Abstract
Carbon Vulcan XC72 is still considered as the standard catalyst support in gas diffusion electrodes for low temperature polymer fuel cell applications. Because of its relatively low specific area and poor corrosion resistance, alternative materials such as carbon nanotubes (CNTs) are currently the focus of numerous investigations. This work reports on the test of single-walled carbon nanotubes (SWCNTSs) as Pt and PtRu catalyst support in the direct methanol fuel cell (DMFC) anode. After the treatment of the CNTs in air at  or in a high concentrated nitric acid solution, power densities of 152 and
or in a high concentrated nitric acid solution, power densities of 152 and  have been reached under DMFC conditions at
have been reached under DMFC conditions at  and
and  . The anodic and cathodic Pt loadings amounted to
. The anodic and cathodic Pt loadings amounted to  . The performance of the membrane electrode assembly (MEA) with a PtRu–SWCNT anode was about 10–15% higher than that of the MEA with a conventional as-prepared PtRu–Vulcan XC72 system. This was principally ascribed to higher catalyst utilization, lower impedance, and methanol crossover of the carbon-nanotube-based electrodes compared to those of the carbon-Vulcan-based one.
. The performance of the membrane electrode assembly (MEA) with a PtRu–SWCNT anode was about 10–15% higher than that of the MEA with a conventional as-prepared PtRu–Vulcan XC72 system. This was principally ascribed to higher catalyst utilization, lower impedance, and methanol crossover of the carbon-nanotube-based electrodes compared to those of the carbon-Vulcan-based one.
Export citation and abstract BibTeX RIS
Methanol has a high energy density, can be produced from biomass, and is more or less compatible with the existing infrastructure for the distribution of petrol or gasoline. For these reasons, its direct conversion into electricity in the direct methanol fuel cell (DMFC) is an interesting alternative process for portable and mobile applications.1, 2 However, high overpotential of the methanol oxidation at Pt3 and high methanol permeation through the polymer membrane4 drastically limit the fuel conversion efficiency and the cell power density of the DMFC, which is related to catalyst mass, still about a hundred times lower than that of the hydrogen fuel cell. The poor kinetics of the methanol oxidation at Pt is principally due to the strong affinity of Pt atoms to intermediate products, such as CO that hinders further methanol oxidation to  5 at the active sites. The efficiency of the reaction layer depends not only on the catalyst activity but also on the structure and morphology of the catalyst support that should have high electronic conductivity, specific area and corrosion resistance, and additionally facilitate optimal mass transport of reactants and products. Carbon nanotubes (CNTs) possess most of the required properties and appear to be an alternative material to the common carbon Vulcan XC72 material.6 They have either one layer [single-walled carbon nanotube (SWCNT)] or multiple layers [multiwalled carbon nanotube (MWCNT)], a tubular graphitelike structure with a diameter from 0.5 to 50 nm depending on the wall number, and a length of a few micrometers up to several millimeters. With the help of a surfactant, SWCNTs can be shaped in a so-called buckypaper.7 During the past decades, diverse processes have been developed for the production of CNTs, such as laser ablation, arc discharge, and chemical vapor phase deposition. For the last two methods, the nanotube growing process is based on the transformation of solid graphite rods or gaseous hydrocarbons on catalyst particles, such as nickel and cobalt, that often have to be removed afterward.8, 9 The surface of the as-prepared CNTs is quite inert and should be activated and functionalized by chemical treatments for further catalyst deposition.10, 11 Despite intensive research activities on CNTs during the past years, only few publications report on the test of CNTs in the DMFC. Liu12 measured
5 at the active sites. The efficiency of the reaction layer depends not only on the catalyst activity but also on the structure and morphology of the catalyst support that should have high electronic conductivity, specific area and corrosion resistance, and additionally facilitate optimal mass transport of reactants and products. Carbon nanotubes (CNTs) possess most of the required properties and appear to be an alternative material to the common carbon Vulcan XC72 material.6 They have either one layer [single-walled carbon nanotube (SWCNT)] or multiple layers [multiwalled carbon nanotube (MWCNT)], a tubular graphitelike structure with a diameter from 0.5 to 50 nm depending on the wall number, and a length of a few micrometers up to several millimeters. With the help of a surfactant, SWCNTs can be shaped in a so-called buckypaper.7 During the past decades, diverse processes have been developed for the production of CNTs, such as laser ablation, arc discharge, and chemical vapor phase deposition. For the last two methods, the nanotube growing process is based on the transformation of solid graphite rods or gaseous hydrocarbons on catalyst particles, such as nickel and cobalt, that often have to be removed afterward.8, 9 The surface of the as-prepared CNTs is quite inert and should be activated and functionalized by chemical treatments for further catalyst deposition.10, 11 Despite intensive research activities on CNTs during the past years, only few publications report on the test of CNTs in the DMFC. Liu12 measured  at a membrane electrode assembly (MEA) with a PtRu SWCNT (
at a membrane electrode assembly (MEA) with a PtRu SWCNT ( catalyst loading) anode compared to
catalyst loading) anode compared to  for the MEA with a common Vulcan catalyst support. Jeng et al.10 used MWCNTs as anode materials and obtained a power density of
for the MEA with a common Vulcan catalyst support. Jeng et al.10 used MWCNTs as anode materials and obtained a power density of  with
with  PtRu at
PtRu at  compared to
compared to  for the commercial system. With only a catalyst loading of
for the commercial system. With only a catalyst loading of  PtRu, Carmo13 reported a cell power density of
PtRu, Carmo13 reported a cell power density of  with a SWCNT-based anode. This work focuses on the study of the influence of the chemical treatment on the behavior of SWCNTs as a catalyst support, especially with respect to a specific area and methanol oxidation under half-cell and fuel cell conditions.
with a SWCNT-based anode. This work focuses on the study of the influence of the chemical treatment on the behavior of SWCNTs as a catalyst support, especially with respect to a specific area and methanol oxidation under half-cell and fuel cell conditions.
Experimental
Preparation and characterization of SWCNTs
The SWCNT material was produced by the conventional arc-discharge evaporation of a 6 mm graphite rod filled with a Ni and Y (4:1 wt %) powder (Yangtze Nanotechnology, Shanghai, China). The reaction took place under a helium atmosphere at 500 mbar, 100 A, and 50 V. The reactor and the reaction products are shown in Fig. 1. The as-prepared raw material contains a significant amount of impurities, such as amorphous carbon and residual Ni and Y catalyst particles. The purification and chemical functionalization of the raw material were performed at the Max-Planck-Institute for Solid State Research (Group of Synthetic Nanostructures, Stuttgart, Germany) by using the following procedures: (i) raw material (SWCNT1), (ii) oxidized in air at  (SWCNT2), (iii) treated with concentrated
(SWCNT2), (iii) treated with concentrated  (SWCNT3), and (iv) oxidized in air at
(SWCNT3), and (iv) oxidized in air at  followed by a treatment with concentrated HCl (SWCNT4).
followed by a treatment with concentrated HCl (SWCNT4).
Figure 1. [(A)–(D)] Reactor parts and (E) reaction products.
The average purity rate of the batch was determined by the solution phase near-infrared (NIR) spectroscopy against a reference sample containing 100% SWCNTs. For this purpose, a suspension of SWCNTs was prepared in  -dimethylformamide (DMF, 99.8%, Sigma-Aldrich) by applying ultrasonic agitation. The absorption spectra were measured with a spectrometer (UV/visible/NIR, type Lambda19, Perkin-Elmer). The amount of metallic impurities left over from the production process in the studied samples was analyzed by simultaneous inductive coupled plasma (ICP) and optical emission spectrometer (type VISTAPROCCD, Varian). Thermogravimetric analysis (TGA) was performed using a thermal analysis setup (type STA449C, Netzsch). The dimension of SWCNTs and catalyst particles was evaluated by transmission electron microscopy (TEM, type EM420, Philips).
-dimethylformamide (DMF, 99.8%, Sigma-Aldrich) by applying ultrasonic agitation. The absorption spectra were measured with a spectrometer (UV/visible/NIR, type Lambda19, Perkin-Elmer). The amount of metallic impurities left over from the production process in the studied samples was analyzed by simultaneous inductive coupled plasma (ICP) and optical emission spectrometer (type VISTAPROCCD, Varian). Thermogravimetric analysis (TGA) was performed using a thermal analysis setup (type STA449C, Netzsch). The dimension of SWCNTs and catalyst particles was evaluated by transmission electron microscopy (TEM, type EM420, Philips).
Preparation and characterization of the SWCNT-based anode
The development of the composite anode includes the following steps: catalyst deposition, ink formulation, electrode coating, characterization in a cell designed for gas diffusion electrodes (GDEs), and, finally, MEA hot pressing and test in the DMFC. The chemical impregnation of the catalyst onto either CNTs or carbon Vulcan XC72 (Cabot GmbH) support took place in a glass cell under strong stirring conditions. The catalyst/support weight ratio was fixed at 60:100. Two different techniques were applied for catalyst deposition depending on the nature of the catalyst. For Pt catalyst deposition, 100 mg support and 180 mg (13% surplus)  (Aldrich GmbH) were mixed in 25 mL of a 37% formaldehyde solution (stabilized in 10% methanol, Merck KGaA) and stirred at
(Aldrich GmbH) were mixed in 25 mL of a 37% formaldehyde solution (stabilized in 10% methanol, Merck KGaA) and stirred at  for 1 h. For the PtRu catalyst preparation, 100 mg support, 180 mg
for 1 h. For the PtRu catalyst preparation, 100 mg support, 180 mg  , and 95 mg
, and 95 mg  (13% surplus) were mixed in 25 mL deionized water. Under stirring conditions, the mixture was heated up to
(13% surplus) were mixed in 25 mL deionized water. Under stirring conditions, the mixture was heated up to  and dropwise stabilized at pH 8.5 with sodium hydroxide.14 After reaching
and dropwise stabilized at pH 8.5 with sodium hydroxide.14 After reaching  , 1.5 mL of 37% formaldehyde (p.a., Merck KGaA) was added. After cooling, the suspension was washed with deionized water, filtered through a
, 1.5 mL of 37% formaldehyde (p.a., Merck KGaA) was added. After cooling, the suspension was washed with deionized water, filtered through a  filter membrane of mixed cellulose esters (Millipore, Inc.), and dried at
filter membrane of mixed cellulose esters (Millipore, Inc.), and dried at  for 5 h. Catalyst yields close to 100% were obtained for the as-prepared catalyst-supported powders. The catalyst ink was homogenized by dipping a titan rod in the catalyst/water/propanol mixture and by applying a high energy ultrasonic technique (type Sonifier, Branson) for 10 min. Then, 10 wt % Nafion and 20 wt % poly(tetrafluoroethylene) (Ion Power, Inc.) were successively added to the suspension that was ultrasonicated for another 15 min. GDEs were prepared by coating the backing layer (Toray carbon paper TGP-H-60) with the air-spray technique. The Pt catalyst loading was fixed at
for 5 h. Catalyst yields close to 100% were obtained for the as-prepared catalyst-supported powders. The catalyst ink was homogenized by dipping a titan rod in the catalyst/water/propanol mixture and by applying a high energy ultrasonic technique (type Sonifier, Branson) for 10 min. Then, 10 wt % Nafion and 20 wt % poly(tetrafluoroethylene) (Ion Power, Inc.) were successively added to the suspension that was ultrasonicated for another 15 min. GDEs were prepared by coating the backing layer (Toray carbon paper TGP-H-60) with the air-spray technique. The Pt catalyst loading was fixed at  both for the anode and the cathode. The coating process included 15–20 air spraying steps, a sintering process at
both for the anode and the cathode. The coating process included 15–20 air spraying steps, a sintering process at  for 4 min between each layer, and, finally, a last sintering step at
for 4 min between each layer, and, finally, a last sintering step at  for 30 min. The electrochemical characterization of the Pt–SWCNT-based GDE was carried out in a three-electrode Plexiglas cell in a
for 30 min. The electrochemical characterization of the Pt–SWCNT-based GDE was carried out in a three-electrode Plexiglas cell in a  or a
or a  solution. The effective geometrical area of the sample was
solution. The effective geometrical area of the sample was  . Electrochemical investigation methods, such as cyclic voltammetry (CV), chronoamperometry, and electrochemical impedance spectroscopy (EIS), were performed with a Princeton Applied Research potentiostat (type 273, EG&G) coupled with a frequency generator (type SI 1255, Schlumberger GmbH). MEAs were fabricated by pressing together the in-house-made anode, the Nafion 117 membrane (Ion-Power, Inc.), and the commercial cathode (Electrochem, Inc.) at 6 bar and
. Electrochemical investigation methods, such as cyclic voltammetry (CV), chronoamperometry, and electrochemical impedance spectroscopy (EIS), were performed with a Princeton Applied Research potentiostat (type 273, EG&G) coupled with a frequency generator (type SI 1255, Schlumberger GmbH). MEAs were fabricated by pressing together the in-house-made anode, the Nafion 117 membrane (Ion-Power, Inc.), and the commercial cathode (Electrochem, Inc.) at 6 bar and  for 4 min and were tested in a
for 4 min and were tested in a  commercial DMFC purchased by Electrochem, Inc. The methanol and oxygen/nitrogen flow feeds was 10 and
commercial DMFC purchased by Electrochem, Inc. The methanol and oxygen/nitrogen flow feeds was 10 and  , respectively. The anode and cathode backpressures were varied between 1 and 3 bar.
, respectively. The anode and cathode backpressures were varied between 1 and 3 bar.
Results and Discussion
Characterization of the arc-discharge reaction products
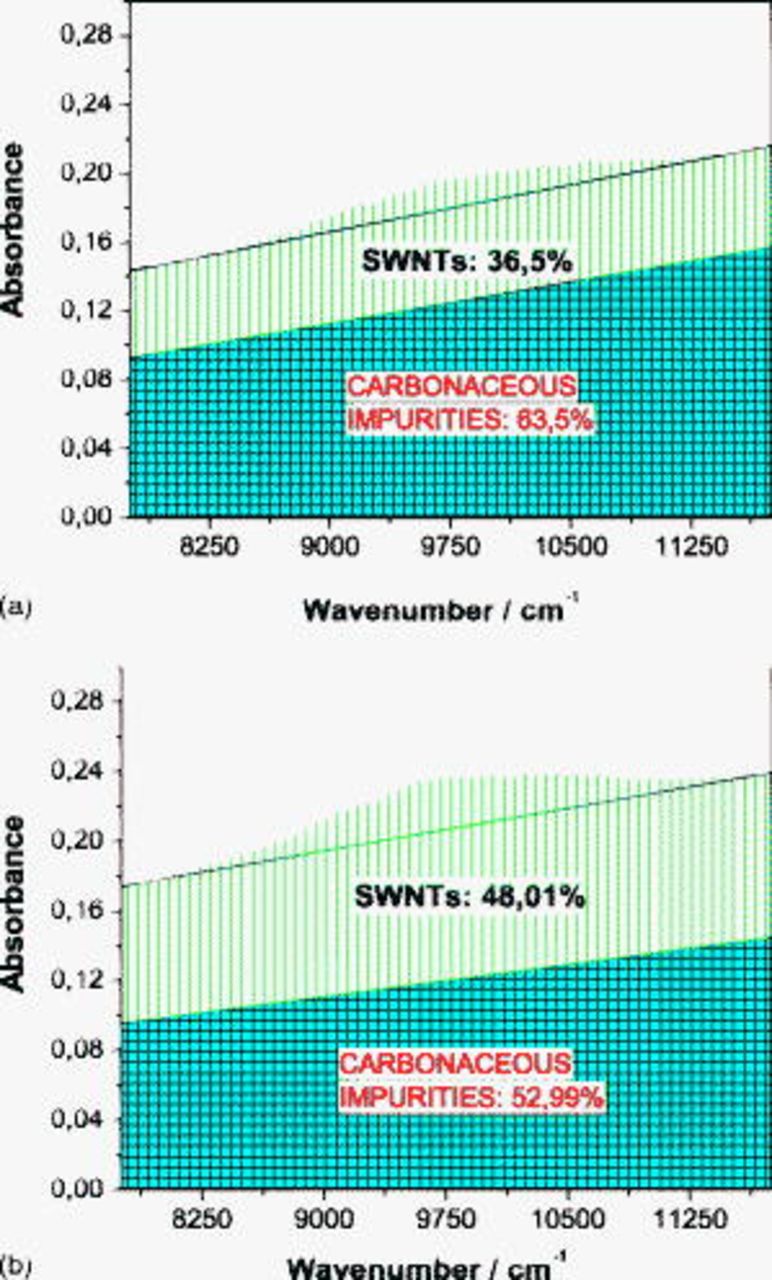
Both NIR spectra of the raw material (SWCNT1) and the material oxidized in air at  (SWCNT2) are shown in Fig. 2. For a quantitative determination of the purity grade with respect to SWCNTs, the absorption band related to the
(SWCNT2) are shown in Fig. 2. For a quantitative determination of the purity grade with respect to SWCNTs, the absorption band related to the  transition in the semiconducting tubes was selected according to the method suggested by Itkis et al.15 The average ratio of the CNTs in the raw soot (SWCNT1) was evaluated at about 36.5% (Fig. 2a). The purification of the raw material by heating in air led to an increase in the SWCNT fraction up to about 48% (Fig. 2b). At the same time, the relative amount of the metal content in SWCNT2 increased, as shown by the ICP analysis (see Table I), because amorphous carbon burned off. The treatment in HCl and
transition in the semiconducting tubes was selected according to the method suggested by Itkis et al.15 The average ratio of the CNTs in the raw soot (SWCNT1) was evaluated at about 36.5% (Fig. 2a). The purification of the raw material by heating in air led to an increase in the SWCNT fraction up to about 48% (Fig. 2b). At the same time, the relative amount of the metal content in SWCNT2 increased, as shown by the ICP analysis (see Table I), because amorphous carbon burned off. The treatment in HCl and  was supposed to remove metallic impurities. However, only the
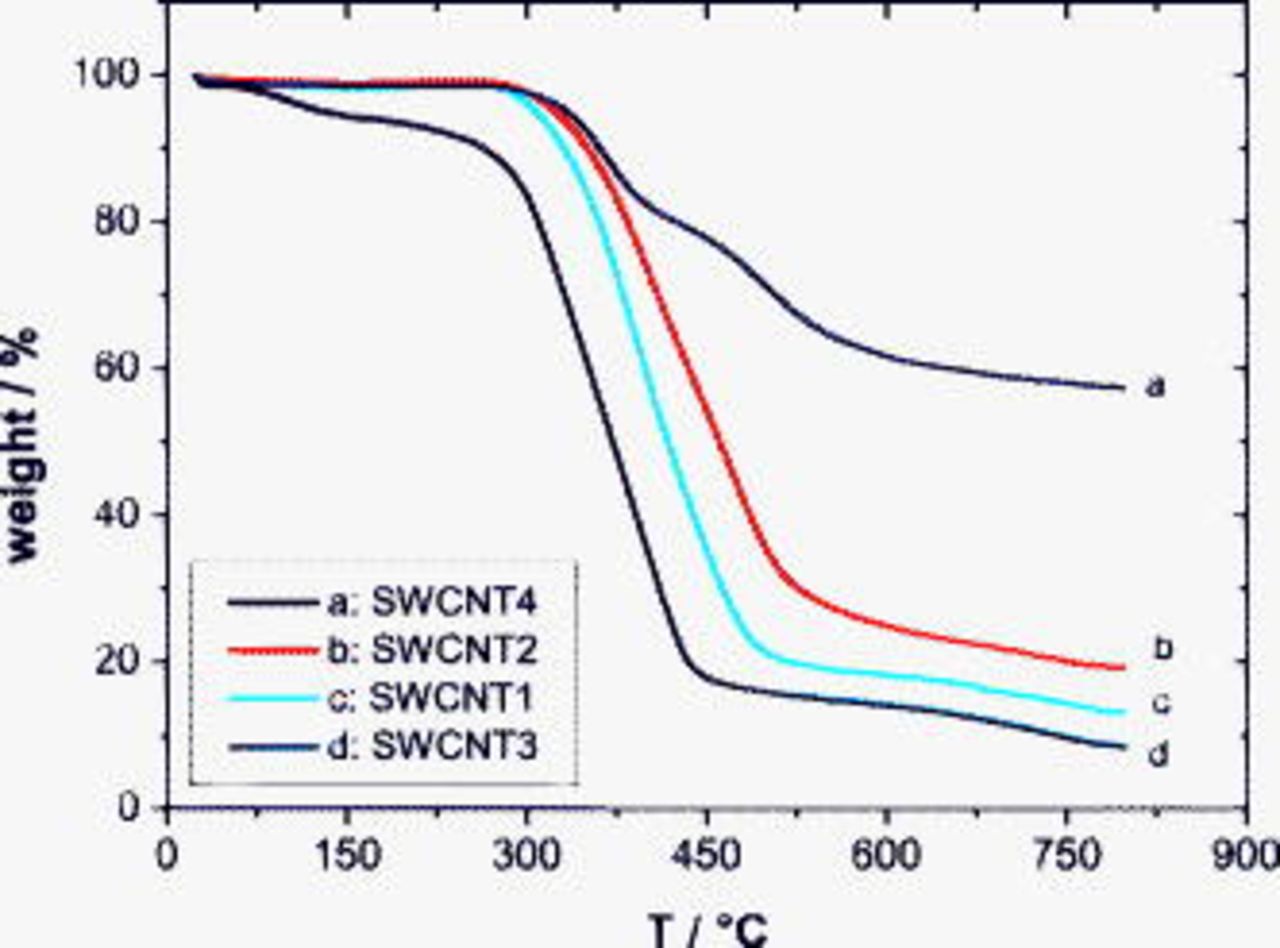
was supposed to remove metallic impurities. However, only the  treatment significantly decreased Ni and Y contents, which is due to the higher penetration of nitric acid into the graphitic layers that encapsulate the metal particles. A wet acid treatment caused a very strong entanglement of the SWCNTs. As a consequence, the chemically modified samples SWCNT3 and SWCNT4 were not dispersible in DMF and not measurable with NIR spectroscopy. The results of the TGA are presented in Fig. 3. SWCNT2 and SWCNT4 show higher thermal stability than the raw material, whereas the sample treated in
treatment significantly decreased Ni and Y contents, which is due to the higher penetration of nitric acid into the graphitic layers that encapsulate the metal particles. A wet acid treatment caused a very strong entanglement of the SWCNTs. As a consequence, the chemically modified samples SWCNT3 and SWCNT4 were not dispersible in DMF and not measurable with NIR spectroscopy. The results of the TGA are presented in Fig. 3. SWCNT2 and SWCNT4 show higher thermal stability than the raw material, whereas the sample treated in  exhibits the highest weight loss. An unusual high content of about 55 wt % for residual ash remains after heating sample 4 (see Table I). Because this sample was prepared from the same batch of pristine nanotube materials, the increased ash amount cannot be explained by the higher content of metallic impurities but is connected with the intrinsic properties of the sample. We have observed that oxidation in air followed by HCl treatment leads to a strong consolidation of the nanotube sample, indicating the formation of intertube covalent junctions. Attempts to disrupt or exfoliate the agglomerates even after a long-time tip sonication in a surfactant solution were not successful. The TGA delivers additional confirmation for the tight-binding nanotube agglomerates in SWCNT4. In comparison to the other three samples, it shows an enhanced thermal stability and a different decomposition behavior at the same experimental conditions (heating rate and oxygen flow rate). The shape of the weight loss curve reveals a two-step burning process where the second step expands over
exhibits the highest weight loss. An unusual high content of about 55 wt % for residual ash remains after heating sample 4 (see Table I). Because this sample was prepared from the same batch of pristine nanotube materials, the increased ash amount cannot be explained by the higher content of metallic impurities but is connected with the intrinsic properties of the sample. We have observed that oxidation in air followed by HCl treatment leads to a strong consolidation of the nanotube sample, indicating the formation of intertube covalent junctions. Attempts to disrupt or exfoliate the agglomerates even after a long-time tip sonication in a surfactant solution were not successful. The TGA delivers additional confirmation for the tight-binding nanotube agglomerates in SWCNT4. In comparison to the other three samples, it shows an enhanced thermal stability and a different decomposition behavior at the same experimental conditions (heating rate and oxygen flow rate). The shape of the weight loss curve reveals a two-step burning process where the second step expands over  . Even though the final temperature achieved
. Even though the final temperature achieved  , the strong bonded aggregates are likely thermally stable and likely contribute to the residual weight. A solid-state reaction of metallic impurities with carbon at an enhanced temperature (starting with
, the strong bonded aggregates are likely thermally stable and likely contribute to the residual weight. A solid-state reaction of metallic impurities with carbon at an enhanced temperature (starting with  ) and the formation of the much stable metal carbides are possible in that sample. The chemical treatment with
) and the formation of the much stable metal carbides are possible in that sample. The chemical treatment with  decreases the thermal stability of the SWNT3 sample. The TGA weight loss curve is shifted by about
decreases the thermal stability of the SWNT3 sample. The TGA weight loss curve is shifted by about  down to a lower temperature in comparison to the measurement of the raw sample. The decoration of the nanotubes by functional groups, such as COOH induced by oxidative acid, supports in this sample the burning process. The results obtained from NIR, ICP, and TGA analyses are summarized in Table I.
down to a lower temperature in comparison to the measurement of the raw sample. The decoration of the nanotubes by functional groups, such as COOH induced by oxidative acid, supports in this sample the burning process. The results obtained from NIR, ICP, and TGA analyses are summarized in Table I.
Figure 2. NIR spectrum of the (a) raw material and (b) sample oxidized in air at  .
.
Table I. Results of the sample analysis with characterization methods NIR, ICP, and TGA.
| Samples | NIR | ICP | TGA | |
|---|---|---|---|---|
| CNTs (%) | Ni (wt %) | Y (wt %) | Ash (wt %) | |
| SWCNT1/raw | 36 | 6.5 | 1.1 | 11 |
| SWCNT2/oxidized in air | 48 | 8.6 | 1.7 | 19 |
SWCNT3/treated with 
| — | 4.4 | 0.7 | 9 |
SWCNT4/oxidized in 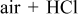
| — | 8.3 | 0.9 | 55 |
Figure 3. TGA curves of different SWCNT-containing samples.
TEM pictures
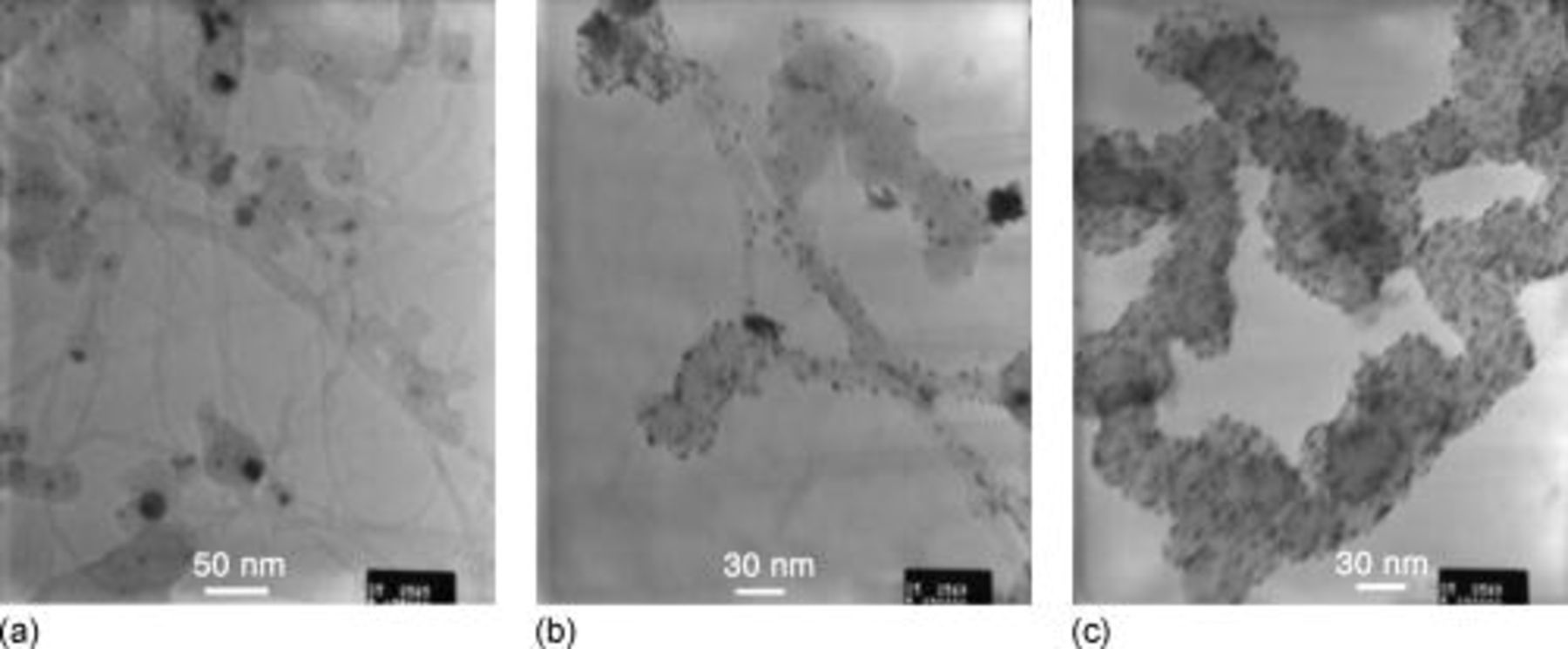
Figure 4 shows TEM pictures of CNT-based samples (a) without and (b) with Pt catalyst particles. For comparison, Pt on carbon Vulcan is presented in Fig. 4c. No isolated SWCNTs are distinctly visible on pictures in Fig. 4a and 4b. The CNT-based sample is heterogeneous and composed of CNT bundles, amorphous carbon, and numerous residual catalyst particles (Ni and Y), which confirms the previous analysis in the Results and Discussion section. Due to their larger diameter that can reach 30 nm compared to 2–7 nm for the Pt particles, residual Ni and Y particles are easily identifiable in Fig. 4a and 4b. The morphologies of CNTs and Vulcan particles differ substantially from each other. SWCNT bundles have quite a regular tubular form with a maximal diameter of about 10 nm, whereas the shape of Vulcan particles is similar to that of a pearl chain with elements varying in diameter from about 10–60 nm. Platinum particles are well dispersed on SWCNTs. Because the catalyst loading is the same for both systems, the density of the Pt particle is obviously higher on Vulcan and is close to saturation by a catalyst/support weight ratio of 60:100.
Figure 4. TEM pictures of CNTs (SWCNT2) (a) without Pt catalyst, (b) with Pt catalyst, and (c) with Pt–Vulcan. Both Pt/support ratios were 60:100.
Characterization of SWCNTs in the half-cell
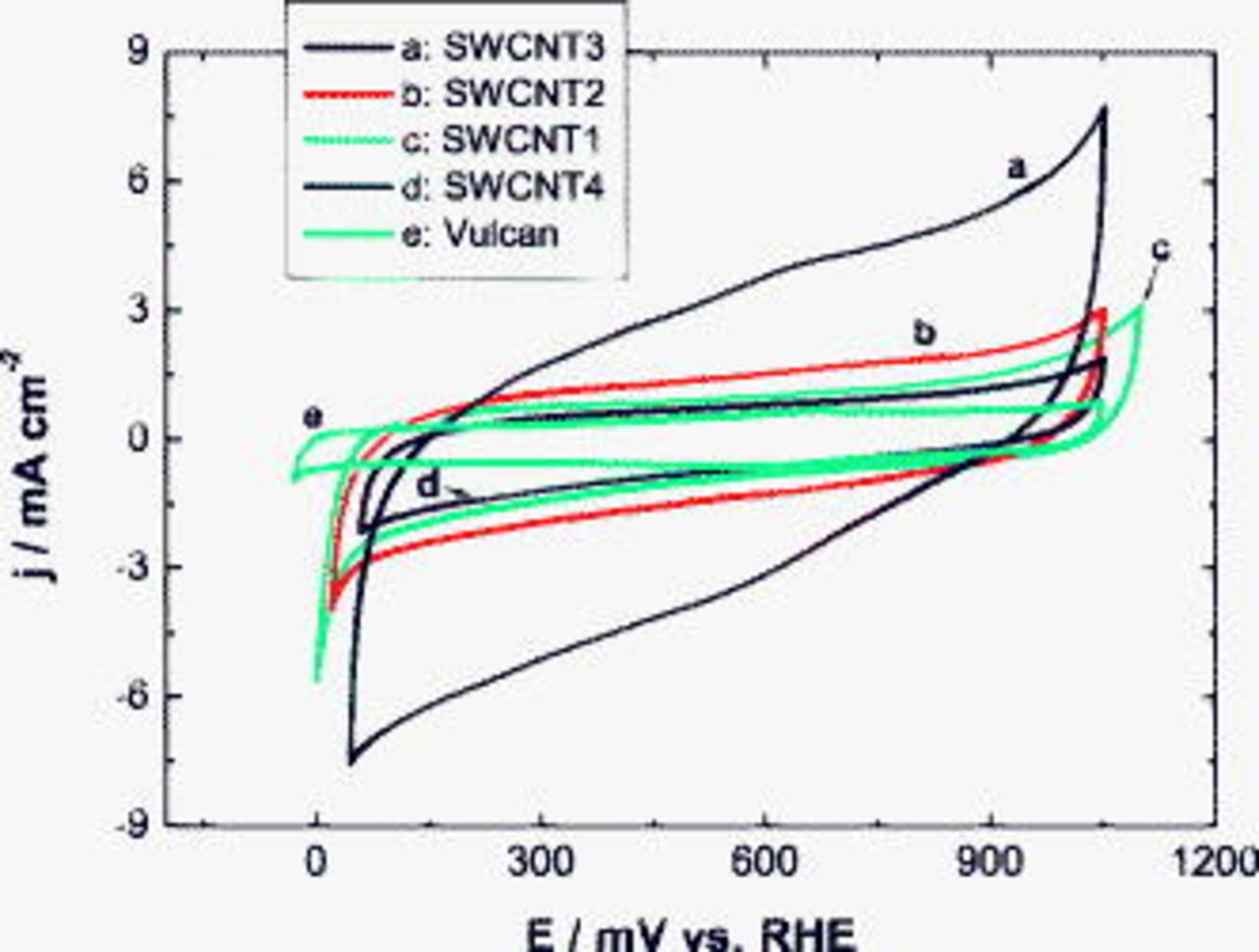
Figure 5 shows cyclic voltammograms of different chemically treated SWCNT GDEs under potentiostatic scanning conditions between hydrogen and oxygen evolution potentials in the  solution. Qualitatively, all SWCNT systems exhibit a similar behavior as carbon Vulcan, which is typical of anodic and cathodic charges of the double layer. The absence of Ni oxidation peaks is an indication of a complete encapsulation of the residual metal catalyst by a corrosion resistant carbon layer. Quantitatively, high current values are observable in the air and especially the nitric acid pretreated sample. By integrating the current over the measuring time, one obtains the charge value in coulomb that is proportional to the active area of the electrode material
solution. Qualitatively, all SWCNT systems exhibit a similar behavior as carbon Vulcan, which is typical of anodic and cathodic charges of the double layer. The absence of Ni oxidation peaks is an indication of a complete encapsulation of the residual metal catalyst by a corrosion resistant carbon layer. Quantitatively, high current values are observable in the air and especially the nitric acid pretreated sample. By integrating the current over the measuring time, one obtains the charge value in coulomb that is proportional to the active area of the electrode material  . Typical values of the specific charge of carbon found in literature are in the range of
. Typical values of the specific charge of carbon found in literature are in the range of  .16 In this work, a middle value of
.16 In this work, a middle value of  was used for the calculation of the active surfaces presented in Table II. It was also assumed that CNTs possess a similar double-layer capacity as carbon. After the chemical treatment in nitric acid, the charge and, consequently, specific active area of SWCNT-based samples increased by factors of 3 and 6 in comparison to the nontreated material and carbon Vulcan, respectively.
was used for the calculation of the active surfaces presented in Table II. It was also assumed that CNTs possess a similar double-layer capacity as carbon. After the chemical treatment in nitric acid, the charge and, consequently, specific active area of SWCNT-based samples increased by factors of 3 and 6 in comparison to the nontreated material and carbon Vulcan, respectively.
Figure 5. Cyclic voltammograms of [(a)–(d)] different SWCNTs and (e) carbon Vulcan in  at
at  and room temperature. The specific carbon loading was
and room temperature. The specific carbon loading was  for all the samples.
for all the samples.
Table II. Charge and corresponding active surface area of the carbon samples calculated from cyclic voltammograms in Fig. 5.
| SWCNT1 | SWCNT2 | SWCNT3 | SWCNT4 | Vulcan | |
|---|---|---|---|---|---|
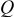 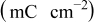
| 26.5 | 34.5 | 78 | 17.4 | 12.8 |
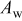 
| 1060 | 1380 | 3120 | 696 | 512 |
Characterization of Pt–SWCNTs in the half-cell and in the DMFC
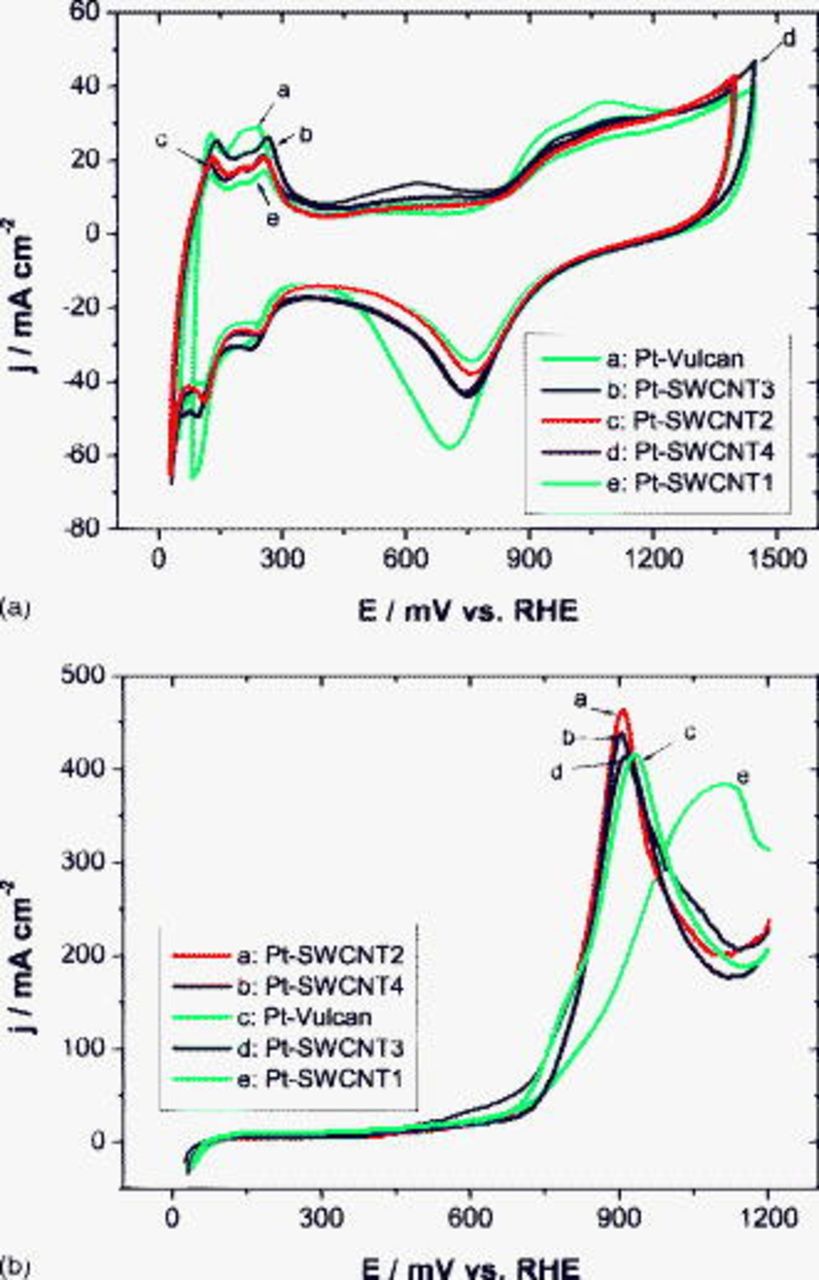
Even for DMFC applications, pre-experiment with pure platinum is a helpful step for the performance evaluation of systems toward methanol oxidation. Electrochemical characterization of Pt-decorated SWCNTs was carried out in the half-cell under potentiostatic conditions in a potential window delimited by hydrogen and oxygen evolution at room temperature. In Fig. 6a, one recognizes the typical behavior of carbon-supported platinum in a sulfuric acid solution for all samples including the nonchemically pretreated SWCNT1 one. Despite catalyst deposition efficiency close to 100% for all these samples, some disparities appear in the hydrogen desorption region that is a precious indicator for an effective catalyst surface. According to the charge of the hydrogen desorption peak, the Pt–Vulcan (103 mC) system should be the most active system followed by CNTs treated in nitric acid (82 mC) and in air (78 mC). The pristine CNT material (66 mC) is a priori the least active system. This is confirmed by the CV measurements in a methanol-containing solution (see Fig. 6b), which were carried out under half-cell conditions at room temperature. All systems exhibit a similar onset potential and a high activity for methanol oxidation lying in the range of  , and they behave quite similarly with the exception of the pristine CNT material that is obviously affected by a high ohmic influence probably due to the highest amount of impurities such as amorphous C, Ni, and Y. During the tests in the DMFC, the MEA with a nitric-acid-treated SWCNT anode showed the best performance, especially with air feeding (see Fig. 7b), followed by MEAs with an as-prepared Pt–Vulcan and an air-treated SWCNT anode. Analogous to measurements in the half-cell, the polarization curves of the MEA with the raw SWCNT1-based anode clearly lie below average.
, and they behave quite similarly with the exception of the pristine CNT material that is obviously affected by a high ohmic influence probably due to the highest amount of impurities such as amorphous C, Ni, and Y. During the tests in the DMFC, the MEA with a nitric-acid-treated SWCNT anode showed the best performance, especially with air feeding (see Fig. 7b), followed by MEAs with an as-prepared Pt–Vulcan and an air-treated SWCNT anode. Analogous to measurements in the half-cell, the polarization curves of the MEA with the raw SWCNT1-based anode clearly lie below average.
Figure 6. CVs of different Pt–SWCNT systems and of Pt–Vulcan (a) in  and (b) in
and (b) in  at
at  and room temperature.
and room temperature.
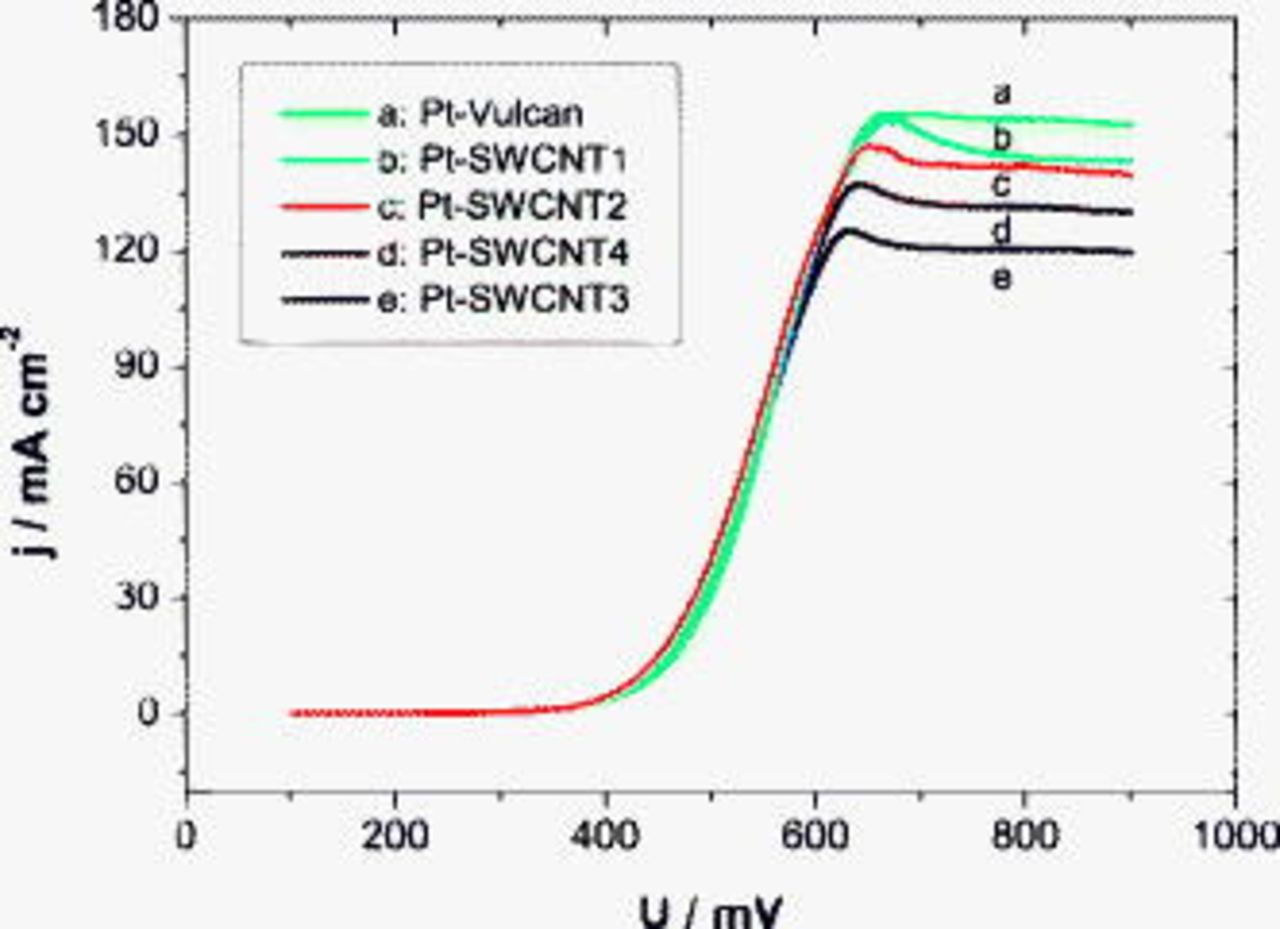
Figure 7. Polarization curves of MEAs with differently chemically treated SWCNT anodes in (a) pure oxygen and (b) air at  anodic and cathodic chamber pressures and
anodic and cathodic chamber pressures and  .
.
To study the influence of the SWCNT catalyst support on the methanol permeation within the composite MEA, the DMFC was connected in the electrolysis mode. After the correction of the electro-osmotic drag, a good approximation of the methanol flux through the Nafion membrane can be evaluated according to the method suggested by Ren et al.17 For this purpose, the cathode and the anode chambers were fed with a  solution and nitrogen gas, respectively. In the Vulcan-XC72-based electrodes, the methanol oxidation rate at the anode is usually limited by the methanol diffusion through the Nafion membrane. Because hydrogen evolution takes place in the cathode chamber fed with
solution and nitrogen gas, respectively. In the Vulcan-XC72-based electrodes, the methanol oxidation rate at the anode is usually limited by the methanol diffusion through the Nafion membrane. Because hydrogen evolution takes place in the cathode chamber fed with  , it can approximately be considered as a reference electrode. This method was primarily applied in this work to obtain some qualitative information about the influence of the SWCNT catalyst support on the methanol permeation within the MEA. Because all tested MEAs are made of the same commercial Nafion 117 membrane and the same commercial Pt–Vulcan electrode (anode in the electrolysis mode), every change in the kinetically controlled or diffusion-controlled region observed during methanol oxidation at the carbon-Vulcan-based electrode is a priori caused by the SWCNT-based counter electrode. In Fig. 8, the limiting current value of the methanol oxidation at the conventional Vulcan-based MEA amounts to
, it can approximately be considered as a reference electrode. This method was primarily applied in this work to obtain some qualitative information about the influence of the SWCNT catalyst support on the methanol permeation within the MEA. Because all tested MEAs are made of the same commercial Nafion 117 membrane and the same commercial Pt–Vulcan electrode (anode in the electrolysis mode), every change in the kinetically controlled or diffusion-controlled region observed during methanol oxidation at the carbon-Vulcan-based electrode is a priori caused by the SWCNT-based counter electrode. In Fig. 8, the limiting current value of the methanol oxidation at the conventional Vulcan-based MEA amounts to  and agrees with the value reported in Ref. 17. In this case, the limiting current is controlled by the methanol flux through the Nafion 117 membrane. The incorporation of CNTs obviously hinders the methanol transport through the reaction layer, which also depends on the CNT chemical treatment and the resulting porosity. MEAs with pristine SWCNT1 and with air/HCl-treated SWCNT4 behave quite similarly and exhibit a plateau at about
and agrees with the value reported in Ref. 17. In this case, the limiting current is controlled by the methanol flux through the Nafion 117 membrane. The incorporation of CNTs obviously hinders the methanol transport through the reaction layer, which also depends on the CNT chemical treatment and the resulting porosity. MEAs with pristine SWCNT1 and with air/HCl-treated SWCNT4 behave quite similarly and exhibit a plateau at about  , whereas the incorporation of SWCNTs treated with hot air or concentrated nitric acid causes further loss in the limiting current density of about 10 and
, whereas the incorporation of SWCNTs treated with hot air or concentrated nitric acid causes further loss in the limiting current density of about 10 and  , respectively. The lower methanol diffusion rate of about 22.5% observed at the MEA with a nitric-acid-treated SWCNT electrode should be responsible for the better performance of this system in the DMFC mode compared to that measured at the Vulcan-based one. All in-house-made electrodes contain 10 wt % Nafion. This judicious amount of Nafion in the reaction layer not only improves ionic transfer but also reduces the limiting current value of the methanol oxidation by about
, respectively. The lower methanol diffusion rate of about 22.5% observed at the MEA with a nitric-acid-treated SWCNT electrode should be responsible for the better performance of this system in the DMFC mode compared to that measured at the Vulcan-based one. All in-house-made electrodes contain 10 wt % Nafion. This judicious amount of Nafion in the reaction layer not only improves ionic transfer but also reduces the limiting current value of the methanol oxidation by about  .18
.18
Figure 8. Linear sweep voltammogram of the methanol oxidation at different MEAs with a commercial Pt–Vulcan anode in nitrogen atmosphere at  and
and  . DMFC is polarized in electrolysis mode. The legend refers to cathode material.
. DMFC is polarized in electrolysis mode. The legend refers to cathode material.
Characterization of PtRu–SWCNTs in the half-cell and in the DMFC
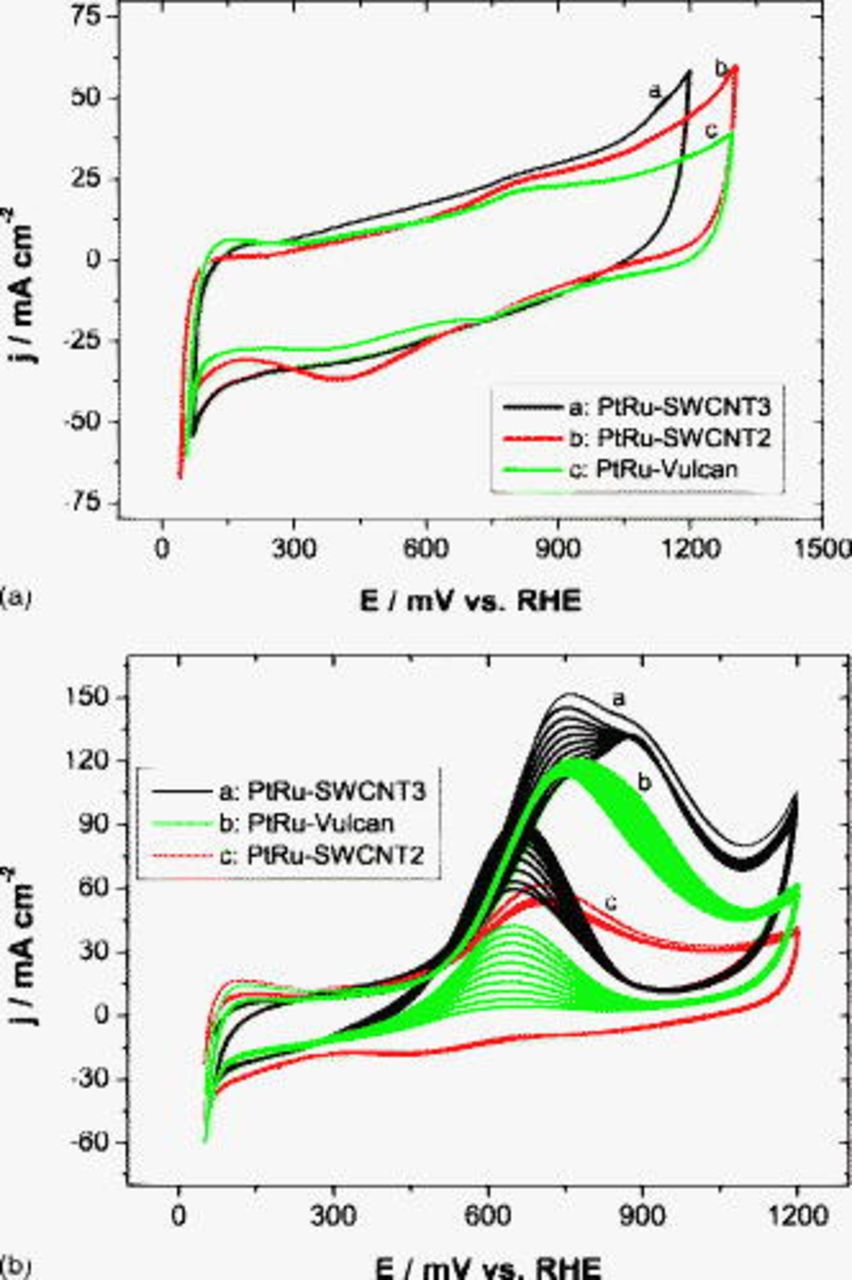
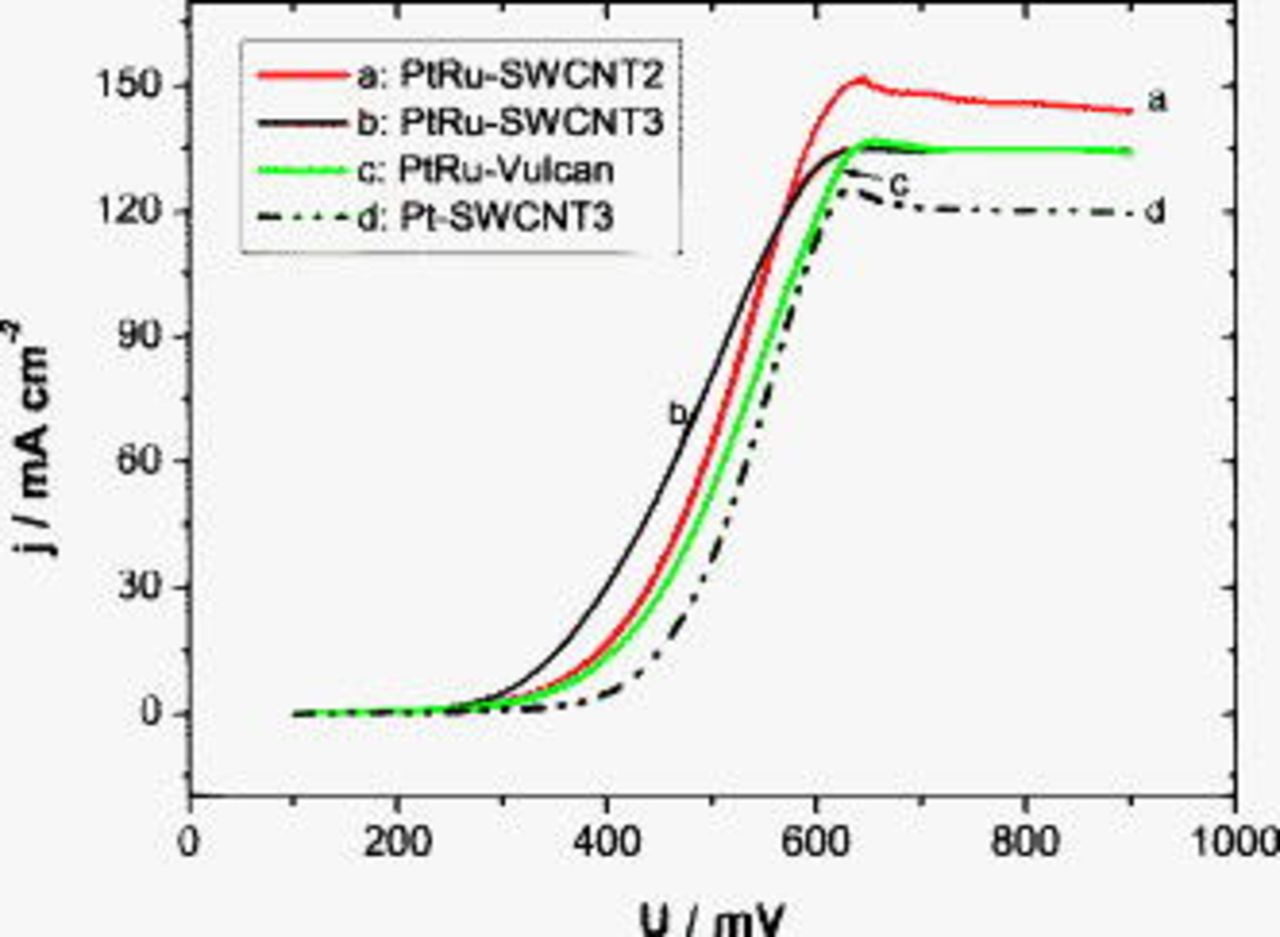
Because of the relative low activity of MEAs in nontreated (SWCNT1) and in HCl/air (SWCNT4)-treated CNT-based anodes, only results obtained at MEAs with anodes made of CNTs treated in hot air (SWCNT2) or immersed in concentrated  are shown. Analogous to the procedure used for the Pt-based anode, the activity of SWCNT-supported PtRu was first examined under half-cell conditions in a potential window limited by hydrogen and oxygen evolution at room temperature. Because ruthenium oxides are not chemically stable and undergo a strong corrosion rate at high positive potential values, only a few voltammograms were recorded in this potential window (see Fig. 9). The electrochemical behavior of PtRu on SWNT2 and SWNT3 in pure sulfuric acid contrasts with that observed at Pt in Fig. 6 and is, in this case, strongly dominated by ruthenium oxide properties. As a consequence, typical Pt characteristics, such as hydrogen and oxide regions, are quasi-inexistent, and methanol oxidation is partially inhibited, especially in the CNT sample treated in hot air. Moreover, the continuous decrease in the methanol oxidation rate during cycling is an indication of a diffusion-controlled process. This is obviously due to the additionally high amount of Ru that may obstruct mesopores and limits methanol diffusion and catalyst utilization. Nonetheless, after the activation period, high power densities of 157 and
are shown. Analogous to the procedure used for the Pt-based anode, the activity of SWCNT-supported PtRu was first examined under half-cell conditions in a potential window limited by hydrogen and oxygen evolution at room temperature. Because ruthenium oxides are not chemically stable and undergo a strong corrosion rate at high positive potential values, only a few voltammograms were recorded in this potential window (see Fig. 9). The electrochemical behavior of PtRu on SWNT2 and SWNT3 in pure sulfuric acid contrasts with that observed at Pt in Fig. 6 and is, in this case, strongly dominated by ruthenium oxide properties. As a consequence, typical Pt characteristics, such as hydrogen and oxide regions, are quasi-inexistent, and methanol oxidation is partially inhibited, especially in the CNT sample treated in hot air. Moreover, the continuous decrease in the methanol oxidation rate during cycling is an indication of a diffusion-controlled process. This is obviously due to the additionally high amount of Ru that may obstruct mesopores and limits methanol diffusion and catalyst utilization. Nonetheless, after the activation period, high power densities of 157 and  with pure oxygen and air as cathode feeds, respectively, were measured at the MEA with the hot-air-treated PtRu–SWCNT2 anode under DMFC conditions (see Fig. 10). Furthermore, in pure oxygen atmosphere, both MEAs with SWCNTs as a catalyst support exhibit a 10–15% higher performance than the MEA with a carbon-Vulcan-based anode.
with pure oxygen and air as cathode feeds, respectively, were measured at the MEA with the hot-air-treated PtRu–SWCNT2 anode under DMFC conditions (see Fig. 10). Furthermore, in pure oxygen atmosphere, both MEAs with SWCNTs as a catalyst support exhibit a 10–15% higher performance than the MEA with a carbon-Vulcan-based anode.
Figure 9. CVs of different Pt–SWCNT systems and Pt–Vulcan in (a)  and (b)
and (b)  at
at  and room temperature.
and room temperature.
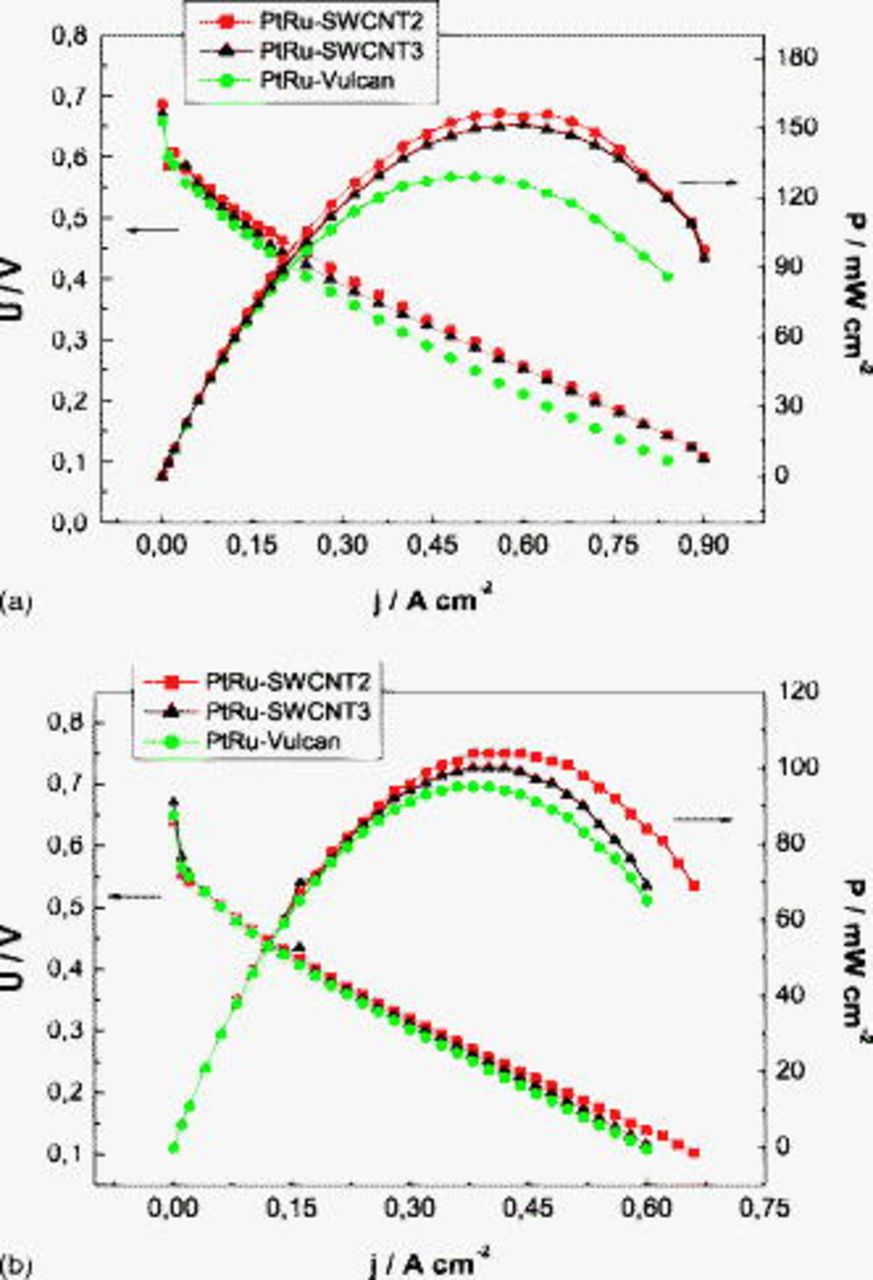
Figure 10. Polarization curves of MEAs with different chemically treated PtRu–SWCNT anodes in (a) pure oxygen and (b) air at  anodic and cathodic chamber pressures and
anodic and cathodic chamber pressures and  .
.
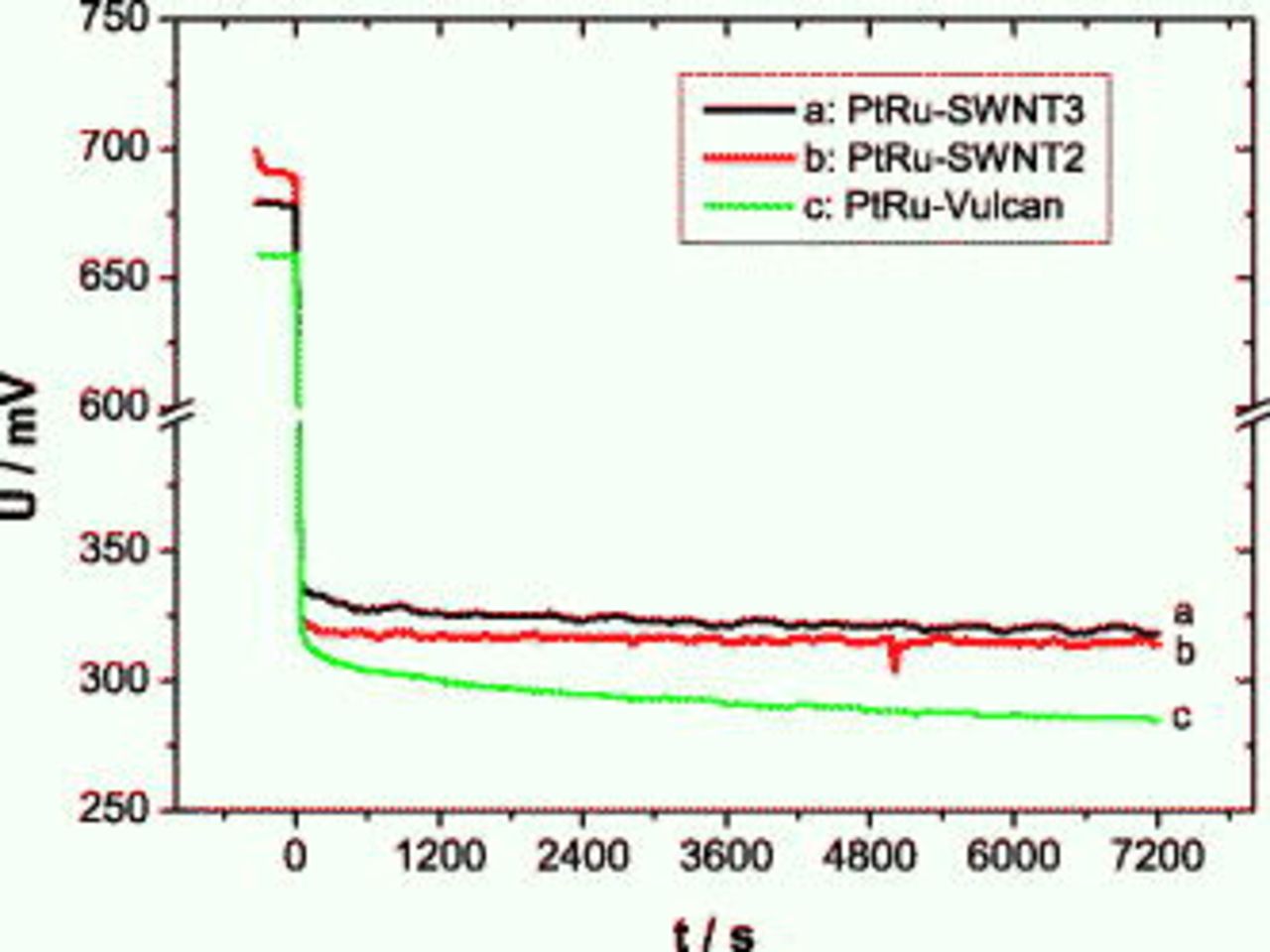
The chronopotentiometric measurements at a current density of  shown in Fig. 11 confirm the trend observed during the current–voltage experiments. The cell voltage of both MEAs with the SWCNT-based anode is pretty stable compared to that of the MEA with the carbon-Vulcan-based one. This can presumably be assigned to the higher specific area of the CNT catalyst support that favors methanol distribution and catalyst accessibility and allows simultaneous transport of
shown in Fig. 11 confirm the trend observed during the current–voltage experiments. The cell voltage of both MEAs with the SWCNT-based anode is pretty stable compared to that of the MEA with the carbon-Vulcan-based one. This can presumably be assigned to the higher specific area of the CNT catalyst support that favors methanol distribution and catalyst accessibility and allows simultaneous transport of  out of the reaction layer.
out of the reaction layer.
Figure 11. Polarization curves of different MEAs in the DMFC at  ,
,  methanol, oxygen back pressure, and
methanol, oxygen back pressure, and  .
.
The methanol permeation behavior of MEAs with PtRu-based cathodes (electrolysis mode), and more precisely linear sweep voltammograms of the methanol oxidation at commercial Pt–Vulcan cathodes, can be seen in Fig. 12. To facilitate the discussion about these experiments and their comparison with those carried out at Pt-based ones, the Pt–SWCNT3 curve from Fig. 8 was also embedded in Fig. 12. No direct correlation between the limiting current density value and the presence of CNTs in the PtRu cathode is discernible. Surprisingly, in the ruthenium-containing Pt–SWCNT3-based cathode, a  higher limiting current density and a 60 mV
higher limiting current density and a 60 mV  potential shift to a lower overpotential are observed in comparison to measurement at the pure Pt– SWCNT3-based MEA. The potential shift in the kinetically controlled region of methanol oxidation at Pt–Vulcan may be attributed to possible Ru migration from the counter electrode. Though the MEA with a PtRu–SWCNT2 exhibits the highest limiting current value of about
potential shift to a lower overpotential are observed in comparison to measurement at the pure Pt– SWCNT3-based MEA. The potential shift in the kinetically controlled region of methanol oxidation at Pt–Vulcan may be attributed to possible Ru migration from the counter electrode. Though the MEA with a PtRu–SWCNT2 exhibits the highest limiting current value of about  , this system shows the best performance in the DMFC. This can be explained by the more pronounced slope of the methanol oxidation in the kinetically controlled region probably due to a smaller ohmic overall resistance of PtRu–SWCNT2-containing MEA because all methanol oxidation measurements shown in Fig. 12 occurred at the same commercial Pt–Vulcan electrodes.
, this system shows the best performance in the DMFC. This can be explained by the more pronounced slope of the methanol oxidation in the kinetically controlled region probably due to a smaller ohmic overall resistance of PtRu–SWCNT2-containing MEA because all methanol oxidation measurements shown in Fig. 12 occurred at the same commercial Pt–Vulcan electrodes.
Figure 12. Linear sweep voltammogram of methanol oxidation at the commercial Pt–Vulcan anodes in nitrogen atmosphere at  ,
,  , and
, and  . The DMFC is polarized in electrolysis mode. The legend refers to the cathode material.
. The DMFC is polarized in electrolysis mode. The legend refers to the cathode material.
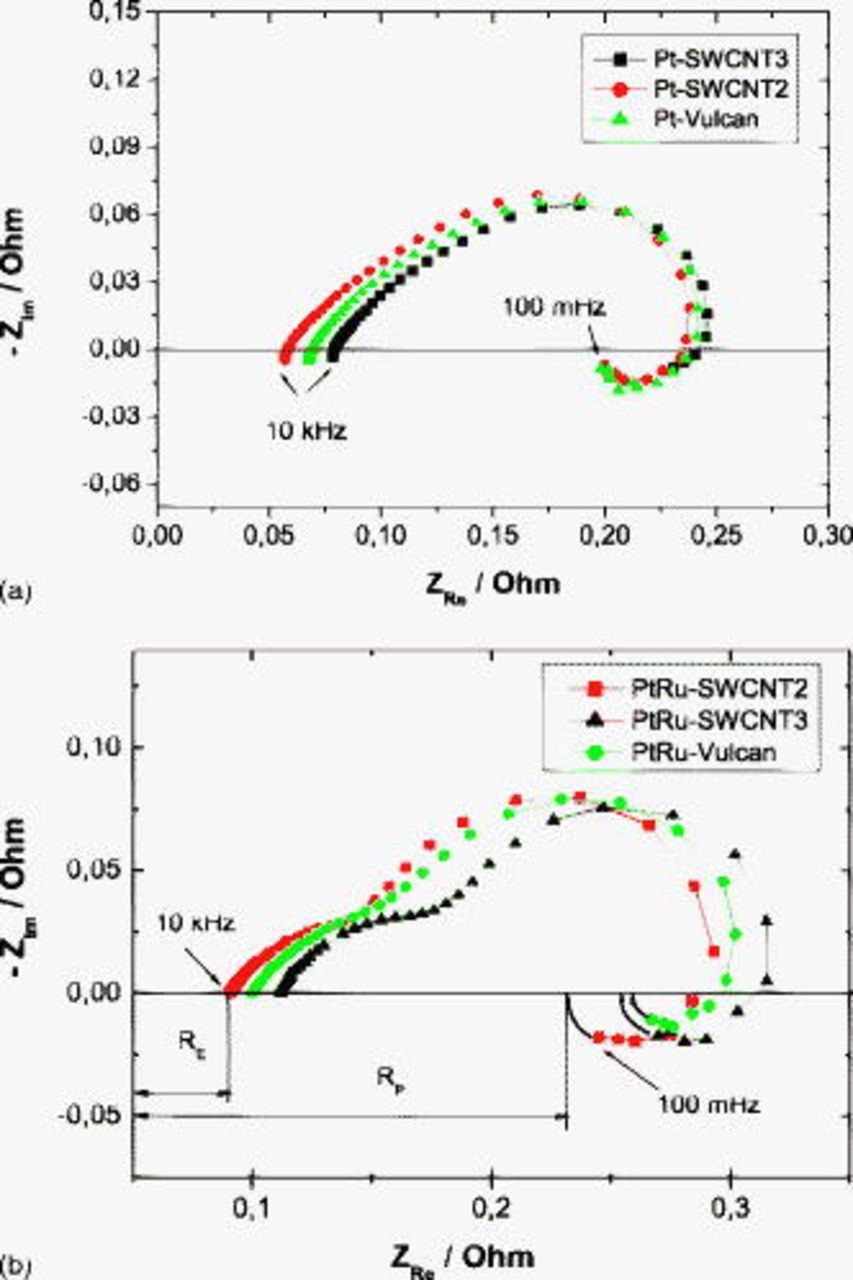
Impedance measurements
CNTs are well known for their high electronic conductivity. In this work, EIS was carried out at MEAs with different anode materials under polarization conditions at a current density of  . Because identical commercial membrane and cathode samples were used, changes in EIS spectra can be attributed to differences in the anode behavior. In the capacitive part in Fig. 13, two time constants are identifiable that can be ascribed to anodic methanol oxidation and cathodic oxygen reduction. The evaluation of EIS spectra is limited to the electrolyte and polarization resistances
. Because identical commercial membrane and cathode samples were used, changes in EIS spectra can be attributed to differences in the anode behavior. In the capacitive part in Fig. 13, two time constants are identifiable that can be ascribed to anodic methanol oxidation and cathodic oxygen reduction. The evaluation of EIS spectra is limited to the electrolyte and polarization resistances  and
and  , respectively, which are determined from the intercept with the origin of the imaginary axis in the Nyquist plot (see Fig. 13b). At high frequencies, the equivalent circuit of a DMFC is reduced to the ohmic resistance of the contact surfaces, electrodes, and membranes that are commonly described as
, respectively, which are determined from the intercept with the origin of the imaginary axis in the Nyquist plot (see Fig. 13b). At high frequencies, the equivalent circuit of a DMFC is reduced to the ohmic resistance of the contact surfaces, electrodes, and membranes that are commonly described as  . The effective geometrical electrode area is
. The effective geometrical electrode area is  . In Fig. 13a, the ohmic impedance values range from about 57–
. In Fig. 13a, the ohmic impedance values range from about 57– for MEAs with the Pt–SWCNT anode treated either in air or in nitric acid. In the MEAs with PtRu anodes in Fig. 13b, an increase of about
for MEAs with the Pt–SWCNT anode treated either in air or in nitric acid. In the MEAs with PtRu anodes in Fig. 13b, an increase of about  is observed, which can be explained by the presence of ruthenium in the reaction layer. Surprisingly, MEAs with SWCNT3 as the catalyst support exhibit about
is observed, which can be explained by the presence of ruthenium in the reaction layer. Surprisingly, MEAs with SWCNT3 as the catalyst support exhibit about  higher ohmic resistance than that measured at the Vulcan-based systems. Impurities, such as amorphous carbon and residual Ni and Y catalysts, seem to affect the overall resistance of the SWCNT-based electrodes. At low frequencies, the imaginary part of the impedance approaches the zero value, and the corresponding real part of the impedance is taken as the polarization resistance
higher ohmic resistance than that measured at the Vulcan-based systems. Impurities, such as amorphous carbon and residual Ni and Y catalysts, seem to affect the overall resistance of the SWCNT-based electrodes. At low frequencies, the imaginary part of the impedance approaches the zero value, and the corresponding real part of the impedance is taken as the polarization resistance  , whereas the inductive loop is ascribed to intermediate adsorptive species on the platinum catalyst, such as carbon monoxide19, 20 and furthermore to cathodic methanol oxidation. The
, whereas the inductive loop is ascribed to intermediate adsorptive species on the platinum catalyst, such as carbon monoxide19, 20 and furthermore to cathodic methanol oxidation. The  and
and  values are listed in Table III. MEAs with hot-air-treated SWCNT2 materials exhibit the lowest
values are listed in Table III. MEAs with hot-air-treated SWCNT2 materials exhibit the lowest  and
and  values, which confirms the hypothesis formulated in the preceding section for explaining the better performance of this system under DMFC conditions.
values, which confirms the hypothesis formulated in the preceding section for explaining the better performance of this system under DMFC conditions.
Figure 13. Nyquist spectra of different MEAs in the DMFC with (a) Pt- and (b) PtRu-based anodes at  ,
,  methanol, oxygen back pressure, and
methanol, oxygen back pressure, and  . The legend refers to the anode material.
. The legend refers to the anode material.
Table III. List of  and
and  values from Fig. 13a and 13b.
values from Fig. 13a and 13b.
| Pt–SWCNT2 | Pt–SWCNT3 | Pt–Vulcan | PtRu–SWCNT2 | PtRu–SWCNT3 | PtRu–Vulcan | |
|---|---|---|---|---|---|---|
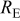 
| 57 | 78 | 68 | 90 | 111 | 100 |
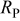 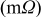
| 200 | 200 | 215 | 232 | 254 | 255 |
aExtrapolated value of the inductive half-circle onto the zero line of  for low frequencies.
for low frequencies.
Conclusion
SWCNTs were produced by the arc-discharge method and were chemically treated to enhance the purity, the active surface area, and the efficiency for catalyst anchoring. The composition and morphology of the CNTs were analyzed by means of NIR, ICP, TGA, and TEM methods. Treatment in hot air at  led to an increase in CNT purity level up to 48%. After immersion in concentrated nitric acid, a partial dissolution of the residual nickel and yttrium catalysts from the fabrication process as well as an impressive increase in surface area by a factor of 3 were achieved. The electrochemical characterization of SWCNT as Pt and PtRu catalyst support was carried out with CV, EIS, polarization, and methanol permeation measurements under half-cell and fuel cell conditions. Both chemical treatments of SWCNTs in hot air and in concentrated nitric acid led to a 10–15% higher performance in the DMFC, especially if PtRu is used as a catalyst compared to that of a MEA with a Vulcan-supported PtRu anode. However, more experimental and simulation data are still necessary to ponder more precisely the contribution of the chemical treatment of the SWCNT on the methanol crossover, catalyst utilization, and impedance behavior under fuel cell conditions.
led to an increase in CNT purity level up to 48%. After immersion in concentrated nitric acid, a partial dissolution of the residual nickel and yttrium catalysts from the fabrication process as well as an impressive increase in surface area by a factor of 3 were achieved. The electrochemical characterization of SWCNT as Pt and PtRu catalyst support was carried out with CV, EIS, polarization, and methanol permeation measurements under half-cell and fuel cell conditions. Both chemical treatments of SWCNTs in hot air and in concentrated nitric acid led to a 10–15% higher performance in the DMFC, especially if PtRu is used as a catalyst compared to that of a MEA with a Vulcan-supported PtRu anode. However, more experimental and simulation data are still necessary to ponder more precisely the contribution of the chemical treatment of the SWCNT on the methanol crossover, catalyst utilization, and impedance behavior under fuel cell conditions.
Acknowledgments
The authors thank the Deutsche Forschungsgemeinschaft (DFG) for financial support (JU201/12-1) and Dr. E. Gyenge from the University of British Columbia in Vancouver for reading this manuscript.
Karl Winnacker Institute assisted in meeting the publication costs of this article.