Ni(IV) because the Ni oxide deposition commenced at ca. 0.65 V. If this is the case, the electron transfer number of Ni oxide deposition (i.e., 2 per Ni atom) is much larger than that of Co oxide deposition (i.e., 2/3 per Co atom). Because  (peak current) of LSV is directly proportional to
(peak current) of LSV is directly proportional to  where
where  is the number of electrons transferred,18 the limiting currents of Co oxide deposition should be only one-ninth of that of Ni oxide deposition. The above speculation is further supported by the fact that the deposition mechanism of Co oxide was found to be more complicated as compared to that of Ni oxide.15 Thus, the much lower peak current on the LSV curve in Fig. 1 for Co oxide deposition may be attributable to its relatively complicated deposition mechanism.
is the number of electrons transferred,18 the limiting currents of Co oxide deposition should be only one-ninth of that of Ni oxide deposition. The above speculation is further supported by the fact that the deposition mechanism of Co oxide was found to be more complicated as compared to that of Ni oxide.15 Thus, the much lower peak current on the LSV curve in Fig. 1 for Co oxide deposition may be attributable to its relatively complicated deposition mechanism.
Typical cyclic voltammograms (CVs) of the  deposit measured at
deposit measured at  in 1 M NaOH at 4, 25, and
in 1 M NaOH at 4, 25, and  are, respectively, shown as curves 1-3 in Fig. 2. On these CV curves, the redox transitions of nickel and cobalt oxides commenced at ca. 0 V and a pair of symmetric but not well-defined peaks was found. In addition, significant currents of oxide oxidation and reduction lasted to the onset potential of oxygen evolution. The above results indicate the fact that the obvious redox transition of
are, respectively, shown as curves 1-3 in Fig. 2. On these CV curves, the redox transitions of nickel and cobalt oxides commenced at ca. 0 V and a pair of symmetric but not well-defined peaks was found. In addition, significant currents of oxide oxidation and reduction lasted to the onset potential of oxygen evolution. The above results indicate the fact that the obvious redox transition of  prior to the oxygen evolution reaction9 was seriously suppressed by mixing in cobalt oxide, which has been employed to store the electrochemical energy of the cathodes in secondary batteries.16 Note that from a comparison of these three curves in Fig. 2, the redox transitions within the binary oxide were not affected by the change in electrolyte temperature although oxygen evolution can be enhanced by an increase of electrolyte temperature. In addition, the voltammetric responses prior to oxygen evolution on the positive sweeps were symmetrical to their counterparts on the negative sweeps. The above results reveal the high electrochemical reversibility of the binary oxide prepared by the anodic deposition. Because the suitability of an electroactive material for EC supercapacitors is strongly dependent upon the electrochemical kinetics of the redox transitions (i.e., reversibility),1
3
4
5
6
12
13 this binary oxide exhibited very suitable electrochemical characteristics for EC supercapacitors.
prior to the oxygen evolution reaction9 was seriously suppressed by mixing in cobalt oxide, which has been employed to store the electrochemical energy of the cathodes in secondary batteries.16 Note that from a comparison of these three curves in Fig. 2, the redox transitions within the binary oxide were not affected by the change in electrolyte temperature although oxygen evolution can be enhanced by an increase of electrolyte temperature. In addition, the voltammetric responses prior to oxygen evolution on the positive sweeps were symmetrical to their counterparts on the negative sweeps. The above results reveal the high electrochemical reversibility of the binary oxide prepared by the anodic deposition. Because the suitability of an electroactive material for EC supercapacitors is strongly dependent upon the electrochemical kinetics of the redox transitions (i.e., reversibility),1
3
4
5
6
12
13 this binary oxide exhibited very suitable electrochemical characteristics for EC supercapacitors.
Figure 2. CVs of the  deposit measured at
deposit measured at  in 1 M NaOH with its temperature of (1) 4, (2) 25, and (3)
in 1 M NaOH with its temperature of (1) 4, (2) 25, and (3) 
The pseudocapacitance of hydrous oxides mainly came from the electrochemical energy stored by the faradaic redox transitions of interfacial oxycation species. In alkaline media, the redox transitions of hydrous cobalt and nickel oxides should include M(III)/M(II) (and/or M(IV)/M(III), where M indicates Co or Ni) in the potential region prior to the oxygen evolution reaction.9
15
16 In addition, these redox transitions, which exchange protons (or  ) with the electrolytes, should obey Eq. 1 or 2 because the redox transitions of superficial oxycations followed a nonstoichiometric ratio9
15
16
19
) with the electrolytes, should obey Eq. 1 or 2 because the redox transitions of superficial oxycations followed a nonstoichiometric ratio9
15
16
19
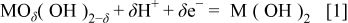
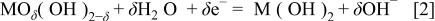
where  indicates the interfacial cobalt and nickel oxides at higher oxidation states. Note that hydrous Ni and Co oxides were proposed to have mixed or nonstoichiometric properties rather than well-defined oxidation states.9 The value of δ is believed to range from 1 to 2, which is strongly dependent upon the average electron transfer number of the redox couples between the higher and lower oxidation states.20
indicates the interfacial cobalt and nickel oxides at higher oxidation states. Note that hydrous Ni and Co oxides were proposed to have mixed or nonstoichiometric properties rather than well-defined oxidation states.9 The value of δ is believed to range from 1 to 2, which is strongly dependent upon the average electron transfer number of the redox couples between the higher and lower oxidation states.20
Because voltammetric currents were mainly due to the faradaic redox transitions of  voltammetric charges,
voltammetric charges,  integrated between 0.54 and 0.1 V on the negative sweeps of CV curves, can be used as an effective index for determining the number of electroactive surface oxycations involving in the redox transitions.4
5
6 Thus, the mean specific capacitance of the oxide can be estimated on the basis of Eq. 3
4
5
6
12
13
integrated between 0.54 and 0.1 V on the negative sweeps of CV curves, can be used as an effective index for determining the number of electroactive surface oxycations involving in the redox transitions.4
5
6 Thus, the mean specific capacitance of the oxide can be estimated on the basis of Eq. 3
4
5
6
12
13
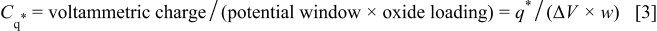
where  is the potential window of the upper and lower limits of integration (i.e., 0.44 V). Based on Eq. 3, the mean specific capacitance of the
is the potential window of the upper and lower limits of integration (i.e., 0.44 V). Based on Eq. 3, the mean specific capacitance of the  deposit obtained from the CV measured at
deposit obtained from the CV measured at  in Fig. 2 is ca.
in Fig. 2 is ca.  This large pseudocapacitance indicates that the
This large pseudocapacitance indicates that the  deposit plated by anodic deposition is a potential candidate for EC supercapacitors.
deposit plated by anodic deposition is a potential candidate for EC supercapacitors.
Typical charging-discharging curves of this  deposit measured at
deposit measured at  in 1 M NaOH at 4, 25, and
in 1 M NaOH at 4, 25, and  are, respectively, shown as curves 1-3 in Fig. 3. The three charging curves are mirror-symmetrical to their discharging counterparts although they were measured at different temperatures. The above results further support the statement that this binary hydrous Co-Ni oxide exhibited highly reversible characteristics in the potential region prior to the oxygen evolution reaction. Thus, this material is more suitable for EC supercapacitors than for the rechargeable batteries. Note that the capacitance of this deposit gradually increased with decrease in temperature of the test electrolytes. The average specific pseudocapacitance of this
are, respectively, shown as curves 1-3 in Fig. 3. The three charging curves are mirror-symmetrical to their discharging counterparts although they were measured at different temperatures. The above results further support the statement that this binary hydrous Co-Ni oxide exhibited highly reversible characteristics in the potential region prior to the oxygen evolution reaction. Thus, this material is more suitable for EC supercapacitors than for the rechargeable batteries. Note that the capacitance of this deposit gradually increased with decrease in temperature of the test electrolytes. The average specific pseudocapacitance of this  deposit can be estimated from Eq. 4
4
5
6
12
13
deposit can be estimated from Eq. 4
4
5
6
12
13
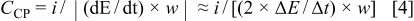
where  and
and  respectively, indicate the constant current that is applied and the slope of these chronopentiometric (CP) curves, which is very close to its mean value when the CP curve is approximately linear and symmetric. The average specific pseudocapacitance of the
respectively, indicate the constant current that is applied and the slope of these chronopentiometric (CP) curves, which is very close to its mean value when the CP curve is approximately linear and symmetric. The average specific pseudocapacitance of the  deposit obtained on the basis of Eq. 4 is ca. 760, 700, and
deposit obtained on the basis of Eq. 4 is ca. 760, 700, and  for the curves measured at 4, 25, and
for the curves measured at 4, 25, and  which is very close to that estimated from the CV curves. This result further supports the fact that the redox transitions of the superficial oxycations exhibit high electrochemical reversibility.
which is very close to that estimated from the CV curves. This result further supports the fact that the redox transitions of the superficial oxycations exhibit high electrochemical reversibility.
Figure 3. Chronopotentiograms of the  deposit measured at
deposit measured at  in 1 M NaOH with its temperature of (1) 4, (2) 25, and (3)
in 1 M NaOH with its temperature of (1) 4, (2) 25, and (3)  between 0.1 and 0.42 V.
between 0.1 and 0.42 V.
The electrochemical reversibility of the redox transitions for this binary hydrous oxide prior to the oxygen evolution reaction was further investigated by CP with changing of the charge-discharge currents. Typical CP diagrams of this  deposit measured at 600, 300, 150, and
deposit measured at 600, 300, 150, and  in 1 M NaOH are, respectively, shown as curves 1-4 in Fig. 4. Note that all anodic charging curves were mirror-symmetrical to their cathodic discharging counterparts although these curves were measured at different applied currents. Moreover, the mean specific capacitance of this
in 1 M NaOH are, respectively, shown as curves 1-4 in Fig. 4. Note that all anodic charging curves were mirror-symmetrical to their cathodic discharging counterparts although these curves were measured at different applied currents. Moreover, the mean specific capacitance of this  deposit measured at 600, 300, 150, and
deposit measured at 600, 300, 150, and  was ca. 700, 680, 690, and
was ca. 700, 680, 690, and  respectively, indicating the fact that the mean specific capacitance of
respectively, indicating the fact that the mean specific capacitance of  is approximately independent of the charging-discharging currents. Therefore, the
is approximately independent of the charging-discharging currents. Therefore, the  prepared in this work is concluded to be an electrochemical energy storage material possessing a high power property.
prepared in this work is concluded to be an electrochemical energy storage material possessing a high power property.
Figure 4. Chronopotentiograms of the  deposit measured at (1) 600, (2) 300, (3) 150, and (4)
deposit measured at (1) 600, (2) 300, (3) 150, and (4)  in 1 M NaOH between 0.1 and 0.42 V.
in 1 M NaOH between 0.1 and 0.42 V.
From all the above results and discussion, the  fabricated by anodic deposition from
fabricated by anodic deposition from  and
and  solution with initial pH 8.0 exhibited ideally capacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) for EC supercapacitors.
solution with initial pH 8.0 exhibited ideally capacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) for EC supercapacitors.
The morphology of this  deposit was examined by means of an atomic force microscope (AFM) and the result is shown in Fig. 5. This deposit showed a very rough morphology with many small spherical grains aggregated to form the larger oxide particles. The mean roughness of this image estimated from the AFM is 576 nm(Ra). The above results indicate that the very large mean specific capacitance of this oxide deposit may be linked to its highly porous nature. Although many obvious grains were congregated to form the deposit, this oxide showed an amorphous structure revealed by the XRD spectrum (not shown here).
deposit was examined by means of an atomic force microscope (AFM) and the result is shown in Fig. 5. This deposit showed a very rough morphology with many small spherical grains aggregated to form the larger oxide particles. The mean roughness of this image estimated from the AFM is 576 nm(Ra). The above results indicate that the very large mean specific capacitance of this oxide deposit may be linked to its highly porous nature. Although many obvious grains were congregated to form the deposit, this oxide showed an amorphous structure revealed by the XRD spectrum (not shown here).
Figure 5. AFM morphology of the  deposit prepared by anodic deposition at 0.75 V from 6 mM
deposit prepared by anodic deposition at 0.75 V from 6 mM 
 with pH 8.0. Img. Z range: the vertical difference between the highest and lowest points within the image. Img. Mean: mean value of data contained within the image. Img. Raw Mean: mean value of image data without application of plane fitting. Img. Rms (Rq): root mean square average of height deviations taken from the mean data plane. Img. Ra: arithmetic average of the absolute values of the surface height deviations measured from the mean plane.
with pH 8.0. Img. Z range: the vertical difference between the highest and lowest points within the image. Img. Mean: mean value of data contained within the image. Img. Raw Mean: mean value of image data without application of plane fitting. Img. Rms (Rq): root mean square average of height deviations taken from the mean data plane. Img. Ra: arithmetic average of the absolute values of the surface height deviations measured from the mean plane.
Conclusions
The  deposit anodically plated from a
deposit anodically plated from a  solution with the Co:Ni ratio of 4:6 and pH 8.0 exhibited a very large pseudocapacitance of ca.
solution with the Co:Ni ratio of 4:6 and pH 8.0 exhibited a very large pseudocapacitance of ca.  in 1 M NaOH. This material showed ideally capacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) at different temperatures for EC supercapacitors. The very large mean specific capacitance of this amorphous hydrous oxide deposit may be linked to its highly porous nature from the AFM morphology.
in 1 M NaOH. This material showed ideally capacitive behavior (i.e., high reversibility, high specific capacitance, and high power property) at different temperatures for EC supercapacitors. The very large mean specific capacitance of this amorphous hydrous oxide deposit may be linked to its highly porous nature from the AFM morphology.
Acknowledgments
The financial support of this work, by the National Science Council of Taiwan under contract no. NSC 90-2214-E-194-007, is gratefully acknowledged.
Dr. Hu assisted in meeting the publication costs of this article.





