Abstract
Single-walled carbon nanotubes (SWNTs) formed gels after being ground with a room-temperature ionic liquid (RTIL). The gels were used as electrodes of electric double-layer capacitors (EDLCs), and the RTIL was used as the electrolyte. The performance of the EDLCs was examined by charge-discharge experiments and was compared with that of the EDLCs using activated carbon electrodes and the RTIL electrolytes. The gels functioned as the electrodes over a wide composition range from 0.02 to 0.12 of SWNT/RTIL (wt/wt), and the retained capacitance increased with increasing the SWNT compositions. The EDLCs with the SWNTs showed higher capacitance than that with the activated carbons in terms of the capacitance per unit surface area, though the gravimetric capacitance was lower. The gel electrodes can be fabricated as thick as  without a severe ohmic-drop problem, which may contribute to a simple cell structure. The changes in the performance of the SWNT-EDLCs, with or without the gelation, were apparent, and the gelation greatly contributed to the high performance. This is due to the formation of continuous SWNT and RTIL paths at the molecular level by the gelation.
without a severe ohmic-drop problem, which may contribute to a simple cell structure. The changes in the performance of the SWNT-EDLCs, with or without the gelation, were apparent, and the gelation greatly contributed to the high performance. This is due to the formation of continuous SWNT and RTIL paths at the molecular level by the gelation.
Export citation and abstract BibTeX RIS
Electric double-layer capacitors (EDLCs) are energy storage devices, which accumulate electric charges at the electric double layers, i.e., at the interface between a polarizable electrode (electronic conductor) and an electrolyte (ionic conductor). The advantages of EDLCs are long cycle life, simple principle and construction, short charging time, safety, and high power density. The key factors determining the performance of the EDLCs are the surface area of electrode materials and the property of electrolytes, such as the electric double-layer capacitance, the ionic conductivity, and the electrochemical window. In this study, we demonstrate that combined use of room-temperature ionic liquids (RTILs) and carbon nanotubes (CNTs) is promising in terms of the fabrication of new EDLCs.
The RTILs are room-temperature molten salts, which generally have high ionic conductivity, high thermal stability, nonvolatility, nonflammability, high electric double-layer capacitance, and a wide electrochemical window. In a sense, they are ideal electrolytes for electrochemical systems.1–6 For the application to the EDLCs, the high electric double-layer capacitance and the wide electrochemical window of the RTILs are quite attractive. The double-layer capacitance (the integral capacitance) at a dropping mercury electrode is larger than that of typical aprotic electrolyte solutions and is close to that of typical aqueous electrolyte solutions, while the electrochemical window is larger than that of the aqueous solutions.6 The nonvolatility and nonflammability may also contribute to improvement of safety and heavy-duty use.
The CNTs have attracted much attention as new materials since their first discovery by Iijima et al.7 As the CNTs have a cylindrical nanostructure of a two-dimensional graphite sheet, which renders them a large π-electronic surface as well as very uniform pores of nanometer size, the CNTs are ideal electron conductors and porous materials. The CNTs have been applied as the electrode materials for electrochemical energy storage systems, such as Li-ion secondary batteries, fuel cells, actuators, and EDLCs.8–15 However, a problem may be encountered when the CNTs are utilized as the electrode materials. The CNTs themselves are heavily entangled with each other to form bundles, and compatibility with electrolyte materials is relatively poor.
Fukushima et al. found that single-walled carbon nanotubes (SWNTs) formed gels after being ground with the RTIL, which were named "bucky gels."16 In these gels, the heavily entangled bundles of the SWNTs are untangled to form much finer bundles. The gels have cross-linked network structures of the fine bundles of the SWNTs and retain the RTIL between the networks. As a result, electronic conduction paths by the SWNTs and ionic conduction paths by the RTIL are formed simultaneously in these gels. Furthermore, these gels can be treated as quasi-solid materials, which seems to be highly advantageous for fabricating electrode materials by using these gels.
In this study, the bucky gels were used as electrodes of the EDLCs. An RTIL, 1-ethyl-3-methylimidazolium bis(trifluoromethane sulfone)imide (EMITFSI), was used for making the gels as well as an electrolyte for the EDLCs. The performance of the EDLCs based on the gel electrodes was examined by a charge-discharge experiment.17 The EDLCs based on SWNT electrodes without gelation and those based on conventional activated carbon electrodes were also fabricated, and the performance was compared with that of the bucky gel EDLCs.
Experimental
Preparation of an ionic liquid
1-Ethyl-3-methylimidazolium bromide was synthesized according to the procedure described in earlier publications.18, 19 The anion exchange reaction to form EMITFSI was carried out using LiTFSI (Morita Chemical) in water at  by metathesis reaction. This ionic liquid is hydrophobic and immiscible with water, and thus, EMITFSI was phase-separated by the metathesis reaction. The ionic liquid was dehydrated under high vacuum, and the structure was identified by
by metathesis reaction. This ionic liquid is hydrophobic and immiscible with water, and thus, EMITFSI was phase-separated by the metathesis reaction. The ionic liquid was dehydrated under high vacuum, and the structure was identified by  nuclear magnetic resonance (NMR) and the fast-atom-bombardment mass spectra.
nuclear magnetic resonance (NMR) and the fast-atom-bombardment mass spectra.
Fabrication of cells for EDLC
SWNTs (HiPco Buckytubes) were purchased from Carbon Nanotechnologies. Specific surface area of the SWNTs was  , which was estimated by
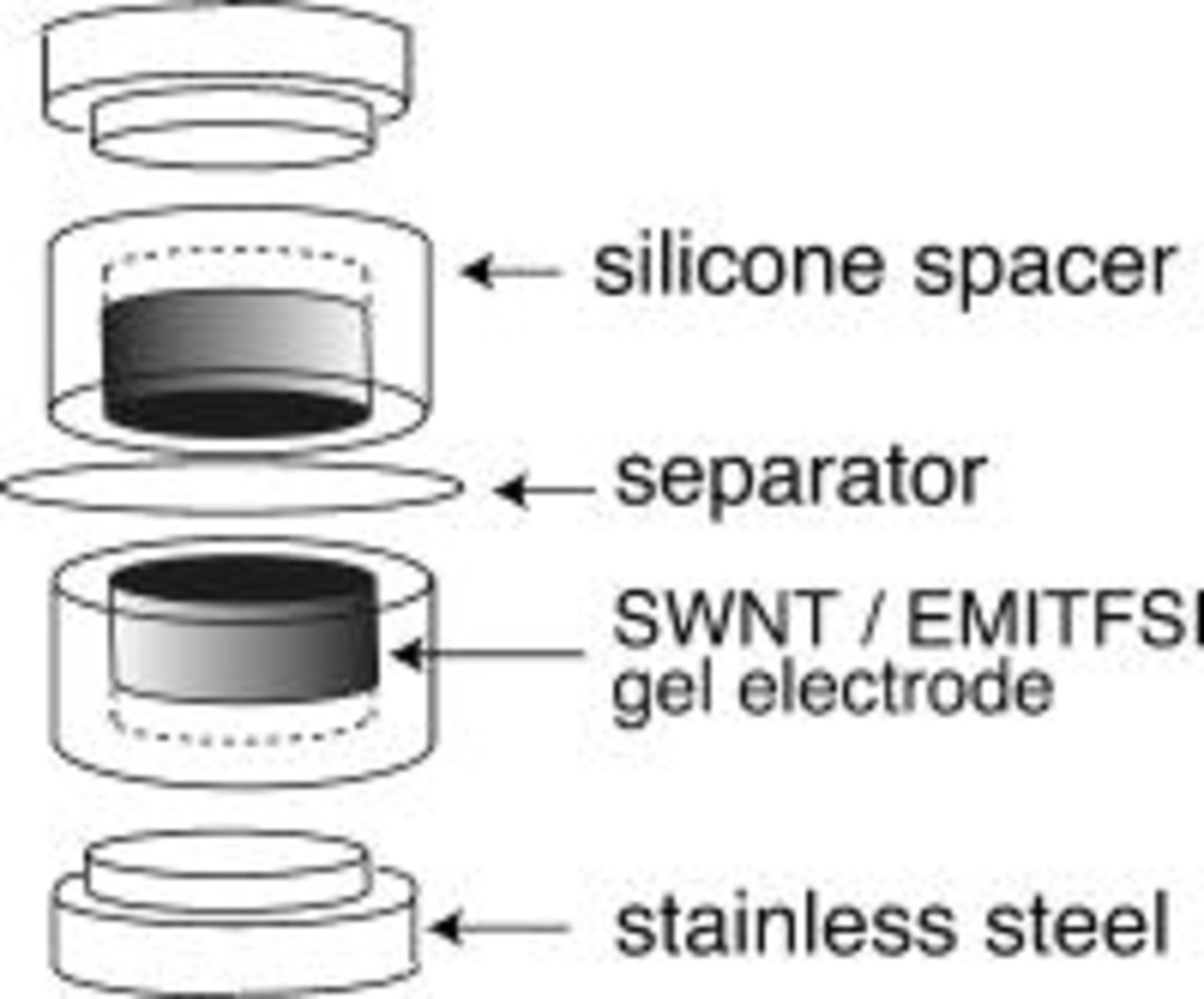
, which was estimated by  Brunauer–Emmet–Teller (BET) adsorption experiments. EMITFSI and the SWNTs were thoroughly mixed by grinding with a pestle for several minutes to form the bucky gels. The mixing ratios of SWNT/EMITFSI by weight ranged from 0.03 to 0.12. When the weight ratio of the SWNTs was low (0.03-0.05), the gels were like toothpaste. When the ratio of the SWNTs was increased (ca. 0.10), the gels became argil-like. A cell was constructed with a pair of the gel electrodes, EMITFSI as an electrolyte, and a piece of viscose rayon paper as a separator. The gels were compressed into silicone spacers, and stainless steel was used as current collector. The EDLC cells were fabricated with the two gel electrodes having an apparent surface area of
Brunauer–Emmet–Teller (BET) adsorption experiments. EMITFSI and the SWNTs were thoroughly mixed by grinding with a pestle for several minutes to form the bucky gels. The mixing ratios of SWNT/EMITFSI by weight ranged from 0.03 to 0.12. When the weight ratio of the SWNTs was low (0.03-0.05), the gels were like toothpaste. When the ratio of the SWNTs was increased (ca. 0.10), the gels became argil-like. A cell was constructed with a pair of the gel electrodes, EMITFSI as an electrolyte, and a piece of viscose rayon paper as a separator. The gels were compressed into silicone spacers, and stainless steel was used as current collector. The EDLC cells were fabricated with the two gel electrodes having an apparent surface area of  with thickness of
with thickness of  , separated by the viscose rayon paper, which had been soaked with EMITFSI. The assembly of the sample cells is shown in Fig. 1.
, separated by the viscose rayon paper, which had been soaked with EMITFSI. The assembly of the sample cells is shown in Fig. 1.
Figure 1. Setup of EDLC cells using the SWNT gels as the electrodes.
The EDLCs consisting of activated carbon (from coconut shell, average diam  ,
,  -BET surface area
-BET surface area  ) electrodes and EMITFSI were also prepared. The activated carbon electrodes were composed of the activated carbon
) electrodes and EMITFSI were also prepared. The activated carbon electrodes were composed of the activated carbon  , carboxymethyl cellulose (binder,
, carboxymethyl cellulose (binder,  ), and acetylene black (conductive reagent,
), and acetylene black (conductive reagent,  ). The composite electrodes (
). The composite electrodes ( thickness) were fabricated onto an aluminum current collector. The activated carbon electrode on an aluminum foil was cut into disks of
thickness) were fabricated onto an aluminum current collector. The activated carbon electrode on an aluminum foil was cut into disks of  diam
diam  , which were impregnated with a few drops of EMITFSI. A piece of the viscose rayon paper that had been soaked in EMITFSI was sandwiched between the activated carbon electrodes to form an EDLC cell.
, which were impregnated with a few drops of EMITFSI. A piece of the viscose rayon paper that had been soaked in EMITFSI was sandwiched between the activated carbon electrodes to form an EDLC cell.
Charge-discharge measurements
Charge-discharge experiments were employed to analyze electrochemical performance of the EDLCs. The assembled cells were charged and discharged in a constant current mode based on the weight of the SWNTs or the activated carbon at  , 0.314, and
, 0.314, and  . All the charge-discharge experiments were conducted by using a Nagano BTS-2004 charge-discharge tester in the voltage range of
. All the charge-discharge experiments were conducted by using a Nagano BTS-2004 charge-discharge tester in the voltage range of  at
at  . The electrochemical potential window of EMITFSI is much larger than
. The electrochemical potential window of EMITFSI is much larger than  .20 The data of the third discharge curves were used to calculate the cell capacitances. The single-electrode cell capacitance was calculated by
.20 The data of the third discharge curves were used to calculate the cell capacitances. The single-electrode cell capacitance was calculated by  , that per unit weight of the electrode materials (the SWNTs and activated carbon) was calculated by
, that per unit weight of the electrode materials (the SWNTs and activated carbon) was calculated by  , and that per the surface area was calculated by
, and that per the surface area was calculated by  , where
, where  is the discharging current,
is the discharging current,  is the discharging current per unit weight of the electrode materials,
is the discharging current per unit weight of the electrode materials,  is the BET surface area per the unit weight, and
is the BET surface area per the unit weight, and  is the inverse of slopes of the discharging curves between 1.0 and
is the inverse of slopes of the discharging curves between 1.0 and  .21
.21
AC impedance measurements
AC impedance measurements were performed by using a Solartron 1255B frequency response analyzer in the frequency range from  at an amplitude of
at an amplitude of  at
at  .
.
Results and Discussion
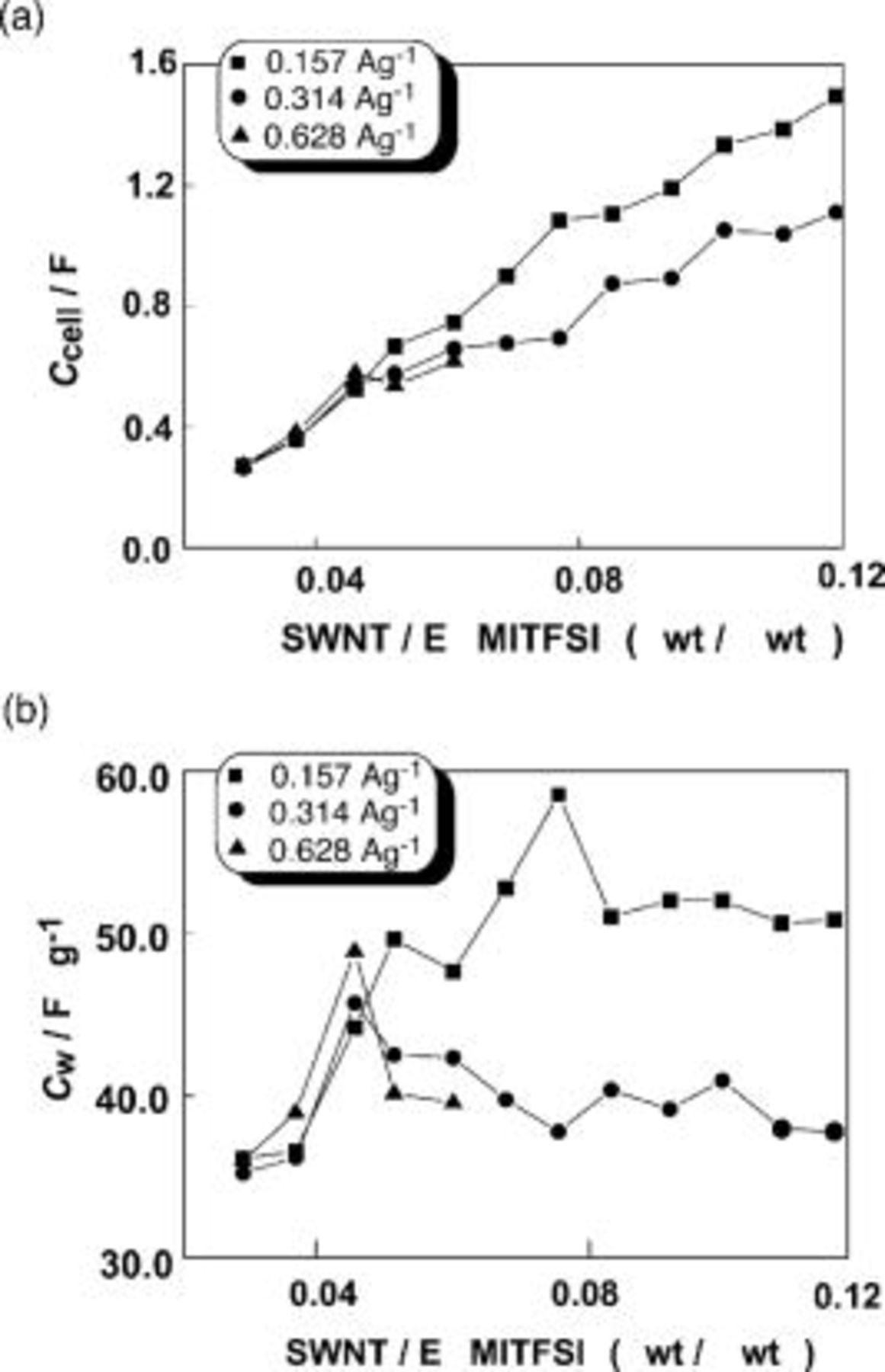
Figure 2a and 2b shows  and
and  , respectively, for EDLCs using the SWNT gel electrodes as a function of gel composition.
, respectively, for EDLCs using the SWNT gel electrodes as a function of gel composition.  (Fig. 2a) increases as the weight of the SWNTs in the electrode increases. It is found that the gels comprised of the SWNTs and EMITFSI function as electrode materials in the EDLCs in the various compositions. When the mixing ratios of the SWNTs are small, the capacitance does not change, independent of the discharging current density. When the mixing ratios become larger, the capacitance decreases with increasing the discharging current density. This indicates that for faster discharging, more ionic liquid is required in the SWNT gels. Figure 2b clearly shows that with increasing the discharge current density, the maximum
(Fig. 2a) increases as the weight of the SWNTs in the electrode increases. It is found that the gels comprised of the SWNTs and EMITFSI function as electrode materials in the EDLCs in the various compositions. When the mixing ratios of the SWNTs are small, the capacitance does not change, independent of the discharging current density. When the mixing ratios become larger, the capacitance decreases with increasing the discharging current density. This indicates that for faster discharging, more ionic liquid is required in the SWNT gels. Figure 2b clearly shows that with increasing the discharge current density, the maximum  is observed at the lower mixing ratios.
is observed at the lower mixing ratios.
Figure 2. (a)  and (b)
and (b)  for EDLCs of the SWNT gel electrodes as a function of composition of the electrodes.
for EDLCs of the SWNT gel electrodes as a function of composition of the electrodes.
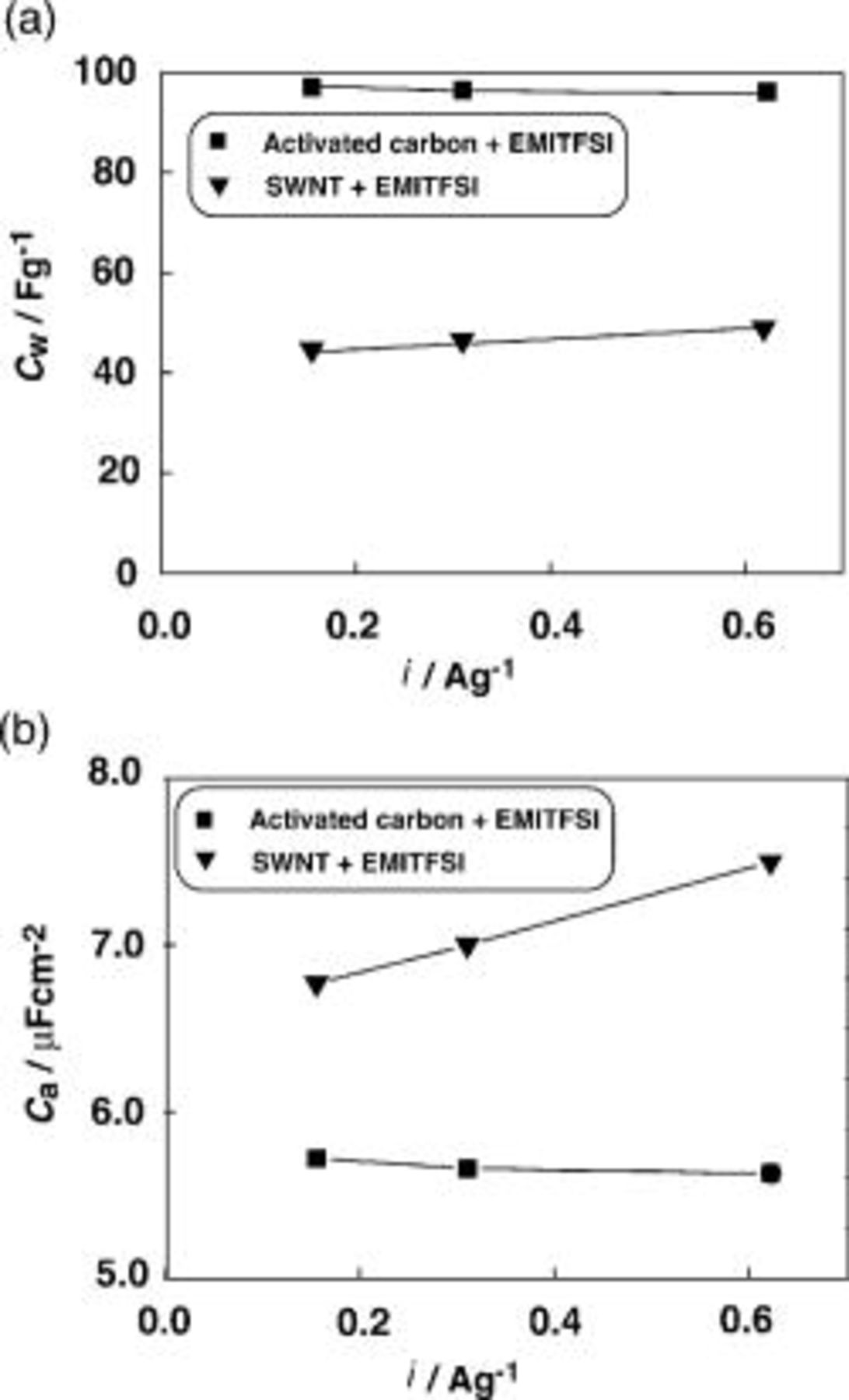
In order to compare the EDLC performances of the SWNT gel electrodes with those of other electrode materials, the activated carbon was selected as the most common electrode material for the EDLC.22–26 Although the activated carbon and EMITFSI were mixed together, as was the case of the SWNTs and EMITFSI, gelation did not occur. Thus, the composite electrodes, consisting of the activated carbon, carboxymethyl cellulose, and acetylene black, were used for this purpose. The comparative results for the EDLC performances are shown in Fig. 3.  (Fig. 3a) for the activated electrodes is ca.
(Fig. 3a) for the activated electrodes is ca.  , while that for the SWNT gel electrodes is ca.
, while that for the SWNT gel electrodes is ca.  .
.  (Fig. 3b) values for the former and the latter are ca. 5.5 and
(Fig. 3b) values for the former and the latter are ca. 5.5 and  , respectively. It is interesting to note that
, respectively. It is interesting to note that  based on the BET surface area becomes larger for the SWNT gel electrodes than for the activated carbon electrodes. The individually cylindrical structure of the SWNTs and the gel formation with the ionic liquid might make use of the surface area of the SWNTs more efficient than that of the activated carbon in terms of the ionic adsorption/desorption during the charging/discharging processes. The ion adsorption/desorption in the SWNT gel electrodes may occur fast without the ion sieving effect, as is the case of the activated carbon electrodes.27 The SWNT gel electrode with the thickness of
based on the BET surface area becomes larger for the SWNT gel electrodes than for the activated carbon electrodes. The individually cylindrical structure of the SWNTs and the gel formation with the ionic liquid might make use of the surface area of the SWNTs more efficient than that of the activated carbon in terms of the ionic adsorption/desorption during the charging/discharging processes. The ion adsorption/desorption in the SWNT gel electrodes may occur fast without the ion sieving effect, as is the case of the activated carbon electrodes.27 The SWNT gel electrode with the thickness of  can be formed without adding any binder and conductive reagent and can be charged and discharged without a severe ohmic drop problem (vide infra), which may contribute to a simple cell structure.
can be formed without adding any binder and conductive reagent and can be charged and discharged without a severe ohmic drop problem (vide infra), which may contribute to a simple cell structure.
Figure 3. A comparison of (a)  and (b)
and (b)  between EDLCs using the SWNT gel electrodes
between EDLCs using the SWNT gel electrodes  or the activated carbon electrodes as a function of the discharge current density.
or the activated carbon electrodes as a function of the discharge current density.
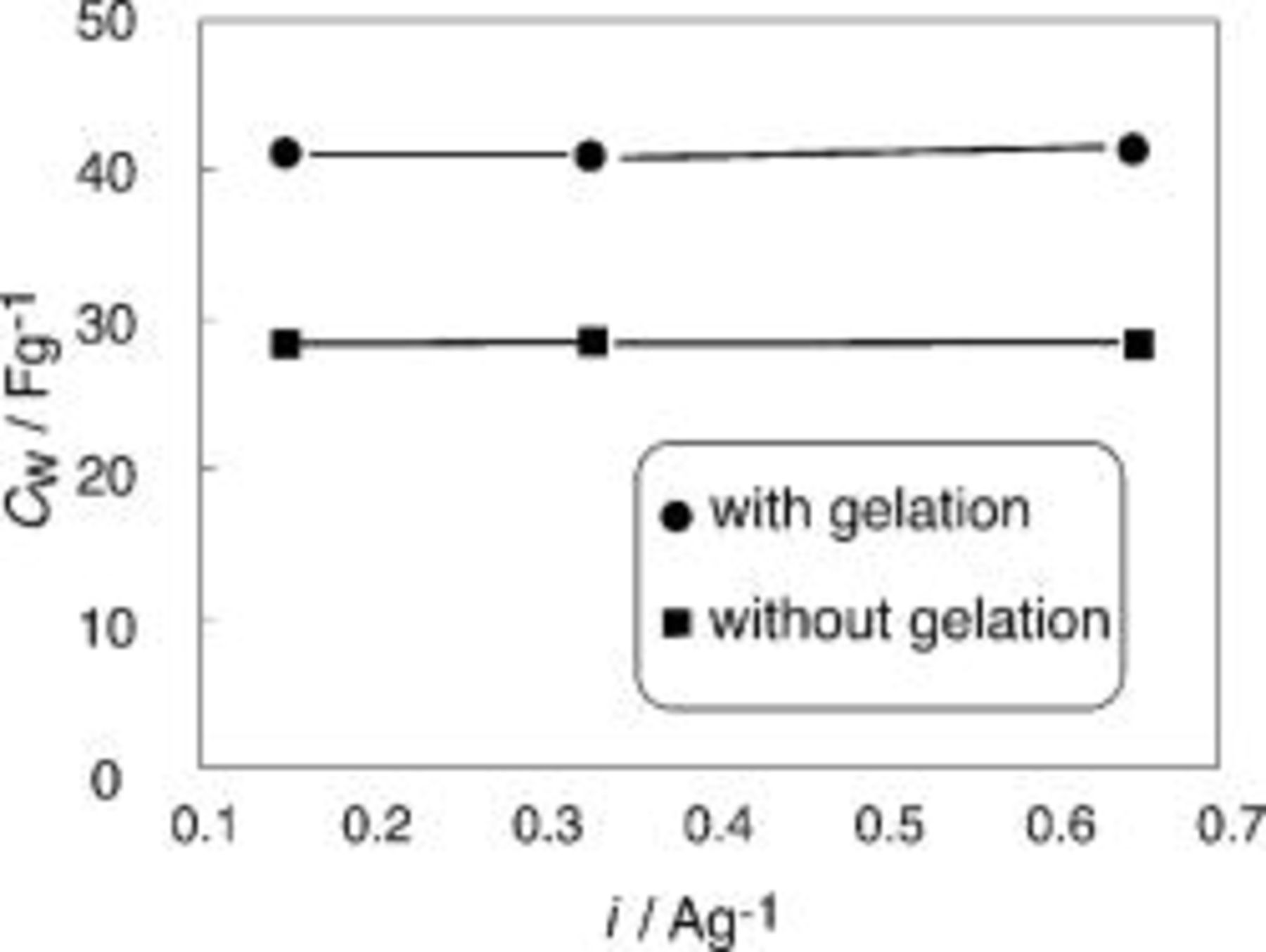
Changes in the capacitance for the EDLCs using the SWNTs, depending on with and without the gelation, were explored to know how the gelation affected the cell capacitance. Figure 4 shows the changes in the  of the EDLCs consisting of the SWNT electrodes with and without the gelation as a function of current density. For the EDLCs using the electrodes without the gelation, the SWNTs were simply packed into the spacer (Fig. 1), and EMITFSI was impregnated into the packed SWNTs. From Fig. 4 it is evident that the gelation greatly contributes to increasing the capacitance. To increase the capacitance of the EDLCs, the gelation turns out to be essential. The gelation did not occur when water and organic solvents were mixed with the SWNTs, which indicated the existence of specific interaction between the SWNTs and the ionic liquid.16
of the EDLCs consisting of the SWNT electrodes with and without the gelation as a function of current density. For the EDLCs using the electrodes without the gelation, the SWNTs were simply packed into the spacer (Fig. 1), and EMITFSI was impregnated into the packed SWNTs. From Fig. 4 it is evident that the gelation greatly contributes to increasing the capacitance. To increase the capacitance of the EDLCs, the gelation turns out to be essential. The gelation did not occur when water and organic solvents were mixed with the SWNTs, which indicated the existence of specific interaction between the SWNTs and the ionic liquid.16
Figure 4. Change in  for EDLCs using the SWNT electrodes with and without the gelation with EMITFSI
for EDLCs using the SWNT electrodes with and without the gelation with EMITFSI  as a function of the discharge current density.
as a function of the discharge current density.
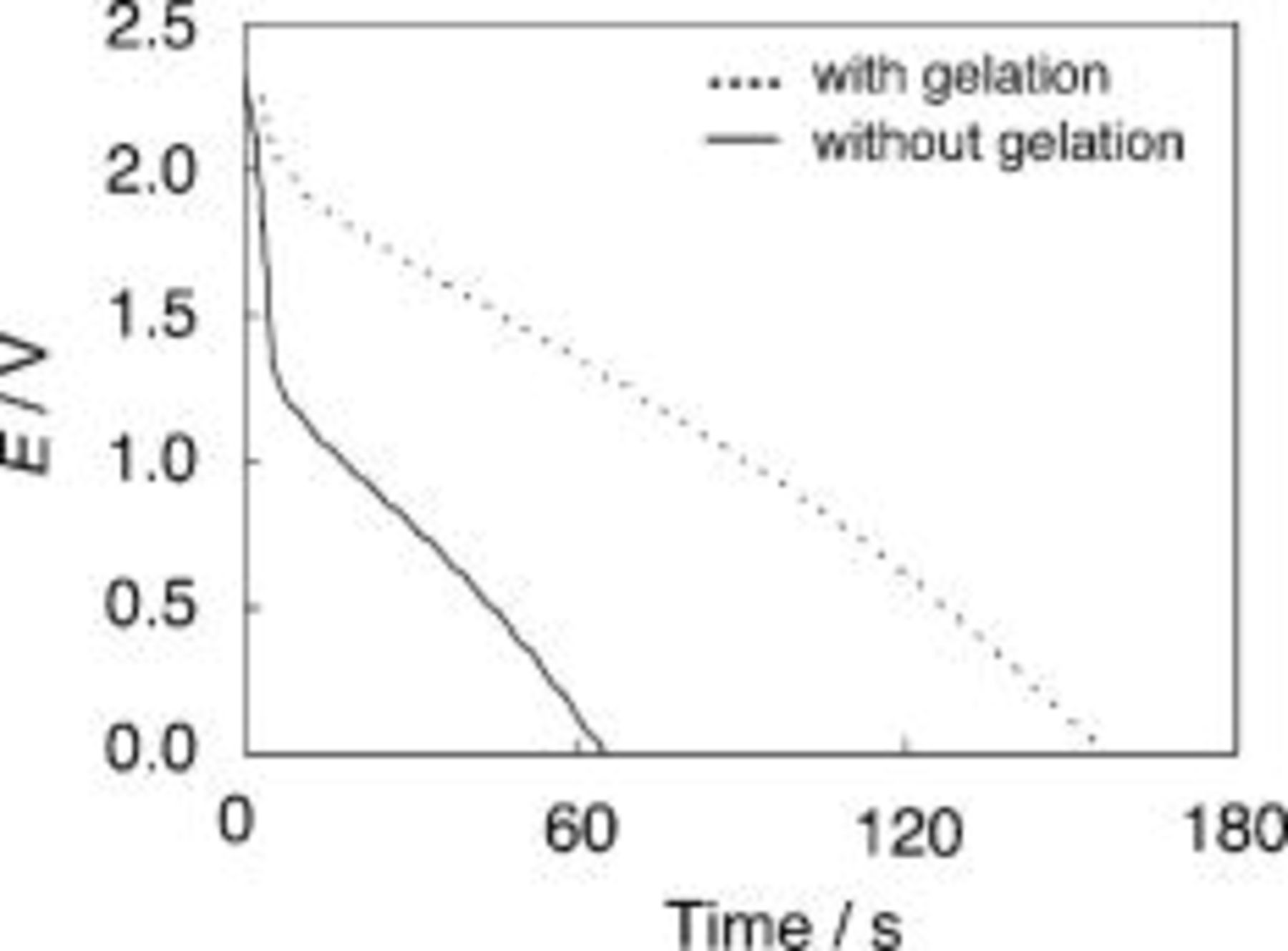
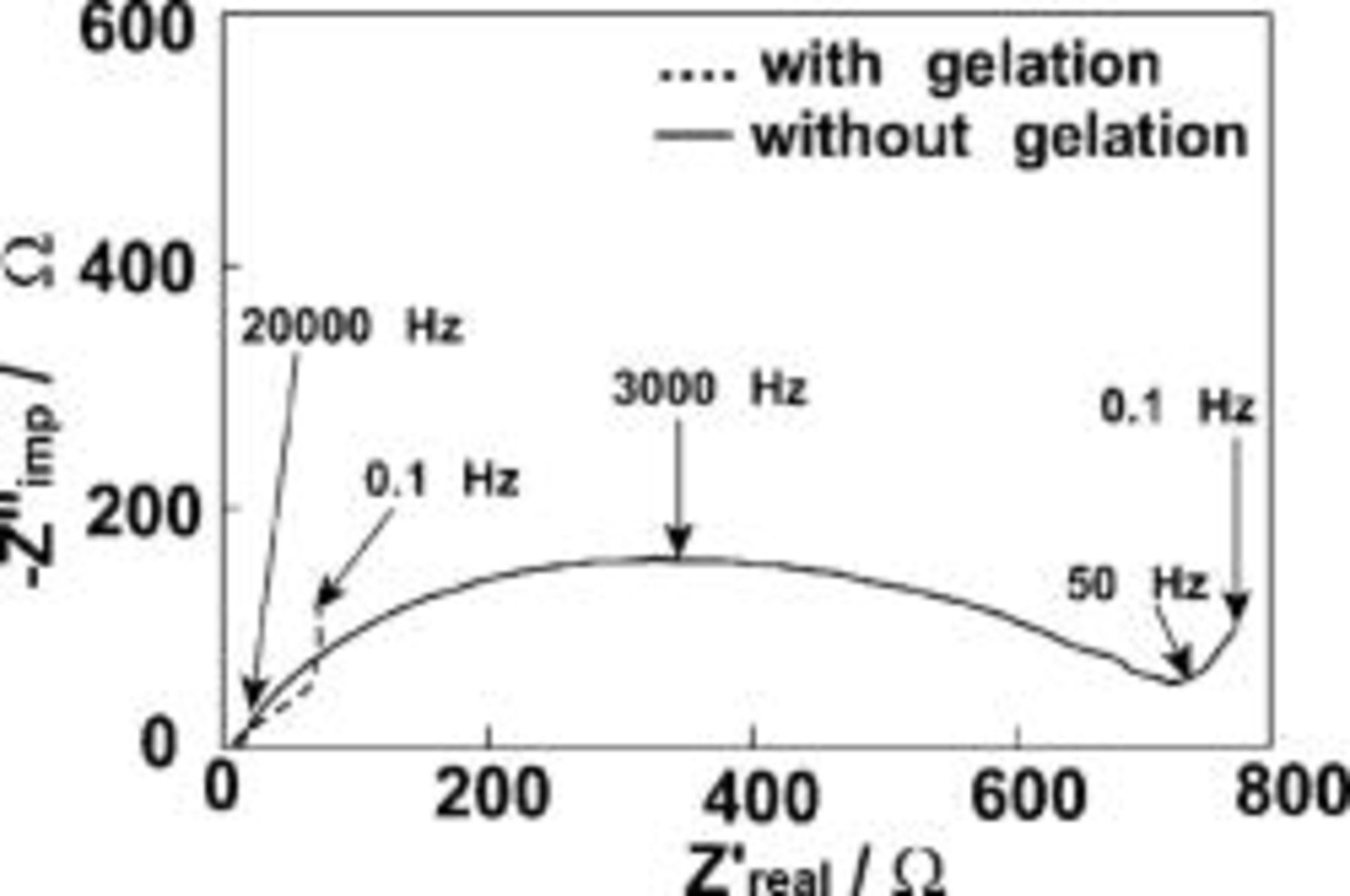
Figure 5 shows the discharge curves of the EDLCs of the SWNT electrodes with and without the gelation. It is evident that the cell capacitance is greatly increased by the gelation, as was mentioned for Fig. 4. The initial voltage drops in the discharging curves in Fig. 5 correspond to the ohmic  drops caused by the internal resistance of the EDLCs. This result indicates that the internal resistance is efficiently decreased by the gelation. Figure 6 shows Nyquist plots of the EDLC cells with and without the gelation. The bulk electrolyte resistance, seen at the high-frequency end, is constant, regardless of the gelation. For the EDLCs of the SWNT electrodes with gelation, the Warburg response and the double-layer capacitive response are seen with decreasing frequency. On the contrary, for the EDLCs without gelation, a large resistive/capacitive response is seen before the Warburg response. The resistive/capacitive response seems to be related to the network formation of the SWNTs in the electrodes. When the SWNTs and EMITFSI are completely mixed to form the gels, the heavily entangled bundles of the SWNTs are untangled to form much finer bundles, which form cross-linked network structures and retain EMITFSI between the networks. As a result, electronic conduction paths by the SWNTs and ionic conduction paths by the RTIL are formed simultaneously in these gels. In the SWNT electrodes without the gelation, on the contrary, the heavily entangled bundles are not in good contact with each other and with the ionic liquid, which causes the large resistive/capacitive impedance response and the large ohmic drop in the discharging curve (Fig. 5). Thus, the simultaneous formation of electronic conduction paths by the SWNTs and ionic conduction paths by the RTIL, possibly at the molecular level, seems to be crucial to reduce the internal resistances of the electrodes.
drops caused by the internal resistance of the EDLCs. This result indicates that the internal resistance is efficiently decreased by the gelation. Figure 6 shows Nyquist plots of the EDLC cells with and without the gelation. The bulk electrolyte resistance, seen at the high-frequency end, is constant, regardless of the gelation. For the EDLCs of the SWNT electrodes with gelation, the Warburg response and the double-layer capacitive response are seen with decreasing frequency. On the contrary, for the EDLCs without gelation, a large resistive/capacitive response is seen before the Warburg response. The resistive/capacitive response seems to be related to the network formation of the SWNTs in the electrodes. When the SWNTs and EMITFSI are completely mixed to form the gels, the heavily entangled bundles of the SWNTs are untangled to form much finer bundles, which form cross-linked network structures and retain EMITFSI between the networks. As a result, electronic conduction paths by the SWNTs and ionic conduction paths by the RTIL are formed simultaneously in these gels. In the SWNT electrodes without the gelation, on the contrary, the heavily entangled bundles are not in good contact with each other and with the ionic liquid, which causes the large resistive/capacitive impedance response and the large ohmic drop in the discharging curve (Fig. 5). Thus, the simultaneous formation of electronic conduction paths by the SWNTs and ionic conduction paths by the RTIL, possibly at the molecular level, seems to be crucial to reduce the internal resistances of the electrodes.
Figure 5. Discharge curves of EDLCs using the SWNT electrodes with and without the gelation  . Discharge current density:
. Discharge current density:  .
.
Figure 6. Nyquist plots of the SWNT electrodes with and without the gelation with EMITFSI  at an ac amplitude of
at an ac amplitude of  .
.
Conclusion
EDLCs need to have several requirements, including high specific capacitance, low electrode and electrolyte resistances, long cycling life, etc. The bucky gels, composed of SWNTs and RTIL, could simply be prepared by physical mixing of the components and were found to function as electrodes of the EDLCs over a wide composition range of the SWNTs and RTIL. The BET surface area normalized capacitance  became larger for the SWNT gel electrodes than for the conventional activated carbon electrodes, though the gravimetric capacitance
became larger for the SWNT gel electrodes than for the conventional activated carbon electrodes, though the gravimetric capacitance  was lower for the former. The performances of the SWNT-EDLCs in terms of the high capacitance and the low electrode resistance were greatly improved by the gelation, which would contribute to the formation of continuous SWNT and RTIL paths at the molecular level. To our knowledge, this is the first time that bucky gels based on the SWNTs and ionic liquid turn out to be useful as EDLC electrodes.
was lower for the former. The performances of the SWNT-EDLCs in terms of the high capacitance and the low electrode resistance were greatly improved by the gelation, which would contribute to the formation of continuous SWNT and RTIL paths at the molecular level. To our knowledge, this is the first time that bucky gels based on the SWNTs and ionic liquid turn out to be useful as EDLC electrodes.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from MEXT (No. 14350452 and 16205024) and by CREST-JST. T.F. and T.A. acknowledge ERATO-JST for financial support.
Yokohoma National University assisted in meeting the publication costs of this article.