Abstract
The electrochemical performance of 3d metal oxide (MO) electrode materials for Li-ion batteries was studied in the form of  half-cells. Reversible capacity in the 750-1000 mAh/g range was achieved and sustained over numerous charge-discharge cycles both at room temperature and at 55°C. The studied oxides were then used as negative-electrode active materials to assemble larger plastic
half-cells. Reversible capacity in the 750-1000 mAh/g range was achieved and sustained over numerous charge-discharge cycles both at room temperature and at 55°C. The studied oxides were then used as negative-electrode active materials to assemble larger plastic  Li-ion cells, which exhibited an average output voltage of 2 V and a stable reversible specific energy of 120 Wh/kg during extended cycling at ambient and elevated temperatures. This value can be compared to 180 Wh/kg obtained for similar
Li-ion cells, which exhibited an average output voltage of 2 V and a stable reversible specific energy of 120 Wh/kg during extended cycling at ambient and elevated temperatures. This value can be compared to 180 Wh/kg obtained for similar  Li-ion cells. Based on modeling, several scenarios involving material considerations present the optimum method for boosting the energy density of such
Li-ion cells. Based on modeling, several scenarios involving material considerations present the optimum method for boosting the energy density of such  Li-ion systems. © 2002 The Electrochemical Society. All rights reserved.
Li-ion systems. © 2002 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Because of their excellent electrochemical performance, large gravimetric and volumetric energy density, and design flexibility, Li-ion batteries are progressively supplanting nickel-metal hydride (Ni-MH) cells, and are the power source of choice for today's portable electronics. With the continuing trend toward lower operating voltage in electronics devices, the 1.2 V Ni-MH battery technology could, within the next few years, catch up again if no progress is made to meet this demand by adjusting the Li-ion cell voltage. This implies the search for negative electrode materials with significantly larger capacities, which would react with Li at a higher voltage than the presently used carbonaceous materials. As a result, they could be used with today's most common Li-based oxides, and lead to lower voltage Li-ion cells. Due to this line of reasoning, there is an increased interest in systems based on carbon alternatives as exemplified by the study of a 2 V Li-ion  system1
2 that provide higher energy density at lower operating voltages than today's
system1
2 that provide higher energy density at lower operating voltages than today's  Li-ion cells. Until now, such promising developments have been slowed down by the restrictive manufacturing requirements needed to protect such compositions from moisture. Thus, more stable negative electrodes are needed.
Li-ion cells. Until now, such promising developments have been slowed down by the restrictive manufacturing requirements needed to protect such compositions from moisture. Thus, more stable negative electrodes are needed.
Numerous systems other than graphitic carbons, such as tin oxide-based electrodes,3
4 composite negative alloy electrodes,5
6 or lithiated vanadium oxide-based electrodes7
8 have received a great deal of attention. However, the poor capacity retention of these new materials during cycling and, more importantly, large irreversible capacity loss observed during the first charge-discharge cycle of these materials, have limited their use in practical cells. The 3d metal oxide negative electrodes recently reported by us9
10
11
12 also suffer from an irreversible capacity loss of about 25% during the first cycle. This is obviously larger than the 15% loss displayed by the coke-type materials used in the first generation of commercial Li-ion cells, but this drawback could be overcome by the large reversible capacities of the metal oxides (750 and 1000 mAh/g for CoO and  respectively) that are about three times as large as those for carbons. Thus, we set out to optimize the performance of Li-ion cells based on 3d metal oxides as the negative electrodes by determining the capacity retention of such materials in half-cells and by finding a solution to their initial large capacity loss, which markedly degrades the performance characteristics of assembled Li-ion cells. While numerous metal oxides-based Li-ion cells (e.g., NiO, FeO,
respectively) that are about three times as large as those for carbons. Thus, we set out to optimize the performance of Li-ion cells based on 3d metal oxides as the negative electrodes by determining the capacity retention of such materials in half-cells and by finding a solution to their initial large capacity loss, which markedly degrades the performance characteristics of assembled Li-ion cells. While numerous metal oxides-based Li-ion cells (e.g., NiO, FeO,  CoO) are being studied in our laboratories, this paper is limited to the discussion of cells using CoO or
CoO) are being studied in our laboratories, this paper is limited to the discussion of cells using CoO or  The resulting experimental 2 V cells were assembled using plastic Li-ion battery technology, and exhibited ca. 66% of the energy density of commercial
The resulting experimental 2 V cells were assembled using plastic Li-ion battery technology, and exhibited ca. 66% of the energy density of commercial  cells.
cells.
Experimental
The Co-based metal oxides in powder form used within this study were obtained from Union Minière (CoO) or synthesized in-house  The latter was prepared by heating CoO at 700°C in air for 12 h with intermediate grinding steps. Alternatively, it was synthesized by the polyol process, which, as recently reported by our group,13 is capable of yielding monodisperse spherical particles with a crystallite size that can be controlled by the firing temperature.
The latter was prepared by heating CoO at 700°C in air for 12 h with intermediate grinding steps. Alternatively, it was synthesized by the polyol process, which, as recently reported by our group,13 is capable of yielding monodisperse spherical particles with a crystallite size that can be controlled by the firing temperature.  was purchased from Seimi Chemical, Japan, while
was purchased from Seimi Chemical, Japan, while  was synthesized in-house according to the previously reported procedure.14 All the active materials were determined by X-ray analysis to be single-phase compounds with a Brunauer-Emmett-Teller method specific surface area smaller than
was synthesized in-house according to the previously reported procedure.14 All the active materials were determined by X-ray analysis to be single-phase compounds with a Brunauer-Emmett-Teller method specific surface area smaller than 
If not otherwise specified, the materials were tested in standard 2035 size coin cells using a plastic positive electrode disk containing 64 wt % of  materials, 8 wt % SP carbon black (MMM Carbon, Belgium), and 28 wt % poly(vinylidene fluoride)-hexafluoropropylene (PVDF-HFP) copolymer binder (the electrode films were cast and processed using a procedure previously reported15), a
materials, 8 wt % SP carbon black (MMM Carbon, Belgium), and 28 wt % poly(vinylidene fluoride)-hexafluoropropylene (PVDF-HFP) copolymer binder (the electrode films were cast and processed using a procedure previously reported15), a  disk of Li foil as the negative electrode member, and a Whatman GF/D borosilicate glass fiber sheet separator saturated with a 1 M
disk of Li foil as the negative electrode member, and a Whatman GF/D borosilicate glass fiber sheet separator saturated with a 1 M  electrolyte solution in a 1:1 (by weight) dimethyl carbonate/ethylene carbonate. The plasticized
electrolyte solution in a 1:1 (by weight) dimethyl carbonate/ethylene carbonate. The plasticized  or
or  positive electrodes were fabricated in a similar way, but using 88, 4, and 8 wt % of oxides, SP carbon black, and PVDF-HFP, respectively. In the three-electrode Swagelok cells,16 the Li metal reference electrode was electrodeposited on a Ni wire, which was previously sandwiched between two electrolyte-soaked glass paper disks, and placed between the positive and the negative electrode disks.
positive electrodes were fabricated in a similar way, but using 88, 4, and 8 wt % of oxides, SP carbon black, and PVDF-HFP, respectively. In the three-electrode Swagelok cells,16 the Li metal reference electrode was electrodeposited on a Ni wire, which was previously sandwiched between two electrolyte-soaked glass paper disks, and placed between the positive and the negative electrode disks.
Both positive (88 wt %  ) and negative (64 wt % CoO or
) and negative (64 wt % CoO or  ) plastic electrodes were cast from acetone slurries using propylene carbonate as a plasticizer, SP carbon as a conductive additive and PVDF-HFP copolymer as a binder. To fabricate plastic Li-ion cells, the two opposite-polarity electrode tapes were bonded to their respective metal grid current collectors (Al on the positive, Cu on the negative side), and then thermally laminated to the opposite sides of the surface-modified microporous polyethylene separator (Celgard 903).17 The resulting plastic battery laminate was then extracted in diethyl ether, dried under vacuum, packaged, and activated with the electrolyte solution described above. All cells were assembled in a helium filled glove box at a dew point of −80°C. The electrochemical tests were performed using a MacPile (BioLogic, France) automatic cycling and data recording system. Unless otherwise specified, the cells were galvanostatically cycled between 3.00 and 0.01 V at a C/5 rate, e.g., one lithium ion per formula unit in 5 h, recording voltage vs. time every 0.02 V. Cycling at elevated temperature was performed with cells placed in an air oven at 55°C.
) plastic electrodes were cast from acetone slurries using propylene carbonate as a plasticizer, SP carbon as a conductive additive and PVDF-HFP copolymer as a binder. To fabricate plastic Li-ion cells, the two opposite-polarity electrode tapes were bonded to their respective metal grid current collectors (Al on the positive, Cu on the negative side), and then thermally laminated to the opposite sides of the surface-modified microporous polyethylene separator (Celgard 903).17 The resulting plastic battery laminate was then extracted in diethyl ether, dried under vacuum, packaged, and activated with the electrolyte solution described above. All cells were assembled in a helium filled glove box at a dew point of −80°C. The electrochemical tests were performed using a MacPile (BioLogic, France) automatic cycling and data recording system. Unless otherwise specified, the cells were galvanostatically cycled between 3.00 and 0.01 V at a C/5 rate, e.g., one lithium ion per formula unit in 5 h, recording voltage vs. time every 0.02 V. Cycling at elevated temperature was performed with cells placed in an air oven at 55°C.
Results and Discussion
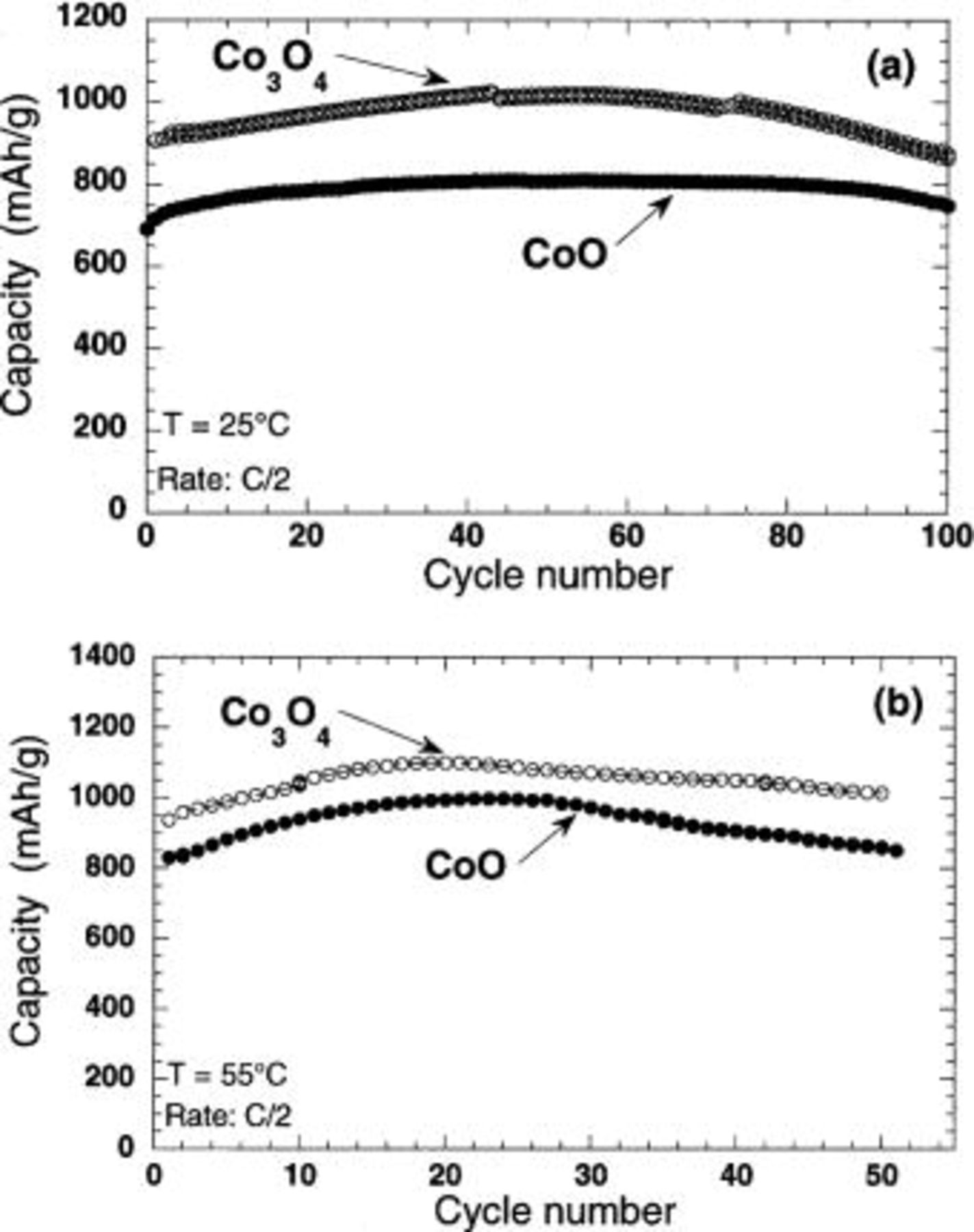
A survey of the electrochemical performances of various metal oxides carried out using Li-metal half-cells indicated that irrespective of the nature of the Co oxide, i.e., CoO or  the best electrochemical performance was obtained for powdered samples with ca. 1 μm particle size. The capacity retention exhibited by these pre-optimized samples is reported on Fig. 1a for both oxides. Note that the first-cycle capacity loss was ca. 25%, but no further capacity loss was observed after more than 100 cycles. However, the two oxides mainly differ in their reversible capacity, which was found to be 750 mAh/g for CoO and 1000 mAh/g for
the best electrochemical performance was obtained for powdered samples with ca. 1 μm particle size. The capacity retention exhibited by these pre-optimized samples is reported on Fig. 1a for both oxides. Note that the first-cycle capacity loss was ca. 25%, but no further capacity loss was observed after more than 100 cycles. However, the two oxides mainly differ in their reversible capacity, which was found to be 750 mAh/g for CoO and 1000 mAh/g for  which is two to three times greater than those for typical carbonaceous materials (330-350 mAh/g), while exhibiting densities that are two to three times greater. The cycling capacity of both Li/CoO and
which is two to three times greater than those for typical carbonaceous materials (330-350 mAh/g), while exhibiting densities that are two to three times greater. The cycling capacity of both Li/CoO and  cells at 55°C initially slightly increases (Fig. 1b), and then levels off at about 30 cycles to reach capacity values as high as 950 and 1100 mAh/g, respectively, and finally decreases slowly during extended cycling. These partially optimized materials were then used in conjunction with
cells at 55°C initially slightly increases (Fig. 1b), and then levels off at about 30 cycles to reach capacity values as high as 950 and 1100 mAh/g, respectively, and finally decreases slowly during extended cycling. These partially optimized materials were then used in conjunction with  and
and  cathodes to assemble Li-ion cells.
cathodes to assemble Li-ion cells.
Figure 1. Capacity retention for Li/CoO and  ion cells at room temperature and 55°C. The electrode area was
ion cells at room temperature and 55°C. The electrode area was  the mass of the electroactive material was 4 mg. The cells were cycled at a C/2 rate between 0.01 and 3 V.
the mass of the electroactive material was 4 mg. The cells were cycled at a C/2 rate between 0.01 and 3 V.
Although the detailed reaction mechanism of Li with 3d oxides differs from that observed for carbon in the well-known Li-ion systems, there are broad similarities between the two. When a rocking chair cell containing  as a positive electrode material and a metal oxide, such as CoO, as the negative electrode, is charged for the first time, lithium is deintercalated from the positive electrode, and reacts with the MO in the negative electrode. Thus, the relative amounts of these two electrode materials are directly correlated. To obtain a rough estimate of the optimum mass ratio
as a positive electrode material and a metal oxide, such as CoO, as the negative electrode, is charged for the first time, lithium is deintercalated from the positive electrode, and reacts with the MO in the negative electrode. Thus, the relative amounts of these two electrode materials are directly correlated. To obtain a rough estimate of the optimum mass ratio  of the two active components, we can use the weight ratio of the two components to equal first-discharge capacities: for CoO vs. Li, 950 mAh/g to 0.01 V, for
of the two active components, we can use the weight ratio of the two components to equal first-discharge capacities: for CoO vs. Li, 950 mAh/g to 0.01 V, for  vs. Li, 1300 mAh/g, and 145 mAh/g the first charge of
vs. Li, 1300 mAh/g, and 145 mAh/g the first charge of  vs. Li (to 4.2 V). This estimate leads to weight ratios
vs. Li (to 4.2 V). This estimate leads to weight ratios  equal to 6.6 and 8.9 for the
equal to 6.6 and 8.9 for the  and
and  Li-ion cells, respectively. However, for safety reasons (the risk of lithium plating at the
Li-ion cells, respectively. However, for safety reasons (the risk of lithium plating at the  electrode or over-delithiation of
electrode or over-delithiation of  ), we cannot rely solely on such simple calculations, especially when CoO and
), we cannot rely solely on such simple calculations, especially when CoO and  display very flat voltage-composition curves in their deintercalated or fully reacted state. Thus, to optimize the electrochemical performance of such Li-ion cell and use both electrodes in an efficient and safe manner, we prepared a series of three-electrode cells with the
display very flat voltage-composition curves in their deintercalated or fully reacted state. Thus, to optimize the electrochemical performance of such Li-ion cell and use both electrodes in an efficient and safe manner, we prepared a series of three-electrode cells with the  and
and  electrode pairs using different
electrode pairs using different  values. The best results were obtained for the values of
values. The best results were obtained for the values of  ranging from 6.0 to 6.5 and from 8.1 to 8.6 for the
ranging from 6.0 to 6.5 and from 8.1 to 8.6 for the  and
and  Li-ion cells, respectively. Using a larger
Li-ion cells, respectively. Using a larger  to MO ratio improves the battery capacity, but decreases its cycle life and safety due to the possibility of Li plating, while a smaller
to MO ratio improves the battery capacity, but decreases its cycle life and safety due to the possibility of Li plating, while a smaller  value leads to a safer, but capacity-limited, battery.
value leads to a safer, but capacity-limited, battery.
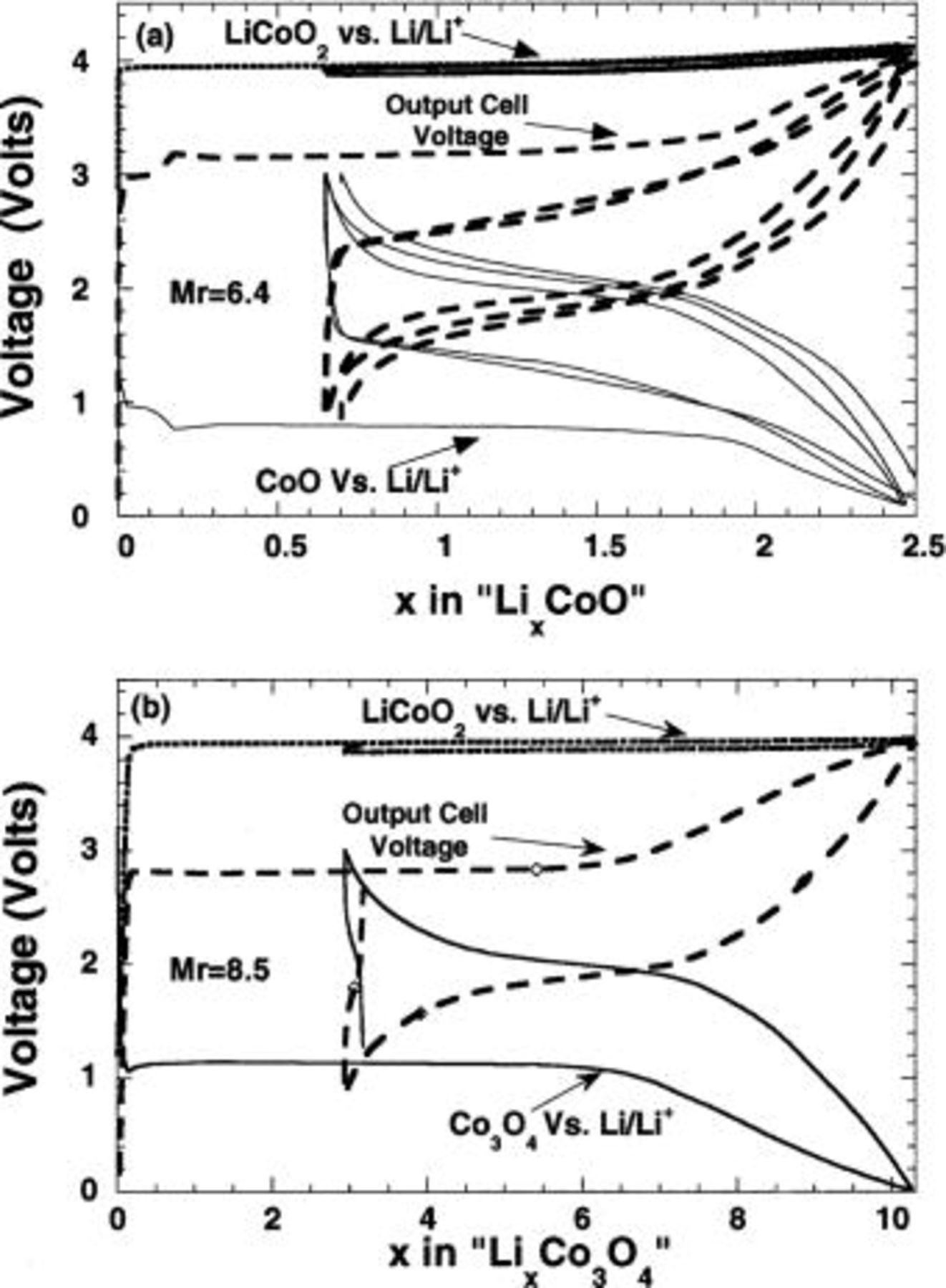
The charge-discharge profiles for three-electrode 
 and
and 
 Li-ion cells are shown in Fig. 2a and b, respectively. In both cases, the first charge takes longer to complete at the same current density than the first discharge as expected from the large irreversible capacity loss of CoO or
Li-ion cells are shown in Fig. 2a and b, respectively. In both cases, the first charge takes longer to complete at the same current density than the first discharge as expected from the large irreversible capacity loss of CoO or  vs. Li during the previously measured first cycle. Once the first cycle concluded, the 2 V cell exhibits excellent reversibility as shown by the equal duration of the charge and discharge segments over numerous cycles (at least 50).
vs. Li during the previously measured first cycle. Once the first cycle concluded, the 2 V cell exhibits excellent reversibility as shown by the equal duration of the charge and discharge segments over numerous cycles (at least 50).
Figure 2. Two- and three-electrode potential data for  (curve a) and
(curve a) and  (b) cells balanced at
(b) cells balanced at  ratios of 6.4 and 8.5, respectively. Note a 2 V cell output voltage and the large irreversible capacity during the first cycle. The cycling was done at a C/5 rate.
ratios of 6.4 and 8.5, respectively. Note a 2 V cell output voltage and the large irreversible capacity during the first cycle. The cycling was done at a C/5 rate.
To compare the properties and performance of these negative metal oxides-based Li-ion cells with those of the benchmark  cells, a number of larger-sized
cells, a number of larger-sized  plastic Liion cells were made using
plastic Liion cells were made using 
 and
and  as the negative/positive electrode materials couples. The thickness of the
as the negative/positive electrode materials couples. The thickness of the  electrode was altered to yield the appropriate previously described matching ratio
electrode was altered to yield the appropriate previously described matching ratio  For example, the densities of the positive and negative electrodes in the Li-ion
For example, the densities of the positive and negative electrodes in the Li-ion  cell were 0.112 and
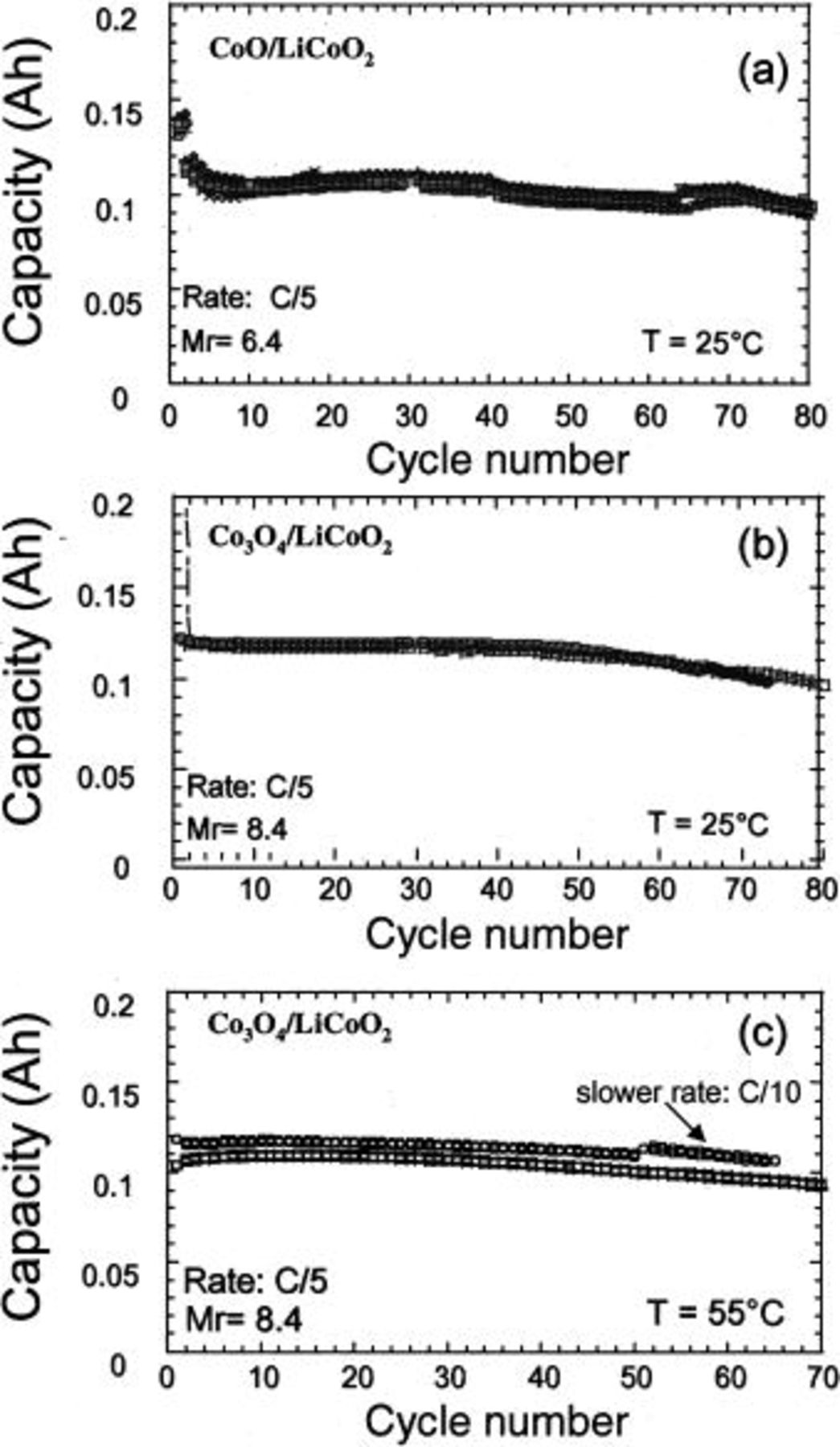
cell were 0.112 and  respectively. The cell capacity for different charge cutoff voltages was determined at a C/5 charge-discharge rate and a lower cutoff voltage of 0.9 V. The best results, without causing a decrease in cycle life, were obtained when the upper cutoff voltage of 4.1 V was used. The capacity retention data for
respectively. The cell capacity for different charge cutoff voltages was determined at a C/5 charge-discharge rate and a lower cutoff voltage of 0.9 V. The best results, without causing a decrease in cycle life, were obtained when the upper cutoff voltage of 4.1 V was used. The capacity retention data for  and
and  Li-ion cells are shown in Fig. 3a, b, and c. For both systems, at least 80 cycles with little capacity decay at room temperature (Fig. 3a and b) and a 100% capacity retention up to 50 cycles could be achieved. The cycle life of the
Li-ion cells are shown in Fig. 3a, b, and c. For both systems, at least 80 cycles with little capacity decay at room temperature (Fig. 3a and b) and a 100% capacity retention up to 50 cycles could be achieved. The cycle life of the  system was not affected at higher temperatures (Fig. 3c), because at least 70 cycles at 55°C were attained with less than 10% capacity loss. Interestingly, the capacity decrease in
system was not affected at higher temperatures (Fig. 3c), because at least 70 cycles at 55°C were attained with less than 10% capacity loss. Interestingly, the capacity decrease in  and
and  Li-ion cells was independent of temperature, and behaved similarly to that in the Li/CoO or
Li-ion cells was independent of temperature, and behaved similarly to that in the Li/CoO or  half-cells, which suggests that the degradation of the MO electrode is most likely responsible for the cycle life limitation. We recently found that the cycle life of such cells is strongly affected by the processing parameters used to prepare plastic electrode laminates, and by using some modifications, more than 200 cycles are achievable.
half-cells, which suggests that the degradation of the MO electrode is most likely responsible for the cycle life limitation. We recently found that the cycle life of such cells is strongly affected by the processing parameters used to prepare plastic electrode laminates, and by using some modifications, more than 200 cycles are achievable.  Li-ion cells exhibited a cycling capacity similar to that of the
Li-ion cells exhibited a cycling capacity similar to that of the  cells, but due to their low average output voltage (2 V vs. 3.65 V for
cells, but due to their low average output voltage (2 V vs. 3.65 V for  Li-ion cells), their energy density was only 120 Wh/kg compared to 180 Wh/kg achievable for
Li-ion cells), their energy density was only 120 Wh/kg compared to 180 Wh/kg achievable for  cells.
cells.
Figure 3. Capacity retention at room temperature for (a) a plastic  Li-ion cells
Li-ion cells  and (b) for a plastic
and (b) for a plastic  Li-ion cell assembled at a
Li-ion cell assembled at a  value of 8.4. The 55°C capacity retention for this
value of 8.4. The 55°C capacity retention for this  ion cell is shown in (c). The cells were cycled at a C/5 rate between 0.9 and 4.1 V. To stress the reproducibility of the experiments the data for 4 and 2 different cells has been reported in (a) and (b, c), respectively.
ion cell is shown in (c). The cells were cycled at a C/5 rate between 0.9 and 4.1 V. To stress the reproducibility of the experiments the data for 4 and 2 different cells has been reported in (a) and (b, c), respectively.
While the recently obtained cycle life results are encouraging, the large irreversible capacity during the first cycle, which is associated with the MO electrode, is an inherent drawback of such a Li-ion systems. This is an issue critical to the acceptance of alternative Li-ion systems, because they do not contain an excess Li metal to make up for the initial irreversible loss. To circumvent this problem, we used positive electrode materials that can serve as a Li reservoir to compensate for the large irreversible loss of the CoO electrode. For example, the chemically lithiated and moisture-stable spinel  having
having  values ranging from 0 to 1 appears to be a suitable candidate. By increasing the Li content from
values ranging from 0 to 1 appears to be a suitable candidate. By increasing the Li content from  to
to  e.g., by creating a reservoir of
e.g., by creating a reservoir of  Li, we barely change the weight of such electrode. To describe the benefits of such an approach, we focused on the electrodes based on
Li, we barely change the weight of such electrode. To describe the benefits of such an approach, we focused on the electrodes based on  and compared the properties of
and compared the properties of  and
and  Li-ion cells. We used a
Li-ion cells. We used a  electrode material prepared by chemically reducing
electrode material prepared by chemically reducing  with a reducing agent, i.e., LiI in acetonitrile as previously reported by us.18 The resulting material was used to fabricate a plasticized electrode film out of which electrode disks were cut. Next, a
with a reducing agent, i.e., LiI in acetonitrile as previously reported by us.18 The resulting material was used to fabricate a plasticized electrode film out of which electrode disks were cut. Next, a  Li-ion cell with an
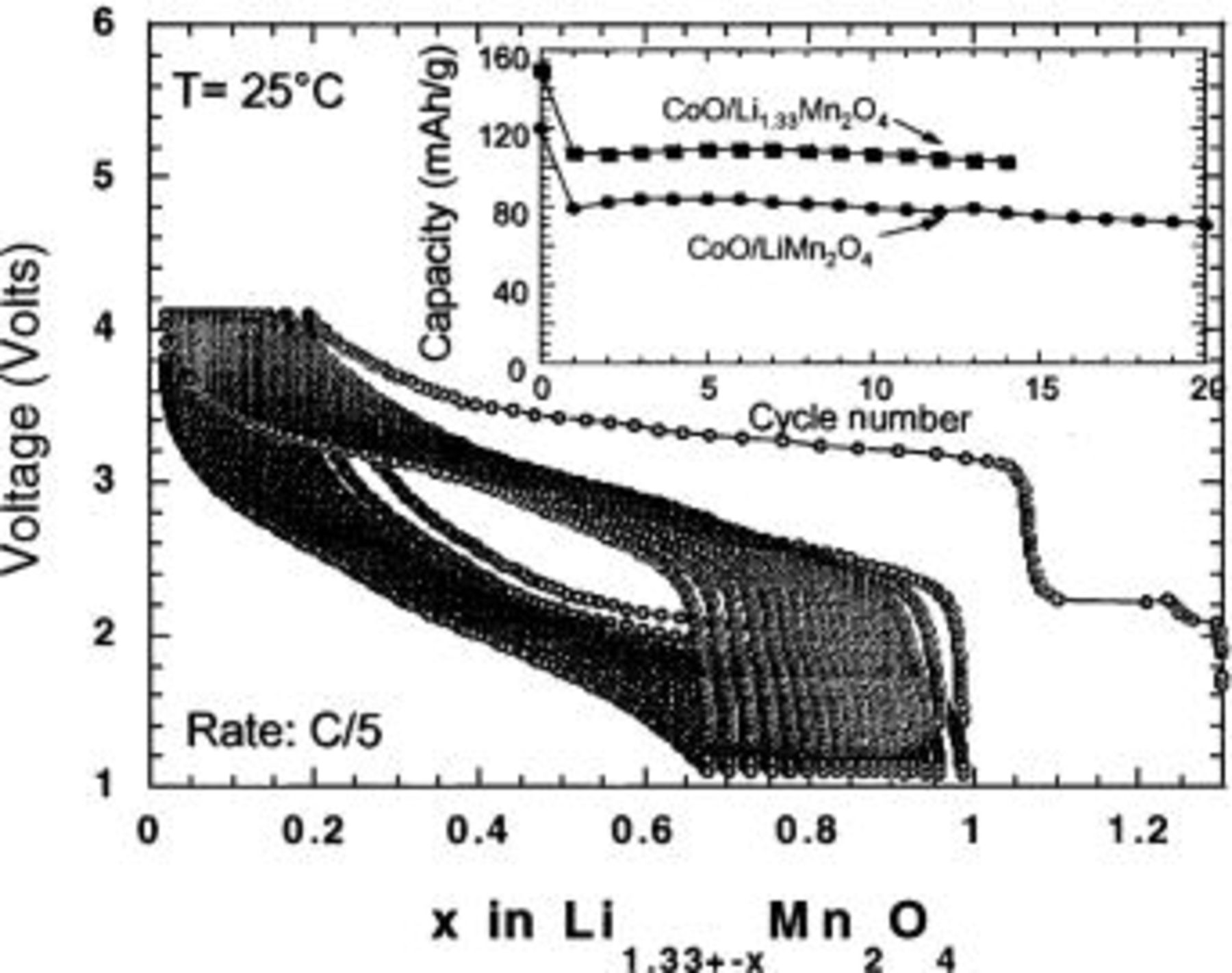
Li-ion cell with an  equal to 6.2 was assembled, and its voltage vs. composition behavior is shown on Fig. 4. Note that during the first charge, the excess of Li removed at 3 V is used to compensate for the initial 25% irreversible loss. During subsequent cycling, the cell was cycled at a capacity of 110 mAh/g of the overlithiated spinel, while a comparable
equal to 6.2 was assembled, and its voltage vs. composition behavior is shown on Fig. 4. Note that during the first charge, the excess of Li removed at 3 V is used to compensate for the initial 25% irreversible loss. During subsequent cycling, the cell was cycled at a capacity of 110 mAh/g of the overlithiated spinel, while a comparable  Li-ion cell containing "regular" spinel
Li-ion cell containing "regular" spinel  cycled at a capacity of 90 mAh/g (Fig. 4 inset). The
cycled at a capacity of 90 mAh/g (Fig. 4 inset). The  cells were by no means optimized, but the above example serves as a demonstration of our premise that the new negative electrode materials, including materials other than metal oxides could, despite their large irreversible loss, be incorporated into future generations of Li-ion batteries. Unfortunately, this approach cannot be applied to the cells based on
cells were by no means optimized, but the above example serves as a demonstration of our premise that the new negative electrode materials, including materials other than metal oxides could, despite their large irreversible loss, be incorporated into future generations of Li-ion batteries. Unfortunately, this approach cannot be applied to the cells based on  because, to our knowledge,
because, to our knowledge,  has not yet been reported.
has not yet been reported.
Figure 4. Voltage vs. composition profile for a  Li-ion cell demonstrating the possible use of
Li-ion cell demonstrating the possible use of  as a Li reservoir. The capacity at a 3 V plateau is used to compensate for the first cycle irreversibility observed with CoO. The inset shows the capacity retention for this cell and illustrates the beneficial effect of using the Li reservoir on cell capacity as compared to
as a Li reservoir. The capacity at a 3 V plateau is used to compensate for the first cycle irreversibility observed with CoO. The inset shows the capacity retention for this cell and illustrates the beneficial effect of using the Li reservoir on cell capacity as compared to  Li-ion cells.
Li-ion cells.
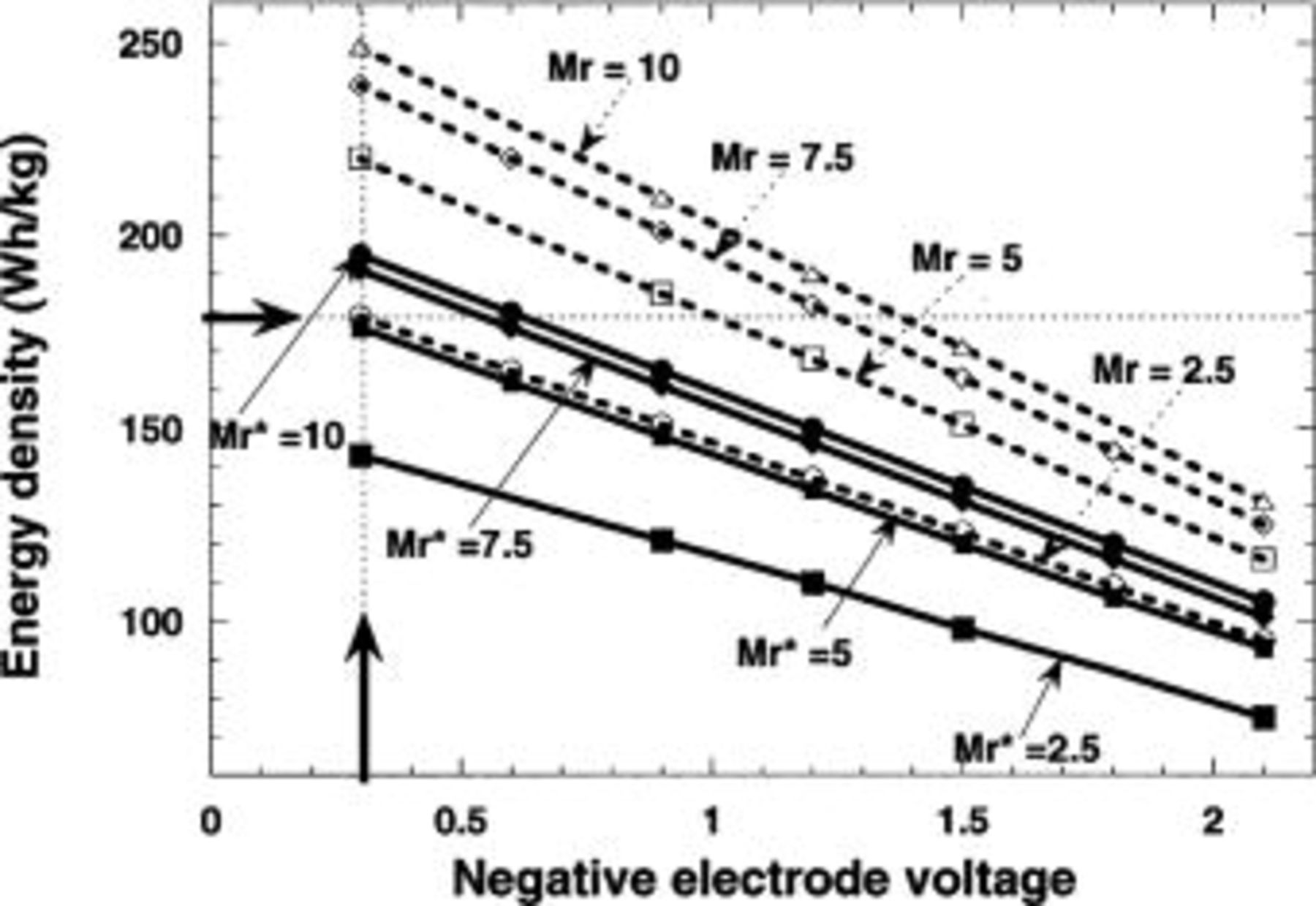
At this point, one may ask whether the proposed  Li-ion system can successfully compete with the present-day Li-ion batteries? We created a specialized battery modeling spreadsheet19 to gain more insight into the relationships between various electrode material properties and the resulting battery characteristics. The relationships of interest included irreversible and reversible capacities of the active materials, the densities of all components, average discharge voltages, and practical electrode compositions, i.e., the respective volumetric fractions of the active materials, binders, conductive additives, and liquid electrolyte, and the resulting specific energy and energy density values. Using this approach, we estimated how the gravimetric energy density of
Li-ion system can successfully compete with the present-day Li-ion batteries? We created a specialized battery modeling spreadsheet19 to gain more insight into the relationships between various electrode material properties and the resulting battery characteristics. The relationships of interest included irreversible and reversible capacities of the active materials, the densities of all components, average discharge voltages, and practical electrode compositions, i.e., the respective volumetric fractions of the active materials, binders, conductive additives, and liquid electrolyte, and the resulting specific energy and energy density values. Using this approach, we estimated how the gravimetric energy density of  Li-ion cells would vary as a function of the voltage and specific capacity of the metal oxide negative electrodes. Two cases were considered using a constant capacity of the positive
Li-ion cells would vary as a function of the voltage and specific capacity of the metal oxide negative electrodes. Two cases were considered using a constant capacity of the positive  electrode of 140 mAh/g; in the first case, the negative electrode voltage was set constant, but its capacity was varying the
electrode of 140 mAh/g; in the first case, the negative electrode voltage was set constant, but its capacity was varying the  value from 2.5 to 10 in steps of 2.5. In the second step, we fixed the capacity of the negative electrode, e.g., the
value from 2.5 to 10 in steps of 2.5. In the second step, we fixed the capacity of the negative electrode, e.g., the  value, but changed the average discharge voltage of the negative electrode from 0.3 to 2.1 in steps of 0.3 V, e.g., the cell output voltage was changed from 2.0 to 3.8 V. Such calculations were performed assuming either a fully reversible negative electrode (dashed line) or a negative electrode having 25% of irreversible capacity (solid lines) as shown in Fig. 5. The performance of the present-day plastic Li-ion batteries is indicated by the intersection of two thin dashed lines (Fig. 5). To the right of this intersection, we marked the voltage and
value, but changed the average discharge voltage of the negative electrode from 0.3 to 2.1 in steps of 0.3 V, e.g., the cell output voltage was changed from 2.0 to 3.8 V. Such calculations were performed assuming either a fully reversible negative electrode (dashed line) or a negative electrode having 25% of irreversible capacity (solid lines) as shown in Fig. 5. The performance of the present-day plastic Li-ion batteries is indicated by the intersection of two thin dashed lines (Fig. 5). To the right of this intersection, we marked the voltage and  ratio ranges (the latter determined by the capacity of the electrode material) required for a metal oxide negative electrode material to successfully compete with carbonaceous electrodes.
ratio ranges (the latter determined by the capacity of the electrode material) required for a metal oxide negative electrode material to successfully compete with carbonaceous electrodes.
Figure 5. Effect of the negative electrode potential vs.  (dashed lines; Mr) and its first-cycle irreversibility (solid lines;
(dashed lines; Mr) and its first-cycle irreversibility (solid lines;  ) on the specific energy of the cell. Estimates were obtained using a battery modeling spreadsheet developed for plastic Li-ion cells.19
) on the specific energy of the cell. Estimates were obtained using a battery modeling spreadsheet developed for plastic Li-ion cells.19
Note that for a fully reversible negative electrode (Fig. 5, dashed lines), the performance of  Li-ion cells can be matched with MO electrodes reacting with Li at a voltage lower than 1.3 V vs.
Li-ion cells can be matched with MO electrodes reacting with Li at a voltage lower than 1.3 V vs.  and having the reversible capacity between 700 mAh/g
and having the reversible capacity between 700 mAh/g  to 1000 mAh/g
to 1000 mAh/g  If we assume a 25% irreversible capacity loss during the first lithiation-delithiation cycle for these MO negative materials, the overall energy density of the cell is reduced as shown by the solid lines in Fig. 5. The important result is that, among the MO negative electrode materials that exhibit a reversible capacity of ca. 1000 mAh/g and the capacity loss discussed above, only those reacting with Li at a potential lower than 0.6 V could result in Li-ion cells with the specific energy comparable to that of
If we assume a 25% irreversible capacity loss during the first lithiation-delithiation cycle for these MO negative materials, the overall energy density of the cell is reduced as shown by the solid lines in Fig. 5. The important result is that, among the MO negative electrode materials that exhibit a reversible capacity of ca. 1000 mAh/g and the capacity loss discussed above, only those reacting with Li at a potential lower than 0.6 V could result in Li-ion cells with the specific energy comparable to that of  Li-ion cells. To this end, we surveyed the reactivity of a wide range of 3d metal oxides towards Li, but we did not find any material that would display an electrochemical activity toward Li at 0.6 V or lower. The closest in this group were MnO and
Li-ion cells. To this end, we surveyed the reactivity of a wide range of 3d metal oxides towards Li, but we did not find any material that would display an electrochemical activity toward Li at 0.6 V or lower. The closest in this group were MnO and  which react with lithium at a potential of ca. 1 V vs.
which react with lithium at a potential of ca. 1 V vs. 
Thus, we conclude that  Li-ion systems, whose MO negative electrodes react with Li at ca. 1 V, are of practical interest only when combined with a positive electrode, such as
Li-ion systems, whose MO negative electrodes react with Li at ca. 1 V, are of practical interest only when combined with a positive electrode, such as  that can serve as a Li reservoir to compensate the inherent irreversible capacity of the MO. In light of these results, as well as due to the fact that a
that can serve as a Li reservoir to compensate the inherent irreversible capacity of the MO. In light of these results, as well as due to the fact that a  phase has not yet been reported, we are currently studying the optimization of MnO-based Li-ion cells with
phase has not yet been reported, we are currently studying the optimization of MnO-based Li-ion cells with  cathodes.
cathodes.
Acknowledgments
The authors thank G. Amatucci, L. Dupont, and P. Poizot for their useful technical discussions.
The Universite de Picardie Jules Verne assisted in meeting the publication costs of this article.